Abstract
Both the canonical and noncanonical nuclear factor κB (NF-κB) pathways have been linked to tumorigenesis. However, it remains unknown whether and how the 2 signaling pathways cooperate during tumorigenesis. We report that inhibition of the noncanonical NF-κB pathway significantly delays tumorigenesis mediated by the viral oncoprotein Tax. One function of noncanonical NF-κB activation was to repress expression of the WWOX tumor suppressor gene. Notably, WWOX specifically inhibited Tax-induced activation of the canonical, but not the noncanonical NF-κB pathway. Mechanistic studies indicated that WWOX blocked Tax-induced inhibitors of κB kinaseα (IKKα) recruitment to RelA and subsequent RelA phosphorylation at S536. In contrast, WWOX Y33R, a mutant unable to block the IKKα recruitment and RelA phosphorylation, lost the ability to inhibit Tax-mediated tumorigenesis. These data provide one important mechanism by which Tax coordinates the 2 NF-κB pathways for tumorigenesis. These data also suggest a novel role of WWOX in NF-κB regulation and viral tumorigenesis.
Introduction
The nuclear factor κB (NF-κB) family of transcription factors plays a central role in regulation of diverse biologic processes, including immune responses, cell growth, and, cell survival.1 The NF-κB factors are usually sequestered in the cytoplasm as latent complexes through physical interaction with the inhibitors of κB (IκB), mainly IκBα and the IκB-like protein p100 (the precursor of the mature form of NF-κB2, p52). Accordingly, inducible degradation of IκBα and selective degradation of the C-terminal IκB-like sequences of p100 (processing) to generate p52 represent 2 major mechanisms leading to NF-κB activation: the canonical and noncanonical NF-κB pathways, respectively.
In the canonical NF-κB pathway, IκBα degradation requires inducible phosphorylation at serines S32 and S36 by a specific IκB kinase (IKK) complex that consists of 2 catalytic components, IKKα (also known as IKK1) and IKKβ (or IKK2), and a regulatory subunit, IKKγ (NEMO). IKK-mediated phosphorylation results in rapid ubiquitination and proteasomal degradation of IκBα, allowing RelA (the prototypic member of NF-κB, also known as p65) and other NF-κB members to localize to the nucleus to induce gene expression.1 In the noncanonical NF-κB pathway, IKKα is specifically recruited into the p100 complex to phosphorylate p100, leading to p100 ubiquitination and processing to p52. The newly generated p52 together with NF-κB binding partners then translocates into the nucleus, where they induce or repress gene expression.2-4
Through deregulation of its target genes, NF-κB has been linked to various malignancies, such as adult T-cell leukemia (ATL) caused by the human T-cell leukemia virus type I (HTLV-I).1 This retrovirus encodes a viral oncoprotein Tax, known to persistently activate both the canonical and noncanonical NF-κB pathways. Tax binds to and activates IKK via IKKγ to phosphorylate IκBα, resulting in IκBα degradation and RelA nuclear translocation.5-9 Recent studies suggested that posttranslational modifications of Tax, such as ubiquitination, are required for Tax binding to IKKγ and subsequent IKK activation.10-12 Another important function of Tax in the activation of the canonical NF-κB pathway is to promote IKKα to phosphorylate S536 of RelA, which is required for RelA transcriptional activity.13,14 In parallel, Tax specifically recruits IKKα into the p100 complex to activate the noncanonical NF-κB pathway.15,16 Although the mechanisms of how the canonical and noncanonical NF-κB pathways are regulated and activated under both physiologic and pathogenic conditions have been extensively studied, it remains largely unknown whether and how the 2 NF-κB signaling pathways cooperate, particularly during tumorigenesis.
The WW domain–containing oxidoreductase (WWOX) gene, also known as WOX1 or FOR, which is located at the second most common human fragile site, FRA16D, has been linked to many different cancer conditions, including leukemia and lymphomas.17,18 Recent evidence from both biochemical and genetic studies has clearly demonstrated WWOX as a bona fide tumor suppressor gene. Although reduction or loss of WWOX expression is strongly associated with tumor genesis and aggressiveness, and its restoration in the WWOX-deficient cancer cells leads to reversal of their tumor phenotypes, targeted deletion of Wwox in mice results in an increased incidence of spontaneous and inducible tumors.17,18 The tumor spectrum in Wwox-deficient mice includes leukemia/lymphoma, lung carcinomas, liver tumors, gastric tumors, and many others. Although it has been suggested that the tumor suppression function requires phosphorylation of Y33 within the N-terminal WW domain,17,18 the mechanisms of how WWOX functions as a tumor suppressor remain unclear. In addition, the role of WWOX in viral tumorigenesis has not yet been investigated.
In the present study, we have demonstrated that the WWOX tumor suppressor gene is a novel molecular link between the canonical and nonanonical NF-κB pathways under HTLV-I/Tax oncogenic regulation. The WWOX gene is a negative target of the noncanonical NF-κB pathway, and a potent suppressor of Tax-activated canonical NF-κB. WWOX specifically prevents Tax-mediated IKKα recruitment to RelA and subsequent IKKα–mediated S536 phosphorylation, but has no effect on Tax-induced IκBα degradation and p100 processing. Interestingly, the WWOX Y33R mutant loses the ability to suppress Tax-mediated tumorigenesis, which is associated with its inability in prevention of Tax-mediated RelA phosphorylation by IKKα. Studies suggest a new tumor suppressor role of WWOX in HTLV-I/Tax-mediated tumorigenesis. These studies also provide an example of how the canonical and noncanonical NF-κB pathways coordinately act together in tumorigenesis.
Methods
Expression vectors and reagents
Expression vectors encoding Tax, Myc-WWOX, and its Y33R mutant p100, serine to alanine (SS/AA) mutant p100SS/AA, p52, IKKα, and hemagglutinin (HA)–IκBαSS/AA have been previously described.15,19 The Tax and WWOX cDNAs were also subcloned into retroviral vectors pCLXSN, pQCXIP, pLHCX, and/or pTRIP by routine cloning strategies as described.20 Tax, Myc, and WWOX antibodies were generated from hybridomas as described.16,21 Antibodies against IKKα, IκBα, or S536 phosphorylated RelA were from Cell Signaling Technology. Heat shock protein 90 (Hsp90) and HA antibodies were from Santa Cruz Biotechnology and Roche, respectively. Proteasome inhibitor MG132 and protein synthesis inhibitor cycloheximide (CHX) were from Biomol.
Cell culture and transfection
Human embryonic kidney 293 cells, HeLa cells, mouse embryonic fibroblasts (MEFs), and Rat-1 fibroblasts were cultured in Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum (FBS) and 2mM l-glutamine.20 Human T-lymphocyte Jurkat cells, CD40+ lymphoma cell lines KM-H2 and L428, HTLV-I–transformed T-cell lines C8166, HuT102, MT-2 and SLB-1, and established ATL cell lines TL-Om-1 and ED40515− were maintained in suspension in RPMI 1640 medium supplemented with 10% FBS and 2mM l-glutamine.22 293, HeLa, and HTLV-I–transformed T cells were transfected, respectively, with diethylaminoethyl-dextran, Transfast reagent (Promega), and Lipofectamine 2000 (Invitrogen) as described previously.20,23
Clinical samples
Peripheral blood was obtained from normal donors and ATL patients after informed consent, approved by the Institutional Review Board of the University of Pittsburgh. The diagnosis of ATL was established hematologically and by demonstration of monoclonal HTLV-I provirus integration into host genome. Subtypes of ATL were defined as described.24 Peripheral blood mononuclear cells (PBMCs) were purified from peripheral blood using Ficoll Paque gradient centrifugation.25 Purified PBMCs contained more than 90% leukemic cells. All patient samples were collected before chemotherapy.
Retroviral transduction and generation of stable cDNA or shRNA transfectants
All the experiments involving virus were performed under Biohazard Safety Level BSL 2+ conditions. MEFs and Rat-1 cells were infected with virus expressing Tax. The cells stably expressing Tax were also reinfected with virus expressing p100, p100SS/AA, p52, HA-IκBαSS/AA, WWOX, or WWOX Y33R mutant for simultaneously expressing Tax and these proteins. The viruses containing empty vector sequences were used as a control. The stable transfectants were obtained by drug selections using a detailed protocol previously described.20,26 Similarly, 293 and Rat-1 cells expressing short hairpin RNA (shRNA) against the wwox gene were generated using the lentiviral vector pLentilox pLL3.7, which expresses green fluorescent protein downstream of the shRNA. The shRNA stable transfectants were obtained by cell sorting based on green fluorescent protein expression. The target sequences were as follows: human WWOX shRNA No. 1 (5′-AAGACTCAGTGGGAACATC-3′), human WWOX shRNA No. 2 (5′-GCAGTGCATCCTGGAAATATG-3′), mouse Wwox shRNA No. 1 (5′-GGATCCAAGATTGGCATTTAC-3′), rat Wwox shRNA No. 1 (5′-GGATCCAAGATTGGCATTTAC-3′), mouse Wwox shRNA No. 2 (5′-GGGCCATGCTGGCTTATAA-3′), rat Wwox shRNA No. 2 (5′-GCATTTACCGTGGATGATAAT-3′), human WWOX shRNA No. 3 (5′-GGCCAAGAATGTGCCTCTTCA-3′), and rat Wwox shRNA No. 3 (5′-GGCCAAGAATGTGCCTCTTCA-3′).
Induction of Tax in Jurkat inducible cell line
For Tax induction, Jurkat-TetOn inducible cells maintained in 10% tetracycline-free FBS were treated with 0.1 μg/mL doxycycline for the indicated time as described previously.25 The cells were then lysed in RIPA buffer (50mM Tris-HCl [pH 7.4], 150mM NaCl, 1mM ethylenediaminetetraacetic acid, 0.25% [wt/vol] sodium deoxycholate, 1% [vol/vol] Nonidet P-40, 1mM dithiothreitol) supplemented with 1mM dithiothreitol and 1mM phenylmethylsulfonyl fluoride or TRIzol reagent for whole-cell lysate and RNA extractions, respectively.
Immunoblotting and immunoprecipitation assays
RT-PCR analysis
Regular reverse transcription–polymerase chain reaction (RT-PCR) or real-time RT-PCR were performed as described.25,29 Primer pairs used for RT-PCR were the following: human WWOX (forward) 5′-CGCGGCCGCCATGGCAGCGCTGCGC, (reverse) 5′-CGAATTCTTAGCCGGACTGGCTGCC; mouse Wwox (forward) 5′-TCATCCCCCGCCCGTGTCAT, (reverse) 5′-CCTCCAGCTCTGGGGCG ACT; rat Wwox (forward) 5′-GGGAGCAGGAGACCGACGA, (reverse) 5′-CACACTACGGAGCACG GCCA; TAX (forward) 5′-GCCGGCCACAACCATGGCCCACTTCCCAGG, (reverse) 5′-GGCGTCGACTCAGACTTCTGTTTCACGGAAAT; and human, mouse, and rat glyceraldehyde-3-phosphate dehydrogenase ([Gapdh] forward) 5′-CACAGTCCATGCCATCACTG, (reverse) 5′-CTTACTCCTTGGAGGCCATG. Primer pairs used for real-time RT-PCR were the following: human WWOX (forward) 5′-CTCCTGCCCGTGTCATTGTG, (reverse) 5′-GCATCGCCCAATAGTCGTTT and human [BETA]-ACTIN (forward) 5′-ATCAAGATCATTGCTCCTCCT, (reverse) 5′-GAGAGCGAGGCCAGGATGGA.
Protein stability assays
Cells were treated with 10μM CHX, followed by chase for the indicated time periods in the presence or absence of MG132, and then subjected to IB to detect the indicated proteins.20
Luciferase gene reporter assays
Cultured 293 cells were transfected with NF-κB–driven firefly luciferase reporter,8,9 TK-driven Renilla luciferase reporter, and Tax in the presence of increasing amounts of WWOX, WWOX Y33R mutant, or different wwox shRNA constructs. For C8166 and MT-2 cells, the luciferase reporters were transfected with increasing amounts of WWOX. At 40 hours after transfection, dual luciferase activities were measured as we described previously.8
Colony formation assays
Soft agar assays were performed as previously described.20,26 Briefly, cell suspensions in culture medium containing 0.6% SeaPlaque low melting agarose were plated on the top of 1% agarose in culture medium. Colony growth was scored after 21 days of cell incubation. All the colony formation assays presented in this study were repeated in at least 3 independent experiments.
In vivo tumor formation
All experiments involving mice were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh. Purchased 5-week-old female severe combined immunodeficient mice (Charles River Breeding Laboratories) were challenged subcutaneously in the hind back with the indicated Rat-1 stable cell lines.20 The recipient mice were monitored daily, and killed for tumor evaluation at day 14 after injection. Long terminal repeat (LTR)-Tax transgenic (Tax+) mice were mated with p100 knockout (p100−/−) mice (generous gifts from J.E. Green and D. Novack, respectively).30,31 The generated Tax+ p100−/− mice and their cohort Tax+ p100+/+ mice were monitored daily for tumor formation for up to 52 weeks and killed when overt tumor development was detected or when signs of morbidity were evident.
Statistical analysis
Results
Knockout of p100/p52 prevents tumorigenesis in Tax transgenic mice
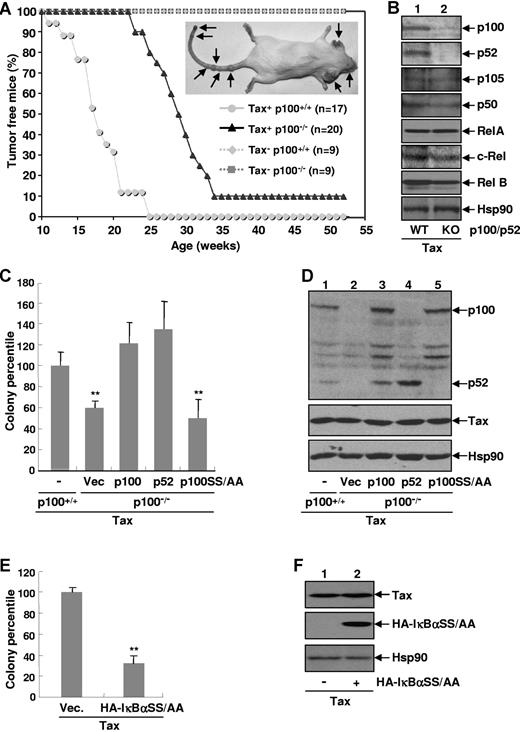
The Tax viral oncoprotein is a potent activator of both the canonical and noncanonical NF-κB pathways. However, the individual role of these 2 protumorigenic pathways in Tax-mediated tumorigenesis remains obscure. To address this important issue, we examined the effect of p100/p52 knockout on tumor formation in transgenic mice expressing the HTLV-I Tax gene (Tax+) driven by HTLV-I viral promoter. In agreement with a previous study,30 Tax+ p100+/+ mice started to develop visible tumors on their ears, mouths, noses, legs, and/or tails at the age of 11 weeks, and all the mice had tumors by 18 weeks of age (Figure 1A). In contrast, the earliest onset of tumor formation in Tax+ p100−/− mice, which lack expression of both the p100 and p52 proteins, started at 24 weeks, and only 10% of mice developed tumors by 25 weeks of age and 10% of mice remained tumor-free during the 52-week observation period. Of note, control mice with no Tax expression (either p100+/+ or p100−/−) did not develop any notable malignancies during the 1-year observation period. Western blot assays further validated that with the exception of p100/p52, expression levels of other NF-κB members were comparable in Tax+ p100+/+ and Tax+ p100−/− tumors (Figure 1B). These data indicated that p100 and/or p52 play an important role in Tax-mediated tumorigenesis in vivo.
Noncanonical NF-κB is involved in Tax-mediated cellular transformation and tumorigenesis. (A) Kaplan-Meier plot of tumor-free mice. Tax+ p100+/+ mice (n = 17), Tax+ p100−/− mice (n = 20), and Tax-free p100+/+ or p100−/− mice (n = 9) were monitored daily for tumor formation for up to 52 weeks. A representative mouse with tumors is shown (right) and the tumors are indicated by arrows. (B) Protein expression levels of NF-κB proteins in Tax+p100+/+ or Tax+p100−/− tumor cells. (C) Involvement of p52 for Tax-mediated colony formation. The p100−/− MEFs stably expressing Tax alone or together with p100, p52, or p100SS/AA were plated in soft agar for colony formation. The colony formation efficiency is represented as a percentile of that in p100+/+ MEFs stably expressing Tax (arbitrarily set as 100). These data represent mean (± SD) for n = 3 samples. **P < .01. (D) Protein expression levels of Tax, p100, p52, and p100SS/AA in the MEFs stably expressing Tax used in panel C. (E) Suppression of Tax-mediated colony formation by IκBαSS/AA. The p100+/+ MEFs stably expressing Tax alone or together with HA-IκBαSS/AA were plated in soft agar and subjected to colony formation as described in panel C. (F) Protein expression levels of Tax and HA-IκBαSS/AA in the MEFs used in panel E.
Noncanonical NF-κB is involved in Tax-mediated cellular transformation and tumorigenesis. (A) Kaplan-Meier plot of tumor-free mice. Tax+ p100+/+ mice (n = 17), Tax+ p100−/− mice (n = 20), and Tax-free p100+/+ or p100−/− mice (n = 9) were monitored daily for tumor formation for up to 52 weeks. A representative mouse with tumors is shown (right) and the tumors are indicated by arrows. (B) Protein expression levels of NF-κB proteins in Tax+p100+/+ or Tax+p100−/− tumor cells. (C) Involvement of p52 for Tax-mediated colony formation. The p100−/− MEFs stably expressing Tax alone or together with p100, p52, or p100SS/AA were plated in soft agar for colony formation. The colony formation efficiency is represented as a percentile of that in p100+/+ MEFs stably expressing Tax (arbitrarily set as 100). These data represent mean (± SD) for n = 3 samples. **P < .01. (D) Protein expression levels of Tax, p100, p52, and p100SS/AA in the MEFs stably expressing Tax used in panel C. (E) Suppression of Tax-mediated colony formation by IκBαSS/AA. The p100+/+ MEFs stably expressing Tax alone or together with HA-IκBαSS/AA were plated in soft agar and subjected to colony formation as described in panel C. (F) Protein expression levels of Tax and HA-IκBαSS/AA in the MEFs used in panel E.
To define whether the defect in p100-deficient mice was a result of deficiency of p100 expression or p52 generation, we took advantage of the MEFs from p100+/+ and p100−/− mice. Consistent with our previous study,20 neither p100+/+ nor p100−/− MEFs were able to form colonies in soft agar (data not shown). Both p100+/+ and p100−/− MEFs acquired the ability to form foci in soft agar when transduced with Tax; however, the colony formation efficiency of Tax expressing p100−/− MEFs was much lower (Figure 1C). The discrepancy of the p100+/+ and p100−/− MEFs in the sensitivity of Tax-induced transformation was not because they were generated from different embryos, as re-expression of exogenous p100 could rescue the transformation of the p100−/− MEFs by Tax. Interestingly, reconstitution of the p100 processed product p52 alone was also able to restore Tax-induced transformation of the p100−/− MEFs. In contrast, reconstitution with p100SS/AA, a serine to alanine point p100 mutant defective in inducible phosphorylation and processing,15,16 failed to do so. It should be pointed out that the expression levels of Tax, p100, p100SS/AA, or p52 were comparable in these MEFs (Figure 1D). To examine the role of the canonical NF-κB pathway in Tax-mediated cellular transformation, we performed the colony formation assay using cells simultaneously expressing Tax and the super repressor form of IκBα (IκBαSS/AA). As shown in Figure 1E-F, coexpression of IκBαSS/AA resulted in a significant decrease of Tax-induced colony formation. These data suggested that both the canonical and noncanonical NF-κB pathways are involved in Tax-mediated cellular transformation and tumorigenesis.
Tax inhibits expression of the WWOX gene largely dependent on p100/p52 expression
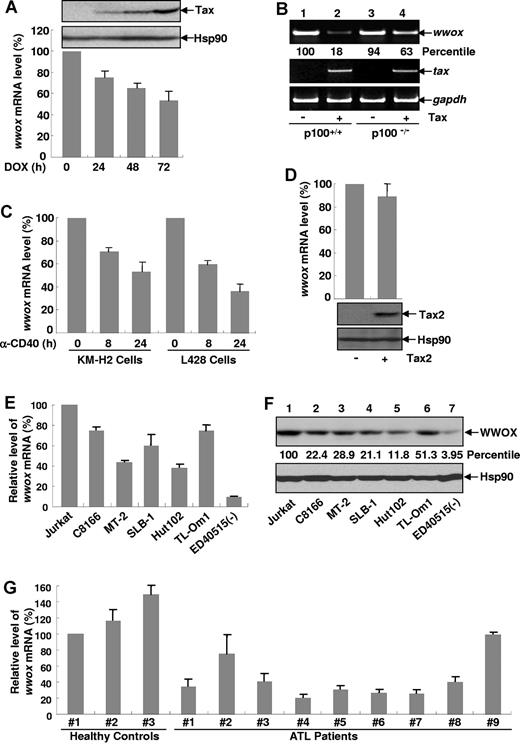
Our previous gene array studies indicated that aberrant expression of p52 resulting from constitutive processing of p100 represses expression of the WWOX tumor suppressor gene.34 Thus, we hypothesized that Tax activation of the noncanonical NF-κB pathway may down-regulate expression of the WWOX gene and thereby may enhance its tumorigenicity. To test this hypothesis, we initially examined whether Tax represses WWOX expression using a Tax-TetOn inducible Jurkat cell line. As shown in Figure 2A, inducible expression of Tax indeed repressed expression of the WWOX gene in a time-dependent manner. In agreement with our previous finding identifying the WWOX gene as a negative target of the noncanonical NF-κB activation, we found that deficiency of p100/p52 resulted in almost complete recovery of the WWOX gene from Tax-mediated repression (Figure 2B). In further support of those findings, CD40 (activator of both canonical and noncanonical NF-κB pathways1 ), but not Tax-2 encoded by HTLV-II (selective activator of the canonical, but not the noncanonical, NF-κB pathway35 ), could significantly down-regulate WWOX mRNA levels (Figure 2C-D). These data suggested that one function of Tax is to activate the noncanonical NF-κB pathway to repress expression of the WWOX tumor suppressor gene.
Tax represses WWOX expression through the noncanonical NF-κB pathway. (A) WWOX repression by Tax. Jurkat Tax-TetOn cells were treated with doxycycline (DOX) for the indicated times to induce Tax expression. The induction of Tax protein was analyzed by direct IB (top). The relative levels of WWOX mRNAs in the Tax inducible cells were analyzed by real-time RT-PCR and normalized according to β-actin mRNA level and represented as a percentile of that in untreated cells (arbitrarily set as 100). These data represent mean (± SD) for n = 3 samples. (B) Requirement of p100/p52 in Tax repression of Wwox. Semiquantitative RT-PCR analysis was performed to detect the expression levels of Wwox, TAX, and Gapdh in the matched ear tissues from p100+/+ mice, Tax+p100+/+ mice, p100−/− mice and Tax+p100−/− mice. The relative levels of Wwox mRNAs based on Gapdh mRNAs in Tax+ cells were also represented as a percentile of the levels in Tax−p100+/+ cells (arbitrarily set as 100). (C) Down-regulation of WWOX gene expression by CD40 activation. The CD40+ cell lines KM-H2 and L428 were treated with anti–CD40 antibodies (10μg/mL) followed by real-time RT-PCR analysis as described in panel A. (D) No significant effect on Wwox gene expression by Tax2. MEFs stably expressing Tax2 or an empty vector were subjected to real-time RT-PCR analysis as described in panel A. Tax 2 expression level was examined by IB. (E) RNA repression of WWOX in HTLV-I–transformed T-cell lines and ATL cell lines. The relative levels of WWOX mRNAs in the indicated HTLV-I–transformed T-cell lines and ATL cell lines were analyzed by real-time RT-PCR analysis as described in panel A. (F) Protein repression of WWOX in HTLV-I–transformed T-cell lines and ATL cell lines. Protein expression levels of WWOX were analyzed in the indicated cell lines by IB. The relative levels of WWOX proteins based on Hsp90 proteins were represented as a percentile of levels in Jurkat cells (arbitrarily set as 100). (G) WWOX repression in freshly isolated ATL cells. The levels of WWOX mRNA in PBMCs directly from ATL patients or healthy donors were analyzed by real-time RT-PCR and represented as a percentile of that in normal control No. 1 (arbitrarily set as 100).
Tax represses WWOX expression through the noncanonical NF-κB pathway. (A) WWOX repression by Tax. Jurkat Tax-TetOn cells were treated with doxycycline (DOX) for the indicated times to induce Tax expression. The induction of Tax protein was analyzed by direct IB (top). The relative levels of WWOX mRNAs in the Tax inducible cells were analyzed by real-time RT-PCR and normalized according to β-actin mRNA level and represented as a percentile of that in untreated cells (arbitrarily set as 100). These data represent mean (± SD) for n = 3 samples. (B) Requirement of p100/p52 in Tax repression of Wwox. Semiquantitative RT-PCR analysis was performed to detect the expression levels of Wwox, TAX, and Gapdh in the matched ear tissues from p100+/+ mice, Tax+p100+/+ mice, p100−/− mice and Tax+p100−/− mice. The relative levels of Wwox mRNAs based on Gapdh mRNAs in Tax+ cells were also represented as a percentile of the levels in Tax−p100+/+ cells (arbitrarily set as 100). (C) Down-regulation of WWOX gene expression by CD40 activation. The CD40+ cell lines KM-H2 and L428 were treated with anti–CD40 antibodies (10μg/mL) followed by real-time RT-PCR analysis as described in panel A. (D) No significant effect on Wwox gene expression by Tax2. MEFs stably expressing Tax2 or an empty vector were subjected to real-time RT-PCR analysis as described in panel A. Tax 2 expression level was examined by IB. (E) RNA repression of WWOX in HTLV-I–transformed T-cell lines and ATL cell lines. The relative levels of WWOX mRNAs in the indicated HTLV-I–transformed T-cell lines and ATL cell lines were analyzed by real-time RT-PCR analysis as described in panel A. (F) Protein repression of WWOX in HTLV-I–transformed T-cell lines and ATL cell lines. Protein expression levels of WWOX were analyzed in the indicated cell lines by IB. The relative levels of WWOX proteins based on Hsp90 proteins were represented as a percentile of levels in Jurkat cells (arbitrarily set as 100). (G) WWOX repression in freshly isolated ATL cells. The levels of WWOX mRNA in PBMCs directly from ATL patients or healthy donors were analyzed by real-time RT-PCR and represented as a percentile of that in normal control No. 1 (arbitrarily set as 100).
To check whether the repression of the WWOX gene is associated with HTLV-I–mediated leukemogenesis, we examined WWOX expressions under different HTLV-I pathogenic conditions. Compared with Jurkat cells, an HTLV-I–negative leukemic cell line, all HTLV-I transformed T-cell lines and established ATL cell lines that we examined expressed significantly lower expression of WWOX at both RNA and protein levels, although to different extents (Figure 2E-F). It should be pointed out that repression of WWOX expression was stronger at the protein level. Our data suggested that WWOX proteins undergo ubiquitination and proteasomal degradation through both Tax-dependent and -independent mechanisms (data not shown and see Figure 6D). To further confirm the pathophysiologic significance of these findings, we examined WWOX expression in primary ATL cells directly isolated from 9 different patients. Compared with normal T cells from healthy donors, approximately 78% (7 of 9) ATL samples showed significantly decreased WWOX expression (Figure 2G). These data strongly suggested that wwox repression is an HTLV-I pathophysiologic event.
The WWOX knockdown increases the transforming potential of Tax
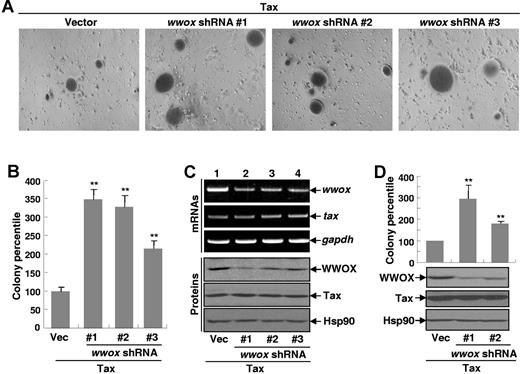
To define the significance of the WWOX repression, we examined the effect of WWOX knockdown by shRNA on Tax-mediated in vitro cellular transformation. In correlation with its tumor suppression function, Wwox knockdown significantly increased Tax-induced anchorage-independent cell growth (Figure 3A-B). Similar results were obtained when 3 different shRNAs were used for the Wwox knockdown (Figure 3A-B). It was noteworthy that the Wwox knockdown had no effect on Tax expression at both RNA and protein levels (Figure 3C). In addition, the cells we used for the colony formation assay did not form colonies in soft agar in the absence of Tax, whether Wwox was knocked down or not (data not shown). Interestingly, Wwox knockdown was able to restore Tax-induced transformation of p100−/− MEFs (Figure 3D). These data suggested that the noncanonical NF-κB–dependent repression of Wwox is an important mechanism contributing to Tax-mediated cellular transformation.
The Wwox knockdown enhances Tax-mediated cellular transformation. (A) Enhancement of Tax-mediated colony formation by Wwox knockdown. Rat-1 cells stably expressing Tax alone or together with different Wwox shRNA constructs were plated in soft agar for colony formation. Photographs were taken at day 21 after plating. Original magnification was ×100. (B) Summary of colony formation efficiency performed in panel A. These data represent mean (± SD), and values for P were obtained using Student t test. **P < .01. (C) Knockdown efficiency of Wwox by shRNAs. Semiquantitative RT-PCR analysis and IB assay were performed, respectively, to examine the RNA and protein expression levels of Wwox and TAX in the Rat-1 stable cell lines used in panel A. (D). Restoration of Tax-mediated transformation of p100−/− MEFs by Wwox knockdown. p100−/− MEFs stably expressing Tax alone or together with different Wwox shRNA constructs were subjected to soft agar colony formation and IB assays as described in panel A.
The Wwox knockdown enhances Tax-mediated cellular transformation. (A) Enhancement of Tax-mediated colony formation by Wwox knockdown. Rat-1 cells stably expressing Tax alone or together with different Wwox shRNA constructs were plated in soft agar for colony formation. Photographs were taken at day 21 after plating. Original magnification was ×100. (B) Summary of colony formation efficiency performed in panel A. These data represent mean (± SD), and values for P were obtained using Student t test. **P < .01. (C) Knockdown efficiency of Wwox by shRNAs. Semiquantitative RT-PCR analysis and IB assay were performed, respectively, to examine the RNA and protein expression levels of Wwox and TAX in the Rat-1 stable cell lines used in panel A. (D). Restoration of Tax-mediated transformation of p100−/− MEFs by Wwox knockdown. p100−/− MEFs stably expressing Tax alone or together with different Wwox shRNA constructs were subjected to soft agar colony formation and IB assays as described in panel A.
WWOX coexpression suppresses Tax-mediated cellular transformation and tumor formation
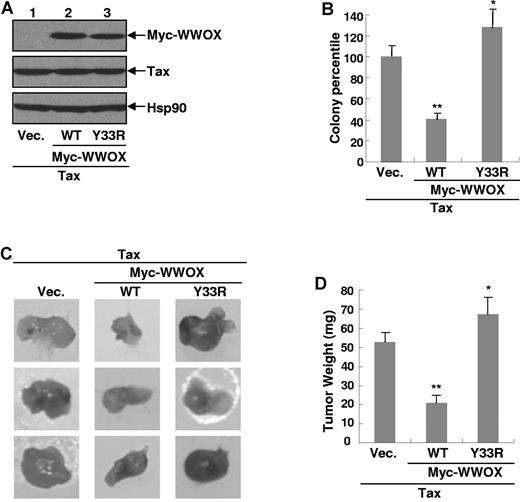
To validate the knockdown studies, we examined whether WWOX expression decreases Tax-mediated cellular transformation. Because it has been reported that the WWOX Y33R mutant loses its tumor suppression function,17,18 we also included this mutant in the experiments. In support of the Wwox knockdown studies suggesting no involvement of WWOX in Tax expression, coexpression of WWOX or its Y33R mutant also did not affect Tax expression (Figure 4A). More importantly, coexpression of WWOX was able to significantly block the anchorage-independent growth of Tax expressing cells (Figure 4B). In sharp contrast, expression of WWOX Y33R mutant actually increased the Tax transforming ability, suggesting a dominant negative function of the mutant in inhibiting endogenous WWOX. Thus, both knockdown and overexpression studies suggested that WWOX suppresses the transforming ability of Tax.
WWOX suppresses Tax-mediated cellular transformation and tumor formation. (A) Generation of Rat-1 stable cell lines expressing Tax alone or together with Myc-WWOX or Myc-WWOX Y33R. IB analysis was used to detect protein expression levels of Tax, Myc-WWOX and Myc-WWOX Y33R in the Rat-1 stable cell lines. (B) WWOX inhibition of Tax-mediated colony formation. The indicated Rat-1 stable cells were plated in soft agar for colony formation. These data represent mean (± SD), and values *P < .05 or **P < .01 were using the Student t test. (C) WWOX inhibition of Tax-mediated tumorigenesis. The Rat-1 stable cells were subcutaneously inoculated into to the left and right hind back of severe combined immunodeficient mice. Photographs were taken at day 14 after inoculation. (D) Summary of tumor weight expressed in panel C. These data represent mean (± SD) for n = 3 samples. *P < .05 or **P < .01 using the Student t test.
WWOX suppresses Tax-mediated cellular transformation and tumor formation. (A) Generation of Rat-1 stable cell lines expressing Tax alone or together with Myc-WWOX or Myc-WWOX Y33R. IB analysis was used to detect protein expression levels of Tax, Myc-WWOX and Myc-WWOX Y33R in the Rat-1 stable cell lines. (B) WWOX inhibition of Tax-mediated colony formation. The indicated Rat-1 stable cells were plated in soft agar for colony formation. These data represent mean (± SD), and values *P < .05 or **P < .01 were using the Student t test. (C) WWOX inhibition of Tax-mediated tumorigenesis. The Rat-1 stable cells were subcutaneously inoculated into to the left and right hind back of severe combined immunodeficient mice. Photographs were taken at day 14 after inoculation. (D) Summary of tumor weight expressed in panel C. These data represent mean (± SD) for n = 3 samples. *P < .05 or **P < .01 using the Student t test.
To extend these in vitro findings, we performed in vivo tumor formation assays using the stably transfected Rat-1 cells. In results consistent with our in vitro colony formation assays, WWOX significantly suppressed Tax-mediated tumorigenesis in mice, whereas the WWOX Y33R mutant dramatically enhanced the oncogenic ability of Tax (Figure 4C-D). Taken together, these data clearly suggested that Tax represses WWOX expression through the noncanonical NF-κB pathway, which in turn contributes to Tax-mediated cellular transformation and tumorigenesis.
WWOX inhibits HTLV-I/Tax-mediated NF-κB activation
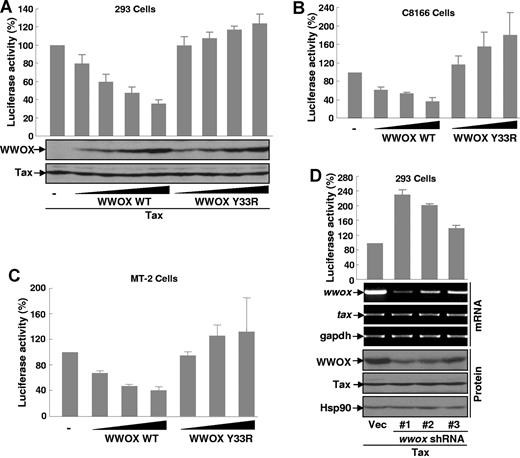
To investigate possible mechanisms by which WWOX suppresses Tax-mediated tumorigenesis, we performed the dual luciferase gene reporter assays to examine the possible role of WWOX in Tax-induced NF-κB transcriptional activation. In accordance with their roles in Tax-mediated tumorigenesis, WWOX inhibited Tax-mediated NF-κB activation in a dose-dependent manner, whereas the WWOX Y33R mutant increased Tax-mediated NF-κB activation also in a dose-dependent manner (Figure 5A). This result was further verified using the HTLV-I-transformed T cell lines C8166 and MT-2 (Figure 5B-C). The inhibition of NF-κB activation by WWOX seemed specific to Tax, as overexpression of WWOX had no effect on TNFα-induced NF-κB activation (supplemental Figure 1; available on the Blood Web site; see the Supplemental Materials link at the top of the online article). In addition to NF-κB activation, WWOX also repressed Tax-mediated HTLV-I LTR activation, another key function of Tax in HTLV-I pathogenesis.20 In results consistent with these overexpression studies, knockdown of endogenous WWOX further up-regulated Tax-mediated NF-κB activation (Figure 5D). As a matter of fact, NF-κB activity induced by Tax was inversely correlated with the knockdown efficiency of WWOX. These data suggested that WWOX, a negative target of NF-κB, is a novel inhibitor of NF-κB induced by Tax. These data also suggested that WWOX suppression of HTLV-I/Tax-mediated tumorigenesis may be mediated through inhibition of NF-κB activation.
WWOX prevents Tax-induced NF-κB transcriptional activation. (A) WWOX prevention of Tax-induced NF-κB activation. 293 cells were transfected with plasmids expressing Tax, A κB-driven firefly luciferase reporter and a TK-driven Renilla luciferase reporter in the presence of an increasing amount of Myc-WWOX or Myc-WWOX Y33R, followed by measure of luciferase activity. The luciferase activity was presented as the percentile of that activated by Tax alone (set as 100). Protein expression levels of transfected Tax, Myc-WWOX, and its mutant were examined by IB. (B-C) WWOX prevention of NF-κB constitutive activation in HTLV-I–transformed T cells. The indicated HTLV-I–transformed T-cell lines were transfected with a κB-driven firefly luciferase reporter and a TK-driven Renilla luciferase reporter in the presence of an increasing amount of Myc-tagged WWOX or WWOX Y33R, followed by measure of luciferase activity as described in panel A. (D) Enhancement of Tax-induced NF-κB activation by WWOX knockdown. The 293 cells were transfected with Tax and κB-driven luciferase reporter in the presence or absence of different WWOX shRNA followed by measure of luciferase activity. The RNA and protein expression level of WWOX and Tax was examined by RT-PCR and IB assays, respectively.
WWOX prevents Tax-induced NF-κB transcriptional activation. (A) WWOX prevention of Tax-induced NF-κB activation. 293 cells were transfected with plasmids expressing Tax, A κB-driven firefly luciferase reporter and a TK-driven Renilla luciferase reporter in the presence of an increasing amount of Myc-WWOX or Myc-WWOX Y33R, followed by measure of luciferase activity. The luciferase activity was presented as the percentile of that activated by Tax alone (set as 100). Protein expression levels of transfected Tax, Myc-WWOX, and its mutant were examined by IB. (B-C) WWOX prevention of NF-κB constitutive activation in HTLV-I–transformed T cells. The indicated HTLV-I–transformed T-cell lines were transfected with a κB-driven firefly luciferase reporter and a TK-driven Renilla luciferase reporter in the presence of an increasing amount of Myc-tagged WWOX or WWOX Y33R, followed by measure of luciferase activity as described in panel A. (D) Enhancement of Tax-induced NF-κB activation by WWOX knockdown. The 293 cells were transfected with Tax and κB-driven luciferase reporter in the presence or absence of different WWOX shRNA followed by measure of luciferase activity. The RNA and protein expression level of WWOX and Tax was examined by RT-PCR and IB assays, respectively.
WWOX selectively blocks Tax-mediated IKKα recruitment and RelA S536 phosphorylation
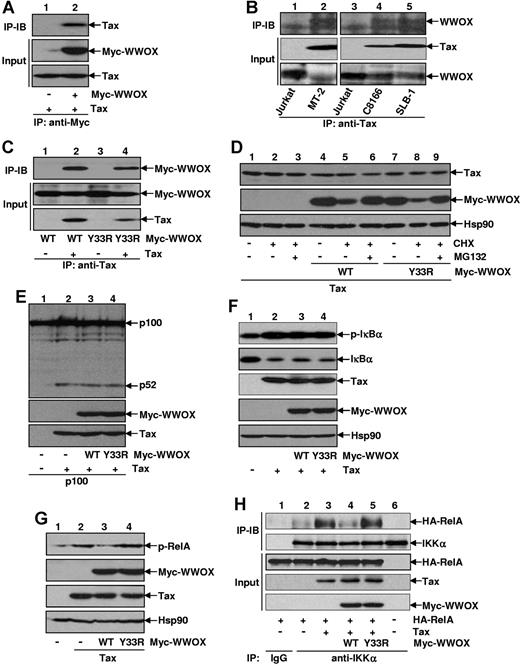
We next examined the possible interaction between WWOX and Tax, as they both are well known for their activities in protein interactions and signaling complex assembly.17,18,36 When coexpressed together in 293 cells, WWOX and Tax formed a stable complex, as evidenced by coimmunoprecipitation assays (Figure 6A). As a matter of fact, endogenous WWOX and Tax also formed a complex in HTLV-I–transformed T cells (Figure 6B). Although the WWOX Y33R mutant loses the ability to suppress Tax-mediated NF-κB activation and tumorigenesis, it still retained the ability to bind to Tax (Figure 6C). These data thus suggested that binding to Tax is not sufficient for WWOX to suppress the oncogenic actions of Tax. Detailed coimmunoprecipitation analysis indicated that the WWOX-Tax interaction occurred both in the cytoplasm and in the nucleus (supplemental Figure 2A). Moreover, WWOX could also interact with Tax M22 and M47, 2 mutants well known for their selective defects in the NF-κB or HTLV-I LTR activation, respectively (supplemental Figure 2B).37 These data provided mechanistic insights into the functions of WWOX on suppression of Tax-induced NF-κB and HTLV-I LTR activations (Figure 5 and supplemental Figure 1B).
WWOX selectively inhibits Tax-mediated IKKα recruitment into RelA and subsequent RelA S536 phosphorylation. (A) Interaction between Tax and WWOX. The 293 cells were transfected with Tax alone or together with Myc-WWOX, followed by IP using Myc antibody and IB using Tax antibody. The inputs of Myc-WWOX and Tax were analyzed by direct IB. (B) In vivo interaction between Tax and WWOX. Endogenous Tax proteins of the indicated HTLV-I–transformed T cells were pulled down by Tax antibody, and the endogenous WWOX proteins that bound to Tax were examined by WWOX antibody. Jurkat cells were used as a negative control. The inputs of endogenous WWOX and Tax were analyzed by direct IB. (C) Interaction between Tax and WWOX Y33R. 293 cells were transfected with Myc-WWOX wild-type or its Y33R mutant in the presence or absence of Tax, followed by IP using Tax antibody and IB using Myc antibody. The inputs of Tax, Myc-WWOX, and its Y33R mutant were analyzed by direct IB. (D) Effect of WWOX on Tax stability. The 293 cells were transfected with Tax in the presence or absence of Myc-WWOX or Myc-WWOX Y33R, and were mock treated or treated with CHX alone or CHX plus MG132 for 5 hours. The expression levels of Tax, Myc-WWOX, Myc-WWOX Y33R, and Hsp90 were detected by IB. (E) Effect of WWOX on Tax-induced p100 processing. The 293 cells were transfected with the indicated plasmids, followed by IB to examine protein levels of p100/p52, Tax, WWOX, and its Y33R mutant. (F) Effect of WWOX on Tax-induced IκBα phosphorylation and degradation. HeLa cells were transfected with Tax in the presence or absence of Myc-WWOX or Myc-WWOX Y33R, followed by IB to examine phosphorylation-modified or unmodified phosphorylated IκBα. Protein levels of Tax, Myc-WWOX, and its Y33R mutant were also examined. (G) Selective inhibition of Tax-mediated RelA S536 phosphorylation by WWOX. The 293 cells were transfected with Tax in the presence or absence of Myc-WWOX or Myc-WWOX Y33R followed by IB to examine S536 phosphorylation of endogenous RelA. (H) Selective inhibition of Tax-mediated IKKα recruitment to RelA by WWOX. The 293 cells were transfected with the indicated plasmids followed by IP using IKKα specific antibody (lanes 2-6) or control IgG (lane 1) and IB using HA antibody or IKKα antibody. The inputs of all related proteins were also shown.
WWOX selectively inhibits Tax-mediated IKKα recruitment into RelA and subsequent RelA S536 phosphorylation. (A) Interaction between Tax and WWOX. The 293 cells were transfected with Tax alone or together with Myc-WWOX, followed by IP using Myc antibody and IB using Tax antibody. The inputs of Myc-WWOX and Tax were analyzed by direct IB. (B) In vivo interaction between Tax and WWOX. Endogenous Tax proteins of the indicated HTLV-I–transformed T cells were pulled down by Tax antibody, and the endogenous WWOX proteins that bound to Tax were examined by WWOX antibody. Jurkat cells were used as a negative control. The inputs of endogenous WWOX and Tax were analyzed by direct IB. (C) Interaction between Tax and WWOX Y33R. 293 cells were transfected with Myc-WWOX wild-type or its Y33R mutant in the presence or absence of Tax, followed by IP using Tax antibody and IB using Myc antibody. The inputs of Tax, Myc-WWOX, and its Y33R mutant were analyzed by direct IB. (D) Effect of WWOX on Tax stability. The 293 cells were transfected with Tax in the presence or absence of Myc-WWOX or Myc-WWOX Y33R, and were mock treated or treated with CHX alone or CHX plus MG132 for 5 hours. The expression levels of Tax, Myc-WWOX, Myc-WWOX Y33R, and Hsp90 were detected by IB. (E) Effect of WWOX on Tax-induced p100 processing. The 293 cells were transfected with the indicated plasmids, followed by IB to examine protein levels of p100/p52, Tax, WWOX, and its Y33R mutant. (F) Effect of WWOX on Tax-induced IκBα phosphorylation and degradation. HeLa cells were transfected with Tax in the presence or absence of Myc-WWOX or Myc-WWOX Y33R, followed by IB to examine phosphorylation-modified or unmodified phosphorylated IκBα. Protein levels of Tax, Myc-WWOX, and its Y33R mutant were also examined. (G) Selective inhibition of Tax-mediated RelA S536 phosphorylation by WWOX. The 293 cells were transfected with Tax in the presence or absence of Myc-WWOX or Myc-WWOX Y33R followed by IB to examine S536 phosphorylation of endogenous RelA. (H) Selective inhibition of Tax-mediated IKKα recruitment to RelA by WWOX. The 293 cells were transfected with the indicated plasmids followed by IP using IKKα specific antibody (lanes 2-6) or control IgG (lane 1) and IB using HA antibody or IKKα antibody. The inputs of all related proteins were also shown.
Although our previous studies showed that WWOX knockdown or expression has no effect on Tax expression at both RNA and protein levels (Figures 3,5), we still examined the potential effect of WWOX and its Y33R mutant on Tax protein stability, as our recent studies have shown that the signaling protein PDLIM2 physically interacts with and recruits Tax to the nuclear matrix for proteasomal degradation.20,38 Consistent with a previous study showing that WWOX is relatively unstable,39 both WWOX and its Y33R mutant underwent proteasomal degradation in the presence of Tax (Figure 6D). But more importantly, either WWOX or its Y33R mutant had no obvious effect on Tax stability. These data suggested that WWOX does not target Tax for degradation.
As WWOX is a negative target of the noncanonical NF-κB activation and WWOX repression provides a feed-forward mechanism for the persistent activation of NF-κB by Tax, we initially compared the potential effects of WWOX with its Y33R mutant on Tax-induced p100 processing. As shown in Figure 6E, both WWOX and its Y33R mutant failed to prevent Tax-mediated p100 processing, suggesting that the noncanonical NF-κB pathway is not a target of WWOX for the suppression.
We next compared the effects of WWOX with its Y33R mutant on Tax-induced phosphorylation and degradation of IκBα, 2 key steps for the activation of the canonical NF-κB pathway. Unexpectedly, neither WWOX nor its Y33R mutant was able to block Tax-mediated IκBα phosphorylation and degradation (Figure 6F). As discussed in the “Introduction,” phosphorylation of RelA at S536 is critical for the transcriptional activation of the canonical NF-κB pathway mediated by Tax.13,14 We thus examined whether WWOX inhibits Tax-induced S536 phosphorylation of RelA. Indeed, coexpression of WWOX resulted in inhibition of Tax-induced S536 phosphorylation of RelA (Figure 6G). Interestingly, the WWOX Y33R mutant lost this ability. These data suggested that the different abilities of WWOX and its Y33R mutant in suppression of inducible RelA phosphorylation may account for their different functions in suppression of Tax-induced NF-κB activation and subsequent tumorigenesis.
It has been reported that IKKα is the kinase that mediates S536 phosphorylation of RelA in response to Tax,13 although the evidence for the interaction between IKKα and RelA is still lacking. Because it is known that Tax and RelA interact with each other, we hypothesized that Tax recruits IKKα to RelA for its S536 phosphorylation. We also hypothesized that WWOX, but not its Y33R mutant, would be able to block the IKKα recruitment of RelA induced by Tax. To test our theories, we performed a series of protein-protein interaction assays. In the absence of Tax, IKKα hardly interacted with RelA (Figure 6H). However, a strong association between IKKα and RelA was detected in the presence of Tax, suggesting an adaptor function of Tax in the IKKα and RelA interaction. Consistent with their different roles in Tax-induced S536 phosphorylation of RelA, WWOX completely prevented Tax-mediated IKKα recruitment to RelA, whereas the WWOX Y33R mutant actually increased, although slightly, IKKα recruitment. These data suggested that WWOX suppresses Tax-mediated NF-κB activation and subsequent tumorigenesis through selectively preventing Tax-induced IKKα recruitment to RelA and subsequent S536 phosphorylation of RelA.
Discussion
Activation of the canonical and noncanonical NF-κB pathways is often associated with human cancer.1 Although many NF-κB target genes have been identified that may contribute to oncogenesis, the individual and coordinated roles of the 2 pathways in tumorigenesis remains obscure and relatively few genes have been identified that link the 2 NF-κB pathways to each other, as well as to cancer. WWOX is a recently demonstrated haploinsufficient tumor suppressor that has been linked to various types of tumors.17,18 Currently, the mechanisms by which WWOX functions as a potent tumor suppressor remain largely unknown. The role of WWOX in viral tumorigenesis has also not been studied. These data show that the noncanonical NF-κB pathway plays an important role in the cellular transformation and tumorigenesis mediated by the HTLV-I–encoded Tax viral oncoprotein. Notably, one function of noncanonical NF-κB activation is to repress transcriptional expression of the wwox tumor suppressor gene, which in turn acts as a specific inhibitor of Tax-mediated canonical NF-κB. These data established a molecular link between the WWOX tumor suppressor and the pro-oncogenic functions of NF-κB.
The Tax oncoprotein is a potent activator of both canonical and noncanonical NF-κB signaling pathways and therefore provides a powerful model system to study the oncogenic actions of NF-κB in tumorigenesis.1 Although the molecular mechanisms by which Tax activates NF-κB have been well defined, the relative significance of the alternative pathways of NF-κB activation in Tax-mediated tumorigenesis has not been determined. Most functional studies have been focusing on the in vitro effects on Tax-induced cell growth and immortalization using different NF-κB inhibitors (most of them not completely NF-κB specific) or Tax mutants defective in NF-κB activation. The most impressive in vivo data supporting a role of NF-κB in Tax-mediated pathogenesis is from studies on transgenic mice conditionally expressing in a lymphocyte-restricted manner either Tax or its mutant forms that selectively compromise NF-κB (M22) or CREB/ATF (M47) activation.37 In these studies, Tax or Tax M47 expressing mice, but not Tax M22 expressing mice, develop aggressive skin disease that shares several features in common with the skin disease occurring during the preleukemic stage in HTLV-I-infected patients. However, these mice do not develop fully malignant phenotypes. Moreover, Tax also has many functions beyond NF-κB and CREB/ATF activation, such as serum response factor activation and induction of genetic instability that may also play a role in oncogenesis.40 Thus, the studies presented here provide the first in vivo evidence that directly demonstrates an important role of NF-κB in Tax-mediated tumorigenesis. Genetic knockout of the nf-kb2 gene can significantly reduce Tax-mediated tumor formation in Tax transgenic mice driven by the viral promoter (Figure 1). Rescue studies further suggest that the processed product p52, but not its precursor p100, is actually involved in the cellular transformation induced by Tax. These findings strongly suggest a critical role of activation of the noncanonical NF-κB pathway in Tax-mediated tumorigenesis.
Until the NF-κB target genes that play a role in oncogenesis are clearly and comprehensively identified, the full significance of the noncanonical NF-κB pathway in tumorigenesis will still be unknown. A plethora of genes have been identified as noncanonical NF-κB target genes, but they are generally positively regulated by noncanonical NF-κB activation and importantly most of them do not have a direct role in tumorigenesis.41 Consistent with the fact that p52 may down-regulate gene expression because of its intrinsic lack of transcriptional activity,1 our data show that Tax represses expression of the wwox tumor suppressor gene through the noncanonical NF-κB pathway (Figure 2). Importantly, wwox repression seems to be one key mechanism of Tax-mediated tumorigenesis because WWOX coexpression is able to prevent Tax-mediated cellular transformation and tumorigenesis, whereas wwox knockdown enhances the tumorigenicity of Tax (Figures 3,4). These findings identify the wwox tumor suppressor gene as a novel noncanonical NF-κB target gene and also make Tax the first viral agent known to target wwox as part of its oncogenic action.
The mechanisms by which WWOX contributes to tumor suppression currently remain elusive. A clue is provided by the structure of WWOX. WWOX harbors 2 N-terminal WW domains and a central short chain deydrogenase/reductase (SDR) domain, that gives WWOX its name.17,18 Although WW domains mediate protein-protein interactions, the known function of the SDR is involved in sex-steroid metabolism. Given the role of WWOX in suppressing both hormone-related and -unrelated tumors,17,18 we can speculate that the SDR enzyme activity is not the critical element for its tumor suppression. Instead, WWOX may bind to and regulate key signaling proteins for tumor suppression. It has been reported that through the WW domain, WWOX physically associates with several known proto-oncogenes such as activator protein 2γ and c-Jun, sequesters them in the cytoplasm and hence inhibits their transcriptional ability.17,18 Recent biochemical studies suggested that WW domain–mediated interactions are regulated by phosphorylation of Y33, present in the first WW domain. Although the involved kinase(s) remain unknown, inhibition of this important phosphorylation by substitution of Y33 with arginine results in loss of WWOX-mediated tumor suppression in certain cellular tumorigenesis models.17,18
Our studies show that the Tax viral oncoprotein is another binding target of WWOX (Figure 6 and supplemental Figure 2). Different from its cellular targets, WWOX does not affect Tax subcellular localization (data not shown and supplemental Figure 2). Moreover, Tax binding activity of WWOX does not require a functional WW domain, as the Y33R mutant still retains the ability to bind to Tax (Figure 6). In further support of this finding, N-terminal deletion of both WW domains does not affect WWOX binding to Tax (data not shown). These data substantiate a WW domain–independent binding activity of WWOX.42 Interestingly, the WWOX Y33R mutant not only completely loses the ability to suppress Tax-mediated tumorigenesis but also has a dominant negative function in this regard. Like the wwox knockdown, expression of the WWOX Y33R mutant increases Tax-mediated cell anchorage-independent growth in soft agar and tumor formation in mice (Figure 4). In agreement with the role of NF-κB in Tax-mediated tumorigenesis, the opposite functions of wild-type WWOX and its Y33R mutant in Tax-mediated tumorigenesis is highly consistent with their different abilities in regulating Tax-induced NF-κB transcriptional activation; wild-type WWOX inhibits Tax-mediated NF-κB activation, whereas Y33R mutant enhances this ability of Tax (Figure 5). Because wwox expression is repressed by Tax-activated noncanonical NF-κB (Figure 2), this finding not only demonstrates WWOX as a new inhibitor of NF-κB activation but also suggests a novel feed-forward mechanism for Tax-mediated NF-κB activation and subsequent tumorigenesis. Notably, WWOX selectively prevents Tax activation of the canonical NF-κB, but has no effect on Tax-mediated activation of the noncanonical NF-κB (Figure 6). Our mechanistic studies further indicate that WWOX does not affect Tax-induced phosphorylation and degradation of IκBα, a key step for the initiation of the canonical NF-κB activation (Figure 6). Instead, WWOX specifically blocks Tax-promoted IKKα recruitment to RelA and subsequent IKKα-mediated S536 phosphorylation of RelA, a posttranslational modification required for the transcriptional activity of the prototypic NF-κB factor. Consistent with its role as a dominant negative form of WWOX, the WWOX Y33R mutant enhances the IKKα recruitment and the IKKα-mediated RelA S536 phosphorylation induced by Tax (Figure 6). These findings suggest a novel mechanism of WWOX tumor suppression activities and provide an additional explanation for the loss-of-function in tumor suppression and the gain-of-function in tumor promotion of the Y33R mutation of WWOX.
In summary, these data demonstrate that an important function of the noncanonical NF-κB activation by the Tax viral oncoprotein is to repress transcriptional expression of the wwox tumor suppressor gene, which in turn facilitates Tax-induced activation of the canonical NF-κB signaling and subsequent tumorigenesis. These studies provide mechanistic insights into the oncogenic actions of Tax, as well as the tumor suppressor function of WWOX. They also provide the first example of how WWOX is involved in the suppression of viral tumorigenesis and how the canonical and noncanonical NF-κB signaling pathways delicately cooperate for tumorigenesis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank J.E. Green for LTR-Tax transgenic mice, D. Novack for p100 knockout mice, N. Mori for ATL primary cell cDNA, M. Fujii for Tax2B constructs and antibody, T. Cheng for the pLL3.7 shRNA lentiviral vector, L.R. Roberts for human wwox shRNA construct No. 1, N. Raab-Traub for Rat-1 cells, M. Mapara for KM-H2 and L428 cells, W.C. Greene and E. Harhaj for TetOn Jurkat cells, and M. Maeda and S. Yamaoka for TL-Om1 and ED40515− cells. We are also grateful to Z. Salah for technical assistance.
This study was supported in part by the National Institutes of Health, National Cancer Institute grant R01 CA116616, by American Cancer Society grant RSG-06-066-01-MGO (G.X.), and by FP7-EU-Marie Curie Re-integration (R.A.).
National Institutes of Health
Authorship
Contribution: J.F., Z.Q., P.Y., C.I., and R.I.A. performed research and analyzed data; R.I.A. and A.B.R. contributed vital new reagents; and G.X. designed the research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gutian Xiao, Hillman Cancer Center Research Pavilion, 5117 Centre Ave, Pittsburgh, PA 15213; e-mail: xiaog2@upmc.edu.
References
Author notes
J.F. and Z.Q. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal