Abstract
Over the past 60 years, the transfusion medicine community has attained significant knowledge regarding transfusion-related acute lung injury (TRALI) through the bedside to bench and back to the bedside model. First, at the bedside, TRALI causes hypoxia and noncardiogenic pulmonary edema, typically within 6 hours of transfusion. Second, bedside studies showed a higher incidence in plasma and platelet products than in red blood cell products (the fatal TRALI incidence for plasma is 1:2-300 000 products; platelet, 1:3-400 000; red blood cells, 1:25 002 000), as well as an association with donor leukocyte antibodies (∼ 80% of cases). Third, at the bench, antibody-dependent and antibody-independent mechanisms have been described, requiring neutrophil and pulmonary endothelial cell activation. Antibodies, as well as alternate substances in blood products, result in neutrophil activation, which, in a susceptible patient, result in TRALI (2-hit hypothesis). Fourth, back to the bedside, policy changes based on results of these studies, such as minimizing use of plasma and platelet products from donors with leukocyte antibodies, have decreased the incidence of TRALI. Thus, steps to mitigate TRALI are in place, but a complete mechanistic understanding of the pathogenesis of TRALI and of which patients are at highest risk remains to be elucidated.
Introduction
Popovsky et al in the 1980s coined the term transfusion-related acute lung injury (TRALI), previously called severe pulmonary hypersensitivity reaction,1 clarified the clinical presentation of hypoxia and bilateral noncardiogenic pulmonary edema (usually occurring within 6 hours of a transfusion), and made the association with leukoagglutinins in the donor.2 In 2005-2009, 48% of the confirmed transfusion-related deaths in the US reported to the Food and Drug Administration (FDA) were secondary to TRALI.3 At that time, and as TRALI was being increasingly recognized as a potentially fatal adverse transfusion event, multiple definitions were in place. Thus, to better understand TRALI, a universally accepted definition was required and was finally created through working groups of the National Heart, Lung, and Blood Institute (NHLBI), and a more universally accepted definition through the 2004 Consensus Panel statement.4 The Consensus Panel definition is currently used in hemovigilance systems, which track transfusion complications, and therefore improve determination of overall and blood product-specific TRALI incidence. The data obtained enables comparison of the incidence of TRALI between countries with different practices, as well as changes in incidence secondary to policy modifications.5
The gain in clinical knowledge of TRALI parallels increased knowledge of its pathophysiology. Pathophysiologic studies have included those at the bedside (hemovigilance data and clinical trials) and the bench (laboratory investigations using in vitro, ex vivo, and in vivo models). Through these studies, it is currently surmised that TRALI results from neutrophil or endothelial activation, or both, by multiple mechanisms in the lung, resulting in pulmonary edema and alveolar damage.6 Blood product substances associated with TRALI include human neutrophil antigen (HNA) and human leukocyte antigen (HLA) class I and II antibodies, CD40-ligand (CD40L), and biologically active lipids. Transfusion of these substances alone usually does not result in TRALI, because a susceptible patient is required. Data from TRALI cases and laboratory studies lead to the hypothesis that a susceptible patient is an individual with activated granulocytes and/or pulmonary endothelium.7
Methods have developed from benchside knowledge to mitigate this untoward reaction. First steps have focused on blood product modifications. Indeed, the US and some other countries have barred donors considered high-risk (ie, those known or highly likely to have white blood cell [WBC] antibodies) from donating products containing a high volume of plasma. Thus, over the past 50 years, the transfusion community learned about TRALI through the “bedside to bench and back to the bedside” approach. Each gain in knowledge has resulted in steps to decrease the incidence of TRALI, but more knowledge is needed to mitigate and treat this potentially fatal transfusion reaction.
Bedside
Recognizing the clinical entity
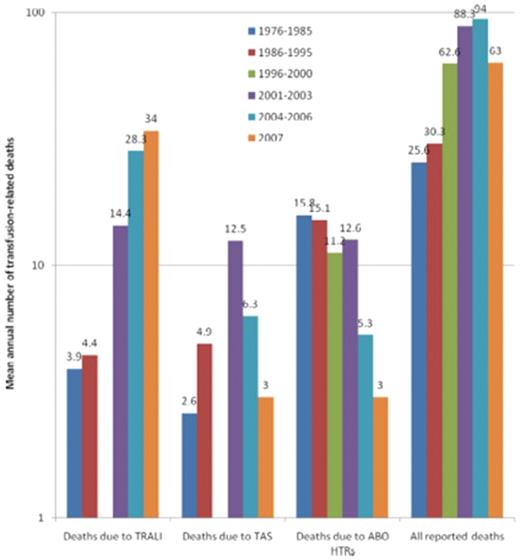
In 1926, WBC incompatibility between the donor and recipient resulting in severe, near-fatal reactions was recognized.8 In 1957, recipient WBC antibodies were associated with febrile transfusion reactions, some leading to cyanosis, weakness, apathy, and prostration. The reactions resulted from the buffy-rich, but not the buffy-poor, fraction of the blood component.9 In 1966, transient noncardiogenic pulmonary edema accompanying fever, cough, and cyanosis without hypervolemia secondary to blood transfusion was described in 3 patients.10 Until the mid-1980s, this reaction was termed “pulmonary hypersensitivity reaction”1,11 and was associated with leukocyte antibodies in the donor against the recipient, or in the recipient against the donor; however, the specifics were not known or understood. Popovsky and Moore in 1985 described 36 cases of TRALI with acute respiratory distress characterized by hypoxemia and pulmonary edema, the onset usually occurring within 4 hours of transfusion and being accompanied by hypotension, with rapid and complete recovery in the majority of patients (81%).2 In 89% of the cases, granulocyte or lymphocytotoxic antibodies were detected in the donor of the implicated product. In 2000, TRALI resulted in 13% of all US transfusion-related fatalities and was the third leading cause of transfusion-related mortality reported; in 2001, the FDA published a letter alerting clinicians to TRALI.12 By 2006, more than 50% of transfusion-related fatalities reported to the FDA were due to TRALI (Figure 1).13,14
Transfusion-related fatalities reported to the FDA.14 The 3 leading causes of known and reported allogeneic blood transfusion-related deaths, based on data reported passively to the US FDA over 32 years (1976-2007). For each of the 5 periods for which data have been made available, the figure shows the mean annual number of deaths deemed to be due to TRALI, transfusion associated sepsis (TAS), or ABO hemolytic transfusion reactions (HTRs), along with the mean total number of deaths reported to the FDA plotted on a logarithmic scale. Deaths reported to the FDA include donor fatalities, recipient fatalities in which allogeneic blood transfusion (ABT) was not deemed to be the probable or major cause of death, and recipient fatalities due to TRALI, TAS, ABO HTRs, and other transfusion complications. Data on TRALI and TAS are not available for the period 1996 to 2000. (Reprinted with permission.)
Transfusion-related fatalities reported to the FDA.14 The 3 leading causes of known and reported allogeneic blood transfusion-related deaths, based on data reported passively to the US FDA over 32 years (1976-2007). For each of the 5 periods for which data have been made available, the figure shows the mean annual number of deaths deemed to be due to TRALI, transfusion associated sepsis (TAS), or ABO hemolytic transfusion reactions (HTRs), along with the mean total number of deaths reported to the FDA plotted on a logarithmic scale. Deaths reported to the FDA include donor fatalities, recipient fatalities in which allogeneic blood transfusion (ABT) was not deemed to be the probable or major cause of death, and recipient fatalities due to TRALI, TAS, ABO HTRs, and other transfusion complications. Data on TRALI and TAS are not available for the period 1996 to 2000. (Reprinted with permission.)
Defining the clinical entity
TRALI was increasingly recognized as a serious adverse transfusion event, but was poorly understood. The NHLBI recognized the need for a common definition to facilitate the understanding and focused study of TRALI, because creating a common and useable definition would permit comparison between reports and clarify diagnosis.15 An NHLBI working group defined TRALI as new acute lung injury (ALI) occurring within 6 hours of the end of transfusion of one or more plasma-containing blood products in patients without other risk factors for ALI, or in patients with other risk factors for ALI if there was no pretransfusion ALI present and if the new ALI was temporally associated with the transfusion.15 NHLBI used the definition of ALI put forth by the North American–European Consensus Conference in 1994 (Table 1).16 In 2004, a Consensus Panel statement titled “Toward an understanding of TRALI” extended the criteria for diagnosing TRALI by expanding the meaning of “the presence of hypoxia” to include other clinical evidence supporting the conclusion of hypoxia, and by creating criteria for “possible TRALI,” which include patients with preexisting ALI and ALI occurring more than 6 hours after transfusion (Table 2).4,17
| Parameter . | Finding . |
|---|---|
| Timing | Acute onset |
| Pulmonary artery occlusion pressure | ≤ 18 mm Hg when measured, or lack of clinical evidence of left atrial hypertension |
| Chest radiograph | Bilateral infiltrates seen on frontal chest radiograph |
| Hypoxemia | Ratio of PaO2/FIO2 ≤ 300 mm Hg regardless of positive end-expiratory pressure level, or oxygen saturation of ≤ 90% on room air (added by NHLBI working group) |
| Parameter . | Finding . |
|---|---|
| Timing | Acute onset |
| Pulmonary artery occlusion pressure | ≤ 18 mm Hg when measured, or lack of clinical evidence of left atrial hypertension |
| Chest radiograph | Bilateral infiltrates seen on frontal chest radiograph |
| Hypoxemia | Ratio of PaO2/FIO2 ≤ 300 mm Hg regardless of positive end-expiratory pressure level, or oxygen saturation of ≤ 90% on room air (added by NHLBI working group) |
Recommended criteria for TRALI and possible TRALI4
| Criteria . |
|---|
| 1. TRALI |
| a. ALI |
| i. Acute onset |
| ii. Hypoxemia |
| Research setting: PaO2/FiO2 ≤ 300 mm Hg, or SpO2 < 90% on room air |
| Nonresearch setting: PaO2/FiO2 ≤ 300 mm Hg, or SpO2 < 90% on room air or other clinical evidence of hypoxemia |
| iii. Bilateral infiltrates on frontal chest radiograph |
| iv. No evidence of left atrial hypertension (ie, circulatory overload) |
| b. No preexisting ALI before transfusion |
| c. During or within 6 h of transfusion |
| d. No temporal relationship to an alternative risk factor for ALI |
| 2. Possible TRALI |
| a. ALI |
| b. No preexisting ALI before transfusion |
| c. During or within 6 h of transfusion |
| d. A clear temporal relationship to an alternative risk factor for ALI |
| Criteria . |
|---|
| 1. TRALI |
| a. ALI |
| i. Acute onset |
| ii. Hypoxemia |
| Research setting: PaO2/FiO2 ≤ 300 mm Hg, or SpO2 < 90% on room air |
| Nonresearch setting: PaO2/FiO2 ≤ 300 mm Hg, or SpO2 < 90% on room air or other clinical evidence of hypoxemia |
| iii. Bilateral infiltrates on frontal chest radiograph |
| iv. No evidence of left atrial hypertension (ie, circulatory overload) |
| b. No preexisting ALI before transfusion |
| c. During or within 6 h of transfusion |
| d. No temporal relationship to an alternative risk factor for ALI |
| 2. Possible TRALI |
| a. ALI |
| b. No preexisting ALI before transfusion |
| c. During or within 6 h of transfusion |
| d. A clear temporal relationship to an alternative risk factor for ALI |
Although the Consensus Panel definition is commonly used to define TRALI,14,18 it lacks validation. A study investigating the applicability of this definition to a cardiac surgery registry demonstrated that approximately 65% of patients on presentation to the intensive care unit (ICU) had a PaO2/FiO2 ratio of less than 300.19 The authors conclude that using this criterion may not be appropriate for cardiac surgery patients.
Incidence, outcome and blood component association
Reports use different definitions of TRALI, different blood products and product modifications, and passive reporting systems; therefore, incidence, outcome, and blood product association data vary from country to country. German hemovigilance data include 44 cases of confirmed TRALI, of which 80% were antibody-mediated and 20% presumed to be nonimmune mediated.20 The fatal 18% of cases were antibody-mediated from female donors. The TRALI risk was 1:260 000 for all blood components. The rate for antibody-mediated TRALI was 1:66 000 for fresh frozen plasma (FFP; 1:285,000 for fatal antibody–mediated TRALI), 1:420 000 for platelet concentrates, and 1:2 860 000 for red blood cell (RBC) products. Hemovigilance data from other countries reported TRALI rates of 1:250 000 (United Kingdom, Denmark and Norway), 1:66 667 (Finland), and 1:55 556 (Sweden) for all transfusions; and 1:66 667 (Denmark), 1:62 500 (United Kingdom), 1:24 390 (Sweden), and 1:11 363 (Finland) for FFP transfusions.5 Of the blood products implicated in TRALI, 49% were FFP, 29% RBCs, 13% platelet concentrates, 2% whole blood, 0% solvent/detergent (SD) plasma, and 7% mixed products. The American Red Cross–estimated risk of fatal TRALI per distributed component was 1:202 673 for plasma, 1:320 572 for apheresis platelets, and 1:2 527 437 for RBC units.21 TRALI mortality rates range from 5%-35% in case series.22
Antibody-mediated TRALI
Leukocyte antibodies in products implicated in TRALI cases.
Leukocyte antibodies were identified in the implicated donor in 65%-90% of TRALI cases.17 A systematic review of case reports with leukocyte antibody testing showed these antibodies contributed to 80% of the cases reported.23 The distributions of the implicated donor antibody targets are similar in 2 large case series. In a series of 36 cases, 31 were antibody-mediated; donors had HLA class I (11%), HLA class II (47%), HLA class I and II (8%), and HNA (33%) antibodies.24 In a series of 44 cases, 35 were antibody-mediated; donors had HLA class I (11%), HLA class II (44%), HLA class I and II (37%), and HNA (23%) antibodies.20 However, because transfusion recipients may receive more than one product, identifying a product with antibodies may overestimate its association with TRALI.
Prevalence of antibodies in blood donors.
Multiple studies determined donor prevalence of leukocyte antibodies. First, in a study of 1043 donors using 5 methods to detect HLA class I, HLA class II, and HNA antibodies, 9.8% of females but no males had antibodies.25 The incidence of antibodies increased with the number of pregnancies: 1 pregnancy, 3.8%; 2, 13.5%; and 3 or more, 24.1%. The antibodies were directed against HLA class I (45.2%), class II (33.9%), and both class I and II (20.9%) antigens; no HNA antibodies were detected. In a second study of 5332 parous donors, 8.9% had HLA class I, HLA class II, HNA, or a combination of antibodies.24 The incidence of antibodies increased with the number of pregnancies: 1 pregnancy, 4.5%; 2, 8.6%; 3, 12.1%; 4, 16.7%; and 5 or more, 15.4%. Ninety-two percent of leukocyte antibodies were against HLA (class I, 61%; class II, 19%; both class I and II, 12%), 5% against HNA, and 2% had a positive granulocyte agglutination test of unknown specificity. In the Leukocyte Antibody Prevalence Study (LAPS I) performed by the Retrovirus Epidemiology Donor Study II (REDS II), 7920 volunteer donors were screened for HLA class I and II antibodies using a multiantigen bead kit.26 HLA antibodies were present in 1.7% of transfused males, 1.0% nontransfused males, and 17.2% females (1.7% nulliparas and 24.4% parous women). Antibody positivity was correlated with the number of pregnancies: 1 pregnancy, 11.2%; 2, 22.3%; 3, 27.5%; and 4 or more, 32.2%. 10.4% of the women had antibodies against class I antigens and 11.7% had antibodies against class II antigens.
Presence of TRALI in patients who receive blood product with antibodies.
Clinical studies investigated occurrence of TRALI in recipients of blood from donors with leukocyte antibodies. In one report, 26 recipients of blood donated by immunized women resulted in no reported TRALI, although 11 of these patients had the cognate antigen.25 A TRALI lookback study of 103 transfusion recipients from an implicated donor with multiple HLA class I and II antibodies revealed one case of TRALI, although 54 of the 55 patients tested had cognate antigens.15 Interestingly, 4 of 62 patients with chest radiographs developed new or worse bilateral infiltrates after transfusion. A fatal case of TRALI from a donor with an antibody to HNA-3a in 36 previous plasma recipient prompted a lookback, which revealed that 36% of the recipients had a transfusion reaction (43% being mild-to-moderate reactions with symptoms of chills, fever, dyspnea, tachycardia, chest pain, and hypotension, and 58% being severe reactions, with symptoms of respiratory failure, dyspnea, tachycardia, pulmonary edema, fever, and hypotension).27 Another lookback study of a TRALI case associated with RBC transfusion from a donor with anti-NB2 and an NB2-positive recipient with thrombotic thrombocytopenic purpura revealed that none of the previous 21 blood donations had resulted in a transfusion reaction.28 Factors additional to antigen-antibody binding probably contribute to the development of TRALI, such as antibody specificity and titer, antigen density, and the patient's underlying condition.25
Plasma from male versus female donors.
A retrospective study of the incidence of acute pulmonary edema after transfusion in 8902 intensive care patients demonstrated 25 cases of transfusion-associated circulatory overload (TACO; incidence 1:356 units transfused), 7 cases of suspected TRALI (1:1271 units transfused), and 14 cases of possible TRALI (1:534 units transfused).29 Patients who developed suspected or possible TRALI received larger amounts of plasma, especially plasma from female donor. In addition, the mortality rate was 67% for suspected or possible TRALI, compared with 20% for TACO and 11% for matched controls. A recent study from Japan demonstrated less posttransfusion pulmonary dysfunction with transfusion from male-only donors versus plasma from mixed-gender donors (P = .022).30 A randomized controlled trial of intensive care unit patients who received one unit of control plasma and one unit of plasma donated from a multiparous woman showed that multipara-donated plasma led to a significant decrease in the PaO2/FiO2 ratio compared with control plasma (P < .01).31 In contrast, a recent retrospective study comparing cardiac surgery patients who received plasma from female versus male donors showed that female compared to male donor plasma was associated with less pulmonary dysfunction and improved short-term outcome, but there was no difference in long-term outcome.32 The number of plasma units transfused was strongly correlated with increased mortality. This analysis did not consider number of platelet or RBC units; or other factors. Thus, the balance of data supports the notion that plasma from parous women is associated with a higher risk for TRALI or pulmonary dysfunction than male- or nullipara-donated plasma.
Autopsy studies.
Autopsy studies aid in the histopathology of pulmonary injury during TRALI. Two autopsy reports of patients who died secondary to TRALI demonstrated pulmonary edema and neutrophil congestion in the alveolar capillaries without the presence of diffuse alveolar damage; case 1 had HLA class I (B50) and class II (DR16) antigens corresponding to the donor's antibodies, and case 2 had HLA class I (A68) antigens corresponding to the donor's antibodies; the donor also had antibodies to HNA-3a, but the recipient was not typed, although more than 90% of whites are positive for HNA-3a.33 In another series of 3 fatal cases of immune-mediated TRALI, the autopsy demonstrated pulmonary edema with cholesterol crystals in the endothelial membranes of venules.34 In this series, patient 1 had HLA class I A2 antigen corresponding to the donor's antibodies; in case 2, no WBC antibodies were identified in the donor, and in case 3 the donor had antibodies to HNA-3a. The proposed pathogenesis based on these findings is that WBCs are coated with antibodies then attached to the pulmonary venule endothelium where WBCs are activated. Activation results in lipid membrane oxidation leading to cholesterol and fatty acid release from membranes so that cholesterol crystals form. These crystals pierce the membrane resulting in pulmonary edema. Autopsy studies question the role of HLA class II antibodies in TRALI. In a fatal case of TRALI, pulmonary tissue immunohistochemistry did not demonstrate the presence of HLA class II antigens on the vascular endothelium or intravascular WBCs, but only on intraalveolar macrophages.35
Bench
Although studies provided significant insight into the clinical characteristics of TRALI, its pathogenesis remained enigmatic. Although anti-HLA and anti-HNA appear to facilitate TRALI development, mechanisms whereby antibodies induce ALI were unknown. Furthermore, antibody-independent mechanisms also probably exist, as many antibody-independent cases have been reported. Clinically, TRALI resembles other forms of ALI, which suggests that TRALI development mechanisms might bear some similarities to other forms of ALI. In other ALI models, neutrophils appear to play a critical role. Indeed, ALI appears to reflect the rapid accumulation and activation of pulmonary neutrophils after significant perturbation of common regulatory pathways.36,37 Whether similar events surround TRALI pathogenesis remain unknown.
Innate inflammatory responses.
Neutrophils provide a critical component of the innate immune response to invading microbial pathogens after tissue injury.38 However, exuberant neutrophil-mediated inflammatory events often induce significant tissue damage and are commonly associated with various deleterious sequelae.38 Neutrophil-mediated inflammation can be associated with liquefactive necrosis, which commonly reflects significant neutrophil necrosis and release of a broad repertoire of factors that can rapidly destroy not only pathogens, but also viable tissue.38 Due to their destructive potential, neutrophils appear to have evolved distinct mechanisms of turnover not found in other leukocyte populations,39,40 which probably reduces the unregulated release of neutrophil contents. In addition to neutrophil necrosis-mediated tissue injury, activation of viable neutrophils in the absence of infection can cause significant tissue injury; reperfusion injuries, for instance, commonly occur in the absence of microbial pathogens after iatrogenic or naturally occurring recanalization of an occluded vessel.41
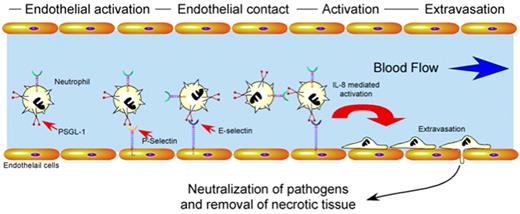
Besides factors regulating neutrophil turnover, proper immunologic homeostasis requires significant regulation of neutrophil activation and recruitment. Endothelial cells play a central role in the proper activation, localization, and extravasation of neutrophils to an area of tissue injury or pathogen invasion42 (Figure 2). Several key mediators appear to be responsible for these events. For example, endothelial cells rapidly mobilize vascular adhesion molecules, such as P-selectin, to the apical surface upon activation after hypoxic challenge, inflammatory cytokines, or pathogen metabolites.42,43 Alterations in the vascular lumen diameter induce margination of leukocytes. This facilitates the engagement of neutrophil ligands, such as PSGL-1, to endothelial and platelet-derived selectins, inducing loose neutrophil adhesion to the vascular wall.44 Cytokine-mediated activation of these neutrophils induces conformational changes in integrin molecules, which allow for firm adhesion to the vascular wall followed by rapid extravasation.42 Chemotactic factors released by pathogens and injured tissue then direct neutrophils to areas of primary tissue damage where they neutralize pathogens and remove necrotic tissue38 (Figure 2).
Regulatory pathways which govern neutrophil activation and extravasation. After tissue injury or pathogen invasion, cytokines produced by resident leukocytes or metabolites generated by pathogens induce endothelial cell activation. Activated endothelial cells mobilize P-selectin and E-selectin to the apical cell surface which facilitates loose neutrophil adhesion through interactions with PSGL-1. Activation of neutrophils during loose adhesion results in conformational changes in integrins that mediate firm adhesion and extravasation. Once extravasated, neutrophils respond to chemotactic stimuli, neutralize pathogens and remove necrotic tissue through the collaboration of a wide variety of factors, including enzymes and free radicals.
Regulatory pathways which govern neutrophil activation and extravasation. After tissue injury or pathogen invasion, cytokines produced by resident leukocytes or metabolites generated by pathogens induce endothelial cell activation. Activated endothelial cells mobilize P-selectin and E-selectin to the apical cell surface which facilitates loose neutrophil adhesion through interactions with PSGL-1. Activation of neutrophils during loose adhesion results in conformational changes in integrins that mediate firm adhesion and extravasation. Once extravasated, neutrophils respond to chemotactic stimuli, neutralize pathogens and remove necrotic tissue through the collaboration of a wide variety of factors, including enzymes and free radicals.
Proposed pathogenesis of antibody-mediated TRALI.
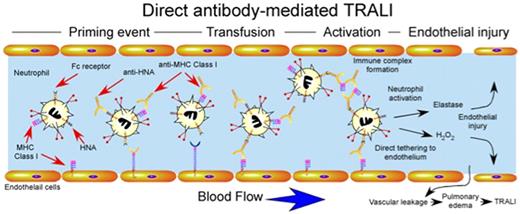
Although several studies suggested that transfusion-induced dysregulation of neutrophils may in part be responsible for the development of TRALI,1,45,46 only recently have several of the mechanisms responsible for these alterations been elucidated. Given the clinical correlations implicating antibodies in the development of TRALI,17,23 early studies examined the potential involvement of antibody-mediated perturbation of normal neutrophil function. Several studies demonstrated that anti–HNA-3a and anti–HLA-A24 possessed the ability to not only recognize and activate fMLP-primed neutrophils, but also cause significant release of granule contents in vitro,47,48 which suggested that significant infusion of neutrophil reactive antibodies may prematurely activate neutrophils intravascularly, resulting in significant neutrophil-mediated damage to the vascular endothelium (Figure 3). In addition to directly activating neutrophils, other studies demonstrated that donors serum negative for neutrophil reactive antibodies, yet positive for HLA class II antibodies, may induce monocytes49,50 and possibly platelets51 to secrete a variety of inflammatory mediators, including LTB4, TNF-α, and IL-8, which subsequently activate neutrophils. In contrast, antibody complexes might activate a variety of leukocyte populations through engagement of cell surface Fc receptors as opposed to direct engagement of antibody target antigens. Consistent with this, several studies demonstrated that antibody-soluble HLA antigen complexes may induce neutrophil activation in vitro through direct engagement of cell surface Fc receptors52 (Figure 3).
Direct antibody-mediated TRALI. Priming events may or may not be required for antibody-induced TRALI, but appear to significantly exacerbate TRALI when present. Transfusion of blood products containing antibodies against HNA and MHC class I can result in direct activation of intravascular neutrophils. Anti-MHC class I antibodies recognizing endothelial MHC class I may also directly tether neutrophils to the endothelium independent of selectin or integrin-mediated events. Immune complexes of anti-HNA or anti-MHC class I and soluble HNA or MHC class I may also be recognized by Fc receptors resulting in neutrophil activation. Intravascular activation of neutrophils results in damage to endothelial cells, vascular leakage and pulmonary edema.
Direct antibody-mediated TRALI. Priming events may or may not be required for antibody-induced TRALI, but appear to significantly exacerbate TRALI when present. Transfusion of blood products containing antibodies against HNA and MHC class I can result in direct activation of intravascular neutrophils. Anti-MHC class I antibodies recognizing endothelial MHC class I may also directly tether neutrophils to the endothelium independent of selectin or integrin-mediated events. Immune complexes of anti-HNA or anti-MHC class I and soluble HNA or MHC class I may also be recognized by Fc receptors resulting in neutrophil activation. Intravascular activation of neutrophils results in damage to endothelial cells, vascular leakage and pulmonary edema.
Antibody-independent mechanisms of TRALI.
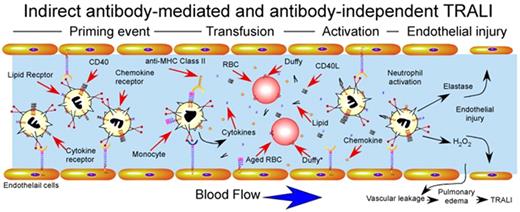
Although early studies primarily focused on potential mechanisms whereby antibodies might induce TRALI, several studies examined only antibody-independent pathways that might also induce neutrophil activation and subsequent TRALI. For example, supernatants harvested from stored platelet units induced significant activation of neutrophils.53 Although several studies failed to identify consistent accumulation of lipid mediators in stored blood products,54,55 several high-performance liquid chromatography (HPLC) analyses demonstrated several fractions capable of mediating neutrophil activation displaying significant lipid content,53,56 similar to those observed in other forms of neutrophil-mediated tissue injury.57 In addition, synthetic lysophosphatidylcholines, analogous to lipids identified in stored units, also induce neutrophil activation, further suggesting that transfusion-associated lipids may in part induce TRALI-like pathways58 (Figure 4).
Indirect antibody–mediated and antibody-independent mechanism of TRALI induction. Priming events secondary to underlying pulmonary pathology, often induced by LPS injection in experimental animal models, activate endothelial cells, resulting in significant sequestration of neutrophils within the pulmonary vasculature. After pulmonary priming events, transfusion-associated factors induce rapid intravascular neutrophil activation with subsequent endothelial damage, vascular compromise and pulmonary edema. Factors responsible for transfusion-induced activation are shown and include soluble CD40L, antibody-mediated monocyte activation and cytokine release, lipid mediators and impaired chemokine scavenging by aged red blood cells. Duffy antigens are specifically shown on the surface of red blood cells, where Duffy* on aged red blood cells indicates Duffy receptors with impaired capacity to bind intravascular chemokines.
Indirect antibody–mediated and antibody-independent mechanism of TRALI induction. Priming events secondary to underlying pulmonary pathology, often induced by LPS injection in experimental animal models, activate endothelial cells, resulting in significant sequestration of neutrophils within the pulmonary vasculature. After pulmonary priming events, transfusion-associated factors induce rapid intravascular neutrophil activation with subsequent endothelial damage, vascular compromise and pulmonary edema. Factors responsible for transfusion-induced activation are shown and include soluble CD40L, antibody-mediated monocyte activation and cytokine release, lipid mediators and impaired chemokine scavenging by aged red blood cells. Duffy antigens are specifically shown on the surface of red blood cells, where Duffy* on aged red blood cells indicates Duffy receptors with impaired capacity to bind intravascular chemokines.
In addition to lipid mediators, several studies identified additional antibody-independent pathways. For example, platelet storage appears to increase levels of CD40L.59 Although commonly thought to primarily regulate adaptive immune responses,60 CD40 also resides on neutrophils, and engagement by storage-derived CD40L induces significant activation.59 Importantly, leukoreduction eliminated accumulation of CD40L, strongly suggesting that CD40L is a leukocyte-derived factor within platelet products59 (Figure 5). In contrast, RBCs themselves appear to possess the ability to scavenge inflammatory mediators,58 a property that may be lost during storage. Consistent with this, aged RBCs display reduced capacity to scavenge CCL2,61 a chemokine capable of recruiting neutrophils in vivo,62 probably because of altered ligand recognition and reduced expression of Duffy antigen61 (Figure 4). Taken together, these results importantly illustrate that several transfusion-associated factors can significantly modulate neutrophil function, with obvious implications in the development of TRALI (Figs. 3, 4).
Transfusion-associated neutrophil activation. Transfusion associated factors ranging from antibodies to lipid mediators engage distinct neutrophil receptors resulting in significant intravascular neutrophil activation. Although the signaling pathways for several receptors, such as the Fc receptors, have been independently examined, the signaling mechanisms responsible for most of these activation events remain unknown.
Transfusion-associated neutrophil activation. Transfusion associated factors ranging from antibodies to lipid mediators engage distinct neutrophil receptors resulting in significant intravascular neutrophil activation. Although the signaling pathways for several receptors, such as the Fc receptors, have been independently examined, the signaling mechanisms responsible for most of these activation events remain unknown.
Animal models of TRALI.
Although several in vitro studies suggested that a variety of mechanisms might be responsible for neutrophil activation,47-50,52 it remained unknown whether similar activation-induced events occur in vivo, in the presence of key variables unique to the in vivo setting. For example, although neutrophils may induce injury and apoptosis of endothelial cells in vitro after significant activation,47,49,50,52 neutrophils commonly extravasate after activation, allowing movement of the potentially deleterious effects of neutrophil activation away from the intravascular compartment and into the extravascular tissue, a process that is significantly limited in vitro.63 Furthermore, neutrophils encounter endothelial cells under the presence of significant shear forces that can significantly affect the signaling and activation of neutrophils in vivo.64 In addition, blood flow itself can modulate the effect of secreted factors by several mechanisms, including directly engaging blood components and immediate dilution of secreted factors.65 Although analysis under flow chambers in vitro may more adequately control for several of these factors,64 in vivo models provide a practical alternative when evaluating the potential influence of these variables.
Early studies used ex vivo lung preparations to evaluate the potential involvement of antibodies and neutrophils in TRALI pathogenesis. Explanted rabbit lungs infused with human anti–HNA-5b induced significant alterations in synthetic arachidonic acid metabolites, endothelial permeability, and lung edema after cotransfusion of 5b-positive but not 5b-negative human neutrophils.66,67 Similar results were obtained using neutrophil reactive antibodies targeting the HNA-2a epitope.68 Development of TRALI using a lung ex vivo was not limited to antibodies, as lipid mediators generated during prolonged platelet storage appeared to likewise mediate significant vascular compromise and increased pulmonary pressure.69 Taken together, these results strongly implied that both antibody and lipid mediators possess the capacity to induce TRALI-like changes in ex vivo lung models.
Although several ex vivo models demonstrated that transfusion factors possessed the ability to directly induce TRALI,66,67 subsequent studies suggested that priming events before transfusion might be required.70 In this 2-hit model, a first hit, secondary to preexisting pulmonary pathology, must occur first, followed by a second, transfusion-associated injury. For example, in one model, lipids generated during prolonged storage of RBCs only induced TRALI-like changes in animals if previously challenged with lipopolysaccharides (LPS).58 Similarly, subsequent studies demonstrated that major histocompatibility complex (MHC) class I antibodies and CD40L only induced TRALI after a similar priming event.59,71 Although several of these studies used ex vivo lungs,69 most of these studies used intact lungs in vivo.61,71-73 Differences in the activation state of the pulmonary vascular endothelium and possible neutrophil priming during contact with synthetic conduits in ex vivo models may alter priming requirements in vivo.74 Alternatively, subtle yet meaningful differences in husbandry practices may also influence outcomes in in vivo models. Animal housing conditions, whether protected by barriers or not, significantly influenced priming requirements for the development of TRALI.72 Furthermore, differences in antibody concentrations used in various animal models may also partially account for variability in priming requirements for the development of TRALI.74 Although LPS and other priming regimens may alter the expression of target antigens in anti-MHC class I models of TRALI, low dose LPS, although capable of priming models, does not appear to alter MHC class I expression, suggesting that other factors, such as toll-like receptor-dependent endothelial cell activation may be required for these priming events to occur.73 As neutrophils and endothelial cells respond to a broad range of activating factors, differences in animal models, priming regimens and distinct activation signals initiated after transfusion probably result in unique thresholds for the development of TRALI in distinct settings.
Mechanisms of TRALI in vivo.
Although the exact molecular mechanisms of TRALI may be difficult to elucidate in vivo, numerous studies suggest that several key players may be involved in distinct models of TRALI. For example, while certain antibodies appear to directly activate neutrophils or endothelial cells in vitro,47,48 whether antibodies possessed the ability to directly activate inflammatory cascades responsible for TRALI remained unknown in vivo. To examine direct antibody as opposed indirect pathways, several animal models using target deletions of putative endogenous factors were used. Injection of MHC class I antibodies induced significant TRALI in a wild-type (wt) animal model, while the same priming and injection regiment failed to induce TRALI in Fc knockout mice.72 Although MHC class I antibodies induced TRALI in Fc null mice transfused with wt neutrophils, antibody infusion into wt mice after transfusion of Fc-null neutrophils failed to result in TRALI,72 strongly suggesting a role for neutrophil Fc receptors in antibody-dependent TRALI. Furthermore, in addition to engaging neutrophil Fc receptors, antibodies appeared to recognize endothelial MHC receptors in vivo,72 implying antibody-mediated tethering of neutrophils in vivo. Consistent with this, antibody-mediated TRALI does not appear to require P-selectin-glycoprotein-1 (PSGL-1) or integrin interactions for neutrophil sequestration and TRALI development,72 leading researchers to believe that Fc-mediated neutrophil interactions may bypass normal adhesion pathways and may in part be responsible for the premature intravascular release of neutrophil derived factors (Figure 3). Although conflicting data exist concerning the requirement for complement in antibody-mediated TRALI,66 C5a-null animals remain sensitive to antibody-induced TRALI,72 strongly suggesting that complement-mediated pathways may not be required. Although several studies demonstrate a central role for neutrophils in TRALI pathogenesis,47,53,56,58,59,69,71,73 some recent studies also propose that platelets, which can mediate neutrophil adhesion to vascular walls,75 might also facilitate neutrophil-mediated injury,73 since depletion of platelets and antiplatelet treatment with aspirin prevented TRALI development after MHC class I antibody injection.73
Although in vitro and in vivo studies clearly implicate neutrophils in TRALI pathogenesis,47,53,56,58,59,69,71,73 few studies have examined actual signaling pathways initiated by putative TRALI-inducing factors. Previous research demonstrated that neutrophils possess multiple signaling pathways capable of inducing robust activation38 (Figure 5) and distinct receptors with unique signaling pathways probably evolved to enable rapid neutrophil response to the wide range of potential pathogens.38 The broad range of putative factors implicated in TRALI suggests that distinct and possibly disparate pathways of activation may be engaged during events surrounding TRALI47,48,57,59,76 Different factors may work synergistically or independently, or require different levels or distinct mechanisms of neutrophil priming in order sufficiently to activate neutrophils to cause injury (Figure 5). As a result, seemingly disparate findings regarding TRALI etiology may simply reflect the multitude of pathways able to cause one common outcome, neutrophil activation. Regardless of the mechanism of neutrophil activation, once activated, neutrophils appear to be able to induce endothelial injury and apoptosis,47,49,50,52 probably through the release of soluble factors,50,52 which ultimately results in loss of vascular integrity and pulmonary edema, hallmarks of ALI. In summary, as neutrophils appear to play a center role in the development of TRALI, additional studies will be needed to develop mechanisms of identifying and mitigating those factors which may increase TRALI risk, both in the patient and product.
Back to the bedside: decreasing the incidence
Through bench studies, antibody- and nonimmune-mediated pathogenesis appear clinically relevant. Yet most clinical studies support transfusion of the antibody as a second hit in a susceptible patient. Because antibodies are present in most TRALI cases, especially severe and fatal cases, most policy changes made to mitigate TRALI have targeted antibody-mediated TRALI.
Decreasing incidence of TRALI through changes in blood products policies is supported through biovigilance data.77 Multiple approaches have been proposed and are in place, including plasma from men only, resuspending pooled buffy coat platelets in plasma from men only, and screening female donors (either all, or only those donors with a history of pregnancy, transfusion, or both) for leukocyte antibodies.78,79 Potential risks of these policies on the available blood supply should be weighed against benefits of implementation.
The International Society for Blood Transfusion (ISBT) published recommendations for screening donors for leukocyte antibodies.80 Antibody detection should include antibodies against HNA (HNA-1a, HNA-1b, HNA-2, and HNA-3a) and HLA class I (HLA-A2) and class II. The ISBT also recommended screening donors at risk for leukocyte antibody formation, that is, parous women, and individuals who have undergone transplantations and transfusions.79 Blood components with high plasma fractions (plasma, apheresis platelets, and whole blood) should not be prepared from these donors. Lastly, those with anti–HNA-3a should not donate because of the high rate of fatality associated with this antibody, even in the receipt of RBCs. Donor testing only needs to be repeated if exposure to leukocyte antigens occurs.
AABB Bulletin #07-03 recommended implementing measures to minimize the preparation of high plasma-volume components from donors known to be leukocyte-immunized or at increased risk of immunization. Date of implementation was November 2007 for plasma components and November 2008 for platelet components.81
Male-only plasma
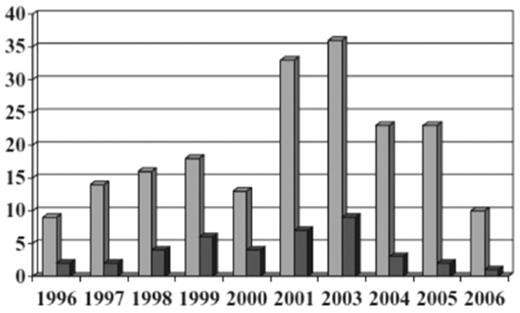
The United Kingdom hemovigilance system from 1996 to 2004 diagnosed TRALI by respiratory distress and hypoxemia associated with transfusion of plasma-containing blood components and in the absence of fluid overload or cardiac failure.82 In 2006, the United Kingdom modified their definition to fit that of the Consensus Panel to limit time of onset from 24 to 6 hours after transfusion.77 In 2003, cases were allocated into 1 of 4 groups: highly likely, probable, possible, and unlikely based on other causes of symptoms and serologic investigations. Reports of TRALI averaged 14 per year through 2001, then rose to 26 per year in 2002 and 36 in 2003 (from 1996 to 2003 there were 93 cases of TRALI reported). TRALI was 5-7 times as likely to be associated with a plasma-rich component (FFP, platelets, and whole blood) than a component containing small amounts of plasma (RBCs). Because TRALI is usually secondary to donor HLA or HNA antibodies, which are more common in females than males, the United Kingdom began using male donor plasma and resuspension of buffy coat-derived platelets in male-donated plasma. Since 2003, 80%-90% of the United Kingdom FFP has been male-donated plasma. In 2004, the United Kingdom started using SD plasma for plasma exchange procedures in thrombotic thrombocytopenic purpura patients. These changes resulted in a decrease in number of TRALI reports and deaths in the United Kingdom (Figure 6); the risk decreased from 1:65 000 to 1:317 000 (P < .001) for FFP, and 1:71 000 to 1:173 000 (P = .068) for platelets; the risks for RBCs (1:949 000) and cryoprecipitate (1:104 000) remained similar.
Decreased incidence of TRALI resulting from male-only plasma.77 All reports of TRALI ( , n = 195) and deaths (■, n = 40) from 1996 through 2006 inclusive. Each reporting year from 1996 until 2000 covers 12 months from October 1 until September 30; the 2001 reporting year covers 15 months from October 1, 2001, to December 31, 2002; and 2003 and subsequent reporting years cover calendar years. (Reprinted with permission.)
, n = 195) and deaths (■, n = 40) from 1996 through 2006 inclusive. Each reporting year from 1996 until 2000 covers 12 months from October 1 until September 30; the 2001 reporting year covers 15 months from October 1, 2001, to December 31, 2002; and 2003 and subsequent reporting years cover calendar years. (Reprinted with permission.)
Decreased incidence of TRALI resulting from male-only plasma.77 All reports of TRALI ( , n = 195) and deaths (■, n = 40) from 1996 through 2006 inclusive. Each reporting year from 1996 until 2000 covers 12 months from October 1 until September 30; the 2001 reporting year covers 15 months from October 1, 2001, to December 31, 2002; and 2003 and subsequent reporting years cover calendar years. (Reprinted with permission.)
, n = 195) and deaths (■, n = 40) from 1996 through 2006 inclusive. Each reporting year from 1996 until 2000 covers 12 months from October 1 until September 30; the 2001 reporting year covers 15 months from October 1, 2001, to December 31, 2002; and 2003 and subsequent reporting years cover calendar years. (Reprinted with permission.)
Likewise, the American Red Cross reported a decrease in fatal and nonfatal TRALI cases after using only male-donated plasma in 2007 (from 26 cases in 2006 to 7 in 2008).83
SD plasma
SD plasma is a pooled human plasma product from 500 to 1600 donations that undergoes viral inactivation. Leukocyte antibodies are not detected in SD plasma because the process dilutes WBC antibodies and soluble HLA antigens are present in the product, neutralizing the antibodies.83,84 Importantly, SD plasma has not been associated with TRALI, although more than 13 million units have been transfused.85
Future directions
Through the last 50 years, knowledge of TRALI has significantly increased, leading to changes that decrease its incidence; yet much remains unknown. First, patients at risk for TRALI are not well characterized, and nonimmune mechanisms are poorly understood. Therefore, blood product modifications to prevent TRALI secondary to these factors have yet to be developed. Second, the Consensus Panel definition of TRALI may need validating. Finally, TRALI incidence and outcome are largely based on passive reporting systems, thus TRALI is probably underreported and its incidence higher than published. Coordinated efforts between bench researchers and translational researchers, clinicians, epidemiologists, and donor centers will be necessary to further minimize the risk of this potentially fatal transfusion complication.
Authorship
Contribution: B.H.S., S.R.S., and C.D.H. have all contributed to the writing of this manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Beth Shaz, MD, New York Blood Center, 310 East 67th St, New York, NY 10065; e-mail: bshaz@nybloodcenter.org.