Abstract
Multiple myeloma (MM) is a malignancy of plasma cells that accumulate in the bone marrow. MM is incurable with approximately 100 000 patients currently in the United States and 20 000 new cases diagnosed yearly. The malignancy causes displacement of hematopoiesis and formation of osteolytic bone lesions also known as myeloma bone disease (MBD). At diagnosis, 79% of patients suffer from MBD associated with severe pain and increased mortality. Wnt inhibitors secreted by MM cells inhibit osteogenesis and promote osteoclastogenesis, therefore rapid targeting of Wnt inhibitors is necessary to prevent potentially irreversible effects on the stroma, which could lead to incurable MBD. Inhibition of glycogen synthetase kinase-3β (GSK3β) causes accelerated Wnt signaling and enhanced osteogenesis in mesenchymal stem/progenitor cells, irrespective of the extracellular concentration of Wnt inhibitors. Our primary goal of this study was to evaluate a GSK3β inhibitor (6-bromoindirubin-3′-oxime BIO) for amelioration of bone destruction in a murine model of MBD. When measured using histomorphometry, peritumoral BIO administration improved bone quality at the bone-tumor interface and, surprisingly, increased histologically apparent tumor necrosis. Furthermore, in vitro assays demonstrated a proapoptotic effect on numerous MM cell lines. These preliminary data suggest that pharmaceutical GSK3β inhibition may improve bone quality in myeloma and other malignant bone diseases.
Introduction
Multiple myeloma (MM) is a malignancy of plasma cells (CD138+/CD38+ B cells) that accumulate in the bone marrow. MM is to date incurable, with approximately 100 000 patients currently in the United States and 20 000 new cases diagnosed nationally each year. The aggregate median survival for MM is 4 years.1 The malignant cells reside primarily in the bone marrow, resulting in displacement of hematopoiesis, production of very high levels of monoclonal immunoglobulin, and formation of osteolytic bone lesions (OLs) also known as myeloma bone disease (MBD). MBD is one of the major challenges in MM therapy. At diagnosis, 79% of patients suffer from OLs, osteoporosis, or bone fractures.2 These occurrences not only reduce quality of life for patients, but they are also associated with approximately 20% increased mortality.3 OLs are formed by MM cells through a change in the cytokine milieu of bone marrow, which causes intensified osteoclastogenesis and inhibits differentiation of mesenchymal stem cells/marrow stromal cells (MSCs), presumptive source of new mature osteoblasts.4-7 For years, the treatment of OLs has focused on the inhibition of osteoclastogenesis by administration of bisphosphonates, but even when osteoclast activity is controlled and successful chemotherapy is achieved, no osteoblastic repair occurs,8 and skeletal events continue to occur in approximately 40% of patients,9 suggesting that MM cells have the capacity to irreversibly disrupt the anabolic axis of bone formation. Indeed, there is an increasing body of literature demonstrating that MM cells secrete factors that cause lingering effects on osteoprogenitor cells such as MSCs. For instance, MM cells secrete factors that inhibit osteogenic differentiation of MSCs such as canonical Wnt inhibitors,4,6,10,11 which in turn cause the release of numerous prosurvival cytokines, such as interleukin-6 (IL-6), from the undifferentiated MSCs.5,12 As well as inhibiting osteogenesis and enhancing stromal support of MM by MSCs, Wnt inhibitors have also been reported to shift the ratio of osteoblastic receptor activator of NF-κB ligand (RANKL) and osteoprotegerin (OPG) secretion in favor of osteoclastogenesis.7 The MM-derived factors seem to have lasting effects on MSCs, even when examined ex vivo in the absence of MM cells,13-15 therefore rapid targeting of Wnt inhibitors is necessary to prevent potentially irreversible effects on the stroma that could lead to intractable MBD.
In the canonical Wnt signaling pathway, secreted Wnt glycoproteins bind to the transmembrane receptor frizzled (Frz) and the coreceptor lipoprotein-related protein 5 and protein 6 on the surface of the target cell. Activation of receptor Frz recruits the cytoplasmic bridging molecule, disheveled, so as to inhibit the action of glycogen synthetase kinase-3β (GSK3β). Inhibition of GSK3β decreases phosphorylation of β-catenin, preventing its degradation by the ubiquitin-mediated pathway. The stabilized β-catenin acts on the nucleus by activating T-cell factor/lymphoid enhancer factor–mediated transcription of target genes that elicits a variety of effects including induction of differentiation and in some cases, proliferation. Canonical Wnt signaling is tightly regulated by a combination of positive induction through the binding of the Wnt ligand and negative regulation through numerous mechanisms by at least 4 classes of the following secreted Wnt inhibitors: the dickkopf (Dkk) inhibitors, sclerostin, soluble Frz receptors, and Wnt inhibitory factor (reviewed in Kawano and Kypta16 and Gregory et al17 ). To date, immunosequestration of Dickkopf-1 (Dkk-1) has been reported to alleviate MBD in animal models.18,19 Dkk-1 acts by blocking the binding of the Wnt ligand to the lipoprotein-related protein receptor, which results in its internalization and degradation.20,21
Given that effective inhibition of GSK3β is necessary for effective canonical Wnt signaling and such signals are necessary for osteogenic differentiation,5,22-27 GSK3β inhibitors are likely candidates for osteoinductive therapy in MM. Because this class of agent acts in the cytoplasm, and the Wnt inhibitors are secreted factors reliant on membrane bound receptors, the therapy would be predicted to function without hindrance in the presence of very high concentrations of Wnt inhibitor. Naturally, there are concerns involving the potential for Wnt modulators to accelerate the expansion of the MM cells,28 but if this effect does occur, it is expected it will be counteracted by antimitotic therapy, or that the putative increase in MM proliferation will be negated by the reduced number of OLs available to serve as a niche for the malignant cells.29 Of additional note are recent studies documenting the antimitotic effect of GSK3β inhibitors on malignant lymphoid cells.30,31
We therefore examined the potential osteoprotective capacity of GSK3β inhibition in a xenograft model of MBD. We chose 6-bromoindirubin-3′-oxime (BIO), a specific inhibitor of GSK3β, and relative of the cyclin-dependent kinase (CDK) inhibitor indirubin-3′-monooxime.32 In our recently published studies, BIO has been shown to improve in vitro osteogenic differentiation by human MSCs,27 block the osteoinhibitory action of Dkk-15 and modestly inhibit the proliferation of cultured osteosarcoma cell lines.33 Our primary goal was to examine whether BIO could block the effects of Wnt inhibitors during bone destruction caused by MBD. For this reason, our measurements focused on histomorphometric parameters at the bone-tumor interface. We demonstrate that systemic injections of BIO improved the percentage of bone volume/tissue volume (BV/TV) of the proximal tibial heads of wild-type (WT) mice. Furthermore, in a novel model of MBD, peritumoral BIO administration slowed bone degradation and increased tumor necrosis when the Dkk-1 expressing MM cell line INA612,34 is engrafted into severe combined immunodeficiency (SCID) beige mice. We further demonstrate that, contrary to predictions of the established dogma, administration of a GSK3β inhibitor did not increase morbidity or tumor burden and caused apoptosis of MM cell lines in vitro. We therefore suggest that GSK3β inhibition should be explored further as a potential adjunct therapy for malignant diseases that destroy bone.
Methods
Additional details on methods can be reviewed in supplemental methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Inhibitor
BIO ((2′Z,3′E)-6-bromoindirubin-3′-oxime) was acquired commercially (GSK3 inhibitor IX; Calbiochem). The 1-mg aliquots were diluted in dimethylsulfoxide (DMSO) to 2.8mM, then diluted in sterile phosphate-buffered saline to 20μM. Aliquots were stored at −80°C.
Tissue culture
We expanded RPMI 8226 and INA6 MM cells, kindly provided by John Shaughnessy (University of Arkansas for Medical Sciences, Little Rock, AR), in medium consisting of RPMI 1640, 10% fetal bovine serum, 10% MarrowMax supplement, 100μM pyruvate, 100 units/mL penicillin G, and 100 μg/mL streptomycin (Invitrogen).
Animal care and use
All animal experiments were performed in accordance with animal use protocols approved by the Tulane University Animal Care and Use committee. For initial experiments involving systemic administration of BIO, male C57BL/6 WT mice at 1 month of age were acquired from The Jackson Laboratory. Mice were caged in groups of 3 to 4 animals and allowed to access standard mouse chow and water ad libitum.
For establishment of the MBD model, male and female C57BL6/Prkdc scid mice (20 per group) were purchased from The Jackson Laboratory and housed 2 to 4 animals per cage under specific pathogen–free conditions. They were allowed access to sterile standard mouse chow and water ad libitum.
Micro computer-aided tomography and conventional x-ray imaging
A Skyscan 1174 specimen imager was used for micro computer-aided tomography (μCT) imaging. A 25-μm filtered beam set to 48 kV was used with a resolution of approximately 6 μm. Conventional x-rays were achieved using a Faxitron specimen imager.
Alkaline phosphatase measurement
Alkaline phosphatase (ALP) levels were measured as described previously5 from serum using a kinetic assay based on the conversion of p-nitrophenol phosphate to nitrophenolate.
ELISA
The pyridinoline (PYD) and tartrate-resistant acid phosphatase (TRAP) 5b enzyme-linked immunosorbent assays (ELISAs) were obtained from Quidel. The β-catenin and Dkk-1 ELISAs were obtained from R&D Systems. Where necessary, values were normalized to cell number using hemacytometric analyses.
Histology
Mice were killed, and leg bones were dissected and placed immediately in 10% (vol/vol) buffered formalin, and stored at 4°C from 24 hours to 3 days. Decalcification was done using 0.5M ethylenediaminetetraacetic acid in phosphate-buffered saline until x-ray scans showed loss of bone opacity. Processing, embedding, and sectioning were performed at the Tulane Histology Core Facility. Sections were stained with hematoxylin and eosin.
Statistics
In vivo statistical tests were performed with groups of 4 mice or more using standard analysis of variance (ANOVA) or 2-tailed Student t test. In vitro assays of cell viability and cell-cycle data were analyzed using ANOVA after arcsine transformation of proportions calculated from sample sets consisting of 3 to 9 replicates. Values were calculated using R Version 2.10.1 software (http://www.R-project.org). Statistical significance was defined at a value P < .05.
Results
Effect of systemic BIO administration on the bone quality of proximal tibial heads of WT mice
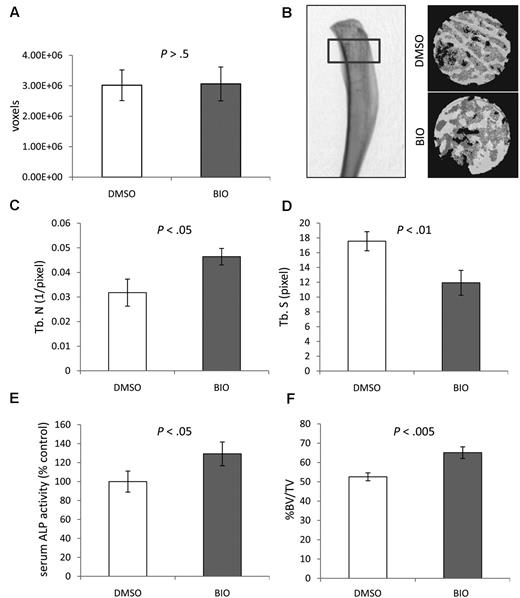
To examine whether BIO could influence bone homeostasis in vivo, 1-month-old male C57BL/6 WT mice (n = 10) received a 100-μL intraperitoneal injection of 2μM BIO every 48 hours for 6 weeks. Controls received the vehicle dimethylsulfoxide (DMSO). No toxicity was apparent by gross inspection, and body masses were comparable among groups. In some instances, the gut and lung tissue were histologically surveyed for signs of neoplasia but none were detected (data not shown). After 6 weeks, the mice were euthanized and the tibias were explanted. Fixed, dried bones were subjected to micro computer-aided tomography (μCT). The length, surface area, and volume of the tibias were not affected by BIO treatment, and remained constant with variation not exceeding 15%. For microanalysis, region of interest (ROI) was defined as the proximal tibial head and diaphysis constituting 10% of the longitudinal length of the bone (Figure 1A-B). Binary representations of the transverse sections (15 μm each) were generated from the scans, and BV/TV values were calculated for each 3-dimensional ROI. Trabecular parameters were significantly affected by BIO treatment with increased trabecular number (Tb N.) and a corresponding decrease in trabecular spacing (Tb Sp., P < .005; Figure 1C-D). Furthermore, the BIO-treated mice had, on average, an 18% (P < .005) increase in BV/TV (Figure 1F). Because BIO has the capacity to up-regulate osteogenic markers such as ALP levels and OPG secretion in some cell types, including stromal cells,27 we analyzed the serum of the test mice. ALP levels were raised in the BIO-treated animals by an average of 30% (P < .005), confirming that the inhibitor had a systemic effect on ALP expression (Figure 1E). Serum RANKL and OPG levels were subject to a high degree of variation, but the ratio of serum RANKL to OPG did not significantly differ from controls (data not shown). Given the modest effect of BIO on the bone, it is possible that these blood markers do not significantly change against a background of high variation. Because intraperitoneally administered BIO had a systemic osteogenic effect in WT, healthy mice, it was decided to test BIO in a model of MM-induced MBD.
Effect of systemic BIO administration compared with vehicle (DMSO) on murine proximal tibial bone parameters as measured by μCT. (A) Mean volume of the ROIs analyzed to confirm comparability between groups. (B) The 3-dimensional μCT rendering (right) of a control proximal tibia with ROI (left). The 3-dimensional reconstruction of ROIs from binary images (right). (C) Mean Tb N. (D) Mean Tb Sp. (E) Relative serum ALP activity. (F) Mean percentage of BV/TV. Statistics analyzed by Student t test for n = 10 mice per group; experiment was performed twice.
Effect of systemic BIO administration compared with vehicle (DMSO) on murine proximal tibial bone parameters as measured by μCT. (A) Mean volume of the ROIs analyzed to confirm comparability between groups. (B) The 3-dimensional μCT rendering (right) of a control proximal tibia with ROI (left). The 3-dimensional reconstruction of ROIs from binary images (right). (C) Mean Tb N. (D) Mean Tb Sp. (E) Relative serum ALP activity. (F) Mean percentage of BV/TV. Statistics analyzed by Student t test for n = 10 mice per group; experiment was performed twice.
Establishing the model of MM-induced bone disease
To establish an OL model to present a measurable bone phenotype, we first administered the human RPMI 8226 MM cell line into the tibial medullary canal of nonlethally irradiated SCID mice. This method was used to facilitate osteogenic-relevant plasmacytoma formation. Human λ antibody fragments secreted by RPMI 8226 cells, were used to track tumor formation because there was a good correlation between soluble λ levels and cell number (R = 0.91). Tumor engraftment, as defined by presence of a palpable tumor (supplemental Figure 1A-B, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) or detection of systemic human λ chains, remained below 50%. Of the mice with engrafted tumors, histologically detectable bone involvement was rare and mild (supplemental Figure 1C), and detection of systemic bone resorption markers TRAP 5b and type I collagen–derived PYD cross-linked products was sporadic.
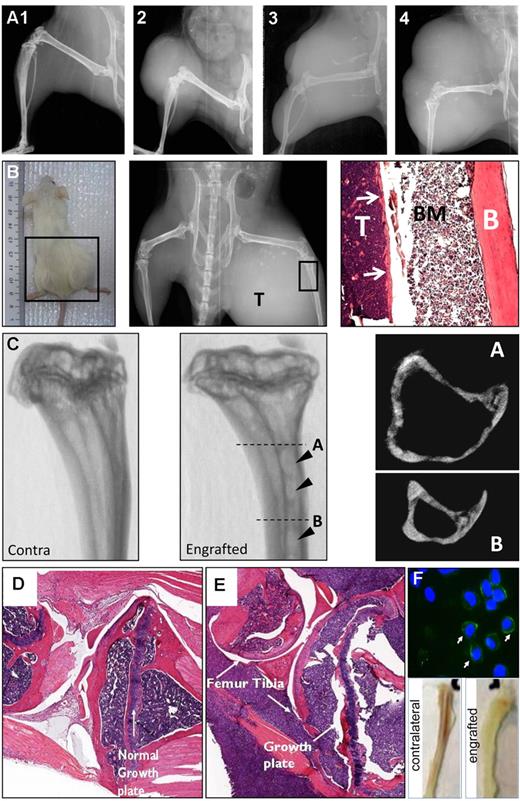
To improve engraftment and initiate bone involvement, an alternative Dkk-1 expressing cell line (INA6) was used. INA6 cells are described as a plasmacytoma-forming plasma cell line established from a patient 80 years of age with plasma cell leukemia. The cells express several plasma cell antigens such as κ light chain, CD75w, and CD138 while being negative for common pan-B cell markers such as CD19 and CD20.12 Although the rate of tumor development varied, with animals developing palpable tumors between 1 and 4 weeks after injection, tumor induction efficiency was 97%. Tumors were localized at the injection site as a solid plasmacytoma (Figure 2A). To standardize treatment strategies and data analyses, a simple staging system was used to categorize tumors based on cross-section (Figure 2A). Established tumors increased in size rapidly, with accompanying lysis of adjacent bone that could be detected radiologically and histologically (Figure 2B). The 3-dimensional μCT scans demonstrated formation of focal lesions in the cortical bone characteristic of MM (Figure 2C arrowhead), which was more apparent in axial images. Additional weakening of the bone tissue was evident, such as invasion of the metaphyses and disruption of the growth plate when examined histologically (Figure 2D-E). Further examination confirmed colonization of the tibial marrow cavity and surrounding muscle tissue with myeloma cells, as well as extensive cortical degradation (Figure 2B,D).
Major characteristics of the INA6 model of MBD. (A) X-ray imaging of the various arbitrary stages (stage 1-4) of plasmacytoma formation. (B) Gross (left), x-ray (center), and histologic (right) appearance of a high grade plasmacytoma and associated bone destruction. The histology shows the fibula with the tumor (T), bone marrow (BM), and cortical bone (B) labeled. (C) The 3-dimensional μCT renderings of contralateral (unaffected left) and engrafted (affected middle) proximal tibias showing focal OLs penetrating deep into the cortices (arrowheads). Axial reconstructions at levels A and B of the affected tibia are presented (right panels) demonstrating the depth of the lesions. (D-E) Histologic analysis of contralateral (D) and engrafted (E) knee joints demonstrating infiltration and disruption of the growth plate architecture. (F) Staining of bone marrow preparations from engrafted long bones with human-specific anti–CD138 antibody (top), demonstrating frequent CD138+ cells. Nuclei are counterstained with 4′,6-diamidino-2-phenylindole. Slides were examined using a Nikon Eclipse 800 upright microscope fitted with a Nikon DXM1200F digital camera. (F) Gross appearance of engrafted and contralateral tibias (bottom), demonstrating displacement of hematopoiesis. Representative images of n = 10 mice per group; experiment was performed twice.
Major characteristics of the INA6 model of MBD. (A) X-ray imaging of the various arbitrary stages (stage 1-4) of plasmacytoma formation. (B) Gross (left), x-ray (center), and histologic (right) appearance of a high grade plasmacytoma and associated bone destruction. The histology shows the fibula with the tumor (T), bone marrow (BM), and cortical bone (B) labeled. (C) The 3-dimensional μCT renderings of contralateral (unaffected left) and engrafted (affected middle) proximal tibias showing focal OLs penetrating deep into the cortices (arrowheads). Axial reconstructions at levels A and B of the affected tibia are presented (right panels) demonstrating the depth of the lesions. (D-E) Histologic analysis of contralateral (D) and engrafted (E) knee joints demonstrating infiltration and disruption of the growth plate architecture. (F) Staining of bone marrow preparations from engrafted long bones with human-specific anti–CD138 antibody (top), demonstrating frequent CD138+ cells. Nuclei are counterstained with 4′,6-diamidino-2-phenylindole. Slides were examined using a Nikon Eclipse 800 upright microscope fitted with a Nikon DXM1200F digital camera. (F) Gross appearance of engrafted and contralateral tibias (bottom), demonstrating displacement of hematopoiesis. Representative images of n = 10 mice per group; experiment was performed twice.
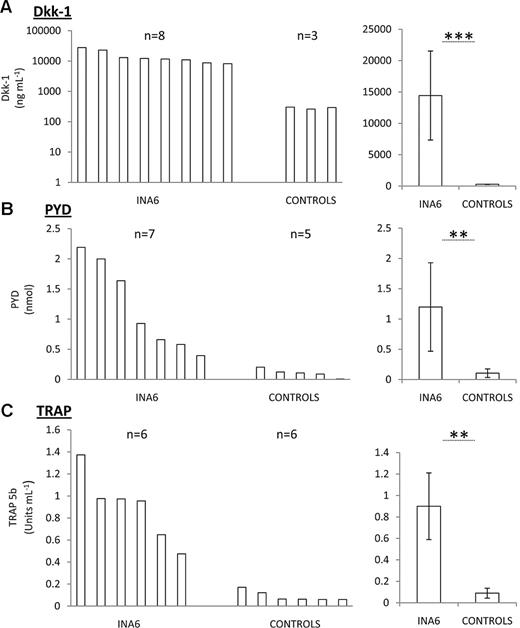
Upon explant, engrafted bones were qualitatively more fragile than the contralateral counterparts with severe tumors causing complete ablation of red marrow from the engrafted bone (Figure 2F bottom). Exudates from the medullary canals of mice with tumors contained an abundance of human CD138+ cells (Figure 2F top). A panel of serum markers of bone resorption was used for following tumor progression from blood samples. Dkk-1,4 PYD cross-links derived from collagen I degradation and TRAP 5b was elevated in sera from tumor bearing mice (Figure 3A,C), but the levels did not correlate with tumor size, suggesting that the angiogenesis in the plasmacytomas might proceed at a different rate than tumor expansion. There was a weak correlation with TRAP and PYD levels and the severity of osteolysis, but unfortunately, the serum markers alone were not sufficient to accurately measure the bone disease nor the size of tumor in this model. Like many naturally occurring MM cases, circulating cells could not be detected in the blood by costaining for CD138 and CD38 followed by flow cytometry, and only under the rarest of circumstances could tumors or lytic lesions be found in distal locations after necropsy. These data therefore suggested that a reproducible model of localized MM-induced bone disease with minimal complications from secondary sites had been developed.
Serum markers of bone disease in the INA6 model of myeloma. Serum levels of human Dkk-1 (A), PYD (B), and TRAP (C) in engrafted and nonengrafted (controls) mice. Range (left) and mean with SD (right) are presented. Error bar represents SD for n = 5 samples per group. **P < .01, ***P < .005 with Student t test. Experiment was performed twice.
Serum markers of bone disease in the INA6 model of myeloma. Serum levels of human Dkk-1 (A), PYD (B), and TRAP (C) in engrafted and nonengrafted (controls) mice. Range (left) and mean with SD (right) are presented. Error bar represents SD for n = 5 samples per group. **P < .01, ***P < .005 with Student t test. Experiment was performed twice.
Effect of peritumoral-administered BIO in experimentally induced MBD mice
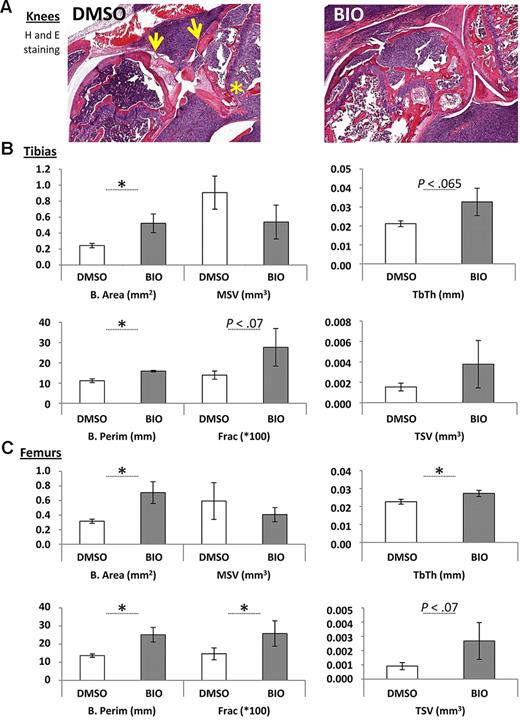
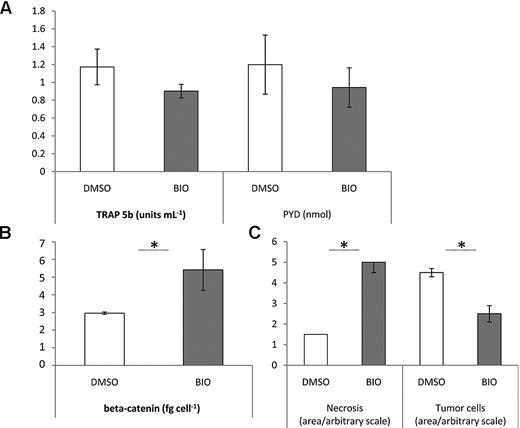
For examination of the effects of BIO on MM-induced bone resorption, mice were randomly divided into treated and untreated groups. Administration of BIO did not begin until tumors developed. Dose and frequency were based on the in vitro studies by Gunn et al5 and Krause et al27 with a peritumoral route to best maintain relevant local concentrations. Although lesions were not apparent in whole body x-ray scans taken at earlier stages, osteolytic lesions and localized bone resorption were detected after approximatelyday 17 of the experiment. The severity of the bone resorption from radiologic data was scored in blinded fashion by an institutional veterinarian according to the degree of osteopenia, periosteal reaction, and bony regeneration. BIO-treated animals had predominantly mild and moderate bone resorption, whereas animals receiving only vehicle had exclusively moderate to severe resorption. Surprisingly, there was no strong relationship between tumor size and radiologic or histologic appearance of bone resorption. Mice that reached endpoint criteria as defined by the animal use protocol were euthanized, and hind limbs were submitted for histopathology and bone histomorphometry. Gross inspection suggested that knee joints treated with BIO appeared to be in better condition than those that received vehicle with improved protection of the subchondral bone and trabecular architecture (Figure 4A). This was supported by static histomorphometry, where statistically significant improvements in bone area, trabecular thickness, bone perimeter, and bone fraction were evident in the BIO-treated specimens for both tibias and femurs compared with DMSO controls (Figure 4B-C). Of note is the observation that all histomorphometric measurements that did not show statistically significant improvement followed a trend in favor of BIO treatment. Similarly, systemic indicators of bone destruction, TRAP 5b and PYD, were not changed in a statistically significant manner by BIO treatment, but in general, BIO-treated mice had lower levels of these factors (Figure 5A).
Histomorphometric scoring of hind limb bones adjacent to tumors in mice treated with a peritumoral dose of BIO or vehicle (DMSO). (A) Example of sections used for the histomorphometric scoring, demonstrating tibial and femoral destruction in untreated but INA6-engrafted knees. Note destruction of the bone at the articular surfaces of the knee (arrowed) and disruption of the trabecular structures that is less apparent in the BIO-treated animals (asterisk). (B) Static histomorphometric assays of peritumoral bone tissue in INA6 bearing mice locally treated with BIO or with vehicle (DMSO). The bone area (B. Area), marrow star volume (MSV), trabecular thickness (Tb. Th.), trabecular star volume (TSV), bone perimeter (B. Perim), and percentage of bone/total tissue (frac × 100) are shown. Bars represent mean (± SEM) for n = 4-5 samples. *P < .05 with Student t test. Where values for P were borderline significant, they are given on the plot. Experiment was performed twice.
Histomorphometric scoring of hind limb bones adjacent to tumors in mice treated with a peritumoral dose of BIO or vehicle (DMSO). (A) Example of sections used for the histomorphometric scoring, demonstrating tibial and femoral destruction in untreated but INA6-engrafted knees. Note destruction of the bone at the articular surfaces of the knee (arrowed) and disruption of the trabecular structures that is less apparent in the BIO-treated animals (asterisk). (B) Static histomorphometric assays of peritumoral bone tissue in INA6 bearing mice locally treated with BIO or with vehicle (DMSO). The bone area (B. Area), marrow star volume (MSV), trabecular thickness (Tb. Th.), trabecular star volume (TSV), bone perimeter (B. Perim), and percentage of bone/total tissue (frac × 100) are shown. Bars represent mean (± SEM) for n = 4-5 samples. *P < .05 with Student t test. Where values for P were borderline significant, they are given on the plot. Experiment was performed twice.
Pathology and biochemical markers in tumor-bearing mice treated with a peritumoral dose of BIO or vehicle (DMSO). (A) Serum PYD and TRAP 5b in response to peritumoral BIO administration. Despite values for P > .05, BIO-treated mice have generally lower levels of circulating resorption markers. (B) ELISA detection of soluble β-catenin in tissue lysates generated from the injection sites of treated and untreated animals. BIO treatment increases β-catenin levels, *P < .05. (C) Pathologic scoring on a 5-point linear scale of bone-tumor interface. Tumor necrosis and tumor cells refer to the sectional area containing dead or live tumor cells, respectively. Error bar represents SEM for n = 4-5 samples, *P < .05. Experiment was performed twice.
Pathology and biochemical markers in tumor-bearing mice treated with a peritumoral dose of BIO or vehicle (DMSO). (A) Serum PYD and TRAP 5b in response to peritumoral BIO administration. Despite values for P > .05, BIO-treated mice have generally lower levels of circulating resorption markers. (B) ELISA detection of soluble β-catenin in tissue lysates generated from the injection sites of treated and untreated animals. BIO treatment increases β-catenin levels, *P < .05. (C) Pathologic scoring on a 5-point linear scale of bone-tumor interface. Tumor necrosis and tumor cells refer to the sectional area containing dead or live tumor cells, respectively. Error bar represents SEM for n = 4-5 samples, *P < .05. Experiment was performed twice.
Given the function of GSK3β in the canonical Wnt signaling pathway is to phosphorylate, and thus destabilize β-catenin, inhibition of GSK3β is predicted to increase soluble β-catenin levels in the tumor and surrounding tissue. To examine the bioavailability of BIO, the tumor and associated stromal cells were extracted from hind limbs and soluble extracts were prepared. The amount of DNA in the digests was used to normalize samples for cell number, and soluble β-catenin was measured by ELISA. Extracts from DMSO-treated animals had a remarkably constant level of soluble β-catenin (Figure 5B), whereas the range of β-catenin values for BIO-treated extracts was higher but variable. Irrespective of the variation, soluble β-catenin levels were significantly higher in BIO-treated extracts, demonstrating that it was having the expected biochemical effect on the tumor and surrounding tissue. Because β-catenin up-regulation is sometimes associated with tumor initiation and expansion, and BIO appears to increase β-catenin levels, there is understandable concern that BIO could promote the malignancy. Nevertheless, BIO caused no increase in tumor morbidity when analyzed by the Kaplan and Meier method (P = .62, n = 20). End point morbidity was defined as a palpable tumor of 1.0 cm in diameter. Tumor growth was similar in both treated and untreated groups with 60% versus 40% animals remaining in the study at day 10, 50% versus 30% at day 20 and 10% versus 5% surviving until day 30 after treatment, respectively. Although reducing tumor burden was not a primary goal of our study, tumor sections were scored for cross-sectional area and area of tumor necrosis. Contrary to expectations, it became apparent that BIO administration substantially increased appearance of necrotic tumor tissue and as a result, reduced viable tumor area in sections from BIO-treated mice (Figure 5C).
The effect of BIO on viability of cultured INA6 WT cells and cells derived from control tumors
Given that indirubin derivatives have additional inhibitory effects on CDK1 and CDK235 and BIO itself has been shown to cause growth inhibition, cell-cycle arrest, and apoptosis in numerous malignant cell lines,33,36-38 including those of lymphoid origin,30,31 we decided to examine the effect of BIO on INA6 viability in more detail in vitro. INA6 sublines were established from tumors explanted from control animals by enzymatic dissociation and culture. The process of in vivo passage selected for those MM cells with greater proliferative potential and adaption to the in vivo microenvironment. The INA6 subline cells were therefore deemed to be a more relevant test line than INA6 cells expanded from parental stocks. One line in particular, designated INA6-10.13, had an in vitro proliferative rate substantially higher than the parental strain. This characteristic could be explained at least in part by a loss of IL-6 dependency in the subline because murine IL-6 does not stimulate the human IL-6 receptor.12
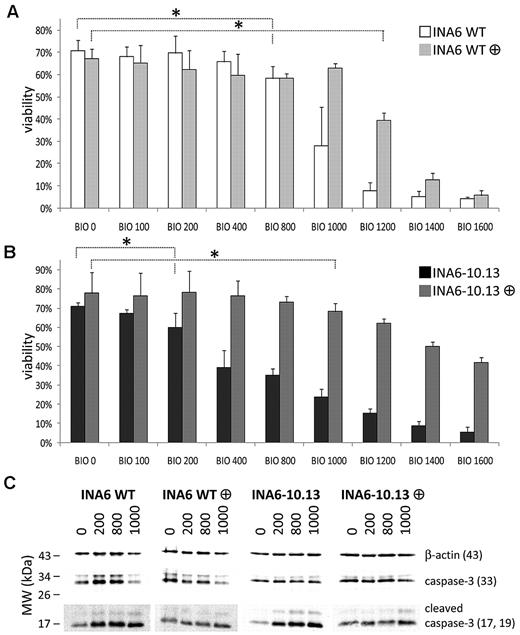
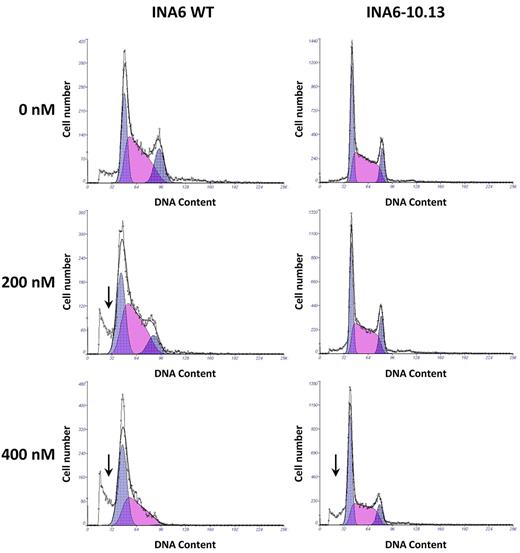
To test whether the INA6 derivatives were more resistant to BIO and whether BIO had a general effect on MM cell survival, we incubated INA6-WT cells, INA6-10.13 cells, and other IL-6–independent cell lines in the presence of 0 to 1600nM BIO. After 72 hours, BIO reduced viable cell yield in all cell lines tested in a dose-dependent manner (supplemental Figure 2 and Figure 6A-B). Guided by previous literature32,35 describing additional affinity for BIO on cyclins, we measured cell-cycle parameters of the BIO-treated cells. We expected cell-cycle rate, as defined by the proportion of cells in S-phase, to be reduced by BIO, accounting for the profound reduction of viable MM cells, but we found that the effect on S-phase was marginal. Surprisingly, the most apparent effect of BIO treatment was cell-cycle blockade at the G2M checkpoint, with subsequent loss of the G2 peak to apoptosis, which could be detected as a sub-G1 peak on the flow cytometric plots (Figure 7). To further examine this observation, INA6-WT and INA6-10.13 cells were incubated in increasing concentrations of BIO for 18 hours and examined for early induction of apoptosis by measurement of cleaved caspase-3 fragments. Both INA6-WT and IL-6–independent subclone INA6-10.13 cells showed a dose-dependent accumulation of cleaved caspase-3 fragments (Figure 6C).
Effect of BIO on viability of INA6 MM cells in vitro. Viability of INA6-WT (A) and INA6-10.13 (B) cells exposed to increasing concentrations of BIO (0-1600nM) for 72 hours with or without addition of 10% MSC-conditioned medium as determined by trypan blue exclusion. Mean (± SD) for n = 6-9 samples is shown. ANOVA was performed after arcsine transformation of proportions. Asterisk indicates cut-off dose of BIO causing statistically significant reduction of viability (*P < .05). There were 2 to 3 sets of independent triplicates analyzed. (C) Western blot for cleaved caspase-3 showing induction of apoptosis in INA6-WT and INA6-10.13 cells by BIO after 18 hours exposure. Note the initial rescuing effect of MSC-conditioned medium, which is overcome by higher concentrations of BIO (1000nM). Membranes were cut and exposed with different sensitivity using an automated imaging system. To ensure comparability, INA6-WT or INA6-10.13 cells were run on the same gel, respectively, and lanes were spliced together for presentation purposes. Experiment was performed twice independently.
Effect of BIO on viability of INA6 MM cells in vitro. Viability of INA6-WT (A) and INA6-10.13 (B) cells exposed to increasing concentrations of BIO (0-1600nM) for 72 hours with or without addition of 10% MSC-conditioned medium as determined by trypan blue exclusion. Mean (± SD) for n = 6-9 samples is shown. ANOVA was performed after arcsine transformation of proportions. Asterisk indicates cut-off dose of BIO causing statistically significant reduction of viability (*P < .05). There were 2 to 3 sets of independent triplicates analyzed. (C) Western blot for cleaved caspase-3 showing induction of apoptosis in INA6-WT and INA6-10.13 cells by BIO after 18 hours exposure. Note the initial rescuing effect of MSC-conditioned medium, which is overcome by higher concentrations of BIO (1000nM). Membranes were cut and exposed with different sensitivity using an automated imaging system. To ensure comparability, INA6-WT or INA6-10.13 cells were run on the same gel, respectively, and lanes were spliced together for presentation purposes. Experiment was performed twice independently.
Effect of BIO on cell cycle of INA6 MM cells in vitro. Cell-cycle analysis of INA6-WT and INA6-10.13 cells exposed to various concentrations of BIO for 72 hours. At higher concentrations, a significant sub-G1 peak is evident (arrow), demonstrating dead cell debris. Note the loss of the G2 population. Experiment was performed twice independently.
Effect of BIO on cell cycle of INA6 MM cells in vitro. Cell-cycle analysis of INA6-WT and INA6-10.13 cells exposed to various concentrations of BIO for 72 hours. At higher concentrations, a significant sub-G1 peak is evident (arrow), demonstrating dead cell debris. Note the loss of the G2 population. Experiment was performed twice independently.
To mimic the putative protective effect by MSCs on MM cells that is presumed to occur in vivo, cultures were supplemented with 10% MSC conditioned medium, a rich source of IL-6 and other gp130 signaling cytokines.5 Although the addition of MSC conditioned medium could rescue MM cells from BIO at lower doses, this could be overcome by higher concentrations of BIO (1000nM). This observation correlates with the finding that in numerous endogenously IL-6–independent cell lines higher doses of BIO are needed for a reduction in cell viability (supplemental Figure 2). Therefore, it appears that BIO reduces MM cell expansion rather than stimulating it. This probably occurs through the promiscuity of the molecule in inhibiting a range of CDKs, a variety of other pathways, and of course, GSK3β.
Discussion
Formation of OLs is a major contributor to morbidity associated with MM, occurring throughout the skeleton and causing severe bone pain and pathologic fractures. Until recently, OLs were assumed to be the result of a cytokine-enriched microenvironment generated by the MM resulting in osteoclastogenesis and local bone resorption. Therefore, the treatment of OLs has focused on the inhibition of osteoclastogenesis, such as by administration of bisphosphonates.1 However, when the tumor load is controlled, and the MM cells are absent from the OLs, they seldom heal,8 suggesting that the anabolic axis of bone formation had also been irreversibly altered. The first study to suggest a mechanism behind the apparent interference of bone repair by MM was from Tian et al4 who observed that secretion of an inhibitor of the canonical Wnt signaling pathway, Dkk-1, by MM cells strongly correlated with the severity of OLs. Given that Wnt signaling has been demonstrated necessary for the differentiation of osteoprogenitors to osteoblasts, the presence of high levels of Wnt inhibitors seemed to explain the deficit in bone repair. Subsequently, it has been shown that exposure to MM cells in vivo and in vitro irreversibly conditions osteoprogenitors to be deficient in osteogenic differentiation, as well as up-regulating some key cytokines that support MM cell survival.5,13-15 The preliminary evidence therefore suggests that early inhibition of the action of Wnt inhibitors could (1) reduce the formation of OLs by enhancing osteogenic signals and (2) reduce the number of undifferentiated stromal cells that provide support for MM cell expansion and survival. In this study, we have attempted to target the activity of Wnt inhibitors that are secreted by many MM tumors by GSK3β inhibition.
To enhance Wnt signaling in vivo, we chose the GSK3β inhibitor BIO, a nucleotide analog related to indirubin-3′-monooxime (IO). Preparations containing IO have been used throughout history for the treatment of leukemic malignancies and continue to be the subject of antimitotic research mainly because of the inhibitory activity of IO on CDKs.32 BIO had been modified to substantially improve its fidelity for GSK3β, but some cross-reactivity with CDKs has been reported. Initial experiments were designed to examine short-term safety of BIO when administered systemically at large doses, and whether steady state bone turnover could be perturbed. We calculated dose based on the IC50 of BIO (2 μM/L) and the LD50 of the parent molecule, indirubin39 (400 mg/kg), which was the only toxicity data available at the time. We administered a total of 60 nmoles of BIO, which was well below the published toxicity. The mice received intraperitoneal injections of the inhibitor well with no detectable complications over the treatment period. After 6 weeks, the mice were euthanized, and bones were analyzed. We hypothesized that GSK3β inhibition, through BIO, would increase Wnt signaling in bone marrow MSCs, and thus increase the proportion of MSCs with a greater propensity for osteogenesis. This finding was supported by our previous observations that BIO accelerates osteogenesis by human MSCs in vitro.27 Superficial histologic examination of the long bones and vertebrae demonstrated that although no gross abnormalities were evident, it was difficult to discern whether BIO had a positive effect on bone quality. Micro-CT analysis was therefore performed to measure the morphometric parameters of the bone tissue. Upon analysis of the proximal tibial head and diaphysis, it was apparent that the BIO had improved trabecular parameters, increasing the Tb N. and reducing Tb Sp. In these experiments, the effects of BIO seemed to be confined to the trabecular bone without detectable changes in cortical bone. Nevertheless, given the much lower rate of turnover in cortical relative to trabecular bone, it is feasible that cortical parameters could also be improved by extended BIO treatment. These data suggest a role for GSK3β inhibition for osteoinductive therapy and agree with previous animal studies24,26 and a human study exploring the effects of chronic lithium use and fracture frequency.40 It is noteworthy that although our results do not unequivocally prove a direct effect of BIO on osteoblast activity, these data described in this study and in related studies strongly suggest that osteoblasts and osteoprogenitors are indeed the target of BIO.
The main goal of this study was to examine the effect of direct GSK3β inhibition on the formation and repair of OLs in MBD by direct histomorphometric evaluation of the bone-tumor interface. Given that Wnt inhibition (through Dkk-1 and soluble Frz-related protein) has been implicated in the formation and persistence of such lesions4-7,10,11 and immunosequestration of Dkk-1 as well as peritumoral Wnt3a administration can reduce MBD in vivo,19,34,41 direct modulation of Wnt signaling through GSK3β inhibition appeared feasible and potentially useful for clinical application. Several excellent in vivo MM assays have been described, but most recapitulate the systemic elements of the disease, resulting in randomly distributed foci. These models effectively reproduce MM pathology, but may have limited utility for specifically examining localized MBD.42-44 A model involving the local implantation of MM cells and a human bone substrate would be particularly suitable, but the procedure requires primary human biomaterials45 that are in short supply for most investigators. Recently, direct implantation models, similar to the procedure described in this study have been used to examine the antitumoral effects of tumor necrosis factor-related apoptosis-inducing ligand involving RPMI 8226 and KMS-11 human MM cell lines.46 The model described in this study produces a solitary plasmacytoma with highly aggressive bone resorption qualities. Although systemic effects are negligible, serum levels of bone resorption markers could be used to follow bone involvement at the single site (Figure 3), making this model extremely convenient for direct study of MBD in a well-defined system. It therefore contrasts with the INA6-based model of disseminated MM disease originally reported by Burger et al.12
Upon detection of INA6-plasmacytomas, peritumoral BIO injections were performed. The dosage corresponded with in vitro studies5 that defined the effective concentration to be approximately 280nM. Because the pharmacokinetics of BIO is currently uncharacterized and bioavailability could not be guaranteed, peritumoral injection was deemed the most reliable way to maintain appropriate local concentrations. Upon conclusion of the study, affected limbs were assigned a code and analyzed by histomorphometry, and pathology was outsourced to Premier Laboratories (Boulder, CO). In all samples, bone involvement was substantial, and localized to the bone-tumor interface. However, in agreement with the systemic injection studies, histomorphometric measurements demonstrated that BIO significantly improved bone quality tibias and femurs (Figure 4). Of note is the observation that contralateral limbs were also affected by BIO administration, although they did not harbor detectable levels of MM cells (data not shown). The results therefore support a potential osteoprotective role for GSK3β inhibitors in MM and probably other malignant diseases of the skeleton. Furthermore, the apparent systemic effect of BIO suggests that testing in disseminated and immune-competent models of MM lines is a necessary course of further study.
One understandable argument against the use of GSK3β inhibitors arises from the observation that Wnt signaling can increase MM cell proliferation in early stages of tumorigenesis (eg,28,47-49 ). However, we found that BIO administration did not adversely affect tumor morbidity. In contrast, we found that BIO increased the necrotic component of the plasmacytoma and as a result, reduced the area of viable tumor observed on pathology slides. Furthermore we could partially reproduce the findings of a previous study30 by demonstrating that BIO could reduce MM cell viability and cell-cycle turnover and thus slow MM cell expansion in vitro. These observations therefore suggest that a less specific inhibitor, with affinity for CDKs as well as GSK3β may indeed be the agent of choice for treatment of severe OLs. Nevertheless, very specific inhibition of GSK3β by thiadiazolidinone has been reported to inhibit in vitro MM cell expansion and cause apoptosis through a mechanism involving the proapoptotic protein Fas ligand.31 Furthermore, fortification of bone tissue by increased Wnt signaling results in displacement and regression of modeled MM due to enhanced osteoblast differentiation without affecting proliferation or differentiation of preosteoclasts.29,41,50 Therefore, BIO is likely to act through several additive or synergizing mechanisms that target MM cells and the surrounding microenvironment.
From the data presented in this study, adjuvant osteoprotective therapy in the form of GSK3β inhibition represents a feasible strategy for the adjunct treatment of MM. Contrary to conventional dogma, GSK3β inhibition did not increase tumor load, but rather induced tumor necrosis. With improved knowledge of its pharmacokinetics and safety profile in humans, this inhibitor could represent a valuable supportive clinical tool for treatment of MBD, and in general, bone destruction occurring through other malignant diseases.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Tara G. Ooms, DVM, DACLAM, Department of Comparative Medicine, Tulane University, New Orleans, LA, for help with the histology scoring and Dina Gaupp, Department of Medicine, Center for Gene Therapy, Tulane University Health Sciences Center, for excellent help with the histology. W.G.G. thanks Tina Kilts, National Institute of Dental and Craniofacial Research, National Institutes of Health, Bethesda, MD, for training and the development and optimization of the injection techniques.
This work was supported in part by National Institutes of Health grants DK071780, and R020152, by the Louisiana Gene Therapy Research Consortium, and by Scott and White Hospital, Texas A&M Health Science Center (to C.A.G.)
National Institutes of Health
Authorship
Contribution: W.G.G., U.K., and C.A.G. designed and performed research, analyzed and interpreted the data and wrote the manuscript; and N.L. performed research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Carl A. Gregory PhD, Institute for Regenerative Medicine, Texas A&M Health Sciences Center, 5701 Airport Rd, Module C, Temple, TX 76502; e-mail: cgregory@medicine.tamhsc.edu.
References
Author notes
W.G.G. and U.K. are equal contributing authors.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal