Abstract
A clinically important adverse drug reaction, heparin-induced thrombocytopenia (HIT), is induced by antibodies specific for complexes of the chemokine platelet factor 4 (PF4) and the polyanion heparin. Even heparin-naive patients can generate anti-PF4/heparin IgG as early as day 4 of heparin treatment, suggesting preimmunization by antigens mimicking PF4/heparin complexes. These antibodies probably result from bacterial infections, as (1) PF4 bound charge-dependently to various bacteria, (2) human heparin-induced anti-PF4/heparin antibodies cross-reacted with PF4-coated Staphylococcus aureus and Escherichia coli, and (3) mice developed anti-PF4/heparin antibodies during polymicrobial sepsis without heparin application. Thus, after binding to bacteria, the endogenous protein PF4 induces antibodies with specificity for PF4/polyanion complexes. These can target a large variety of PF4-coated bacteria and enhance bacterial phagocytosis in vitro. The same antigenic epitopes are expressed when pharmacologic heparin binds to platelets augmenting formation of PF4 complexes. Boosting of preformed B cells by PF4/heparin complexes could explain the early occurrence of IgG antibodies in HIT. We also found a continuous, rather than dichotomous, distribution of anti-PF4/heparin IgM and IgG serum concentrations in a cross-sectional population study (n = 4029), indicating frequent preimmunization to modified PF4. PF4 may have a role in bacterial defense, and HIT is probably a misdirected antibacterial host defense mechanism.
Introduction
The chemokine platelet factor 4 (PF4, CXCL4) is stored within platelet α-granules1 and released during platelet activation. Although its biologic role is poorly understood, PF4 commands attention in clinical medicine because it binds charge-dependently to the anticoagulant heparin, one of the most frequently used anticoagulants in clinical medicine, thereby neutralizing heparin's anticoagulant effect,2,3 but also forming highly antigenic multimolecular complexes.4-6 The resulting antibody response7 induces the most frequent immune-mediated adverse drug reaction involving human blood cells, heparin-induced thrombocytopenia (HIT).8 The pathogenic antibodies bind to PF4/heparin complexes at the platelet surface, and the resulting immune complexes induce Fc- receptor–mediated platelet activation and enhanced thrombin generation.8 In a subset of patients, this causes thrombocytopenia and triggers paradoxical thrombosis, which is aggravated by continuation of heparin treatment.
The immune response of HIT has several atypical features. Even in patients who receive heparin for the first time, there is rapid induction (as early as 4 days) of anti-PF4/heparin antibodies of IgG isotype. Moreover, IgG antibodies are the predominant class of immunoglobulins formed.9,10 We and others discovered that up to 50% of patients after cardiopulmonary bypass surgery and 20% to 30% of orthopedic surgery patients (many of whom have not been previously exposed to heparin) developed anti-PF4/heparin IgG antibodies in the second week after heparin exposure.11-13
These findings are unusual for a primary immune response, in which predominant formation of IgM, followed by later and weaker response of IgG, would be expected. In addition, serum from apparently healthy persons can contain anti-PF4/heparin antibodies, even without previous exposure to pharmacologic heparin.14,15 Recently “spontaneous” HIT has been described, in which patients develop an illness clinically and serologically mimicking HIT with strongly reacting anti-PF4/heparin IgG antibodies in the absence of any exposure to heparin.16-18 All these clinical observations suggest exposure to the antigen induced by a binding partner of PF4 different from pharmacologic heparin.
It is known that anti-PF4/heparin antibodies are not heparin-specific, as they also recognize PF4 bound to certain other polyanions in vitro.19-21 Anti-PF4/heparin antibodies can also be induced in vivo by certain nonheparin polyanions, such as hypersulfated chondroitin sulfate.22 These observations raise the possibility that in HIT the immune system might have encountered PF4 complexes earlier, perhaps because of binding of PF4 to naturally occurring nonheparin polyanions. It was therefore an intriguing observation that 3 of the 5 reported patients with the spontaneous HIT syndrome had histories of recent bacterial infection. The cell surface of bacteria could be a potential natural source for PF4-binding polyanions.23,24 In this study, we assessed whether PF4 can bind to bacteria, thereby exposing the epitope recognized by antibodies induced in patients during heparin treatment, and whether bacteria can induce anti-PF4/heparin antibodies in a mouse model.
Methods
Bacterial strains
Escherichia coli JM109 (Promega), Neisseria meningitidis MC58ΔsiaD,25 Staphylococcus aureus SA113spa (protein A–deficient strain, provided by Dr Goerke, Universität Tübingen, Tübingen, Germany), Streptococcus pneumoniae NCTC 10319 (serotype 35A),26 and Listeria monocytogenes L028 Sr 1/2c27 were grown in Todd-Hewitt broth (0.5% yeast extract; Roth) until mid-log phase.28 Fluorescein isothiocyanate (FITC)–labeled E coli (K-12 strain) were obtained from Invitrogen.
PF4 biotinylation
We biotinylated human (hu)-PF4 from platelets and recombinant murine (mu)-PF4 (Chromatec GmbH) as described.29 Briefly, Heparin Sepharose (GE Healthcare) was washed 3 times with phosphate-buffered saline (PBS), pH 7.4, mixed with PF4 for 15 minutes, and then left overnight at 4°C. The PF4-heparin-Sepharose suspension was mixed and incubated with biotin-XX (1 hour at room temperature; Invitrogen), washed (0.8M NaCl, 20mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 5mM ethylenediaminetetraacetic acid, pH 7.4), and biotinylated PF4 eluted with high salt buffer (2M NaCl, 20mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 5mM ethylenediaminetetraacetic acid, pH 7.4). The concentration of biotinylated PF4 was determined by a bicinchoninic acid protein assay kit using bovine serum albumin as standard (Sigma-Aldrich).
PF4 binding to bacteria
We incubated E coli, N meningitidis, S aureus, S pneumoniae, and L monocytogenes (30 minutes; 4°C) with biotinylated hu-PF4 (0, 1.25, 2.5, 5, 10, 20, and 40 μg/mL) or mu-PF4 (E coli, S aureus), washed the bacteria (PBS + 0.05% bovine albumin; 3000g, 5 minutes, 4°C), incubated the bacteria (30 minutes; 4°C) with peridinin chlorophyll protein-Cy5.5 conjugated streptavidin (BD Biosciences), washed them again, and fixed them (1% paraformaldehyde; 20 minutes, 4°C). We analyzed PF4 binding by flow cytometry (Cytomics FC 500, Beckman Coulter). The geometric mean fluorescence intensity multiplied by the percentage of labeled bacteria constituted binding activity. As a control, we also assessed binding of nonbiotinylated PF4 to bacteria, which was visualized with an FITC-labeled polyclonal rabbit anti–hu-PF4 antibody.30 Results did not differ from the ones obtained with biotinylated PF4.
In addition, we tested biotinylated hu-PF4 binding (20 μg/mL) to S aureus SA113spa in the presence of increasing concentrations (0, 12.5, 25, 50, and 100 μg/mL) of heparin (150 IU/mg; Braun) or dextran sulfate (Sigma-Aldrich).
PF4/heparin antibody assays
We performed the hu-PF4/heparin-enzyme immunoassay (EIA) with human sera of healthy volunteers, of patients who were immunized during heparin treatment and with eluates and adsorbates of PF4-coated or uncoated bacteria as described.31
In addition, we analyzed mouse sera using recombinant mu-PF4/heparin complexes as antigen and the respective mouse antibody specific conjugates: goat anti–mouse IgG Fc-specific peroxidase (Sigma-Aldrich) or goat anti–mouse IgM μ-chain–specific peroxidase (Dianova). All sera were tested for inhibition by soluble excess unfractionated heparin (100 IU/mL), which disrupts PF4/heparin complexes and for binding to PF4 alone.
The heparin-induced platelet activation (HIPA) test was performed as described.32
Antibody purification (adsorption and elution experiments)
We preincubated S aureus SA113spa or E coli JM109 with hu-PF4 (20 μg/5 × 107 bacteria, 30 minutes, 4°C) or buffer (PBS, pH 7.4), washed (3000g, 5 minutes, 4°C) and incubated (30 minutes, 4°C) them with diluted human serum of patients known to contain anti-PF4/heparin IgG antibodies (n = 5 per strain), and washed them again to remove unbound antibodies. We eluted bound antibodies with glycine buffer (0.1M, pH 2.7; 5 minutes at room temperature), centrifuged the bacteria, and neutralized the supernatant (eluate) with Tris buffer (1M, pH 9). We tested untreated sera and eluates by hu-PF4/heparin IgG EIA and HIPA test. To exclude nonspecific antibody binding, we also processed human sera containing anti–human leukocyte antigens–class I antibodies (n = 1), anti–human platelet antigen-1a alloantibodies (n = 2), and an anti-glycoprotein Ib/IX platelet autoantibody (n = 1) identically and measured IgG binding of the eluates to human platelets expressing the respective antigens assessed by flow cytometry and by a glycoprotein-specific EIA, the monoclonal antibody immobilization of platelet antigens method monoclonal antibody–specific immobilization of platelet antigens.33
Phagocytosis assay
This assay was modified from the phagotest kit (Orpegen Pharma). We diluted heat-inactivated (56°C, 45 minutes) human sera (1:50) of patients containing anti-PF4/heparin IgG antibodies or control sera and preadsorbed them with E coli–FITC (non–PF4-coated; 15 minutes, 37°C; 4 times), incubated the preadsorbed sera (n = 4) with hu-PF4–coated or –noncoated E coli–FITC (16 hours, 4°C), and then incubated (10 minutes, 37°C) the bacteria with whole blood cells, which we obtained by washing hirudinized blood of healthy volunteers twice (PBS; 300g, 5 minutes, 4°C) and then incubated on ice for 10 minutes. We quenched fluorescent signals from E coli–FITC attached to the cell surface with trypan blue (5 minutes, ice bath; Sigma-Aldrich). After this incubation step, we washed the cells twice and lysed the erythrocytes with FACS lysing solution (7 minutes at room temperature; BD Biosciences), washed the remaining cells with PBS (300g, 5 minutes, 4°C), stained their DNA with propidium iodide (10 minutes, ice bath; Fluka BioChemica), and analyzed the cells by flow cytometry. We recorded the mean fluorescence intensity (MFI) of FITC-positive polymorphonuclear leukocytes (PMNs) as a measure for bacterial phagocytosis.
Polymicrobial sepsis model
We implanted an 18-G stent (Ohmeda AB) into the ascending colon of ketamine-anesthetized female C57BL/6 mice (age 7 weeks; Charles River), which induced colon ascendens stent peritonitis (CASP) by allowing controlled leakage of feces into the abdominal cavity.34,35 At day 1, 3, 7, and 14 after CASP, we drew blood from the retroorbital sinus. In sham operations, we fixed the stent outside the ascending colon and took blood at days 1, 7, and 14.
SHIP
Study of Health in Pomerania (SHIP) is a cross-sectional population-based survey conducted in northeast Germany.36 Details of the population assessed in this study are given elsewhere.37 Of 4310 participants, we assessed the sera of 4029 persons of whom serum was available by hu-PF4/heparin-EIA. Heparin administration or any in-hospital treatment within the last 12 months before blood drawing was excluded for all participants. This time period is sufficient to exclude antibody persistence induced by earlier application of pharmacologic heparin, as anti-PF4/heparin antibodies usually disappear within 3 to 6 months after the immune response.38
Statistical analysis
We calculated differences between the eluates of PF4 and buffer-preincubated bacteria in the adsorption/elution experiments by paired t test. For the phagocytosis assay, we compared samples by paired t test. We analyzed antibody production in the mouse model using Wilcoxon rank-sum test. All samples tested in PF4/heparin-EIA were compared with their reaction in the presence of excess heparin and in binding to PF4 alone using paired t test. P less than .05 was considered statistically significant.
Ethics
All volunteers gave written informed consent in accordance with the Declaration of Helsinki. All experiments were performed according to the German animal safety regulations and approved by the local animal protection authority.
Results
PF4 binding to bacteria
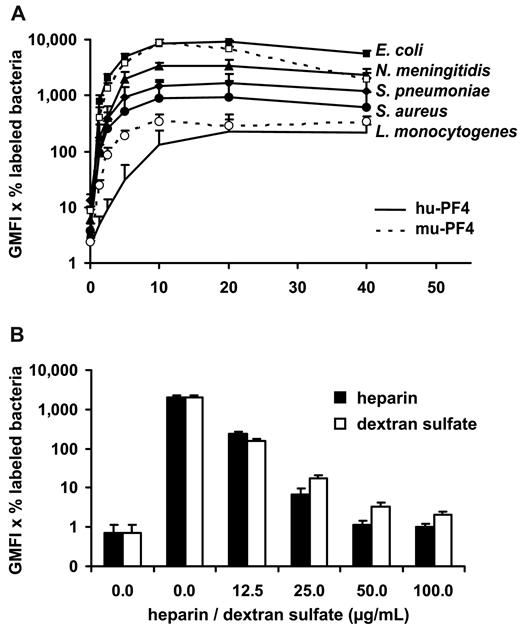
As bacteria display negatively charged molecules on their surfaces, we assumed that these molecules could be binding partners for PF4. We found that hu-PF4 bound in a dose-dependent manner to Gram-positive bacteria, such as S aureus, S pneumoniae, and L monocytogenes, and Gram-negative bacteria, such as E coli and N meningitidis. Saturation of binding was reached at 20 μg/mL (n = 3 each) of PF4 (Figure 1A), with E coli showing the highest binding capacity. Similarly to hu-PF4, mu-PF4 bound to S aureus and E coli (Figure 1A). Binding of hu-PF4 to S aureus was inhibited by heparin (n = 3) and dextran sulfate (n = 3), suggesting a charge-dependent interaction between PF4 and the bacteria (Figure 1B).
PF4 binds to Gram-positive (S aureus, S pneumoniae, and L monocytogenes) and Gram-negative bacteria (E coli and N meningitidis). (A) PF4 binding to Gram-positive and Gram-negative bacteria is dose-dependent and saturates at 20 μg/mL. Binding capacity for PF4 differs between bacterial strains, with E coli showing the highest binding capacity. Each bacterial strain was incubated with increasing concentrations of biotinylated hu-PF4 (solid symbols, all bacteria) or mu-PF4 (open symbols, E coli and S aureus). PF4 binding was detected with peridinin chlorophyll protein–Cy5.5-labeled streptavidin using flow cytometry and expressed as geometric mean fluorescence intensity (GMFI) multiplied by the percentage of labeled bacteria. Data are mean ± SD of 3 independent experiments. (B) PF4 binding to S aureus SA113spa is inhibited in a dose-dependent manner by heparin and dextran sulfate demonstrating charge dependency. S aureus SA113spa was incubated with buffer or with 20 μg/mL biotinylated hu-PF4 alone or in the presence of increasing concentrations of heparin or dextran sulfate (0, 12.5, 25, 50, and 100 μg/mL). PF4 binding was detected with peridinin chlorophyll protein–Cy5.5-labeled streptavidin using flow cytometry and expressed as GMFI multiplied by the percentage of labeled bacteria. Data are mean ± SD of 3 independent experiments.
PF4 binds to Gram-positive (S aureus, S pneumoniae, and L monocytogenes) and Gram-negative bacteria (E coli and N meningitidis). (A) PF4 binding to Gram-positive and Gram-negative bacteria is dose-dependent and saturates at 20 μg/mL. Binding capacity for PF4 differs between bacterial strains, with E coli showing the highest binding capacity. Each bacterial strain was incubated with increasing concentrations of biotinylated hu-PF4 (solid symbols, all bacteria) or mu-PF4 (open symbols, E coli and S aureus). PF4 binding was detected with peridinin chlorophyll protein–Cy5.5-labeled streptavidin using flow cytometry and expressed as geometric mean fluorescence intensity (GMFI) multiplied by the percentage of labeled bacteria. Data are mean ± SD of 3 independent experiments. (B) PF4 binding to S aureus SA113spa is inhibited in a dose-dependent manner by heparin and dextran sulfate demonstrating charge dependency. S aureus SA113spa was incubated with buffer or with 20 μg/mL biotinylated hu-PF4 alone or in the presence of increasing concentrations of heparin or dextran sulfate (0, 12.5, 25, 50, and 100 μg/mL). PF4 binding was detected with peridinin chlorophyll protein–Cy5.5-labeled streptavidin using flow cytometry and expressed as GMFI multiplied by the percentage of labeled bacteria. Data are mean ± SD of 3 independent experiments.
Binding of anti-PF4/heparin antibodies to PF4-coated bacteria
To assess whether human anti-PF4/heparin antibodies bind to PF4/polyanion complexes on the bacterial surface, we used hu-PF4–coated bacteria to affinity purify (by adsorption and elution) IgG antibodies of sera (n = 5) from patients with HIT. These patients were known to contain anti-PF4/heparin IgG antibodies. After 3 or 4 adsorption steps, anti-PF4/heparin antibodies were depleted from the human sera (Figure 2A-B insets). The IgG antibodies eluted from hu-PF4-coated bacteria reacted with hu-PF4/heparin complexes in the PF4/heparin-EIA (Figure 2). All controls showed the expected results (Figure 2): (1) the affinity-purified antibodies did not react with hu-PF4 alone; (2) excess heparin (100 IU/mL), which disrupts PF4/heparin complexes, inhibited anti-PF4/heparin antibody binding; (3) non–PF4-coated bacteria did not bind the anti-PF4/heparin antibodies; and (4) platelet-reactive antibodies with other specificities (antihuman leukocyte antigens-class I antibodies, n = 1; anti–human platelet antigen-1a alloantibodies, n = 2; and an anti–glycoprotein Ib/IX platelet autoantibody, n = 1) were not affinity-purified by PF4-coated bacteria. The eluates of PF4-coated bacteria incubated with these antiplatelet antibodies showed negligible IgG binding to platelets as analyzed by flow cytometry (1.6%–4.2% binding of the original serum) and all optical densities (ODs) were below the cutoff of 0.2 in the glycoprotein specific EIA.
Anti-PF4/heparin IgG from sera of patients with HIT binds to PF4-coated bacteria but not to bacteria alone. From sera of 5 patients with HIT, known to contain anti-PF4/heparin antibodies, IgG antibodies were affinity purified using hu-PF4-precoated bacteria. (Insets) Depletion of the anti-PF4/heparin antibodies from the original serum (n = 5) by sequential incubation with hu-PF4–coated bacteria (mean ± SD). In the main panels, each symbol represents the reactivity of the affinity-purified antibodies of one serum; mean values are given as horizontal lines. Reactivities of antibodies affinity purified by hu-PF4-coated E coli JM 109 (A) or by hu-PF4-coated S aureus SA113spa (B). Reactivity of the purified antibodies with hu-PF4/heparin complexes (first column) was inhibited by excess heparin (100 IU/mL unfractionated heparin [UFH], column 2), which disrupts PF4/heparin complexes. Accordingly, antibodies did also not react with hu-PF4 alone (column 3). Non–PF4-coated bacteria served as control for unspecific binding of the antibodies to bacteria alone (column 4).
Anti-PF4/heparin IgG from sera of patients with HIT binds to PF4-coated bacteria but not to bacteria alone. From sera of 5 patients with HIT, known to contain anti-PF4/heparin antibodies, IgG antibodies were affinity purified using hu-PF4-precoated bacteria. (Insets) Depletion of the anti-PF4/heparin antibodies from the original serum (n = 5) by sequential incubation with hu-PF4–coated bacteria (mean ± SD). In the main panels, each symbol represents the reactivity of the affinity-purified antibodies of one serum; mean values are given as horizontal lines. Reactivities of antibodies affinity purified by hu-PF4-coated E coli JM 109 (A) or by hu-PF4-coated S aureus SA113spa (B). Reactivity of the purified antibodies with hu-PF4/heparin complexes (first column) was inhibited by excess heparin (100 IU/mL unfractionated heparin [UFH], column 2), which disrupts PF4/heparin complexes. Accordingly, antibodies did also not react with hu-PF4 alone (column 3). Non–PF4-coated bacteria served as control for unspecific binding of the antibodies to bacteria alone (column 4).
The affinity-purified anti-PF4/heparin antibodies also activated platelets in a functional test: HIPA assay, at low (0.2 IU/mL) but not at high heparin concentrations (100 IU/mL; 5 of 5 eluates of hu-PF4-coated E coli and 3 of 5 eluates of hu-PF4-coated S aureus). Probably the concentration of the antibodies in 2 of 5 eluates of PF4-coated S aureus was not high enough for providing a solid functional response in the HIPA test. Because S aureus had a lower PF4 binding capacity than E coli, it is possible that also less anti-PF4/heparin antibodies bound.
A mouse model for anti-PF4/heparin antibody induction by bacterial infection
To test whether bacteria can induce anti-PF4/heparin antibodies in vivo, we used a mouse model of polymicrobial sepsis. Sublethal polymicrobial bacterial peritonitis was induced by insertion of a stent into the ascending colon of mice (CASP). Non–heparin-treated C57BL/6 mice developed anti–mu-PF4/heparin IgM starting at day 3 (OD: mean ± SD, 0.738 ± 0.191 vs 0.234 ± 0.117 at day 1, P = .0051; Figure 3A). IgM concentrations increased at day 7 (OD: mean ± SD, 1.389 ± 0.457, P = .0051 vs day 1); subsequently, titers decreased somewhat to day 14 but remained elevated throughout the observation period (OD: mean ± SD, 0.914 ± 0.215, P = .0081 vs day 1). Antibody binding was significantly lower in the presence of excess heparin and also when mu-PF4 alone was coated. Sham-operated mice also showed a slight increase in OD levels, which, however, were significantly lower compared with those of CASP mice (day 7, P = .0082; day 14, P = .0003), whereas the untreated control mice showed negligible anti–mu-PF4/heparin IgM production.
Mice with polymicrobial sepsis develop anti-PF4/heparin antibodies. C57BL/6 mice underwent CASP, which caused polymicrobial sepsis, or were sham-operated. At 1, 3, 7, and 14 days after surgery, sera were tested for anti–mu-PF4/heparin IgM (A) and IgG (B) as described in “P4/heparin antibody assays.” Mice with polymicrobial sepsis (black bars) developed anti–mu-PF4/heparin IgM from day 3 and anti–mu-PF4/heparin IgG from day 14. Binding of IgM and IgG was reduced by excess heparin (100 IU/mL, striped bars). When mu-PF4 alone was coated (open bars), IgM antibodies showed minimal binding, but the IgG antibodies also reacted. Antibody titers of mice with CASP were always higher than those of sham-treated mice (gray bars). At each time point, at least 6 mice were assessed (days 1, 3, and 7: n = 6 each for CASP and sham-operated mice; day 14: n = 8 for CASP, n = 11 for sham). Data represent mean OD ± SD. All comparisons of OD values were performed between the OD values obtained at the same experimental day using as reference the values obtained when the sera of CASP mice where incubated with PF4/heparin complexes (black bars). *P < .05; **P < .01; ***P < .001.
Mice with polymicrobial sepsis develop anti-PF4/heparin antibodies. C57BL/6 mice underwent CASP, which caused polymicrobial sepsis, or were sham-operated. At 1, 3, 7, and 14 days after surgery, sera were tested for anti–mu-PF4/heparin IgM (A) and IgG (B) as described in “P4/heparin antibody assays.” Mice with polymicrobial sepsis (black bars) developed anti–mu-PF4/heparin IgM from day 3 and anti–mu-PF4/heparin IgG from day 14. Binding of IgM and IgG was reduced by excess heparin (100 IU/mL, striped bars). When mu-PF4 alone was coated (open bars), IgM antibodies showed minimal binding, but the IgG antibodies also reacted. Antibody titers of mice with CASP were always higher than those of sham-treated mice (gray bars). At each time point, at least 6 mice were assessed (days 1, 3, and 7: n = 6 each for CASP and sham-operated mice; day 14: n = 8 for CASP, n = 11 for sham). Data represent mean OD ± SD. All comparisons of OD values were performed between the OD values obtained at the same experimental day using as reference the values obtained when the sera of CASP mice where incubated with PF4/heparin complexes (black bars). *P < .05; **P < .01; ***P < .001.
IgG binding to mu-PF4/heparin increased at day 14 in sera of CASP mice (OD: mean ± SD, 0.543 ± 0.287, P = .0024 vs day 1; Figure 3B) and was significantly higher than in sera of sham-operated mice (P = .0149). Binding was reduced by excess heparin (P = .0280). The untreated control group showed no anti–mu-PF4/heparin IgG production. This pattern is compatible with a primary immune response, with early IgM and a delayed weak IgG response.
The IgG antibodies also bound to mu-PF4 alone, a feature seen in some patients with HIT.39
Biologic effects of anti-PF4/heparin antibodies
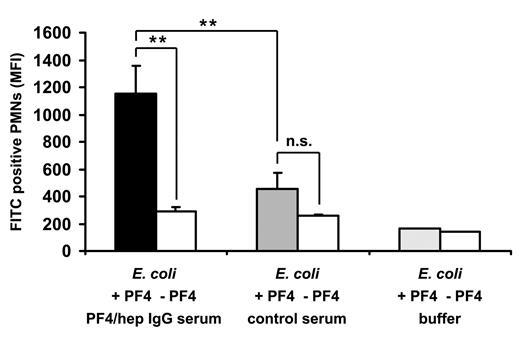
To address the question of whether anti-PF4/heparin antibodies have biologic effects on the clearance of bacteria, we incubated hu-PF4–coated bacteria with hu-PMNs. Anti-PF4/heparin-IgG containing human serum significantly enhanced phagocytosis of PF4-coated E coli by PMNs (n = 4, MFI, 1151.3 ± 207.8) compared with noncoated E coli (n = 4; MFI, 286.7 ± 34.9, P = .0052) (Figure 4). Control serum did not increase phagocytosis of hu-PF4-coated E coli (n = 4; 453.1 ± 122.3) compared with noncoated E coli (n = 4; 259.5 ± 7.4, P = .0541). Phagocytosis of hu-PF4-coated E coli was significantly higher after preincubation with anti-PF4/heparin-IgG-containing human serum than with control serum, which did not contain anti-PF4/heparin antibodies (P = .0012). hu-PF4 alone, without serum, had no effect on phagocytic activity (MFI < 200).
PF4 and anti-PF4/heparin antibodies enhance bacterial phagocytosis. FITC-labeled E coli were preincubated with hu-PF4 (black and gray bars) or with buffer (open bars) and additionally with heat-inactivated human serum, which did (black bar) or did not (gray bar) contain anti-PF4/heparin IgG (preadsorbed with E coli alone), or with buffer alone (open bar). After preincubation, the bacteria were subjected to a whole blood phagocytosis assay using flow cytometry. The figure shows the MFI of FITC-positive PMNs, which is a marker for phagocytosed E coli. Data are mean ± SD of 4 different sera representative of 2 independent experiments. **P < .01. n.s. indicates not significant.
PF4 and anti-PF4/heparin antibodies enhance bacterial phagocytosis. FITC-labeled E coli were preincubated with hu-PF4 (black and gray bars) or with buffer (open bars) and additionally with heat-inactivated human serum, which did (black bar) or did not (gray bar) contain anti-PF4/heparin IgG (preadsorbed with E coli alone), or with buffer alone (open bar). After preincubation, the bacteria were subjected to a whole blood phagocytosis assay using flow cytometry. The figure shows the MFI of FITC-positive PMNs, which is a marker for phagocytosed E coli. Data are mean ± SD of 4 different sera representative of 2 independent experiments. **P < .01. n.s. indicates not significant.
Prevalence of anti-PF4/heparin antibodies in the general population
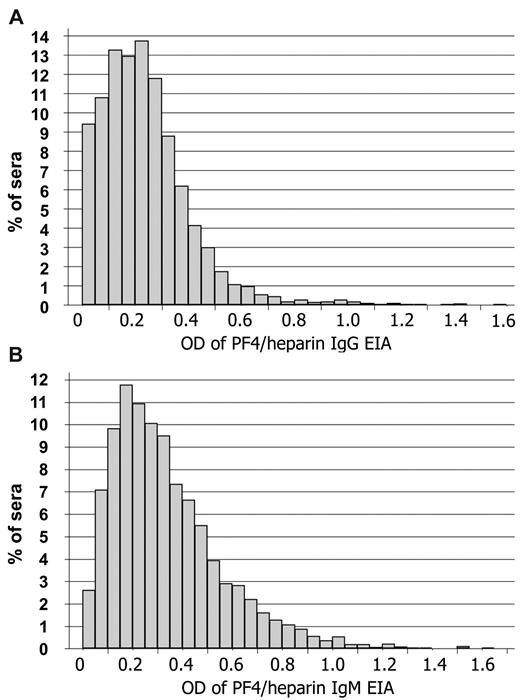
To provide further evidence that anti-PF4/heparin antibodies can be induced by means other than heparin treatment (eg, PF4/bacteria complexes), we systematically assessed the presence of anti-PF4/heparin antibodies in the general population with a very low likelihood of recent heparin treatment. We tested serum samples obtained in a cross-sectional population study37 (n = 4029) using a hu-PF4/heparin-EIA. The normal population showed a continuous, rather than a dichotomous, distribution of reactivities (Figure 5) with no significant effect of age (β = −0.002, P = .128) or sex (β = 0.031, P = .472). In total, 6.1% of the normal population showed an OD higher than 0.5 for IgG (Figure 5A) and 18.8% for IgM (Figure 5B). In 72% of sera with OD higher than 0.5 for IgG (total 4.4%) and in 42% of sera with OD higher than 0.5 for IgM (total 7.9%), the antibody binding to hu-PF4/heparin was inhibited by a high concentration of heparin (100 IU/mL) by more than 30%. Inhibition by high heparin indicates specificity for hu-PF4/heparin complexes because an excess of heparin disrupts the hu-PF4/heparin complexes.4,40,41
Anti-PF4/heparin antibodies are present in the normal population. Sera of participants (n = 4029) from the SHIP were tested for IgM- and IgG-binding to hu-PF4/heparin complexes using EIA as described in “P4/heparin antibody assays.” The results for anti-PF4/heparin IgG (A) and IgM (B) antibodies are shown. The distribution of antibody reactivities as measured by OD values represented a semilogarithmic continuum rather than 2 separate populations of immunized and nonimmunized persons. This is consistent with the concept that the general population is frequently exposed to PF4/heparin-like antigen complexes, which results in a continuum of the immune response. The OD values are arbitrary units. For comparison: with the same assay, strong reacting anti-PF4/heparin antibodies from patients with HIT reacted in an OD range of 1.0 to 2.0.
Anti-PF4/heparin antibodies are present in the normal population. Sera of participants (n = 4029) from the SHIP were tested for IgM- and IgG-binding to hu-PF4/heparin complexes using EIA as described in “P4/heparin antibody assays.” The results for anti-PF4/heparin IgG (A) and IgM (B) antibodies are shown. The distribution of antibody reactivities as measured by OD values represented a semilogarithmic continuum rather than 2 separate populations of immunized and nonimmunized persons. This is consistent with the concept that the general population is frequently exposed to PF4/heparin-like antigen complexes, which results in a continuum of the immune response. The OD values are arbitrary units. For comparison: with the same assay, strong reacting anti-PF4/heparin antibodies from patients with HIT reacted in an OD range of 1.0 to 2.0.
Discussion
In this study, we have shown that PF4 binds to bacteria and forms antigenic complexes on bacterial surfaces. These complexes are recognized by anti-PF4/heparin antibodies from heparin-treated patients, who developed the adverse drug effect HIT. The epitopes on bacterial-bound PF4 are thus similar or identical to those formed by PF4/heparin complexes on administration of pharmacologic heparin. These findings may therefore explain the unusual time course of the immune reaction underlying one of the most frequent and dangerous antibody-mediated adverse drug reactions, HIT, with occurrence of PF4/heparin IgG antibodies as early as day 5 after start of heparin treatment.9,10,38 We propose that antibodies induced in a primary immune response (eg, against PF4-coated invasive bacteria) cross-react with PF4/heparin complexes formed on the platelet surface during heparin treatment. As a consequence, treatment with this anticoagulant boosts the original antibody response and causes a secondary immune reaction to PF4/heparin-coated platelets.
It is well known that the epitopes recognized by anti-PF4/heparin antibodies are formed on multimolecular, linear complexes of PF4 and heparin.4,6 These antigenic epitopes on PF4 are exposed when the positive charge of PF4 is neutralized by polyanions, such as heparin, leading to linear, ridge-like complexes, in which the distance between single PF4 tetramers is narrowed to 3 to 5 nm.5,42 Here we show that PF4 binds to various nonpathogenic and pathogenic Gram-positive and -negative bacteria, including S aureus and E coli (Figure 1). This interaction is charge-dependent because both dextran sulfate and heparin are able to displace PF4 from the bacterial surface. Strikingly, PF4 attached to bacterial cell surfaces exposes epitopes that bind anti-PF4/heparin antibodies from sera of patients who developed these antibodies during heparin treatment (Figure 2). The antibodies can even be “affinity purified” from patient sera using PF4-coated bacteria, which indicates that the same PF4 epitopes are formed on the bacterial surface.
In a standard mouse model of polymicrobial bacterial sepsis (CASP),34 which permits assessment of the immune response over several weeks, we tested whether bacteria could induce the immune response to PF4/polyanions. Although mice did not receive any heparin before or during the experiment, they generated anti-PF4/heparin–reactive IgM antibodies within 3 days and low titers of anti-PF4/heparin IgG within 14 days after induction of bacterial infection (Figure 3). This reflects the typical features of a primary immune response in 7-week-old mice, which probably did not experience major bacterial infection before the experiment.
The conducted experiments do not rule out any other cause of preimmunization against PF4/polyanion complexes in humans. For instance, human cell surfaces also expose negatively charged polyanions, such as heparan sulfate and chondroitin sulfate. However, these polyanions have a much lower charge density than pharmacologic heparin.43 Although PF4 binds to these human cell surface glycosaminoglycans, their lower charge density does not neutralize the strong positive charge of PF4 to an extent that would allow formation of the antigenic clusters recognized by anti-PF4/heparin antibodies. Indeed, heparan sulfate even disrupts the PF4/heparin complexes,30 and heparan sulfate is the main constituent of an alternative anticoagulant (danaparoid) approved for further anticoagulation in patients with HIT.44 Other than endothelial cell heparan sulfate, heparin stored in mast cells is more negatively charged. However, the amount of mast cell heparin released into the circulation is minimal under physiologic conditions. Indeed, if released in higher doses, it is inducing anaphylactoid reactions. Thus, the concentrations of mast cell-derived heparin in the circulation reached under physiologic conditions are probably not high enough to provide the necessary molar ratio for formation of the PF4/heparin complexes. Several groups have found these complexes to occur in vitro at a molar ratio of heparin to PF4 of 1:1 to 1:4.5,6
The anti-PF4/heparin antibodies may even have a biologic function as they significantly enhanced phagocytosis of PF4-coated bacteria compared with uncoated bacteria (Figure 4). Thus, the immune response to PF4/polyanion complexes could be an example for a host defense mechanism. PF4 mediates this response by acting as a bacteria-targeting marker protein. The PF4-coated bacteria are able to induce a humoral immune response against PF4 complexes, which compose a relatively narrow range of specificities. The resulting antibodies, however, can bind to a wide variety of bacterial species, even when not previously encountered by the host immune system. This is achieved through the ability of the highly cationic PF4 molecules to bind to the anionic cell walls of numerous bacterial species. It is conceivable that this anti-PF4/heparin immune response is an ancient host defense mechanism at the interface between innate and adaptive immunity. Once these anti-PF4/polyanion antibodies are formed, they can react with other bacteria even if not previously encountered by the host immune system. To our knowledge, this is the first example of a mechanism based on “labeling” pathogens with an endogenous human protein by forming linear complexes after binding to bacteria, thereby triggering a specific humoral immune response. The immune response in HIT has further interesting features, which would be consistent with an evolutionary old type of immune response. There is no clear restriction to IgG, but IgM and IgA antibodies are generated at the same time.9 Furthermore, the antibody titers rapidly decrease within several weeks.9,38 This might also explain the lack of memory B cells for anti-PF4/heparin IgG in patients who developed anti-PF4/heparin antibodies after cardiac surgery.45 A potential B-cell population that might show these features are marginal zone B cells. However, this requires further studies, and our mouse model might be instrumental for identifying the involved B-cell population as it is the only model with “natural” formation of anti-PF4/heparin antibodies.
Further indirect evidence for the involvement of endogenous PF4 in sepsis has also recently been shown by enhancing survival after LPS challenge depending on activated protein C generation.46
However, the biologic function of the PF4/polyanion immune response induced by bacteria was not the primary focus of our study, and future studies have to address the biologic role of these antibodies in bacterial sepsis. Because mechanisms of bacterial defense are highly redundant, the absolute contribution of anti-PF4/heparin antibodies to survival is probably relatively low.
Preimmunization by PF4-coated bacteria could also explain previous observations of natural anti-PF4/heparin antibodies in non–heparin-treated persons.14,15 The systematic assessment of natural anti-PF4/heparin antibodies in a large cross-sectional population study, the SHIP study,37 indicated the presence of these antibodies in the normal population in a continuum of reactivities from negative to positive, without a dichotomous pattern (Figure 5). This suggests that a considerable proportion of the normal population has been exposed to PF4/heparin-like antigens. It is well known that anti-PF4/heparin antibodies are very transient and disappear within 3 months.9,38 This is a possible reason that the prevalence of anti-PF4/heparin antibodies in the general population (of the SHIP study) does not increase with age. The antibodies found in the normal population rather result from repeated challenge with bacteria, which elicits transient production of the anti-PF4/heparin antibodies. A further interesting finding is the absence of differences between age groups regarding the presence of anti-PF4/heparin IgG and IgM antibodies. This parallels the immune response to blood group A and B, which is also induced by bacteria and in which IgM antibodies are also persistent throughout life despite continuous exposure to the antigen.
We are aware of the limitations of the present study. We could not test whether the antibodies induced in the mice can provoke thrombocytopenia, as platelet activation in HIT is dependent on the presence of the Fc-receptor IIa on platelets and murine platelets do not express this receptor. However, sepsis by itself is causing thrombocytopenia. Even using a transgenic mouse model, which is expressing human PF4 and the Fc-receptor IIa on platelets,47 it will be extremely difficult to differentiate between sepsis-induced thrombocytopenia and anti-PF4/heparin antibody-induced thrombocytopenia. We also cannot exclude that other strongly negatively charged molecules interact with PF4, thereby exposing the preimmunizing antigen. Thus, the proposed mechanism of preimmunization by PF4-coated bacteria might be just one of several mechanisms.
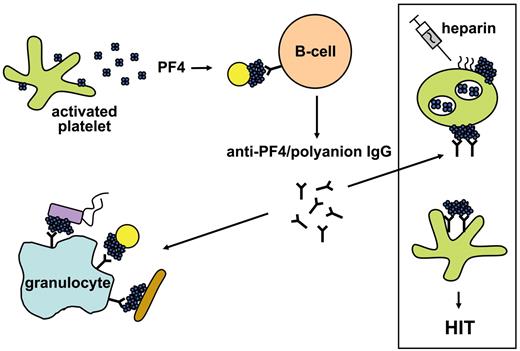
In conclusion, our experiments indicate that the endogenous chemokine PF4 exposes neoepitopes after charge-related binding to bacteria (Figure 6). This results in the formation of immunogenic clusters of PF4, inducing antibodies with specificity for complexed PF4. These antibodies are able to target a large variety of PF4-coated bacteria and to facilitate their phagocytosis. This indicates a potential new function for PF4, namely, triggering an antibody-mediated immune response against different bacteria. Unfortunately, this antigen is also generated when patients are treated with heparin. Parenteral heparin application leads to binding of heparin to platelets.48-50 These heparin-coated platelets then expose the PF4/heparin complex antigens, which mimic the epitopes of PF4 complexes formed on bacterial cell surfaces. We propose that HIT represents a misdirected bacterial defense mechanism.
Schematic representation of the mechanism of how PF4 mediates antibacterial host defense and concurrently primes for HIT. Bacteria activate platelets, which release positively charged PF4 interacting with polyanions at the bacterial surface. This generates clusters of PF4, which initiate antibody production by B cells. Once antibodies are induced, they can bind to all bacterial strains, which form PF4 clusters on their surface. Antibody binding to PF4-coated bacteria facilitates binding to granulocytes and subsequently phagocytosis. On the other hand, these antibodies can induce a severe adverse drug reaction, HIT. During heparin treatment, heparin binds to platelets. This mediates formation of PF4/heparin complexes on the platelet surface. These platelet/PF4/heparin complexes mimic PF4 bound to bacteria, and the anti-PF4/polyanion antibodies bind to the platelet surface by their Fab part where they activate platelets by crosslinking the platelet Fc-receptors with their Fc parts. This finally results in massive thrombin generation via a cascade involving activated platelets, platelet microparticles, endothelial cells, and monocytes leading to HIT and new thrombosis. Yellow circles, brown rods, and violet rectangles represent different bacterial species.
Schematic representation of the mechanism of how PF4 mediates antibacterial host defense and concurrently primes for HIT. Bacteria activate platelets, which release positively charged PF4 interacting with polyanions at the bacterial surface. This generates clusters of PF4, which initiate antibody production by B cells. Once antibodies are induced, they can bind to all bacterial strains, which form PF4 clusters on their surface. Antibody binding to PF4-coated bacteria facilitates binding to granulocytes and subsequently phagocytosis. On the other hand, these antibodies can induce a severe adverse drug reaction, HIT. During heparin treatment, heparin binds to platelets. This mediates formation of PF4/heparin complexes on the platelet surface. These platelet/PF4/heparin complexes mimic PF4 bound to bacteria, and the anti-PF4/polyanion antibodies bind to the platelet surface by their Fab part where they activate platelets by crosslinking the platelet Fc-receptors with their Fc parts. This finally results in massive thrombin generation via a cascade involving activated platelets, platelet microparticles, endothelial cells, and monocytes leading to HIT and new thrombosis. Yellow circles, brown rods, and violet rectangles represent different bacterial species.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Christiane Goerke (Universität Tübingen, Germany) for providing the S aureus protein A-deficient strain SA113spa, Jessica Bagemühl for technical assistance, and Antje Wessel (Ernst-Moritz-Arndt-Universität, Greifswald, Germany) for help with the statistical analysis.
This work was supported by Deutsche Forschungsgemeinschaft (Graduiertenkolleg 840), Bundesministerium für Bildung und Forschung (ZIK-HIKE 03Z2CK1), and Forschungsverbund Molekulare Medizin of the Ernst-Moritz-Arndt-Universität Greifswald (FOMM 2008).
Authorship
Contribution: K.K. designed and performed the in vitro experiments, analyzed the data, and wrote the manuscript; C.P., B.M.B., and W.K. performed the CASP experiments and provided the murine sera; S.M. established the CASP model; B.F. performed the phagocytosis experiments and analyzed the data; S.H. and C.W. provided the bacteria, contributed to the bacterial binding experiments, and revised the manuscript; T.I. evaluated the data of the SHIP study; and A.G. developed the concept, designed the experiments, reviewed and analyzed the results, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Prof Dr Andreas Greinacher, Ernst-Moritz-Arndt Universität, Institut für Immunologie und Transfusionsmedizin, Sauerbruchstr, D 17475 Greifswald, Germany; e-mail: greinach@uni-greifswald.de.

![Figure 2. Anti-PF4/heparin IgG from sera of patients with HIT binds to PF4-coated bacteria but not to bacteria alone. From sera of 5 patients with HIT, known to contain anti-PF4/heparin antibodies, IgG antibodies were affinity purified using hu-PF4-precoated bacteria. (Insets) Depletion of the anti-PF4/heparin antibodies from the original serum (n = 5) by sequential incubation with hu-PF4–coated bacteria (mean ± SD). In the main panels, each symbol represents the reactivity of the affinity-purified antibodies of one serum; mean values are given as horizontal lines. Reactivities of antibodies affinity purified by hu-PF4-coated E coli JM 109 (A) or by hu-PF4-coated S aureus SA113spa (B). Reactivity of the purified antibodies with hu-PF4/heparin complexes (first column) was inhibited by excess heparin (100 IU/mL unfractionated heparin [UFH], column 2), which disrupts PF4/heparin complexes. Accordingly, antibodies did also not react with hu-PF4 alone (column 3). Non–PF4-coated bacteria served as control for unspecific binding of the antibodies to bacteria alone (column 4).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/4/10.1182_blood-2010-08-301424/4/m_zh89991063860002.jpeg?Expires=1767702569&Signature=wm2X2kZKRKlZbw2RfVGWXQxc-yOSJnlKlJogxkpd3pocuwqdN4YZYtUe9AI8NAfUS-axsRm3f2sBrD72EE-47PNbtv9pL9zdCos~ki9pXF4b0DiefOiKL0APPQ2v6zIy6x2qq6j8tH-bjVjiRJQEUguq58VP7mhKpkbuXkv17pc3ob~PYqkGqa52OZwUUY5fYVL7-HpzGg16pW9BTK33-miy~ke3rdmUzK3~tqqENRS2E6AaqvjJCgEoz4WFArbd7dbn5-eAH-iHIPRq1rIJWuQDDRlVGq9kmD0XXGmZuLt2pYtrInKX60KIk8kmNu4IunuX1RSlSWBeXkE8wj4RhA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)