Abstract
Whether long-term use of vitamin K antagonists (VKAs) might affect the incidence of cancer is a longstanding hypothesis. We conducted a population-based study including all cancer- and thromboembolism-free patients of our health area; study groups were defined according to chronic anticoagulant use to VKA-exposed and control groups. Cancer incidence and cancer-related and overall mortality was assessed in both groups. 76 008 patients (3231 VKA-exposed and 72 777 control subjects) were followed-up for 8.2 (± 3.2) years. After adjusting for age, sex, and time-to-event, the hazard ratio of newly diagnosed cancer in the exposed group was 0.88 (95% confidence interval [95% CI] 0.80-0.98; P < .015). VKA-exposed patients were less likely to develop prostate cancer, 0.69 (95% CI 0.50-0.97; P = .008). The adjusted hazard ratio for cancer-related and overall mortality was 1.07 (95% CI 0.92-1.24) and 1.12 (95% CI 1.05-1.19), respectively. These results support the hypothesis that anticoagulation might have a protective effect on cancer development, especially prostate cancer.
Introduction
There is evidence that warfarin has an inhibitory effect on tumor growth and metastasis.1 Tumor-mediated activation of the hemostatic system has been associated with both tumor stroma formation and metastasis.2-5 These findings raise the question of whether the use of anticoagulant therapies to down-regulate coagulation activation might not only serve to reduce the risk of venous thromboembolism but also to directly influence cancer cell biology and tumor development. Long-term use of vitamin K antagonists (VKAs) has been associated with a lower incidence of cancer, specifically prostate cancer.6,7 We conducted a population-based observational cohort study that sought to assess the impact of long-term VKA use (warfarin or acenocoumarol) on the development of newly diagnosed malignancies and on cancer-related and overall mortality.
Methods
All residents of the Health Area 16 of the Veneto Region (Italy) ages 65-90 years in the period January 1, 1996, to December 31, 2002, were included in this study. The Public Health Service system, which covers this area, keeps record of all discharge diagnoses from public or private hospitals (through use of the International Classification of Diseases system codes [ICD-9-CM]), hospital outpatient visits, and medications dispensed (by use of the Anatomical Therapeutic Chemical [ATC] classification).
We identified and excluded from the study all patients who had ongoing or previous history of neoplastic disease (ICD-9-CM: 140-239) and superficial thrombophlebitis (ICD-9-CM: 451) or venous thromboembolism (ICD-9-CM: 452-453) during the year of enrollment or that before.8,9 The period of observation began with the reaching of the 65th birthday during the enrolment period and ended with the reaching of the 90th birthday, diagnosis of neoplastic disease, death, emigration, or the end of the follow-up period, that is, December 31, 2007, whichever came first.
VKA exposure was assessed on the basis of ATC codes on the drug prescriptions (ATC code B01AA03 for warfarin and ATC code B01AA07 for acenocoumarol) and used to define the study groups: VKA-exposed patients and controls. Chronic exposure was evaluated in the 3 years preceding enrollment and defined as at least 2 prescriptions per year and at least 1 prescription during the following 2 years. Ascertainment of anticoagulant exposure was validated by randomly extracting a sample of 300 patients (≈1%) from the exposed group and reviewing their medical records and by conducting a phone interview of the patients or their caregivers.
The end points of the study were the incidence of previously undiagnosed malignancies identified at the 3-digit coding level of the ICD-9-CM and overall and cancer-related mortality. The study was approved by the Veneto Region privacy and data-protection board and by the local ethics committee.
The incidence of cancer is expressed per 1000 patients. The predicted proportion of cancer incidence in the exposed group was calculated by applying the observed proportion in the control group. The difference between the number of expected and observed cancers was expressed as avoided cancers.
We used the Koopman approximate method10 to compute cancer development, total and cancer-related mortality relative risk (RR), and their associated 95% confidence intervals (95% CI).
Cox proportional-hazards regression analysis was used to estimate hazard ratio (HR) of cancer development adjusted for age at enrollment (as continuous variable), sex, and time to event. Data analysis was performed by the use of SPSS Version 18.0 (SPSS Inc).
Results and discussion
Of the 89 787 eligible subjects, 13 779 were excluded because they had been diagnosed with cancer or experienced superficial thrombophlebitis or venous thromboembolism during the year of entry or that before, died, or moved during the year of enrollment or thereafter. Of the remaining cohort, 3231 were included in the VKA-exposed group, and 72 777 served as control subjects. After validation of the medical records, 94% of the patients were found to be correctly included in the exposed group.
The patients in the VKA-exposed group were significantly older (76.4 ± 6.8 years vs 74.8 ± 7.2 years) and were followed for a longer time (9.1 ± 2.4 years vs 8.8 ± 2.6 years); there were more females in both groups (50.4% in the VKA-exposed group and 59.9% in the control group).
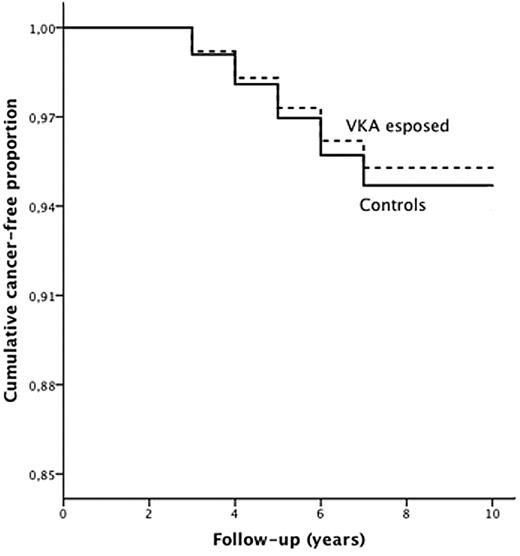
During follow-up, 421 new malignancies were recorded among the VKA-exposed patients (130/1000) and 9741 among the control subjects (134/1000), yielding a crude RR of 0.97 (95% CI 0.88-1.06). After adjusting for age and sex (Figure 1), the incidence of cancer was significantly lower in the VKA-exposed patients (HR 0.88; 95% CI 0.80-0.98; P < .015). Among specific tumors (Table 1), the incidence of prostate cancer was significantly reduced in the exposed group, 0.69 (95% CI 0.50-0.97; P = .008).
Incidence of cancer in VKA-exposed patients (dotted line) and in control patients (continuous line) during follow-up (HR 0.88; P = .015).
Incidence of cancer in VKA-exposed patients (dotted line) and in control patients (continuous line) during follow-up (HR 0.88; P = .015).
Risk ratios of specific cancer types among VKA-treated patients and control population
| Site or type of cancer (ICD-9-CM code) . | Cancer . | Cancer RRs . | ||||||
|---|---|---|---|---|---|---|---|---|
| Observed . | Predicted . | Avoided . | ||||||
| Control subjects (n = 72 777) . | c/1000 . | Exposed (n = 3231) . | c/1000 . | Exposed . | Exposed . | Crude RR . | Age- and sex-adjusted HR . | |
| Upper gastrointestinal (140-145,146-149,150-151) | 504 | 7 | 26 | 8 | 23 | -3 | 1.16 (0.78-1.71) | 1.07 (0.68-1.49) |
| Lower gastrointestinal (152-154) | 1454 | 20 | 66 | 20 | 65 | -1 | 1.02 (0.80-1.30) | 0.91 (0.71-1.17) |
| Liver, gallbladder (155-156) | 364 | 5 | 15 | 5 | 16 | 1 | 0.93 (0.56-1.54) | 0.85 (0.51-1.43) |
| Pancreatic (157) | 238 | 3 | 12 | 4 | 10 | -2 | 1.14 (0.64-2.01) | 1.03 (0.58-1.84) |
| Respiratory tract (160-162) | 1004 | 14 | 40 | 12 | 45 | 5 | 0.90 (0.66-1.23) | 0.78 (0.57-1.07) |
| Skin cancer including melanoma (172-173) | 1108 | 15 | 60 | 19 | 48 | -12 | 1.22 (0.94-1.58) | 0.99 (0.76-1.29) |
| Breast (174-175) | 1146 | 16 | 38 | 12 | 52 | 14 | 0.64 (0.54-1.02) | 0.88 (0.64-1.22) |
| Uterus and other female reproductive (in 45 237 women; 179-184) | 304 | 7 | 12 | 7 | 12 | 0 | 1.06 (0.60-1.86) | 1.02 (0.57-1.81) |
| Renal and urinary tract (188-189) | 1189 | 16 | 50 | 15 | 52 | 2 | 0.95 (0.72-1.25) | 0.80 (0.60-1.06) |
| Prostate (in 30 771 men; 185) | 993 | 34 | 36 | 22 | 55 | 19 | 0.66 (0.47-0.91)* | 0.69 (0.50-0.97)* |
| Leukemia and other blood (200-208) | 730 | 10 | 38 | 12 | 32 | -6 | 1.17 (0.85-1.62) | 1.06 (0.76-1.47) |
| Other (158-159,163-165,170-171,176, 186-187, 190-195, 199) | 528 | 7 | 18 | 6 | 23 | 5 | 0.77 (0.48-1.22) | 0.69 (0.44-1.12) |
| Metastatic disease (196-198) | 1354 | 19 | 55 | 17 | 61 | 6 | 0.91 (0.70-1.19) | 0.85 (0.65-1.12) |
| Any cancer (140-208) | 9741 | 134 | 421 | 130 | 433 | 12 | 0.97 (0.88-1.06) | 0.88 (0.80-0.98)* |
| Site or type of cancer (ICD-9-CM code) . | Cancer . | Cancer RRs . | ||||||
|---|---|---|---|---|---|---|---|---|
| Observed . | Predicted . | Avoided . | ||||||
| Control subjects (n = 72 777) . | c/1000 . | Exposed (n = 3231) . | c/1000 . | Exposed . | Exposed . | Crude RR . | Age- and sex-adjusted HR . | |
| Upper gastrointestinal (140-145,146-149,150-151) | 504 | 7 | 26 | 8 | 23 | -3 | 1.16 (0.78-1.71) | 1.07 (0.68-1.49) |
| Lower gastrointestinal (152-154) | 1454 | 20 | 66 | 20 | 65 | -1 | 1.02 (0.80-1.30) | 0.91 (0.71-1.17) |
| Liver, gallbladder (155-156) | 364 | 5 | 15 | 5 | 16 | 1 | 0.93 (0.56-1.54) | 0.85 (0.51-1.43) |
| Pancreatic (157) | 238 | 3 | 12 | 4 | 10 | -2 | 1.14 (0.64-2.01) | 1.03 (0.58-1.84) |
| Respiratory tract (160-162) | 1004 | 14 | 40 | 12 | 45 | 5 | 0.90 (0.66-1.23) | 0.78 (0.57-1.07) |
| Skin cancer including melanoma (172-173) | 1108 | 15 | 60 | 19 | 48 | -12 | 1.22 (0.94-1.58) | 0.99 (0.76-1.29) |
| Breast (174-175) | 1146 | 16 | 38 | 12 | 52 | 14 | 0.64 (0.54-1.02) | 0.88 (0.64-1.22) |
| Uterus and other female reproductive (in 45 237 women; 179-184) | 304 | 7 | 12 | 7 | 12 | 0 | 1.06 (0.60-1.86) | 1.02 (0.57-1.81) |
| Renal and urinary tract (188-189) | 1189 | 16 | 50 | 15 | 52 | 2 | 0.95 (0.72-1.25) | 0.80 (0.60-1.06) |
| Prostate (in 30 771 men; 185) | 993 | 34 | 36 | 22 | 55 | 19 | 0.66 (0.47-0.91)* | 0.69 (0.50-0.97)* |
| Leukemia and other blood (200-208) | 730 | 10 | 38 | 12 | 32 | -6 | 1.17 (0.85-1.62) | 1.06 (0.76-1.47) |
| Other (158-159,163-165,170-171,176, 186-187, 190-195, 199) | 528 | 7 | 18 | 6 | 23 | 5 | 0.77 (0.48-1.22) | 0.69 (0.44-1.12) |
| Metastatic disease (196-198) | 1354 | 19 | 55 | 17 | 61 | 6 | 0.91 (0.70-1.19) | 0.85 (0.65-1.12) |
| Any cancer (140-208) | 9741 | 134 | 421 | 130 | 433 | 12 | 0.97 (0.88-1.06) | 0.88 (0.80-0.98)* |
HR indicates hazard ratio; ICD-9-CM, International Classification of Diseases, 9th Edition, Clinical Modification; RR, relative risk; and VKA, vitamin K antagonist.
Statistically significant difference.
Overall, during the follow-up period, there were 1023 deaths (317/1000) among the VKA-exposed patients and 15 349 (211/1000) among controls (crude RR 1.50; 95% CI 1.42-1.58). Age- and sex-adjusted mortality was greater among the VKA-exposed patients (HR 1.12; 95% CI 1.05-1.19). Among the newly diagnosed malignancies, 184 deaths (437/1000) were recorded in the VKA-exposed group and 3683 among the controls (378/1000), yielding a crude RR of 1.16 (95% CI 1.03-1.29). The rate of age- and sex-adjusted mortality was similar in both groups (HR 1.07; 95% CI 0.92-1.24).
These results support the proposed hypothesis that chronic VKA use has a protective role on cancer incidence. Among specific cancer types, statistical significance was reached for prostate cancer only. Proposed mechanisms for this antineoplastic effect involve thrombin relation to protease-activated receptors GAS6 and AXL.11-13 GAS6/AXL axis in turn regulates prostate cancer invasion, proliferation, and survival.14 Coumarin given to rats bearing the R-3327H prostate adenocarcinoma decreased the size of the primary tumor.15 The possible protective role of chronic oral anticoagulation was assessed in human studies16-18 with favorable results, although the validity can be questionable because of the low number of the events recorded. Some researchers6,7 support the hypothesis that chronic anticoagulation has a protective effect on cancer incidence, especially on prostate cancer. The fact that a reduced risk of cancer was observed years after exposure suggests a probable protective effect of warfarin at the stage of tumor initiation or promotion rather than an effect on an established tumor.
Other studies found no significant difference in urogenital cancer incidence between warfarin users and nonusers, although the increased risk of cancer in smokers might have overweighed any potential benefit of warfarin in one study,19 whereas the short follow-up period might have influenced the results on the other.20
Although retrospective, this is a large population-based study with long follow-up. Selection bias in our study is unlikely: first, our cohort included all residents; second, because cancers were identified through the Public Health System database, it is difficult that events might have been overlooked; third, recall bias in terms of exposure to VKA could hardly occur since the computerized approach was validated in 300 randomly extracted patients.
A potential limitation of this study is that other important risk factors for neoplastic diseases such as changes in diet, decrease in alcohol consumption, smoking cessation, more frequent medical visits, could not be taken into consideration. However, because the whole population of the Area was included, these factors were probably balanced between the groups. In conclusion, our results support the hypothesis that chronic VKA exposure has a protective role on cancer incidence, especially prostate cancer, in patients 65 years or older. Other large-scale prospective, long follow-up studies are needed to confirm these results.
Presented as abstract at the XXII Congress of the International Society on Thrombosis and Haemostasis, Boston, MA, July 11-16, 2009.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: V.P., F.N., and P.P. planned the study and contributed to the writing of the manuscript; G.D., M.F.P., U.G., and A.M.G. managed the study database and analyzed the data; S.I. organized the study; and all authors critically reviewed the article and approved the final version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Vittorio Pengo, MD, Department of Cardiothoracic and Vascular Sciences, Clinical Cardiology, Thrombosis Centre, University of Padova, School of Medicine, Via Giustiniani 2, 35128 Padova, Italy; e-mail: vittorio.pengo@unipd.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal