Abstract
Lunatic Fringe (Lfng) enhances Notch1 activation by Delta-like 4 (DL4) to promote Notch1-dependent T-lineage commitment of thymus-seeding progenitors. Subsequently, Notch1 and T-cell receptor-β (TCRβ)–containing pre-TCR complexes signal CD4/CD8 double-negative 3 (DN3) committed T-cell progenitors to survive, proliferate, and differentiate into CD4/CD8 double-positive (DP) αβ T-cell precursors. Few DP thymocytes develop without Notch1 or pre-TCR signals, whereas ectopic Notch1 activation causes T-cell leukemia. However, mechanisms of a Notch-pre-TCR collaboration during this “β-selection” process are poorly understood. We genetically manipulated Lfng to attenuate or enhance Notch1 activation in DN3 thymocytes without inducing leukemogenesis. We show that Lfng temporally sustains DL-induced Notch1 signaling to prolong proliferative self-renewal of pre-DP thymocytes. Pre-TCR signaling greatly augmented Notch trophic functions to promote robust proliferation of pre-DP progenitors. In contrast, in the absence of DL/Notch signaling, pre-TCR-expressing progenitors rapidly atrophied and differentiated into DP thymocytes. Thus, Lfng prolongs Notch1 signaling to promote self-renewal more than differentiation during the early stages of β-selection. Our data provide novel insights into the Notch-pre-TCR collaboration, and suggest that decreasing Lfng expression during the DN3-DP transition minimizes the potent leukemogenic potential of Notch1 signaling.
Introduction
Notch signaling provides a highly conserved mechanism of intercellular communication that regulates a wide spectrum of developmental processes including cell self-renewal, cell differentiation, and, in particular, binary cell fate decisions. Some Notch-induced developmental processes are regulated by Fringe proteins, which are Golgi-localized glycosyltransferases that add N-acetylglucosamine to O-fucose moieties to the extracellular domains of Notch receptors.1 Fringe modifications enhance Notch activation by ligands belonging to the Delta-like (DL) family, and diminish Notch activation by Serrate/Jagged family ligands, thus modulating many Notch-dependent cell fate decisions.2-6 In the immune system, Lfng plays a key role in the early stages of T-lymphocyte development, critically enhancing DL4/Notch1-dependent suppression of alternative B-lineage potential and promoting T-lineage specification.7-10 Furthermore, Lfng and Manic Fringe cooperatively enhance DL1-induced Notch2 activation to promote marginal zone B-cell development.11
Lfng continues to be highly expressed after thymus-seeding progenitors develop into T cell–committed DN2 (CD117+ CD25+) and then DN3a (CD117− CD25+) pro–T cells,10 but its functions during the postcommitment stages of T-cell development have not been defined. Notch1 activation is essential for thymocyte β-selection, during which pre–T-cell receptor (TCR)α (pTα) and TCRβ-containing pre-TCR complexes signal DN3a pro-T cells to survive, proliferate, and differentiate into CD4/CD8 double-positive (DP) thymocytes, the immediate precursors of mature αβ T cells.12 During the earliest step in β-selection, small, resting DN3a pro-T cells are induced to survive and develop into TCRβ+ CD27hi DN3b blasts.13,14 These progenitors then proliferate extensively as they transit through the DN4 (CD44−CD117−CD25−) and CD8 immature single-positive (ISP) stages,15,16 eventually differentiating into nonproliferative TCRβ+ DP thymocytes. It has been estimated that thymocytes undergo 8-10 rounds of cell division during the DN3-DP transition,17 ensuring the generation of a large pool of DP thymocytes from small numbers of pre-TCR–expressing progenitors. Large numbers of DP thymocytes are required, because only a small fraction express TCRαβ heterodimers capable of promoting their differentiation into mature CD4 or CD8 T cells.18
TCRβ+ DN3 and DN4 progenitors generate very few DP thymocytes in the absence of Notch1 signaling,19-23 revealing that Notch signaling is required for efficient β-selection of TCRβ+ DN progenitors. Notch signaling is required to maintain cell size and glucose uptake in preselection (TCRβ−) DN3a thymocytes,24 revealing a trophic function for Notch activation. Notch signaling also regulates the expression of nutrient receptors such as CD71 (transferrin receptor) and CD98 (neutral amino acid transporter heavy chain) in DN4 progenitors,25 but it remains unclear if the trophic functions of Notch differ in preselection versus pre-TCR+ DN3 and DN4 thymocytes. Furthermore, the precise contributions of Notch versus pre-TCR signaling to the survival, proliferation, and differentiation phases of β-selection are not well understood.
Lfng and Notch1 are coexpressed at relatively high levels in DN3 and DN4 thymocytes, but the expression of both genes and Notch signaling sharply decline in DP thymocytes.10,14,26 Ectopic maintenance of high Notch1 activity in post-DN3 thymocytes causes T-cell leukemia in mice, and mutations leading to ectopic Notch1 activation are frequent in human T-cell leukemia.27,28 The sharp down-regulation in Lfng expression during the DN3-DP transition could contribute to reducing Notch1 activation in DP thymocytes, but this notion has not been tested experimentally. Furthermore, the Notch ligand that drives β-selection in vivo has not been identified. DL4 but not DL1 is expressed at high levels in cortical epithelial cells,29,30 but expression of DL4 in subcapsular niches where β-selection occurs31 has not been evaluated.
We have used complementary genetic approaches to determine how Lfng affects Notch1-dependent β-selection of DN3 thymocytes to provide insights into the nature of the Notch ligand that regulates β-selection, as well as the specific functions of Notch signaling in the DN3-DP transition. We demonstrate that Lfng critically enhances and temporally sustains Notch1-DL interactions to prolong proliferation of DN3b and DN4 thymocytes, thus expanding the number of TCRβ-expressing progenitors before they differentiate into nonproliferative DP thymocytes. Lfng expression declines during the late stages of β-selection, decreasing Notch1 responsiveness to DL ligands to facilitate pre-TCR–induced differentiation. These data define an important new function for Lfng during β-selection that is distinct from its earlier known role in promoting Notch1-dependent T-lineage commitment, and provide novel insights into how Notch and pre-TCR signaling collaborate in T-cell development.
Methods
Mice
The mouse strains used in this study are described in supplemental data (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Immunofluorescence staining and flow cytometry
Immunofluorescence was analyzed on FACSCalibur or LSR-II (BD Biosciences) flow cytometers using standard techniques as described previously.8,10 Statistical significance for all population comparisons was calculated using the randomization test (2-tailed probability) except where specified otherwise. Flow cytometric analysis of intracellular TCRβ expression in OP9 or OP9-DL4/thymocyte progenitor cocultures was performed as per the BD Cytofix/Cytoperm Kit protocol after 24 hours of coculture.
Adoptive transfer experiments
DN3 thymocytes were sorted with > 98% purity from 4- to 8-week-old mice as described in Tan et al.8 Enhanced green fluorescent protein–positive (EGFP+) DN3 cells were sorted from ROSA26-EGFP+Lck-Cre+Lfngfl/fl (B6.CD45.2) or ROSA26-EGFP+Lck-Cre+Lfng+/+(B6.CD45.2) mice, whereas EGFP− DN3 cells were sorted from wild-type (WT) B6.CD45.1; CD45.2 (F1) mice. Sorted cells were intrathymically injected as a 50:50 mixture (105 cells from each donor type/lobe) into WT B6.CD45.1 host mice as described in Visan et al.10 Thymocytes from individual lobes were analyzed 10 days after intrathymic injection.
OP9 cultures
Methods for the generation of OP9-DL4 cells can be found in supplemental data. OP9-GFP and OP9-DL1 cell lines were provided by Juan-Carlos Zuniga-Pflucker (Toronto) and used as described previously.19,32 Briefly, 2 × 103 DN3 or DN4 thymocytes (or up to 5 × 104 for short-term cultures of < 48 hours) were seeded into 24-well plates containing 80% confluent monolayers of OP9-GFP, OP9-DL1, or OP9-DL4 cells in 1 mL of α-minimum essential medium (GIBCO) supplemented with 20% fetal bovine serum (GIBCO), penicillin (100 U/mL), and streptomycin (100 μg/mL, GIBCO), 5 ng/mL recombinant human Flt-3 ligand (R&D Systems), and 5 ng/mL murine interleukin-7 (IL-7; StemCell Technologies). Cells were incubated at 37°C in a humidified atmosphere with 5% CO2. Where indicated, 10μM bromo-deoxyuridine (BrdU) was added for the last 2 hours of an 8-day coculture of DN3 or DN4 progenitors with OP9-DL1. BrdU was detected using the BrdU Flow Kit as per the manufacturer's instructions (BD Biosciences).
Histology
Whole-mount X-Gal (4-bromo-5-chloro-3-indoyl-β-D-galactopyrosanide) staining was performed on thymic fragments (1-2 mm) from 3- to 6-month-old mice as described previously.11 Image acquisition of X-Gal–stained tissue sections and visualization of DL4-expressing cells by electron microscopy is described in supplemental data.
Results
Conditional deletion of Lfng at the DN3 stage of T-cell development impairs β-selection
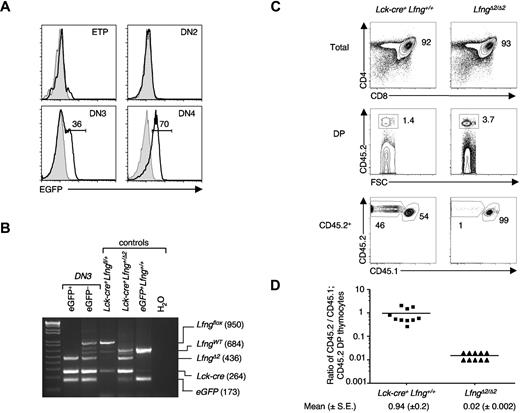
Because Lfng deficiency compromises Notch1-dependent T-cell development from thymus-seeding progenitors,10 we used a conditional knockout strategy to specifically ablate Lfng function in committed DN3 thymocytes. Briefly, we used mice in which exon2, encoding the catalytic domain of the Lfng glycosyltransferase gene, was flanked by LoxP sites (Lfngfl/fl),5 and intercrossed them with Lck-cre transgenic mice. We detected partial exon2 deletion in DN2 and DN3 thymocytes from Lck-cre+Lfngfl/fl mice, whereas only LfngΔ2 alleles were detected in DN4 thymocytes (supplemental Figure 1). Lck-cre+Lfngfl/fl thymi were frequently hypocellular and showed an accumulation of DN3 thymocytes at the expense of DN4 and DP cells, suggesting a profound block in β-selection of DN3 thymocytes (supplemental Figure 1). However, this phenotype was variable from mouse to mouse, likely reflecting variable Lfng exon2 deletion in DN3 thymocytes.
This notion was confirmed by experiments using Lck-cre+Lfngfl/fl ROSA26-EGFP Cre reporter mice.33 Consistent with previous studies on the transcriptional regulation of Lck by its proximal promoter,34 we observed minimal Lck-cre-mediated EGFP expression in DN1 and DN2 thymocytes (Figure 1A). In contrast, EGFP was bimodally expressed in DN3 thymocytes, whereas most DN4 thymocytes expressed EGFP. Exon2 deletion was complete in EGFP+ but not in EGFP− DN3 thymocytes from Lck-cre+Lfngfl/fl ROSA26-EGFP mice (Figure 1B). The percentage of EGFP+ DN3 cells ranged from 23%-59%, and we found that mice with more than 40% EGFP+ DN3 thymocytes had hypocellular thymi (supplemental Table 1), reflecting a small DP thymocyte pool.
ROSA26-EGFP+Lck-cre+LfngΔ2/Δ2 DN3 thymocytes fail to reconstitute the DP thymocyte pool in mixed chimeras. (A) Detection of Cre-recombinase activity in Lck-cre+Lfngfl/fl mice using ROSA26-EGFP Cre-recombinase reporter mice. Histograms show EGFP expression in each indicated subset from ROSA26-EGFP+Lck-cre+ (black) versus ROSA26-EGFP+Lck-cre− (gray) mice. No EGFP+ B cells or myeloid cells were detected in the spleen or bone marrow (data not shown). (B) Validation of Lfng exon2 excision by Lck-Cre in EGFP+ DN3 thymocytes. PCR analysis of genomic DNA from sorted EGFP+ and EGFP− DN3 (CD117−CD25+) thymocytes isolated from ROSA26-EGFP+Lck-cre+Lfngfl/fl mice. PCR primers used amplify WT Lfng (LfngWT; 684 bp), exon2-floxed Lfng (LfngloxP; 950 bp), and exon2-deleted Lfng (LfngΔ2; 436 bp) alleles from genomic DNA. (C) Loss of Lfng dramatically impairs the generation of DP thymocytes. DN3 thymocytes sorted from ROSA26-EGFP+Lck-cre+LfngΔ2/Δ2 or Lck-cre+ B6.CD45.2 mice were mixed with an equal number of WT DN3 (B6.CD45.1; CD45.2) thymocytes and intrathymically injected into WT (B6.CD45.1) hosts. Ten days later, thymocytes were analyzed by immunofluorescence staining and flow cytometry for CD4 versus CD8 expression on total thymocytes (top), the proportion of DP thymocytes that were CD45.2+ donor-derived cells (middle), and the proportion of CD45.2+ donor cells that coexpressed CD45.1 (bottom), indicating that they were derived from the WT DN3 donor. The range of B6.CD45.2-derived DP thymocytes was 21%-67% for Lck-cre+Lfng+/+ chimeras and 0.3%-2% for LfngΔ2/Δ2 chimeras. (D) Relative contribution of donor-derived cells to the DP thymocyte pool of control vs LfngΔ2/Δ2 chimeras. The frequency of donor-derived thymocytes was calculated using the gating scheme defined in (C). Each symbol represents the percentage of B6.CD45.2 DP thymocytes divided by the percentage of WT B6.CD45.1; CD45.2 DP thymocytes in each thymic lobe. Mean ratios (± SE) are shown beneath the plot and are portrayed as horizontal bars.
ROSA26-EGFP+Lck-cre+LfngΔ2/Δ2 DN3 thymocytes fail to reconstitute the DP thymocyte pool in mixed chimeras. (A) Detection of Cre-recombinase activity in Lck-cre+Lfngfl/fl mice using ROSA26-EGFP Cre-recombinase reporter mice. Histograms show EGFP expression in each indicated subset from ROSA26-EGFP+Lck-cre+ (black) versus ROSA26-EGFP+Lck-cre− (gray) mice. No EGFP+ B cells or myeloid cells were detected in the spleen or bone marrow (data not shown). (B) Validation of Lfng exon2 excision by Lck-Cre in EGFP+ DN3 thymocytes. PCR analysis of genomic DNA from sorted EGFP+ and EGFP− DN3 (CD117−CD25+) thymocytes isolated from ROSA26-EGFP+Lck-cre+Lfngfl/fl mice. PCR primers used amplify WT Lfng (LfngWT; 684 bp), exon2-floxed Lfng (LfngloxP; 950 bp), and exon2-deleted Lfng (LfngΔ2; 436 bp) alleles from genomic DNA. (C) Loss of Lfng dramatically impairs the generation of DP thymocytes. DN3 thymocytes sorted from ROSA26-EGFP+Lck-cre+LfngΔ2/Δ2 or Lck-cre+ B6.CD45.2 mice were mixed with an equal number of WT DN3 (B6.CD45.1; CD45.2) thymocytes and intrathymically injected into WT (B6.CD45.1) hosts. Ten days later, thymocytes were analyzed by immunofluorescence staining and flow cytometry for CD4 versus CD8 expression on total thymocytes (top), the proportion of DP thymocytes that were CD45.2+ donor-derived cells (middle), and the proportion of CD45.2+ donor cells that coexpressed CD45.1 (bottom), indicating that they were derived from the WT DN3 donor. The range of B6.CD45.2-derived DP thymocytes was 21%-67% for Lck-cre+Lfng+/+ chimeras and 0.3%-2% for LfngΔ2/Δ2 chimeras. (D) Relative contribution of donor-derived cells to the DP thymocyte pool of control vs LfngΔ2/Δ2 chimeras. The frequency of donor-derived thymocytes was calculated using the gating scheme defined in (C). Each symbol represents the percentage of B6.CD45.2 DP thymocytes divided by the percentage of WT B6.CD45.1; CD45.2 DP thymocytes in each thymic lobe. Mean ratios (± SE) are shown beneath the plot and are portrayed as horizontal bars.
To assess the impact of Lfng deficiency on β-selection, we purified EGFP+ thymocytes (with complete exon2 deletion) from Lck-cre+Lfngfl/fl ROSA26-EGFP mice and intrathymically injected equal mixtures of these LfngΔ2/Δ2 DN3 and WT DN3 thymocytes into sublethally irradiated WT (CD45.1) hosts. Ten days later, we analyzed the contribution of LfngΔ2/Δ2 (CD45.2+) versus WT (CD45.1+ CD45.2+) DN3 donors to the DP thymocyte pool. As expected, in control chimeras, WT (CD45.1+ CD45.2+) and Lck-cre+Lfng+/+ (CD45.2+) DN3 donors contributed equally to the donor-derived DP thymocyte pool (Figure 1C). In striking contrast, < 2% of donor-derived DP thymocytes were derived from LfngΔ2/Δ2 DN3 thymocytes in experimental chimeras (Figure 1C-D). Because only very small numbers of DP thymocytes were generated from LfngΔ2/Δ2 DN3 progenitors, we conclude that Lfng deficiency permits DN3 differentiation to the DP stage, but severely impairs clonal expansion during this developmental transition.
DL4 is selectively expressed in subcapsular thymic epithelial cells
The Notch ligand that drives β-selection in vivo has not been determined, but the Lfng dependence of β-selection strongly suggests the involvement of one or more DL ligands. We therefore compared the pattern of DL1, DL4, and Jagged1 expression in thymi from DL1+/LacZ, DL4+/LacZ, and Jagged1+/LacZ reporter mice using X-Gal staining to identify LacZ-expressing cells. We observed weak, primarily vascular X-Gal staining in DL1+/LacZ and Jagged1+/LacZ thymi (supplemental Figure 2), although strong vascular staining in spleens from both strains of reporter mice were seen previously.11 In contrast, vascular and epithelial cells in DL4+/LacZ thymi were strongly X-Gal–stained (Figure 2). However, DL4 expression in the subcapsular niche where β-selection occurs31 was not evaluated in previous studies.29,30 Subcapsular regions of DL4+/LacZ thymi contained darkly X-Gal–stained cells that also expressed cytokeratin-8 (Figure 2B). These LacZ+ cells exhibited epithelial morphology and were found in close contact with thymocytes (Figure 2C). Thus, DL4 but not DL1 or Jagged1 is highly expressed in subcapsular cortical niches where Notch1-dependent β-selection occurs.
DL4 is expressed abundantly in subcapsular thymic epithelial cells. (A) Thymi from 3- to 6-month-old DL4+/LacZ mice were whole-mount X-Gal–stained, paraffin-embedded, sectioned, and then counterstained with neutral red (i-ii, original magnifications, 100× and 800×). (B) Thymi from 3- to 6-month-old DL4+/LacZ mice were whole-mount X-Gal–stained, sucrose-infused, and embedded in Tissue-Tek optimal cutting temperature compound. Cryosections were stained with anti-cytokeratin 8 (original magnification, 600×). (C) Thymi from 3- to 6-month-old DL4+/LacZ mice stained in whole-mount preparations with Bluo-Gal that was visualized by scanning electron microscopy (original magnification, 10 000×). Images are representative of those obtained from at least 3 mice of each genotype analyzed in a minimum of 3 different experiments.
DL4 is expressed abundantly in subcapsular thymic epithelial cells. (A) Thymi from 3- to 6-month-old DL4+/LacZ mice were whole-mount X-Gal–stained, paraffin-embedded, sectioned, and then counterstained with neutral red (i-ii, original magnifications, 100× and 800×). (B) Thymi from 3- to 6-month-old DL4+/LacZ mice were whole-mount X-Gal–stained, sucrose-infused, and embedded in Tissue-Tek optimal cutting temperature compound. Cryosections were stained with anti-cytokeratin 8 (original magnification, 600×). (C) Thymi from 3- to 6-month-old DL4+/LacZ mice stained in whole-mount preparations with Bluo-Gal that was visualized by scanning electron microscopy (original magnification, 10 000×). Images are representative of those obtained from at least 3 mice of each genotype analyzed in a minimum of 3 different experiments.
Lfng overexpression enhances proliferative expansion of DN3 and DN4 thymocytes in response to DL ligands in vitro
We next sought to determine how sustaining Lfng at high levels across the DN3-DP transition affects the proliferation and differentiation of DN3 and DN4 thymocytes in response to DL ligands. In initial experiments, we used an OP9 bone marrow stromal cell line expressing high levels of DL1.19 First, we compared proliferative expansion of Lfng Tg+ versus Tg− DN3 thymocytes after 4, 6, and 9 days of coculture with OP9-DL1. Lfng overexpression only marginally enhanced expansion of DN3 progeny over 9 days (Figure 3A). However, Lfng Tg+ DN4 cells produced 3-4 times more progeny than Tg− DN4 progenitors (Figure 3A). Interestingly, Lfng Tg+ DN3 and DN4 progeny included higher proportions of immature CD4− cells (DN and CD8 ISP) thymocytes relative to those initiated with Tg− progenitors at every time point examined (supplemental Figure 3). We repeated these experiments comparing expansion of WT and Lfng Tg+ DN4 thymocytes on OP9-DL1 versus OP9-DL4. On both stromal lines, Lfng Tg+ DN4 progeny continued to expand from day 6 to day 9, whereas expansion of Tg− DN4 progeny leveled off or declined during this period (Figure 3B).
Lfng overexpression enhances proliferative expansion of DN3 and DN4 thymocytes on OP9-DL1 and OP9-DL4. (A) Proliferative expansion of DN3 and DN4 thymocytes on OP9-DL1. Graphs display the fold expansion of Lfng Tg+ (diamonds) versus Lfng Tg− (squares) DN3 or DN4 progeny at each time point relative to the number of cells seeded on day 0. (B) Proliferative expansion of DN4 thymocytes from Lfng Tg+ (diamonds) versus Lfng Tg− (squares) mice on OP9-DL1 versus OP9-DL4 stromal cells. Graphs display the -fold expansion at each time point relative to the number of cells seeded. Similar results were obtained in 3 independent experiments. (C) Prolonged proliferative expansion of Lfng Tg+ DN3 and DN4 progeny in OP9-DL1 stromal cell cultures. DN3 and DN4 thymocytes from Lfng Tg− (top row) and Lfng Tg+ (bottom row) mice were cultured on OP9-DL1 stromal cells for 8 days. BrdU was added to each culture 2 hours before harvesting cells and staining with anti-BrdU–fluorescein isothiocyanate (FITC) and anti-CD4–allophycocyanin (APC) plus anti-CD8–APC. Histograms depict percentage of BrdU+ DN and DP cells generated from cultures initiated with DN3 (left) or DN4 (right) thymocytes. Similar results were obtained in 2 independent experiments.
Lfng overexpression enhances proliferative expansion of DN3 and DN4 thymocytes on OP9-DL1 and OP9-DL4. (A) Proliferative expansion of DN3 and DN4 thymocytes on OP9-DL1. Graphs display the fold expansion of Lfng Tg+ (diamonds) versus Lfng Tg− (squares) DN3 or DN4 progeny at each time point relative to the number of cells seeded on day 0. (B) Proliferative expansion of DN4 thymocytes from Lfng Tg+ (diamonds) versus Lfng Tg− (squares) mice on OP9-DL1 versus OP9-DL4 stromal cells. Graphs display the -fold expansion at each time point relative to the number of cells seeded. Similar results were obtained in 3 independent experiments. (C) Prolonged proliferative expansion of Lfng Tg+ DN3 and DN4 progeny in OP9-DL1 stromal cell cultures. DN3 and DN4 thymocytes from Lfng Tg− (top row) and Lfng Tg+ (bottom row) mice were cultured on OP9-DL1 stromal cells for 8 days. BrdU was added to each culture 2 hours before harvesting cells and staining with anti-BrdU–fluorescein isothiocyanate (FITC) and anti-CD4–allophycocyanin (APC) plus anti-CD8–APC. Histograms depict percentage of BrdU+ DN and DP cells generated from cultures initiated with DN3 (left) or DN4 (right) thymocytes. Similar results were obtained in 2 independent experiments.
We also performed mixing experiments to confirm that the differences between Tg− and Tg+ progenitors that we observed in the OP9-DL cocultures resulted from cell-autonomous effects of Lfng overexpression. Although similar numbers of cells were recovered from single 9-day cultures of Tg− and Tg+ DN3 cells (Figure 3A), in mixed cultures, we consistently observed that the relative proportion of Tg+ progeny increased between days 7 and 9 (supplemental Figure 4A), suggesting higher proliferation of Lfng Tg+ DN3 progeny during late phases of the cultures. In cultures initiated with equal mixtures of Tg− and Tg+ DN4 progenitors,we observed an even more profound skewing toward Tg+ DN4 progeny between days 7 and 9 of cultures (supplemental Figure 4B), in agreement with the greater proliferation of Tg+ DN4 progeny in the single cultures. Finally, most Tg− DN3 and DN4 progeny in mixed cultures had differentiated into DP thymocytes, whereas Tg+ progeny contained mostly pre-DP cells (supplemental Figure 4). These experiments show that Lfng overexpression prolongs proliferative expansion induced by DL1 or DL4 during β-selection and selectively maintains pre-DP subsets.
Lfng overexpression prolongs proliferative self-renewal of DN3 and DN4 progeny
We next conducted experiments to determine how Lfng overexpression alters the cell-cycle kinetics of DN3 and DN4 thymocytes in response to DL Notch ligands. Because Lfng Tg+ and Tg− DN3 and DN4 progeny diluted carboxyfluorescein diacetate succinimidyl ester (CFSE), a cell-tracing dye, to similar extents during the first 4 days of culture on OP9-DL1, we concluded that overexpression of Lfng did not increase cell-cycle rates in the short term (data not shown). However, Lfng Tg+ DN3 and DN4 progeny contained many more BrdU+ thymocytes than their Tg− counterparts in the late stages of these cultures (Figure 3C), and most were DN (Figure 3C), suggesting that transgenic Lfng could not maintain proliferation after progenitors differentiated into DP thymocytes.
The enhanced proliferation of Lfng Tg+ DN3 and DN4 progenitors was not limited to the initial culture period, but continued for more than 3 weeks in serial passage experiments (Figure 4). The effect of Lfng overexpression on DN4 thymocytes was particularly striking, because Lfng Tg+ DN4 cultures could be serially passaged for 24 days, whereas Tg− DN4 progeny could not be repassaged after the initial 7-day culture (Figure 4B and supplemental Table 2). Higher percentages of blast cells were present in serially passaged cultures of Lfng Tg+ relative to Tg− DN3 and DN4 thymocytes, suggesting a prolonged phase of proliferative self-renewal. Interestingly, the effect of Lfng overexpression was IL-7 dependent in these serial passage experiments (supplemental Figure 5A). These findings demonstrate that Lfng overexpression prolongs the amount of time during which β-selected pre-DP progenitors undergo DL-induced proliferation before differentiating into nonproliferative DP thymocytes.
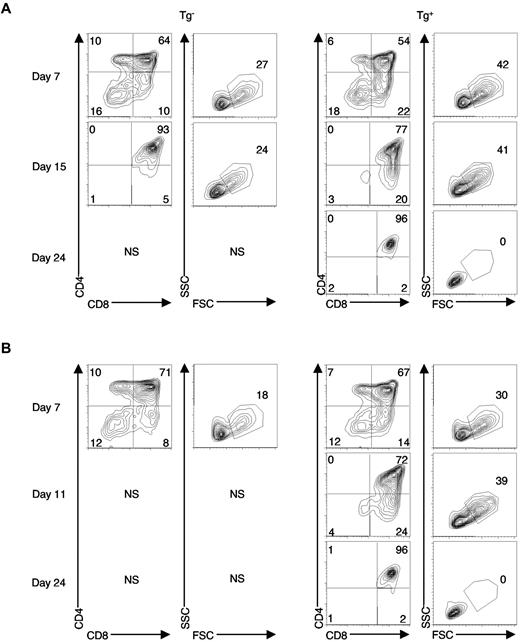
Lfng overexpression sustains proliferation of DN3 and DN4 thymocytes on OP9-DL1. DN3 (A) or DN4 (B) thymocytes were sorted from Lfng Tg+ or WT mice and cultured on OP9-DL1 cells. Beginning at day 7, cells were passaged every 4-5 days onto fresh OP9-DL1 monolayers. At each time point, an aliquot of cells was harvested and stained with anti-CD4–FITC versus anti-CD8–phycoerythrin (PE; left panels). The 2-parameter contour plots (5% probability) depict CD4 versus CD8 expression (left panels) or forward scatter (FSC) versus side scatter (SSC, right panels) of progeny at each time point. Numbers depict the percentage of cells in each quadrant or gate. NS, not sufficient (the number of cells present was not sufficient to be analyzed). The fold expansion for each time point is summarized in supplemental Table 2. Similar results were obtained in 2 independent experiments.
Lfng overexpression sustains proliferation of DN3 and DN4 thymocytes on OP9-DL1. DN3 (A) or DN4 (B) thymocytes were sorted from Lfng Tg+ or WT mice and cultured on OP9-DL1 cells. Beginning at day 7, cells were passaged every 4-5 days onto fresh OP9-DL1 monolayers. At each time point, an aliquot of cells was harvested and stained with anti-CD4–FITC versus anti-CD8–phycoerythrin (PE; left panels). The 2-parameter contour plots (5% probability) depict CD4 versus CD8 expression (left panels) or forward scatter (FSC) versus side scatter (SSC, right panels) of progeny at each time point. Numbers depict the percentage of cells in each quadrant or gate. NS, not sufficient (the number of cells present was not sufficient to be analyzed). The fold expansion for each time point is summarized in supplemental Table 2. Similar results were obtained in 2 independent experiments.
Lfng overexpression prolongs responsiveness to DL ligands during thymocyte β-selection
We hypothesized that Lfng prolongs the proliferation of DN3 and DN4 progenitors by increasing their responsiveness to DL ligands. To test this hypothesis, we compared the ability of Lfng Tg+ versus Tg− thymocytes to bind soluble fusion proteins that contain the extracellular portion of DL4 or DL1 fused to the Fc portion of human immunoglobulin 1 (IgG1). WT hematopoietic stem cells (HSCs) and DN1-DN4 thymocytes bound 10- to 20-fold more DL4-Fc than DL1-Fc (Figure 5A), as reported in other studies.35,36 Tg− CD8 ISP and DP progenitors also bound substantially more DL4-Fc (∼ 2.5- to 6-fold higher, Figure 5B, supplemental Table 3). DL1-Fc binding by Tg− DP and by CD4 or CD8 SP thymocytes was not detected above the background binding of Fc protein alone, although we observed considerably more DL4-Fc binding to mature SP thymocytes than was seen in previous studies.35,36 This dynamic developmental regulation of DL4-Fc binding parallels the expression of Notch1 but not Notch2 protein in thymocytes.37
Lfng overexpression augments binding of DL1 and DL4 by DN and DP thymocytes. (A) Binding of Fc, DL1-Fc, and DL4-Fc fusion proteins by HSC and DN thymocytes. Total fetal liver cells from E12.5-16.5 WT mice were stained with a cocktail of biotinylated lineage markers along with anti-CD117–APC and anti–Sca-1–FITC to identify Lin− Sca-1hi CD117hi HSCs. Total thymocytes from WT mice were depleted of lineage-positive (Lin+) cells and stained with anti-CD117–APC and anti-CD25–FITC to identify DN1 (Lin−c-kit+CD25−), DN2 (Lin−c-kit+CD25+), DN3 (Lin−c-kit−CD25+), and DN4 (Lin−c-kit−CD25−) cells. Cells were subsequently stained with either human-Fc (control protein consisting of only the hinge and Fc portions of human IgG1; solid lines), DL1-Fc (dashed lines), or DL4-Fc (dotted lines) followed by anti–human IgG1-PE. (B) Effect of Lfng overexpression on binding of DL1-Fc and DL4-Fc in thymocyte subsets. Total thymocytes were stained with anti-CD4–FITC, anti-CD8–Alexa Fluor 633, and either human-Fc (control protein consisting of only the hinge and Fc portions of human IgG1), DL1-Fc, or DL4-Fc followed by anti–human IgG1-PE. Histograms compare binding of each protein to indicated thymocyte subsets from Lfng Tg+ (solid lines) versus WT (dashed lines) mice. Similar results were obtained in 3 independent experiments. (C) Lfng overexpression prolongs DL-induced Notch signaling in DN4 thymocytes. Sorted DN4 thymocytes from Lfng Tg+ and WT mice were cultured on OP9-DL1, and progeny were harvested and stained with anti-CD4–FITC, anti-CD8-PE, and biotinylated anti-CD25 (avidin Alexa Fluor 633) after 4 (top row) and 9 (bottom row) days of culture. Histograms display CD25 expression on DN and DP progeny from cultures initiated with Lfng Tg+ (solid line) versus WT (dotted line) DN4 thymocytes at each time point.
Lfng overexpression augments binding of DL1 and DL4 by DN and DP thymocytes. (A) Binding of Fc, DL1-Fc, and DL4-Fc fusion proteins by HSC and DN thymocytes. Total fetal liver cells from E12.5-16.5 WT mice were stained with a cocktail of biotinylated lineage markers along with anti-CD117–APC and anti–Sca-1–FITC to identify Lin− Sca-1hi CD117hi HSCs. Total thymocytes from WT mice were depleted of lineage-positive (Lin+) cells and stained with anti-CD117–APC and anti-CD25–FITC to identify DN1 (Lin−c-kit+CD25−), DN2 (Lin−c-kit+CD25+), DN3 (Lin−c-kit−CD25+), and DN4 (Lin−c-kit−CD25−) cells. Cells were subsequently stained with either human-Fc (control protein consisting of only the hinge and Fc portions of human IgG1; solid lines), DL1-Fc (dashed lines), or DL4-Fc (dotted lines) followed by anti–human IgG1-PE. (B) Effect of Lfng overexpression on binding of DL1-Fc and DL4-Fc in thymocyte subsets. Total thymocytes were stained with anti-CD4–FITC, anti-CD8–Alexa Fluor 633, and either human-Fc (control protein consisting of only the hinge and Fc portions of human IgG1), DL1-Fc, or DL4-Fc followed by anti–human IgG1-PE. Histograms compare binding of each protein to indicated thymocyte subsets from Lfng Tg+ (solid lines) versus WT (dashed lines) mice. Similar results were obtained in 3 independent experiments. (C) Lfng overexpression prolongs DL-induced Notch signaling in DN4 thymocytes. Sorted DN4 thymocytes from Lfng Tg+ and WT mice were cultured on OP9-DL1, and progeny were harvested and stained with anti-CD4–FITC, anti-CD8-PE, and biotinylated anti-CD25 (avidin Alexa Fluor 633) after 4 (top row) and 9 (bottom row) days of culture. Histograms display CD25 expression on DN and DP progeny from cultures initiated with Lfng Tg+ (solid line) versus WT (dotted line) DN4 thymocytes at each time point.
Lfng overexpression greatly enhanced binding of both DL1-Fc (up to ∼3-fold higher) and DL4-Fc (up to ∼ 4.5-fold higher) by DN, CD8 ISP, and DP subsets (Figure 5B, supplemental Table 3). However, as expected based on the decline in Lck proximal promoter activity after the DP stage,38 Tg− and Lfng Tg+ SP thymocytes bound similar levels of both fusion proteins. These data show that HSCs and all thymocyte subsets exhibit much stronger binding of DL4 than DL1, and that Lfng overexpression via the Lck-proximal promoter enhances binding of both DL ligands from the DN to DP stages of thymocyte development.
To determine whether Lfng overexpression prolongs DL/Notch signaling, we evaluated the expression of CD25, a direct Notch1 transcriptional target.22 Interestingly, although Lfng Tg+ DN4 thymocytes were isolated as CD25− cells, they rapidly re-expressed CD25 after culture with OP9-DL1 or OP9-DL4. Thus, the CD25 locus remains Notch inducible in DN4 thymocytes. Both the DN and DP progeny of Lfng Tg+ DN4 thymocytes expressed higher levels of CD25 than Tg− DN4 progeny throughout the 9-day culture period (Figure 5C). These data provide direct evidence that Lfng enhances and prolongs DL-induced Notch signaling during the DN3-DP transition.
Expression of the IL-7 receptor α chain (also known as CD127) is highly expressed in DN2 and DN3 thymocytes but is normally down-regulated by the DP thymocyte stage.39 A recent study reported that CD127 is a direct Notch1 target in human DN2 thymocytes and T-cell leukemia cell lines.40 Although DP thymocytes generated from Tg− and Tg+ DN4 thymocytes in OP9-DL cultures expressed CD25, which is indicative of active Notch signaling, they down-regulated CD127 normally (supplemental Figure 5B). Thus, Notch activation is not sufficient to maintain CD127 expression in nonmalignant DP thymocytes, and Lfng overexpression does not induce aberrant CD127 expression in DP thymocytes.
Pre-TCR and Notch signaling collaboratively induce blastogenesis and proliferation of TCRβ+ DN thymocytes
Our findings suggested that a major function of DL-induced Notch signaling during β-selection is to promote transient proliferative self-renewal of TCRβ+ DN thymocytes before they differentiate into nonproliferative DP thymocytes. This model predicts that withdrawal of Notch signals should lead to rapid differentiation of TCRβ+ DN thymocytes into DP cells, whereas maintenance of DL/Notch activation should promote proliferative expansion of TCRβ+ DN thymocytes. However, published studies have shown that few viable progeny can be recovered after 1 week of culture of DN3 or DN4 thymocytes on OP9 cells lacking DL Notch ligands,20,24 so this notion has not been carefully evaluated. Therefore, we examined the fate and phenotype of DN3 or DN4 thymocytes after only 24 hours of coculture on OP9 versus OP9-DL4. A previous study used this approach to show that DN3 and DN4 thymocytes require both pre-TCR and Notch signaling to up-regulate nutrient receptors such as CD71 and CD98 and undergo blastogenesis.25 However, pre-TCR signaling has been implicated in the survival of TCRβ+ DN thymocytes,41 and these experiments did not determine whether, in the absence of Notch signaling, pre-TCR–expressing thymocytes died or survived but failed to undergo blastogenesis. Accordingly, in our experiments we evaluated cultured DN3 and DN4 thymocytes for intracellular TCRβ expression to determine the fate of pre-TCR+ versus pre-TCR− cells after 1 day of culture.
High expression of CD25 was maintained on DN3 and DN4 progeny in both the pre-TCR+ and pre-TCR− subsets during 24 hours of culture on OP9-DL4 but not OP9 (Figure 6A-B), confirming a rapid loss of Notch signaling in OP9 cocultures. Surprisingly, Notch signaling minimally affected the ratio of pre-TCR− to pre-TCR+ cells, suggesting that it did not induce preferential survival of pre-TCR+ DN thymocytes over the first 24 hours of culture. Similar to previous studies,24 we observed minor trophic effects of Notch signaling on pre-selection (TCRβ−) DN3 progeny, because cell size and expression of CD71 and CD98 nutrient receptors were slightly higher after culture on OP9-DL4 (Figure 6). Strikingly, however, TCRβ+ DN3 progeny cultured on OP9-DL4 were much larger and expressed much higher levels of CD71 and CD98 than TCRβ− DN3 progeny cultured on OP9-DL4 (Figure 6C-E). These data demonstrate that Notch signaling had a more robust trophic effect on TCRβ+ relative to TCRβ− DN3 progeny, revealing that Notch activation collaborates with pre-TCR signaling to enhance the trophic effects of Notch activation in pre-DP thymocytes.
Pre-TCR and Notch signaling collaboratively induce blastogenesis and proliferation of TCRβ+ DN thymocytes. DN3 or DN4 thymocytes (2 × 104) from WT mice were sorted onto OP9 versus OP9-DL4 monolayers and cultured for 24 hours. DN3 progeny (A) and DN4 progeny (B) were harvested and stained with anti-CD4–PE, anti-CD8–APC, anti-CD25– FITC, and anti-TCRβ CyCHR. Contour plots display expression of CD25 versus TCRβ on total progeny (left) or CD4 versus CD8 expression on TCRβ+ progeny (right). (C-E) DN3 progeny (left) and DN4 progeny (right) were harvested and stained with anti-CD71–FITC, CD98-PE, and anti-TCRβ CyCHR. Histograms display FSC (C), CD98 (D), and CD71 (E) profiles on TCRβ+ or TCRβ− progeny 24 hours after culture on OP9 (dotted histograms) versus OP9-DL4 (solid histograms).
Pre-TCR and Notch signaling collaboratively induce blastogenesis and proliferation of TCRβ+ DN thymocytes. DN3 or DN4 thymocytes (2 × 104) from WT mice were sorted onto OP9 versus OP9-DL4 monolayers and cultured for 24 hours. DN3 progeny (A) and DN4 progeny (B) were harvested and stained with anti-CD4–PE, anti-CD8–APC, anti-CD25– FITC, and anti-TCRβ CyCHR. Contour plots display expression of CD25 versus TCRβ on total progeny (left) or CD4 versus CD8 expression on TCRβ+ progeny (right). (C-E) DN3 progeny (left) and DN4 progeny (right) were harvested and stained with anti-CD71–FITC, CD98-PE, and anti-TCRβ CyCHR. Histograms display FSC (C), CD98 (D), and CD71 (E) profiles on TCRβ+ or TCRβ− progeny 24 hours after culture on OP9 (dotted histograms) versus OP9-DL4 (solid histograms).
Interestingly, DP progeny were generated from TCRβ+ DN3 thymocytes on OP9 but not on OP9-DL4 after 24 hours (Figure 6A). The generation of DP progeny on OP9 required TCR expression, but up-regulation of CD27, a marker of β-selection,14 occurred only when DL/Notch signaling was maintained (supplemental Figure 6). Furthermore, DN4 thymocytes cultured on OP9 shrank and lost CD71, and nearly all became DP, whereas most DN4 progeny remained large and few differentiated to the DP stage after culture on OP9-DL4 (Figure 6B). These data suggest that in addition to the profound trophic effect of Notch signaling on TCRβ+ DN thymocytes, Notch activation maintains them at a pre-DP stage of differentiation, at least in the short term.
Minimal proliferation and rapid differentiation of TCRβ+ DN thymocytes in the absence of DL-induced Notch signaling
To follow the longer-term consequences of maintaining DL-induced Notch signaling in DN4 progeny, we quantified the number of DN, CD8 ISP, and DP progeny generated from 5000 Tg− DN4 thymocytes after 2, 4, and 6 days of coculture with OP9 versus OP9-DL4 stromal cells. We observed striking differences in both total cell recoveries and phenotypes of DN4 progeny after only 2 days of coculture with OP9 versus OP9-DL4 (Figure 7). There was no net increase in cell number when DN4 cells were cultured on OP9, and > 70% differentiated to the DP stage by day 2 (Figure 7A). At this time, all DN4 progeny from OP9 cultures were CD25− (Figure 7B), attesting to a rapid loss of active Notch signaling. Approximately 5300 progeny were recovered from OP9 cocultures after 2 days, and ∼ 4200 of these had differentiated to the DP stage, compared with roughly 500 DN and 500 CD8 ISP progeny (Figure 7). Only ∼ 1300 viable progeny (95% DP) were recovered from OP9 cocultures after 4 days, and none were recovered by day 6. Thus, in the absence of Notch signals, most DN4 progeny did not proliferate but rapidly differentiated into DP thymocytes, which then died during days 4-6, which is in keeping with the known short lifespan of this subset.
Minimal proliferation and rapid differentiation of TCRβ+ DN thymocytes in the absence of DL-induced Notch signaling. (A) DN4 thymocytes (5 × 103) from Lfng Tg− mice were sorted and cultured on OP9 versus OP9-DL4 monolayers. Progeny were harvested, counted, and stained with anti-CD4–PE, anti-CD8–APC, and anti-CD25–FITC to measure the number of DN (diamonds), CD8 ISP (squares), and DP (circles) progeny at each time point. (B) Contour plots display expression of CD4 versus CD8 on progeny harvested on day 2. Histograms display CD25 expression on DN (solid), CD8 ISP (dotted), and DP (dashed) progeny. (C) DN3 or DN4 thymocytes from WT mice were sorted, labeled with CFSE, and cultured on OP9 versus OP9-DL4 monolayers. Progeny were harvested after 2 days and stained with anti-CD4–APC and anti-CD8–APC. Contour plots depict CD4 + CD8 versus CFSE expression.
Minimal proliferation and rapid differentiation of TCRβ+ DN thymocytes in the absence of DL-induced Notch signaling. (A) DN4 thymocytes (5 × 103) from Lfng Tg− mice were sorted and cultured on OP9 versus OP9-DL4 monolayers. Progeny were harvested, counted, and stained with anti-CD4–PE, anti-CD8–APC, and anti-CD25–FITC to measure the number of DN (diamonds), CD8 ISP (squares), and DP (circles) progeny at each time point. (B) Contour plots display expression of CD4 versus CD8 on progeny harvested on day 2. Histograms display CD25 expression on DN (solid), CD8 ISP (dotted), and DP (dashed) progeny. (C) DN3 or DN4 thymocytes from WT mice were sorted, labeled with CFSE, and cultured on OP9 versus OP9-DL4 monolayers. Progeny were harvested after 2 days and stained with anti-CD4–APC and anti-CD8–APC. Contour plots depict CD4 + CD8 versus CFSE expression.
We observed strikingly different kinetics of both proliferation and differentiation when DN4 thymocytes were cocultured with OP9-DL4 cells. More than 61 000 DN4 progeny (∼ 12-fold more than input) were recovered after 2 days, but only 18 000 (28%) had differentiated to the DP stage. The remaining cells were DN (∼ 19 000) and CD8 ISP (∼ 24 000) progeny. All day-2 progeny from OP9-DL4 cocultures expressed CD25 (Figure 7B), but CD25 levels declined as DN4 differentiation progressed through the CD8 ISP to DP stages, likely reflecting the down-regulation of both Lfng10 and Notch126,42 during the DN3-DP transition. Thus, sustained DL4-induced Notch signaling promotes pre-DP thymocytes to undergo 3-4 rounds of cell division with minimal differentiation to the DP stage during the first 2 days of culture.
To track cell division in these cocultures, we carried out additional experiments in which DN3 or DN4 progenitors were labeled with CFSE before culturing on OP9 versus OP9-DL4. We observed considerable dilution of CFSE when DN3 or DN4 cells were cultured for 2 days on OP9-DL4, but very little when they were cultured on OP9 (Figure 7C). Furthermore, most DN4 progeny expressed high levels of CD4 and CD8 when cultured on OP9, whereas they remained DN or CD8 ISP (indicated by lower expression of CD4 and CD8) after culture on OP9-DL4. Thus, sustaining Notch signaling greatly enhanced proliferation of pre-DP progenitors during the first 2 days of culture. Pre-DP thymocytes continued to expand on OP9-DL4 between days 2 and 4. During days 4-6, the total numbers of DN and CD8 ISP progeny slowly declined and the numbers of DP progeny increased.
These short-term culture experiments demonstrate that in the absence of DL/Notch signaling, pre-DP thymocytes proliferate very little and instead rapidly differentiate into DP thymocytes, whereas maintenance of DL/Notch signaling maintains metabolism and promotes proliferation of pre-DP progenitors. Differentiation of pre-DP into DP thymocytes occurs primarily in the later phases of OP9-DL4 cultures, and is presumably facilitated by the declining levels of Notch activation due to the death of DL4-expressing cells. Due to the robust proliferation of pre-DP thymocytes during days 2-4, the cumulative production of DP thymocytes over 6 days was 100-fold greater in OP9-DL4 than in OP9 cultures.
Discussion
In this study, we have shown that Lfng is required to enhance Notch-dependent clonal expansion of committed αβΤ cell DN3b and DN4 thymocytes during thymocyte β-selection. DN3 thymocytes conditionally lacking Lfng generated very few DP thymocytes in vivo. Furthermore, when DN3 and DN4 thymocytes were cultured without DL Notch ligands in vitro, they rapidly differentiated into DP thymocytes without proliferating. Conversely, Lfng overexpression in DN3 and DN4 thymocytes prolonged responsiveness to DL ligands and maintained IL7-responsive pre-DP subsets in OP9-DL cultures for much longer periods of time than in cultures of WT DN3 or DN4 progenitors. These studies reveal that Lfng-enhanced DL/Notch signaling prolongs DL/Notch-induced survival and proliferation of IL7-responsive pre-DP subsets without inducing aberrant IL-7 responsiveness in DP thymocytes. Thus, Lfng-enhanced DL/Notch signaling greatly increases the number of DP thymocytes that can be generated from each pre-TCR–expressing DN3b or DN4 progenitor. Given the relatively low frequency of DP thymocytes that make positively selectable αβ TCRs,18 maximizing the number of DP thymocytes generated is critically important to ensuring the development of a sufficiently diverse repertoire of αβ T cells to provide immunity against a wide range of microorganisms.
Even though Notch1 has relatively high affinity for DL4 when Lfng is expressed at low levels,36 our data demonstrate that Lfng is essential to producing a normal number of DP thymocytes during β-selection in vivo. Furthermore, our data implicate a DL rather than a Jagged Notch ligand in promoting the DN3-DP transition. Although Jagged2 is highly expressed in the thymic cortex,35 we have recently shown that Fringe proteins suppress Notch activation by Jagged2 in T-cell progenitors (J.S.Y., J.B.T., I.V., P. Stanley, T. Taghon, S.E.E., and C.J.G., manuscript in preparation). Therefore, if Jagged2 played an important role in β-selection, then conditional deletion of Lfng in DN3 thymocytes would have been expected to enhance rather than compromise the DN3-DP transition in vivo. These data therefore indicate that β-selection is driven by DL rather than Jagged ligands. Because DL1 is not essential for T-cell development,43 and only DL4 was highly expressed by subcapsular thymic epithelial cells, we suggest that DL4 drives β-selection in vivo.
DN4 thymocytes rapidly differentiated into DP thymocytes without proliferating when cultured in the absence of DL Notch ligands. This finding is in agreement with older studies showing that purified DN3b and DN4 thymocytes rapidly generate DP thymocytes in the absence of Notch signaling in vitro.14,44 Although this suggests that stromal cell and Notch independence emerges by the DN3b stage, our data clearly show that DN3b thymocytes are highly dependent on Lfng-enhanced Notch signaling to generate the large number of DP thymocytes needed in vivo. Furthermore, we showed that overexpression of Lfng prolongs Notch-induced self-renewal of early post–β-selection thymocytes while delaying pre-TCR–induced differentiation in vitro. Thus, over the longer term, many more DP thymocytes develop when Notch signaling is maintained in DN3b and DN4 progenitors than when it is inactive, as illustrated by the results of the present study and those of others.19,20,45
Our studies provide novel insights into the functions of Notch and pre-TCR signaling and mechanisms by which these pathways collaborate during β-selection. We and others24 have observed a weak trophic effect of DL-induced Notch activation in pre-selection TCRβ− DN3 thymocytes. However, DL-induced Notch signaling induced a much greater increase in cell size and nutrient receptor expression in TCRβ+ DN thymocytes. Transferrin uptake via CD71 is essential for the proliferative burst that occurs during the DN3-DP transition.46 These data suggest that pre-TCR signaling greatly augments weak trophic functions of Notch to drive rapid proliferation of TCRβ+ pre-DP thymocytes. The inability of pre-TCR–expressing DN thymocytes to maintain nutrient receptor expression, metabolism, and proliferation in the absence of DL-induced Notch activation may reflect down-regulation of pre-TCR signaling, because pTα expression is regulated by Notch signaling in DN2 thymocytes.47
Ectopically maintaining Lfng expression at high levels across the DN3-DP transition keeps DN4 and CD8 ISP progenitors more responsive to DL ligands and maintains them for longer periods of time as pre-DP cells when cultured on OP9-DL4. Therefore, our data imply that in normal thymocytes, decreasing Lfng expression during the DN3-DP transition10 is important for attenuating DL/Notch1-induced self-renewal to allow differentiation into DP thymocytes that express very low levels of Notch1.21,37 Pre-TCR signals also contribute to the eventual attenuation of Notch signaling by inducing inhibitors of E proteins that positively regulate Notch1 transcription.26 Notch1 signaling also positively regulates Notch1 transcription in an auto-feedback loop,26 which will be disrupted as Notch1 transcription declines. Thus, maintaining Lfng expression at high levels in pre-TCR–expressing cells may maintain Notch1-induced Notch1 transcription for longer periods of time, delaying differentiation into postmitotic DP thymocytes.
Activating Notch1 mutations are frequent in human and murine T-cell leukemias, and in both species the malignant blasts are often arrested during the DN3-DP transition.27,28 We suggest that the potent leukemogenic activity of dysregulated Notch1 signaling derives from its robust ability to delay differentiation and promote self-renewal of committed αβ T-cell progenitors during normal T-cell development. While murine T-cell leukemias induced by retroviral or transgenic overexpression of active Notch are typically DP, we have found that CD8 ISP cells are often prominent in T-cell leukemias harboring spontaneous Notch1 mutations in Atm−/− mice (I.R.M., P. C. Wong, J.S.Y., R. Haw, V. Y. Ling, I. Grandal, J. Ellis, J. Beyene, A. Canty, J.S.D., and C.J.G., manuscript in preparation). Furthermore, CD8 ISP but not DP thymocytes contained pre-leukemic progenitors 2 weeks after retroviral transduction of active Notch into HSCs.48 Thus, leukemogenic Notch signaling can promote the aberrant survival and proliferation of pre-DP thymocytes, although in some cases they eventually progress to the DP stage of development. Interestingly, a large-scale retroviral mutagenesis screen revealed that ectopic expression of Lfng and full-length Notch1 are often coselected during T-cell leukemogenesis,49 so Lfng may enhance leukemogenic Notch1 signaling in some cases. Our study suggests that the precipitous decline in Lfng expression and Notch1 activation that occurs during the late stages of the DN3-DP transition may protect against leukemic transformation of committed T-cell progenitors by limiting the duration of DL/Notch-induced self-renewal of pre-DP thymocytes.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Chris Paige, Juan-Carlos Zuniga-Pflucker, Janet Rossant, Ellen Richie, Freddy Radtke, and Conrad Bleul for reagents and Mike Gellalchew for technical support.
This work was supported by operating grants from the Canadian Institutes of Health Research (FRN 11 530 to C.J.G.) and the Canadian Cancer Society Research Institute (CCRS 20 490 to S.E.E.). J.S.Y., J.B.T. and I.V. were supported by the RESTRACOMP fund from the Hospital for Sick Children Research Institute. I.V. was also supported by a postdoctoral fellowship award from the Canadian Institutes of Health Research.
Authorship
Contribution: J.S.Y., J.B.T., I.V, I.R.M., and P.U. performed experiments and analyzed data; K.X. and S.E.E. contributed mice and expertise; C.J.G. and J.S.D. supervised the experimental work; and C.J.G. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Cynthia J. Guidos, Hospital for Sick Children Research Institute, TMDT Bldg (East Tower), Rm 14-312, 101 College St, Toronto, ON M5G 1L7 Canada; e-mail: cynthia.guidos@sickkids.ca.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal