Abstract
The heterogeneity and mechanisms for the generation of CD4 memory T (CD4 Tm) cells remain elusive. Distinct subsets of dendritic cells (DCs) have been found to regulate a distinct T-helper (Th)–cell subset differentiation by influencing cytokine cues around CD4 T cells; however, whether and how the regulatory DC subset can regulate Tm-cell differentiation remains unknown. Further, there is no ideal in vitro experimental system with which to mimic the 3 phases of the CD4 T-cell immune response (expansion, contraction, memory generation) and/or to culture CD4 Tm cells for more than a month. By analyzing CD4 T cells programmed by long-term coculture with regulatory DCs, we identified a population of long-lived CD4 T cells with a CD44hiCD62L−CCR7− effector memory phenotype and rapid, preferential secretion of the Th2 cytokines interleukin-4 (IL-4), IL-5, IL-10, and IL-13 after antigenic stimulation. These regulatory DC-programmed Tm cells suppress CD4 T-cell activation and proliferation in vitro via IL-10 and inhibit the delayed-type hypersensitivity response once infused in vivo. We also identify their natural counterpart, which is up-regulated by regulatory DC transfusion and negatively regulates the recall response in vivo. Different from interferon-γ–producing conventional Tm cells, these IL-4–producing CD4 Tm cells act as alternative Tm cells with a regulatory function, suggesting a new way of negative immune regulation by memory T cells.
Introduction
Immunological memory, the hallmark of the immune system, can benefit the host by initiating a rapid and more effective immune response against invading pathogens, but it can also damage host tissues by mediating inflammation in response to allergens. Although the generation and maintenance of CD8 T-cell memory against infectious diseases or after vaccination has been extensively studied, little is known about the generation and maintenance of CD4 T-cell memory and its underlying mechanisms. One of major challenges for studying CD4 T-cell memory is the low precursor frequency of CD4 memory T (CD4 Tm) cells in vivo and the rare Tm cells induced and maintained in vivo to track or detect during a normal immune response or after vaccination. There have been various models developed for the study of Tm cells, such as the adoptive transfer of T-cell receptor (TCR)–transgenic CD4 T cells followed by the induction of CD4 T-cell activation by the delivery of antigen in vivo or the induction of endogenous antigen-specific CD4 T cells activated by antigen co-administrated with adjuvant.1 In the latter, genuine Tm cells could be induced, but this is difficult to detect by major histocompatibility complex class II tetramers or by cytokine production. In addition, up to now there has been no ideal in vitro experimental system with which to culture or expand Tm cells. Thus, establishing a long-term in vitro culture system for Tm cells will contribute to further identification of the precise mechanisms for their generation and maintenance, thus providing a better understanding of the immune response in diseases and after vaccination.
After activation, naive CD4 T cells can expand and differentiate into a variety of effector subsets, including T-helper 1 (Th1), Th2, Th17, follicular helper T cells, and inducible regulatory T (Treg) cells, all of which have different cytokine profiles and distinct effector functions.2-4 Furthermore, a small population of antigen-experienced CD4 T cells differentiate into Tm cells, providing systemic immune surveillance to react promptly in case of re-attack. The heterogeneous Tm cells can be divided into effector memory T cells (TEM) and central memory T cells (TCM) on the basis of their distinct cell phenotype, effector function, and capacity to migrate to secondary lymphoid organs or sites of tissue inflammation.5-7 TCM cells, which patrol secondary lymphoid organs, undergo homeostatic proliferation in response to the cytokines interleukin-7 (IL-7) and IL-15, and can differentiate into effectors. Meanwhile, TEM cells, which act as sentinels in peripheral tissues, display a reduced proliferative capacity and can initiate immediate effector functions once restimulated with antigen. TEM and TCM can be further divided into Th1 or Th2 cell subsets that are prone to producing Th1 or Th2 cytokines, respectively, after TCR triggering.5,8 It is still unclear whether heterogeneous Tm cells prone to producing Th1 or Th2 cytokines are precommitted before Tm-cell formation or if this is determined by recall response conditions.9 The Th2 memory CD4 T cells, which preferentially produce IL-4, IL-5, IL-10, and IL-13, have been found to be accumulated in the inflammatory tissues and act as an important mediator in allergic diseases.10,11 Th2 memory CD4 T cells can be also induced in Th2-prone BALB/c mice infected with intestinal nematode parasites, and were found to mediate protective function against parasite infection by inducing the generation of alternatively activated macrophages.12
Recent studies have focused on the classical Th2 cytokine function of Th2 memory CD4 T cells. Although thymic stromal lymphopoietin-dendritic cells (TSLP-DCs; CD11c+ DCs activated by TSLP) have been recently shown to promote the maintenance of human Th2 central memory T cells,13 to date, little is known about the mechanisms for the generation and function of Th2 effector memory CD4 T cells. Because of the lack of long-term culture methods and an expansion system to provide enough purified Th2 memory CD4 T cells for functional study in vitro or adoptive transfer in vivo, there have been no studies showing whether Th2 memory CD4 T cells may have other new functions in addition to the mediation of allergic inflammation.
DCs, which link innate and adaptive immunity for preventive and therapeutic responses against diseases, also play important roles in tolerance induction and maintenance.14-16 Regulatory or tolerogenic DCs with regulatory properties have attracted much attention due to their potential important role in preventing autoimmune diseases and transplantation rejection or in controlling chronic inflammation.17 Regulatory DCs can suppress the T-cell response by inhibiting T-cell proliferation, inducing regulatory T cells, or inducing T-cell anergy.17-21 In addition, distinct subsets of DCs can regulate distinct Th cell subset differentiation by influencing cytokine cues around CD4 T cells.22 Cellular and molecular mechanisms underlying the regulation of Th1- and Th2-cell differentiation by DC subsets have been widely investigated; however, whether and how regulatory DCs can regulate the differentiation and maintenance of Th cells, especially Th2 memory cells, remains unknown.
Our previous study showed that endothelial splenic stroma could drive mature DCs to proliferate and differentiate into one new population of CD11bhi Ialow DCs (diffDCs) which could significantly suppress T-cell proliferation, thus outlining a new way to control the immune response.20 Our subsequent experiment showed that the supernatant derived from endothelial splenic stroma could prolong the lifespan of this kind of regulatory DCs in vitro for up to 30 days (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Interestingly, we also observed that some mature DC-activated CD4 T cells could survive > 30 days after coculture with regulatory diffDCs in the presence of low-dose antigen (supplemental Figure 2). Therefore, we investigated how these long-lived CD4 T cells were generated by regulatory DCs and what their functional characteristics were. We demonstrate that OX-40L expressed by regulatory DCs is responsible for the generation of these long-lived CD4 T cells, which distinctively produce Th2 cytokines, especially IL-4 and IL-10, and suppress the CD4 T-cell response both in vitro and in vivo. Their natural counterpart is identified in vivo, which, up-regulated by regulatory DC adoptive transfer, can regulate the recall response by negative feedback. Our experiments provide a new method with which to control immune homeostasis by Th2 memory CD4 T cells.
Methods
Mice
C57BL/6J and BALB/c mice were obtained from Joint Ventures Sipper BK Experimental Animal Co. EGFP (enhanced green fluorescent protein)–transgenic mice, ovalbumin323-339 (OVA323-339)–specific TCR-transgenic DO11.10 mice or OT-2 mice, B6.SJL-Ptprca Pep3b/BoyJ mice (CD45.1 mice), and B6.PL-Thy1a/CyJ (Thy1.1 mice) were obtained from The Jackson Laboratory and bred in pathogen-free conditions. All experimental manipulations were undertaken in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, with the approval of the Scientific Investigation Board of Second Military Medical University, Shanghai.
Reagents
Recombinant mouse granulocyte-macrophage colony-stimulating factor and IL-4 were from PeproTech. Fluorescence-conjugated monoclonal antibodies (mAbs) to CD4, CD45.1, Thy1.1, CD11c, B7-H1, B7-DC, OX-40L, 4-1BBL, CD25, CD69, CD44, CD62L, Foxp3, CD45RB, CD127, Bcl-2, T-bet, GATA-3, PD-1, CD122, Ly-6C, CD94, CD27, CCR7, CTLA-4, OX-40, GITR, IL-2, interferon-γ (IFN-γ), IL-4, IL-5, or IL-10; biotin-conjugated mAb to Fas, FasL, or transforming growth factor-β (TGF-β); and phycoerythrin (PE)–conjugated streptavidin were from BD Pharmingen or eBioscience. Microbead-conjugated mAbs to CD4 or CD11c were from Miltenyi Biotec. Biotin-conjugated mAb to IL-15Rα, neutralizing antibodies against mouse OX-40L, 4-1BBL, IL-4, IL-10, TGF-β, B7-H1, and CTLA-4, and the isotype control antibodies were from R&D Systems. OVA323-339 was synthesized by Sangon Company. 7-Amino-actinomycin D, lipopolysaccharide, OVA protein, complete Freund adjuvant (CFA), and nitro-L-arginine methyl ester (L-NAME) were from Sigma-Aldrich.
Preparation of mouse mature DCs and diffDCs
Bone marrow–derived mature DCs (mDCs) and diffDCs from C57BL/6J or EGFP mice were generated as described in Zhang et al.20
Long-term coculture of effector CD4 T cells with regulatory DCs
Splenic naive CD4 T cells (1 × 106/mL) from DO11.10 × C57BL/6J F1 hybrid mice were cocultured with 1 × 105/mL of mDCs in the presence of 200nM OVA323-339 for 4 days, then purified using anti-CD4 microbeads as effector CD4 T cells. Then 1 × 106/mL of effector CD4 T cells were cocultured with 1 × 105/mL of diffDCs in the presence of 10nM OVA323-339 and 50% (vol/vol) endothelial splenic stromal cell (ESSC) supernatant (maintaining the long-term survival of diffDCs, as shown in supplemental Figure 1). Half of the medium was removed and replaced with fresh medium containing 50% ESSC supernatant and 10nM OVA323-339 every 7 days. After 30 days or more, nonadherent cells were harvested and positively selected with anti-CD4 microbeads. Purified CD4 T cells were used for further analysis or adoptive transfer in delayed-type hypersensitivity (DTH) assays. In some experiments, neutralizing anti–OX-40L or anti–4-1BBL antibody was added into the coculture system.
Assays for T-cell activation and proliferation
Splenic naive CD4 T cells from DO11.10 × Thy1.1 F1 hybrid mice were used as antigen-specific responders. Thy1.1+ CD4 T cells (1 × 105 in 200 μL/well) were cocultured for 3 days with mDCs (1 × 104 in 200 μL/well) and/or various numbers of Thy1.1−-purified long-lived CD4 T cells in the presence of 200nM OVA323-339. On day 3, supernatants were collected for assay of cytokines by enzyme-linked immunosorbent assay (ELISA), and cells were stained with anti-CD4–fluorescein isothiocyanate, Thy1.1-PE, 7-amino-actinomycin D, and CD25-allophycocyanin (CD25-APC) for flow cytometry. For analysis of CD4 T-cell activation, Thy1.1+ CD4+ 7AAD− live T cells were gated for analyzing CD25 expression. For analysis of CD4 T-cell proliferation, the number of Thy1.1+ CD4+ 7AAD− cells was counted as described in Zhang et al.20 In Transwell experiments, purified long-lived CD4 T cells (5 × 105/well) were cultured in the upper chamber of culture inserts with 0.4-μm pore size, and mDCs (1 × 105/well) and CD4 T cells (1 × 106/well) were added to the lower chamber and incubated in the presence of 200nM OVA323-339. In some experiments, anti–IL-4, anti–IL-10, anti–TGF-β, anti–B7-H1, or anti–CTLA-4 neutralizing antibody or the arginine analog L-NAME was added. Intracellular cytokine staining was performed as described in Zhang et al.20 Flow cytometry was done with a FACSCalibur or LSR II (BD Bioscience), and data were analyzed with CellQuest Version 3.3 software (BD Bioscience) or FlowJo Version 5.7.2 software (TreeStar).
Induction and assessment of DTH response
BALB/c × C57BL/6J F1 mice were each immunized by injecting subcutaneously 60 μL of OVA/CFA emulsion (30 μL of 300 μg OVA plus 30 μL of CFA) into the left footpad on day 0, and then challenged with 300 μg of heat-aggregated OVA (10 mg/mL) in right footpad on day 7. On days −1, 3, and 6, purified long-lived CD4 T cells (3 × 106 cells/mouse) were intravenously transferred into the immunized mice. DTH responses were assessed by measuring the thickness of the challenged footpad prior to and 24 hours after challenge, as described in Tang et al.18 Unimmunized naive mice that were injected with heat-aggregated OVA in both footpads were included as controls for nonspecific swelling.
Induction and detection of antigen-specific CD4 T-cell response in vivo
OVA323-339-specific TCR-transgenic splenic CD4+ T cells from OT-2 × CD45.1 F1 hybrid mice were intravenously injected into C57BL/6J mice (2 × 106 cells/mouse) on day −1. On day 0, 200 μL of OVA/CFA emulsion (100 μL of 500 μg of OVA + 100 μL of CFA) were injected intraperitoneally into C57BL/6J mice adoptively transferred with CD45.1+ OT-2 CD4 T cells. On days 1, 3, 5, 7, 9, 15, 20, and 30, the percentage of CD45.1+ cells in peripheral blood CD4+ T cells was assayed by flow cytometry. On day 6, diffDCs were intraperitoneally injected into the immunized mice (5 × 106 cells/mouse). On days 9, 16, and 30, the splenocytes from these mice were restimulated ex vivo with 400nM OVA323-339 for 18 hours, and for the last 5 hours 10 μg/mL Brefeldin A was added. Cells were then harvested and stained with surface markers including CD62L-fluorescein isothiocyanate, CD4-PerCP, CD45.1-APC and CD44-APC-Cy7, and with PE-conjugated cytokine antibodies using a Cytofix/Cytoperm Kit (BD Bioscience). CD45.1+ CD44hi CD62L− CD4+ T cells were gated for analyzing intracellular cytokine expression. Gated CD44hi CD62L− CD4+ T cells from unimmunized C57BL/6J mice were used as control. To detect recall responses in immunized mice, on day 30 after immunization, the mice were rechallenged by injecting mature DCs (2 × 106 cells/mouse) loaded with 1μM OVA323-339. After 4 days, the percentage of CD45.1+ cells in the peripheral blood and in splenic CD4+ T cells was assayed by flow cytometry.
Statistical analysis
All data analysis was performed using a 2-tailed Student t test. A P value < .05 was considered statistically significant.
Results
Regulatory DCs maintain long-term survival of effector CD4 T cells in vitro via OX-40L
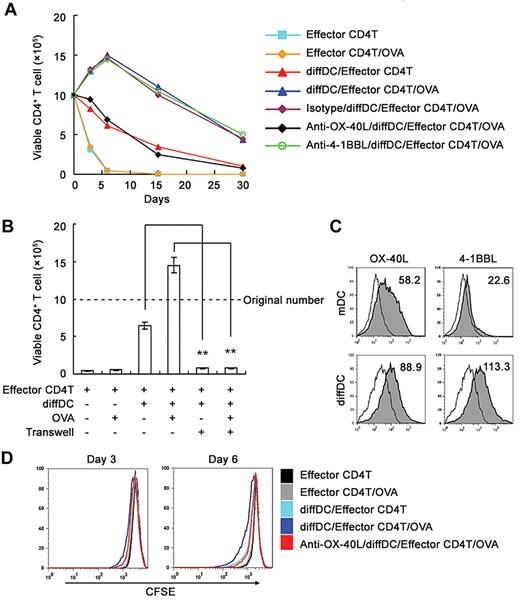
First, OVA323-339-specific CD4 T cells were cocultured with mature DCs in the presence of OVA323-339 for 4 days, then the activated and proliferating CD4 T cells were purified (used as effector T cells) and cocultured with regulatory diffDCs in the presence or absence of OVA323-339. As a control, the effector CD4 T cells cultured in the medium all died within 6 days. Whereas some of those effector T cells cocultured with regulatory DCs survived for > 30 days, most of them died. In the presence of low-dose antigen OVA323-339, more CD4 T cells survived (Figure 1A and supplemental Figure 2). These data demonstrate that regulatory DCs could maintain the survival of effector CD4 T cells for > 1 month. In this experimental system, the 4 phases of CD4 T-cell response, including the priming phase, expansion phase, contraction phase, memory generation, and maintenance phase, could be established and mimicked in vitro. Thus, this experimental system is a good model with which to investigate the mechanisms for the generation and functions of memory CD4 T cells.
Regulatory DCs maintain survival of antigen-specific CD4+ T cells in vitro for > 30 days via OX-40L. (A) Naive CD4+ T cells were activated by mDCs in the presence of antigen OVA323-339 for 4 days, and then the effector CD4+ T cells were purified to coculture with diffDCs at a ratio of 10:1 for various times in the presence of 50% ESSC supernatant and 10nM OVA323-339. In some experiments, anti–OX-40L or anti–4-1BBL blocking antibody (5μg/mL) was added into the coculture system. Viable CD4+ T cells were counted by flow cytometry. (B) The Transwell system was used to separate effector CD4 T cells from diffDCs and then viable CD4 T cells were counted. (C) OX-40L and 4-1BBL expression on diffDCs and mDCs were detected by flow cytometry. Numbers in the histograms indicate the geometric mean fluorescence of the test samples. (D) The purified effector CD4+ T cells labeled with 5μM CFSE were cocultured with diffDCs at a ratio of 10:1 for 3 or 6 days in the presence of 50% ESSC supernatant and 10nM OVA323-339 with or without anti–OX-40L blocking antibody. Viable CD4+ T cells were gated for analyzing CFSE content of CD4 T cells by flow cytometry. **P < .01. Similar results were obtained in at least 3 independent experiments.
Regulatory DCs maintain survival of antigen-specific CD4+ T cells in vitro for > 30 days via OX-40L. (A) Naive CD4+ T cells were activated by mDCs in the presence of antigen OVA323-339 for 4 days, and then the effector CD4+ T cells were purified to coculture with diffDCs at a ratio of 10:1 for various times in the presence of 50% ESSC supernatant and 10nM OVA323-339. In some experiments, anti–OX-40L or anti–4-1BBL blocking antibody (5μg/mL) was added into the coculture system. Viable CD4+ T cells were counted by flow cytometry. (B) The Transwell system was used to separate effector CD4 T cells from diffDCs and then viable CD4 T cells were counted. (C) OX-40L and 4-1BBL expression on diffDCs and mDCs were detected by flow cytometry. Numbers in the histograms indicate the geometric mean fluorescence of the test samples. (D) The purified effector CD4+ T cells labeled with 5μM CFSE were cocultured with diffDCs at a ratio of 10:1 for 3 or 6 days in the presence of 50% ESSC supernatant and 10nM OVA323-339 with or without anti–OX-40L blocking antibody. Viable CD4+ T cells were gated for analyzing CFSE content of CD4 T cells by flow cytometry. **P < .01. Similar results were obtained in at least 3 independent experiments.
Next we investigated how regulatory DCs maintained long-term survival of the effector CD4 T cells. Using a Transwell system, we found that cell-cell contact was required for the process, because the CD4 T cells died quickly once separated from regulatory DCs (Figure 1B), indicating the involvement of membrane molecules on regulatory DCs. It has been shown that TNF family ligands such as OX-40L and 4-1BBL can provide additional signals for maximal maintenance of memory CD4 and CD8 T cells.23 Regulatory DCs expressed membrane OX-40L and 4-1BBL (Figure 1C). Neutralizing Abs against OX-40L, but not anti–4–1BBL, could significantly reduce the long-term survival of effector CD4 T cells maintained by regulatory DCs and low-dose antigen (Figure 1A), indicating that OX-40L played a key role in this process.
We also found that blockade of OX-40L did not affect cell death and Bcl-2 expression of effector CD4 T cells (supplemental Figure 3), but did reduce the CD4 T-cell divisions maintained by regulatory DCs and low-dose antigen (Figure 1D and supplemental Figure 4). The data indicated that maintaining the long-term survival of effector CD4 T cells by regulatory DCs and low-dose antigen in the ex vivo system we established was similar to T-cell “homeostatic proliferation.”
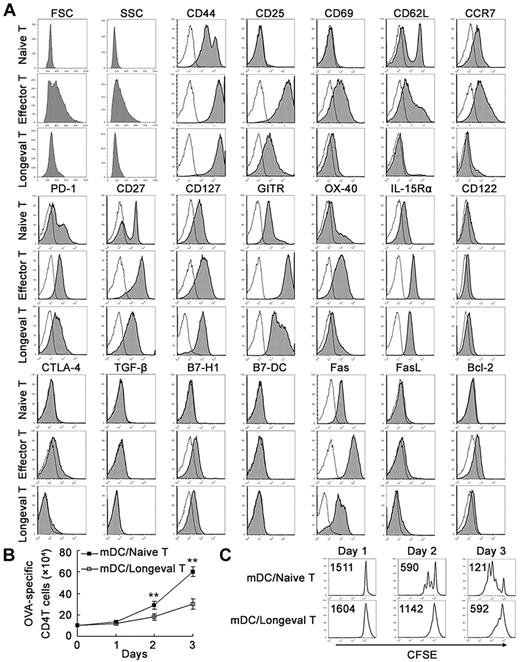
Regulatory DC-maintained, long-lived CD4 T cells display the effector memory T-cell phenotype with low proliferative ability
Next we investigated the characteristics of the long-lived CD4 T cells arrested from the activated CD4 T cells and then maintained by regulatory DCs and low-dose antigen. We analyzed the phenotype of these CD4 T cells cultured for > 30 days and found that they were FSClow SSClow CD25low/− CD44hi CD69− CD62L− CCR7− CD45RBhi CD27low CD127hi GITRlow/int PD-1int IL-15Rαhi CD122− OX-40low Ly-6C− CD94− CTLA-4− TGF-β− B7-H1+ B7-DC− Faslow FasL− and Bcl-2low (Figure 2A and supplemental Figure 5), closely resembling memory T cells with small size (low FSC/SSC), high expression of CD44, down-regulated expression of most effector-associated markers, low expression of the proapoptotic marker Fas, and up-regulated expression of the antiapoptotic marker Bcl-2. According to their phenotype (CD44hi CD62L− CCR7−), these T cells were just like effector memory CD4 T cells. To confirm this, we detected their capacity to proliferate, because it is accepted that effector memory T cells show rapid effector function but reduced proliferation capacity after antigenic stimulation compared with the potent proliferation capacity of central memory CD4 T cells.7,24 As shown in Figure 2B-C, these CD4 T cells proliferated less significantly than naive CD4 T cells, as determined by cell count and carboxyl fluorescein succinimidyl ester (CFSE) division, further indicating that these cells have reduced proliferation capacity similar to that of effector memory CD4 T cells.
Phenotype and proliferative ability of regulatory DC-maintained long-lived CD4 T cells. (A) Naive CD4+ T cells, effector CD4+ T cells, and the long-lived CD4 T cells maintained by regulatory DCs for > 30 days (Longeval CD4+ T cells) were stained for the indicated markers and analyzed by flow cytometry. Slim solid line, background staining. Shaded histograms, specific antibodies. (B) Long-lived CD4 T cells or naive CD4 T cells were stimulated with mDCs and 200nM OVA323-339, and viable CD4+ T cells were counted by flow cytometry on days 1, 2, and 3. (C) The long-lived CD4 T cells or naive CD4 T cells were labeled with 5μM CFSE and then cultured with mDCs in the presence of 200nM OVA323-339. Viable CD4+ T cells were then gated for analyzing the CFSE content of CD4 T cells by flow cytometry on days 1, 2, and 3. Numbers in the histograms indicate the geometric mean fluorescence of the test samples. **P < .01. Similar results were obtained in at least 3 independent experiments.
Phenotype and proliferative ability of regulatory DC-maintained long-lived CD4 T cells. (A) Naive CD4+ T cells, effector CD4+ T cells, and the long-lived CD4 T cells maintained by regulatory DCs for > 30 days (Longeval CD4+ T cells) were stained for the indicated markers and analyzed by flow cytometry. Slim solid line, background staining. Shaded histograms, specific antibodies. (B) Long-lived CD4 T cells or naive CD4 T cells were stimulated with mDCs and 200nM OVA323-339, and viable CD4+ T cells were counted by flow cytometry on days 1, 2, and 3. (C) The long-lived CD4 T cells or naive CD4 T cells were labeled with 5μM CFSE and then cultured with mDCs in the presence of 200nM OVA323-339. Viable CD4+ T cells were then gated for analyzing the CFSE content of CD4 T cells by flow cytometry on days 1, 2, and 3. Numbers in the histograms indicate the geometric mean fluorescence of the test samples. **P < .01. Similar results were obtained in at least 3 independent experiments.
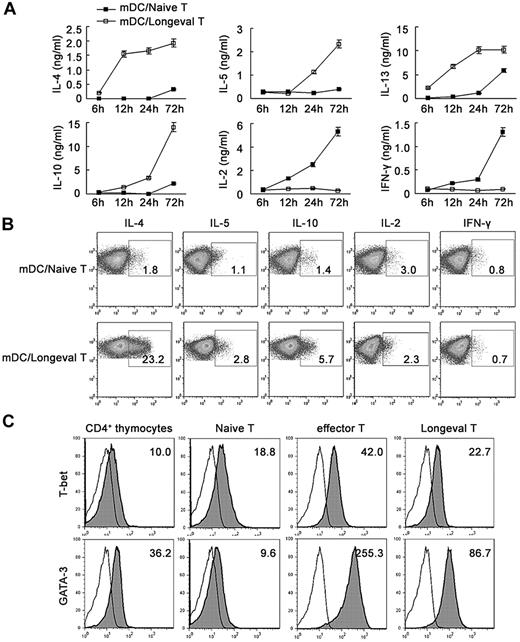
Regulatory DC-maintained, long-lived CD4 T cells preferentially produce Th2 cytokines
We also analyzed cytokine production by these CD4 T cells. In response to antigenic stimulation with mature DCs and OVA323-339, these CD4 T cells produced Th2 cytokines, including IL-4, IL-5, IL-13, and IL-10, faster and more strongly than naive CD4 T cells. These CD4 T cells secreted very low levels of IL-2 and Th1 cytokine IFN-γ compared with naive CD4 T cells (Figure 3A). On a per-cell basis, these CD4 T cells secreted more IL-4, IL-5, and IL-10 than naive CD4 T cells, as determined by intracellular cytokine staining (Figure 3B). In addition, these CD4 T cells expressed high levels of GATA-3 but low levels of T-bet (Figure 3C). The unique patterns of cytokine production of these cells, together with the effector memory phenotype and low proliferative capacity, raised the possibility that these regulatory DC-induced CD4 T cells were more similar to effector memory Th2 cells. Therefore, regulatory DCs could program the generation of a population of memory CD4 T cells displaying effector memory Th2 cell properties.
Cytokine profile and transcription factor expression of regulatory DC-maintained long-lived CD4 T cells. (A) Long-lived CD4 T cells (Longeval CD4 T cells) or naive CD4 T cells were stimulated with mDCs and 200nM OVA323-339 for various times, and the supernatants were collected for assay of cytokines by ELISA. (B) Long-lived CD4 T cells or naive CD4 T cells were cultured with mDCs in the presence of 200nM OVA323-339 for 18 hours and assayed by intracellular cytokine staining for IL-2, IFN-γ, IL-4, IL-5, and IL-10 expression. Numbers in plots indicate the percentage of various cytokine-producing CD4+ T cells. (C) Various CD4+ T cells were detected for T-bet and GATA-3 expression by intracellular staining. CD4+ thymocytes were used as a positive control of GATA-3 expression. Thin solid line, background staining. Shaded histograms, specific antibodies. Numbers in the histograms indicate the geometric mean fluorescence of the test samples. Similar results were obtained in at least 3 independent experiments.
Cytokine profile and transcription factor expression of regulatory DC-maintained long-lived CD4 T cells. (A) Long-lived CD4 T cells (Longeval CD4 T cells) or naive CD4 T cells were stimulated with mDCs and 200nM OVA323-339 for various times, and the supernatants were collected for assay of cytokines by ELISA. (B) Long-lived CD4 T cells or naive CD4 T cells were cultured with mDCs in the presence of 200nM OVA323-339 for 18 hours and assayed by intracellular cytokine staining for IL-2, IFN-γ, IL-4, IL-5, and IL-10 expression. Numbers in plots indicate the percentage of various cytokine-producing CD4+ T cells. (C) Various CD4+ T cells were detected for T-bet and GATA-3 expression by intracellular staining. CD4+ thymocytes were used as a positive control of GATA-3 expression. Thin solid line, background staining. Shaded histograms, specific antibodies. Numbers in the histograms indicate the geometric mean fluorescence of the test samples. Similar results were obtained in at least 3 independent experiments.
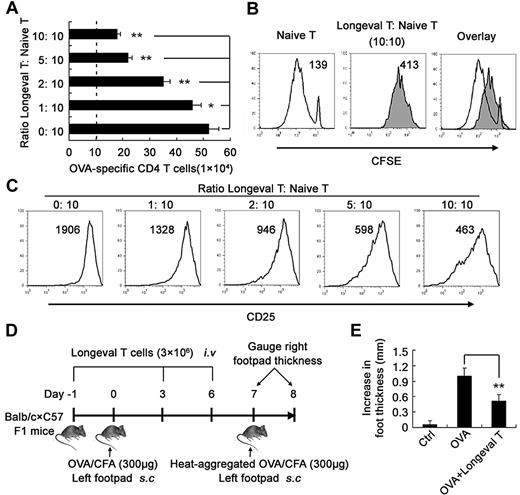
Regulatory DC-programmed memory CD4 T cells suppress CD4 T-cell response both in vitro and in vivo
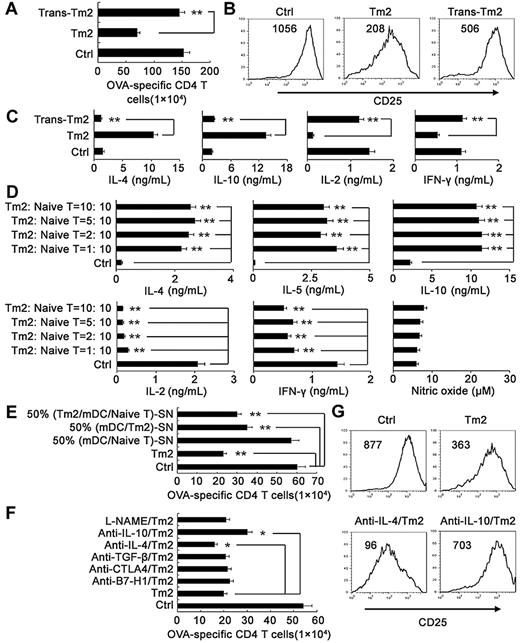
Next we determined the functional characteristics of regulatory DC-programmed memory CD4 T cells. The results showed that these memory CD4 T cells could significantly suppress mature DC-initiated, antigen-specific CD4 T-cell proliferation (Figure 4A), division (Figure 4B), and CD25 expression (Figure 4C). They also significantly suppressed anti-CD3/mature DC-induced nonspecific CD4 T-cell proliferation and activation (data not shown). The data indicated that these regulatory DC-programmed memory CD4 T cells could exert unique regulatory functions in vitro. To exclude the possibility that Treg cells might be induced and involved in the suppression of the CD4 T-cell response, we analyzed the CD25+ Foxp3+ cells in the effector CD4+ T cells cocultured by regulatory DCs plus low-dose antigen for 3 days, but did not find any increase in the number of conventional Treg cells (supplemental Figure 6). We also detected Treg cells in memory CD4 T cells maintained by regulatory DCs plus low-dose antigen for > 30 days, and did not find the presence of CD25+ Foxp3+ conventional Treg cells among these memory CD4 T cells. Furthermore, these memory CD4 T cells did not increase the number of CD25+ Foxp3+ conventional Treg cells in mDC-activated CD4 T cells (supplemental Figure 7). Therefore, conventional Treg cells were not induced or involved in the suppression of CD4 T-cell response by these memory CD4 T cells that we observed. To confirm their regulatory effect in vivo, we adoptively transferred these memory CD4 T cells into recipient mice during OVA antigen sensitization and the challenge phases of DTH. As shown in Figure 4D, adoptive transfer of these memory CD4 T cells could significantly inhibit OVA antigen-induced DTH responses in vivo, indicating their important regulatory roles during the recall response (Figure 4E). Thus, this kind of memory CD4 T cells, programmed and generated by long-term coculture with regulatory DCs in vitro, suppressed the T-cell response both in vitro and in vivo. Based on these observations and also inspired by the classification of Th1 and Th2, we propose designating these regulatory DC-programmed, immunosuppressive memory CD4 T cells as type 2 memory T cells (Tm2) as distinct from the conventional IFN-γ–producing CD4 T cells, which might be designated as type 1 memory T cells (Tm1).
Regulatory DC-maintained long-lived CD4 T cells suppress antigen-specific CD4 T-cell activation and proliferation both in vitro and in vivo. (A-C) The suppressive effect of the long-lived CD4 T cells (Thy1.1−, Longeval CD4 T cells) on proliferation and CD25 expression of Thy1.1+ naive CD4 T cells activated by mDCs for 3 days in the presence of 200nM OVA323-339. On day 3, Thy1.1+ CD4+ 7AAD− viable T cells were counted (A) and gated for analyzing CD25 expression (C) by flow cytometry. For analyzing the influence of the long-lived CD4 T cells on CD4 T-cell division initiated by mDCs and OVA323-339, Thy1.1+ CD4 T cells were labeled with CFSE, and Thy1.1+ CD4+ 7AAD− viable T cells were gated for analyzing CFSE content (B). (D) Schematic of the experimental design of the DTH response. (E) BALB/c × C57BL/6J F1 mice were sensitized with OVA/CFA on day 0. On days −1, 3, and 6, the long-lived CD4 T cells (3 × 106 cells/mouse) were adoptively transferred into the immunized mice via intravenous injection. On day 7, the mice were challenged with heat-aggregated OVA, and then DTH responses were assessed over the following 24 hours. The mice of the unimmunized control group (Ctrl) were challenged with heat-aggregated OVA. The DTH responses are expressed as the mean ± SDs of the increase in footpad thickness for 5 mice per group. *P < .05; **P < .01. Similar results were obtained in at least 3 independent experiments.
Regulatory DC-maintained long-lived CD4 T cells suppress antigen-specific CD4 T-cell activation and proliferation both in vitro and in vivo. (A-C) The suppressive effect of the long-lived CD4 T cells (Thy1.1−, Longeval CD4 T cells) on proliferation and CD25 expression of Thy1.1+ naive CD4 T cells activated by mDCs for 3 days in the presence of 200nM OVA323-339. On day 3, Thy1.1+ CD4+ 7AAD− viable T cells were counted (A) and gated for analyzing CD25 expression (C) by flow cytometry. For analyzing the influence of the long-lived CD4 T cells on CD4 T-cell division initiated by mDCs and OVA323-339, Thy1.1+ CD4 T cells were labeled with CFSE, and Thy1.1+ CD4+ 7AAD− viable T cells were gated for analyzing CFSE content (B). (D) Schematic of the experimental design of the DTH response. (E) BALB/c × C57BL/6J F1 mice were sensitized with OVA/CFA on day 0. On days −1, 3, and 6, the long-lived CD4 T cells (3 × 106 cells/mouse) were adoptively transferred into the immunized mice via intravenous injection. On day 7, the mice were challenged with heat-aggregated OVA, and then DTH responses were assessed over the following 24 hours. The mice of the unimmunized control group (Ctrl) were challenged with heat-aggregated OVA. The DTH responses are expressed as the mean ± SDs of the increase in footpad thickness for 5 mice per group. *P < .05; **P < .01. Similar results were obtained in at least 3 independent experiments.
Regulatory DC-programmed memory CD4 T cells suppress CD4 T-cell response via cell contact-dependent IL-10 production
We sought to determine the underlying mechanisms of how Tm2 cells exert their regulatory function. CD4 Tm2 cells could not suppress CD4 T-cell activation and proliferation once CD4 Tm2 cells were separated from mDC/naive CD4 T cells in the Transwell system, indicating that the regulatory function of CD4 Tm2 cells required cell-cell contact between CD4 Tm2 cells and mDC/CD4 T cells (Figure 5A-B). Interestingly, once CD4 Tm2 cells were added to the mDC/naive CD4 T-cell coculture system, there were higher levels of Th2 cytokines, including IL-4, IL-5, and IL-10, but lower levels of IL-2 and the Th1 cytokine IFN-γ in the supernatant (Figure 5D). In addition, the abundant IL-4 and IL-10 production in the supernatant of CD4 Tm2/mDC/naive CD4 T-cell coculture system also depended on cell-cell contact between CD4 Tm2 cells and mDC/CD4 T cells, indicating that Th2 cytokines might be involved in the regulatory function of CD4 Tm2 cells (Figure 5C). Further experiments showed that the supernatant derived from the mDC/CD4Tm2 coculture system significantly suppressed T-cell proliferation, and the supernatant derived from CD4Tm2/mDC/CD4T cell coculture system suppressed T-cell proliferation more significantly (Figure 5E). Considering the high amount of the Th2 cytokines IL-4 and IL-10 in the supernatants, we added anti–IL-4 or anti–IL-10 blocking antibody, and found that the anti–IL-10 antibody significantly reversed the suppression of activation and proliferation of CD4 T cells. The anti–IL-4 antibody did not reverse the suppression of naive CD4 T-cell proliferation, but instead promoted the suppression of naive CD4 T-cell activation and proliferation (Figure 5F-G). Furthermore, other soluble factors such as TGF-β or nitric oxide and membrane molecules such as CTLA-4 or B7-H1 were shown not to be involved in the regulatory function of CD4 Tm2 cells (Figure 5F). These results suggest that these regulatory DC-programmed memory CD4 T cells suppress the CD4 T-cell response via cell-cell contact-dependent IL-10 production.
Regulatory DC-maintained long-lived CD4 T cells suppress CD4 T-cell response via cell contact-dependent IL-10 production. (A-C) In Transwell experiments, the long-lived CD4 T cells (CD4 Tm2) were separated from the mDC/naive CD4 T cell/OVA323-339 coculture system. On day 3, Thy1.1+ CD4+ T-cell proliferation (A) and CD25 expression (B) were assayed by flow cytometry. The supernatants were collected for assay of cytokines by ELISA (C). (D) Various numbers of Thy1.1− CD4 Tm2 were added into the mDC/naive CD4 T cell/OVA323-339 coculture system. On day 3, supernatants were collected for assay of cytokines by ELISA. (E-G) Thy1.1+ CD4+ T-cell proliferation (E-F) and CD25 expression (G) were assayed when the supernatants from mDC/CD4 T, mDC/Tm2, or Tm2/mDC/CD4 T, or neutralizing antibodies (anti–IL-4, anti–IL-10, anti–TGF-β, anti–B7-H1, or anti–CTLA-4), or arginine analog L-NAME were added into the coculture system. Numbers in the histograms indicate the geometric mean fluorescence of the test samples. *P < .05; **P < .01. Similar results were obtained in at least 3 independent experiments.
Regulatory DC-maintained long-lived CD4 T cells suppress CD4 T-cell response via cell contact-dependent IL-10 production. (A-C) In Transwell experiments, the long-lived CD4 T cells (CD4 Tm2) were separated from the mDC/naive CD4 T cell/OVA323-339 coculture system. On day 3, Thy1.1+ CD4+ T-cell proliferation (A) and CD25 expression (B) were assayed by flow cytometry. The supernatants were collected for assay of cytokines by ELISA (C). (D) Various numbers of Thy1.1− CD4 Tm2 were added into the mDC/naive CD4 T cell/OVA323-339 coculture system. On day 3, supernatants were collected for assay of cytokines by ELISA. (E-G) Thy1.1+ CD4+ T-cell proliferation (E-F) and CD25 expression (G) were assayed when the supernatants from mDC/CD4 T, mDC/Tm2, or Tm2/mDC/CD4 T, or neutralizing antibodies (anti–IL-4, anti–IL-10, anti–TGF-β, anti–B7-H1, or anti–CTLA-4), or arginine analog L-NAME were added into the coculture system. Numbers in the histograms indicate the geometric mean fluorescence of the test samples. *P < .05; **P < .01. Similar results were obtained in at least 3 independent experiments.
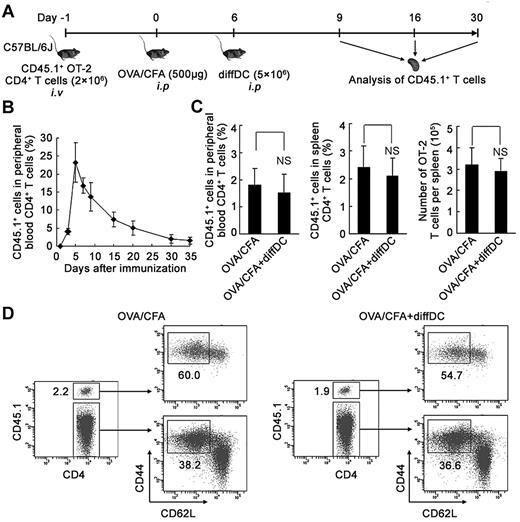
Phenotypic and functional identification of the in vivo natural counterpart of regulatory DC-programmed memory CD4 T cells
To confirm the existence of the in vivo natural counterparts of CD4 Tm2 cells that were IL-4-producing CD44hi CD62L− effector memory CD4 T cells, we established an in vivo model with an antigen-specific CD4 T-cell immune response, which is depicted in Figure 6A. Briefly, mice transferred with CD45.1+ OT-2 naive CD4 T cells 1 day before being immunized by OVA/CFA to induce an OVA323-339-specific CD4 T-cell response. By detecting the percentage of peripheral blood CD45.1+ OT-2 CD4 T cells among CD4+ T cells, the OVA323-339-specific CD4 T-cell response was found to be induced efficiently by OVA/CFA intraperitoneal immunization, reaching a peak on day 5 after immunization and then gradually decreasing (Figure 6B). On day 6, diffDCs were adoptively transferred to the mice and then the memory CD4 T-cell generation was observed by analyzing the CD4 T-cell phenotype and cytokine patterns during the memory phase (> 30 days after immunization). Adoptive transfer of diffDCs did not affect the percentage and number of CD45.1+ memory CD4 T cells in the peripheral blood and spleen (Figure 6C). Further, diffDCs did not affect the percentage of the effector memory CD4 T cells among CD45.1+ memory CD4 T cells (Figure 6D).
Influence of adoptive transfer of regulatory DCs on the frequency and phenotype of memory CD4 T cells in vivo. (A) Schematic of experimental design of the induction of antigen-specific CD4 T-cell response in vivo with or without adoptive transfer of diffDCs. (B) Frequency of peripheral blood CD45.1+ CD4+ OT-2 T cells after adoptive transfer into C57BL/6J mice immunized with OVA, presented as the percentage of total CD4+ T cells. (C) The percentage of peripheral blood and splenic CD45.1+ CD4+ OT-2 memory T cells among total CD4+ T cells was assessed by flow cytometry in the mice 30 days after immunization. Absolute numbers of CD45.1+ CD4+ OT-2 memory T cells present in spleen were counted. NS indicates no statistical significance. (D) The expression of CD62L and CD44 on CD45.1+ CD4+ OT-2 memory T cells and endogenous CD4+ T cells from the spleen of immunized mice 30 days after immunization. Numbers in the plots indicate the percentage of gated cells. Similar results were obtained in at least 3 independent experiments.
Influence of adoptive transfer of regulatory DCs on the frequency and phenotype of memory CD4 T cells in vivo. (A) Schematic of experimental design of the induction of antigen-specific CD4 T-cell response in vivo with or without adoptive transfer of diffDCs. (B) Frequency of peripheral blood CD45.1+ CD4+ OT-2 T cells after adoptive transfer into C57BL/6J mice immunized with OVA, presented as the percentage of total CD4+ T cells. (C) The percentage of peripheral blood and splenic CD45.1+ CD4+ OT-2 memory T cells among total CD4+ T cells was assessed by flow cytometry in the mice 30 days after immunization. Absolute numbers of CD45.1+ CD4+ OT-2 memory T cells present in spleen were counted. NS indicates no statistical significance. (D) The expression of CD62L and CD44 on CD45.1+ CD4+ OT-2 memory T cells and endogenous CD4+ T cells from the spleen of immunized mice 30 days after immunization. Numbers in the plots indicate the percentage of gated cells. Similar results were obtained in at least 3 independent experiments.
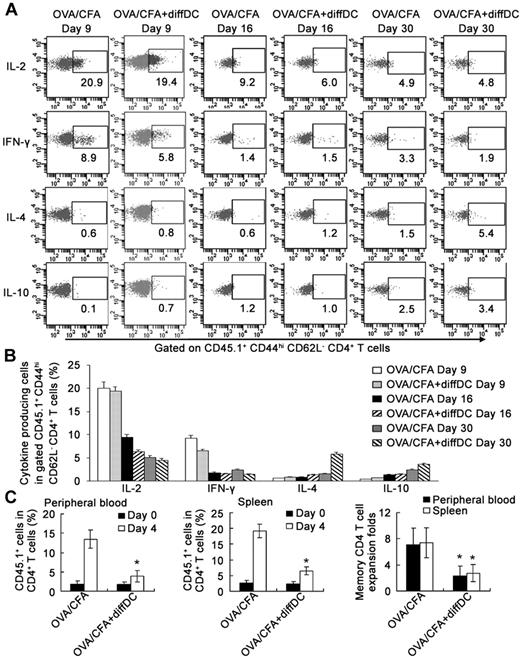
By analyzing the intracellular cytokine patterns of the gated CD45.1+ CD44hi CD62L− CD4 T cells at different times during the immune response, we found that IL-2– or IFN-γ–producing cells were high, but IL-4– or IL-10–producing cells were very rare in the mice on day 9 after immunization. After that, IL-2– or IFN-γ–producing cells decreased, but IL-4– or IL-10–producing cells increased. IL-4– or IL-10–producing CD45.1+ CD44hi CD62L− cells increased significantly in the mice 30 days after OVA/CFA immunization, indicating that the natural counterpart of CD4 Tm2 cells might exist in vivo during the memory phase of the immune response (Figure 7A-B). diffDCs transferred adoptively on day 6 after immunization could increase IL-4– or IL-10–producing cells more significantly on day 30 after immunization, although they had no notable effects on the number of IL-2– or IFN-γ–producing cells (Figure 7A-B). Furthermore, diffDCs transferred adoptively on day 6 after immunization could not increase the percentage of conventional regulatory CD4 T cells among CD45.1+ OT2 CD4 T cells or CD45.1− endogenous CD4 T cells on days 9, 16, and 30 after immunization (supplemental Figure 8). These data indicate that diffDCs can promote the in vivo generation of IL-4–producing CD44hi CD62L− CD4 T cells, which may be generated naturally during the late or memory phase of the antigen-specific immune response.
Detection of in vivo natural counterpart of Tm2 during recall response. (A-B) On days 9, 16, and 30 after immunization (as described in Figure 6), various cytokine-producing cells in CD44hi CD62L− CD45.1+ CD4+ OT-2 cells from splenocytes were analyzed by intracellular staining after ex vivo antigenic restimulation with OVA323-339. Numbers in the plots indicate the percentage of various cytokine-producing cells among gated CD44hi CD62L− CD45.1+ CD4+ OT-2 cells (A) and these are summarized in (B). Each group contained 3 mice. (C) On day 30 after immunization, mice with or without adoptive transfer of diffDCs were rechallenged with 2 × 106 OVA323-339-loading mature DCs via intravenous injection. The percentage of peripheral blood and splenic CD45.1+ CD4+ OT-2 memory T cells among total CD4+ T cells was assessed by flow cytometry in the mice before and 4 days after rechallenge. Memory CD4 T-cell expansion folds were defined as the ratio of the percentage of CD45.1+ CD4+ OT-2 memory T cells among total CD4+ T cells 4 days after and before rechallenge. Each group contained 5 mice. *P < .05, “OVA/CFA+diffDC” group compared with “OVA/CFA” group. Similar results were obtained in at least 3 independent experiments.
Detection of in vivo natural counterpart of Tm2 during recall response. (A-B) On days 9, 16, and 30 after immunization (as described in Figure 6), various cytokine-producing cells in CD44hi CD62L− CD45.1+ CD4+ OT-2 cells from splenocytes were analyzed by intracellular staining after ex vivo antigenic restimulation with OVA323-339. Numbers in the plots indicate the percentage of various cytokine-producing cells among gated CD44hi CD62L− CD45.1+ CD4+ OT-2 cells (A) and these are summarized in (B). Each group contained 3 mice. (C) On day 30 after immunization, mice with or without adoptive transfer of diffDCs were rechallenged with 2 × 106 OVA323-339-loading mature DCs via intravenous injection. The percentage of peripheral blood and splenic CD45.1+ CD4+ OT-2 memory T cells among total CD4+ T cells was assessed by flow cytometry in the mice before and 4 days after rechallenge. Memory CD4 T-cell expansion folds were defined as the ratio of the percentage of CD45.1+ CD4+ OT-2 memory T cells among total CD4+ T cells 4 days after and before rechallenge. Each group contained 5 mice. *P < .05, “OVA/CFA+diffDC” group compared with “OVA/CFA” group. Similar results were obtained in at least 3 independent experiments.
To investigate whether the in vivo counterpart of CD4 Tm2 cells promoted by diffDCs have regulatory functions during recall response, we detected recall responses in immunized mice with or without the adoptive transfer of diffDCs via injecting OVA323-339-loaded mature DCs, and found that recall responses in the immunized mice with adoptive transfer of diffDCs were significantly lower than those of immunized mice without adoptive transfer of diffDCs (Figure 7C). These data indicate that CD4 Tm2 cells promoted by diffDCs play important regulatory roles during the recall response in vivo.
Discussion
The immune system is balanced by generating different immune subsets with reciprocal interactive functions, such as Th1/Th2, type 1 macrophage/type 2 macrophages (M1/M2), or reciprocal control between activating receptors or inhibitory receptors such as NKG2D/NKG2A. We identified one population of IL-4–/IL-10–producing memory CD4 T cells with immunosuppressive function in vitro and in vivo, which may contribute to immune balance with conventional IL-2–/IFN-γ–producing CD4 Tm cells.
This is the first report that memory CD4 T cells can be programmed in vitro by culturing for > 1 month. In our system, if these long-lived CD4 Tm2 cells were isolated and continued to coculture with regulatory diffDCs, they could be maintained for > 2 months. For the convenience of our experiments, memory CD4 T cells maintained by regulatory DCs for 30 days were used. Compared with regulatory DCs, mature DCs survived for < 7 days in vitro, and could not maintain CD4 T cells for long when cocultured with effector CD4 T cells (data not shown). This unique experimental system will be useful for the future study of memory CD4 T cells because it easily identifies various factors influencing memory CD4 T-cell generation and tracks memory CD4 T cells more conveniently than that in vivo.
As the most potent antigen-presenting cells, DCs play crucial roles in priming primary CD4 T-cell responses via presenting antigen peptide on major histocompatibility complex class II molecules to antigen-specific naive CD4 T cells.16 Increasing evidence indicates that various DC subsets may be involved in memory T-cell generation and functions during various phases of memory T-cell formation.13,25-27 TSLP-DCs not only prime the Th2 response,28 but also promote the maintenance and further polarization of Th2 central memory cells via OX-40L.13 Human plasmacytoid DCs can rapidly induce IL-10 production of memory T cells to regulate recall responses.27 An in vivo CD11chi DC depletion system showed that CD11chi DCs played important roles in reactivating CD8 memory T cells during various pathogen infections.26 One recent study indicated that human tolerogenic DCs could differently regulate the functions of naive and memory CD4 T cells in vitro.29 Our results demonstrated that regulatory DCs could program the generation of memory CD4 T cells, adding new insight into the roles of DC subsets in regulating memory T-cell generation.
The linear differentiation model of the transition of effectors to memory CD4 T cells has been intensively studied,30-32 but the mechanisms underlying the generation of memory CD4 T cells remain elusive. IL-7 and IL-15 are key factors regulating the survival and generation of memory CD4 T cells,33-37 but IL-7 and IL-15 were found not to be involved in promoting memory CD4 T-cell maintenance by regulatory DCs, because no IL-7 and IL-15 was found in the long-term coculture system (data not shown). With the exception of the soluble cytokines, some membrane molecules such as OX-40/OX-40L play critical roles in the homeostasis of memory CD4 T cells by promoting Bcl-xL and Bcl-2 expression.38 Furthermore, OX-40/OX-40L interactions are pivotal in contributing to the generation of memory Th2 cells that drive lung inflammation during experimental asthma.10 Consistent with these findings, we showed that OX-40L expressed on regulatory DCs contributed to the long-term survival of CD4 T cells. Our previous study demonstrated that regulatory DCs alone could induce cell division of naive CD4 T cells weakly, although regulatory DCs inhibited the antigen-pulsed, mDC-induced potent division of naive CD4 T cells.20 Thus, the regulatory DC-induced weak division of effector CD4 T cells in the presence of low-dose antigen may contribute to their regulatory functions. Blockade of OX-40L/OX-40 during regulatory DC–T-cell interaction inhibited regulatory DC-induced cell division of the CD4 T cells, which was similar to the effect of OX-40L on TSLP-DCs.13
The major characteristics of long-lived memory CD4 T cells programmed by regulatory DCs are as follows: effector memory phenotype; rapid and preferential secretion of the Th2 cytokines IL-4, IL-5, IL-10, and IL-13 after antigenic stimulation; and immunosuppressive function. The in vivo natural counterpart of this memory population with regulatory function could be identified and up-regulated by regulatory DC transfusion. Considering the possible effect of different antigen doses on the generation of this memory population in vivo, further experiments need to be performed in the future to confirm this point, although we found that the strength of the primary response induced by different antigen concentrations (including 100, 250, and 500 μg of OVA protein) was similar (data not shown). Compared with classical memory Th2 cells, these cells possess some similarities, although there is a major difference in immunosuppressive function, indicating that, like memory Th2 cells, long-lived memory CD4 T cells programmed by regulatory DCs may play important roles in allergy or parasite infections. These questions remain to be further addressed in the future.
In conclusion, regulatory DCs could induce the generation of Th2 memory CD4 T cells with regulatory functions. CD4 Tm2 cells programmed by regulatory DCs could suppress naive CD4 T-cell activation and proliferation via cell contact-dependent IL-10 in vitro and inhibit antigen-induced DTH response in vivo. CD4 Tm2 cells, up-regulated by regulatory DCs, can negatively regulate the recall response in vivo. The existence of memory cells with suppressive functions has physical significance in that they may control the strength of the recall response mediated by other memory CD4 T cells, especially central memory CD4 T cells, thus finely regulating the balance of the recall response.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Ms Rui Zhang for her excellent technical assistance.
This study was supported by grants from the National Key Basic Research Program of China (2009CB521902, 2007CB512403), the National Natural Science Foundation of China (30721091, 30771963 and 30872303), and the National Key Project for Hepatitis B Research (2008ZX10002-008).
Authorship
Contribution: X.X, Z.G., X.J., Y.Y., Q.G., and Y.D. performed the experiments; and X.X., Z.G., and X.C. designed the experiments and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Xuetao Cao, National Key Laboratory of Medical Immunology and Institute of Immunology, Second Military Medical University, 800 Xiangyin Rd, Shanghai 200433, China; e-mail: caoxt@immunol.org.
References
Author notes
X.X., Z.G., and X.J. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal