Abstract
We present results of a phase 3 randomized trial of autografting in chronic lymphocytic leukemia versus observation for responding patients after first- or second-line treatment. The primary objective was to demonstrate that autografting improves the 5-year event-free survival (EFS) from 30% to 50%. There were 223 enrolled patients, 72% men and 28% women, 83% after first and 17% after second-line treatment. Binet stages were progressive A 13%, B 67%, C 20%; at randomization, 59% were in complete remission, and 41% in less than complete remission. Patients were randomized between autografting (n = 112) and observation (n = 111). Median EFS was 24.4 months (range, 16.7-32 months) in the observation group and 51.2 months (39.8-62.5 months) in the autografting group; the 5-year EFS was 24% and 42%, respectively (P < .001). Accordingly, the 5-year relapse incidence was 76% versus 54% (P < .001). Median time to relapse requiring therapy or death was 40 months (25-56 months) in the observation arm and 65 months (59-71 months) after autografting (P = .002). Cox modeling confirmed that autografting significantly improved EFS (hazard ratio 0.44, 95% confidence interval 0.30-0.65; P < .001). At 5 years, the probability of OS was 85.5% and 84.3% for autografting and observation, respectively (P = .77). In chronic lymphocytic leukemia, consolidating autografting reduces the risk of progression by more than 50% but has no effect on overall survival.
Introduction
Chronic lymphocytic leukemia (CLL) is the most common form of leukemia in adults. Although most patients are older than 65 years of age and many will not require treatment, 40% of patients are younger than 65 years of age at presentation. For this group of younger adults, standard therapies may not provide sustained responses, and most of these patients are destined to die of their disease.1 CLL is incurable with conventional treatment.2 The outlook has improved with the use of combination therapy with fludarabine and cyclophosphamide3 and is further improved by the addition of rituximab to the combination.4 Allogeneic stem cell transplantation by the use of a graft versus leukemia effect5,6 may offer the potential for cure but is hampered by the availability of donors and the high proportion of nonrelapse deaths.7
Autologous stem cell transplantation has several attractions. Donor availability is not a problem, and transplantation-related mortality is much less than in allogeneic transplantation. The result of early phase 2 studies were encouraging, and several studies have been published demonstrating the feasibility of autografting in patients with CLL.8-11 Subsequently larger series have been reported from the United States,12 Germany,13 and the United Kingdom.14 Although the phase 2 data demonstrated the ability to eradicate disease measured either by sensitive polymerase chain reaction methods or flow cytometry, it was unclear as to the degree to which this influenced clinical outcomes. The authors of a German matched-pair analysis attempted to answer this question and apparently showed that autografted patients fared better than those treated with conventional chemotherapy in both overall and progression-free survival, but of course this finding is subject to the usual caveats about unintended bias and the choice of the control arm.15 Although there was initial optimism that autografting would result in sustained clinical and molecular responses this was not borne out by later results. These results demonstrated that although most patients undergoing autografting achieved a complete molecular response, it was not maintained and that subsequent clinical progression was inevitable.12,14,16,17 In addition there were other problems. Peripheral progenitor cells were not easy to harvest from patients with CLL, perhaps as the result of marrow involvement with CLL or previous treatment with fludarabine,18 which has been shown to inhibit the mobilization of stem cells in acute myeloid leukemia patients.19 The early experience more closely identified those patients likely to gain a benefit from autografting: those who achieved a complete remission (CR) before transplantation doing best.20 There has been limited information on the results of autografting patients with CLL who have adverse prognostic risk factors such as 11q- or 17p- genetic abnormalities or unmutated VH gene.21,22
Finally, concerns have been raised about the reported high incidence of myelodysplasia (MDS) as a consequence of previous chemo(radiotherapy) in some series; this was estimated at 12% at 5-8 years after autograft in the United States and United Kingdom12,23 but was 5% in the German series.13
In view of the uncertainties concerning the clinical role of autografting in younger patients with CLL, several European CLL research groups (France, United Kingdom, Germany, Switzerland) and the European Group for Blood and Marrow Transplantation agreed to collaborate in an Intergroup trial. The trial opened in October 2001 and closed in July 2007.
Methods
Criteria for eligibility
This phase 3 randomized European Group for Blood and Marrow Transplantation trial included patients 18 years of age or older with a diagnosis of CLL according to the National Cancer Institute-Sponsored Working Group24 who were in stage A progressive, B, or C according to the Binet classification25 at the initiation of first line treatment. Patients had to be in CR, in nodular partial remission (nPR), or in very good partial remission (VGPR) after first- or second-line treatment. Patients with poor performance status (World Health Organization grade > 2), or having one of the following criteria were not considered eligible: T-cell leukemia, non-Hodgkin lymphoma, Richter syndrome, mantle cell lymphoma, prolymphocytic leukemia, HIV seropositivity, inadequate renal or liver function, severe heart failure, severe concomitant neurologic or psychologic disease, pregnancy or lactation, or planned allografts.
The trial was conducted in accordance with the Declaration of Helsinki. The study protocol was approved by the local ethics committees at all participating institutions. Written informed consent was obtained for all participants.
Protocol design and treatment modalities
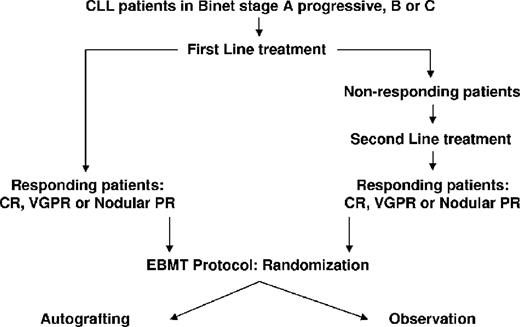
Because this trial was not testing the induction therapy, the first- and second-line induction treatments were left to the discretion of investigators. Eligible patients were randomly assigned to receive either autologous stem cell transplantation with the use of peripheral stem cells (autografting arm) or to surveillance without additional treatment (observation arm) before stem cell mobilization. Patients randomized to the observation arm had peripheral blood stem cells (PBSCs) harvested and stored for possible use in the future. It was recommended that the PBSC mobilization be performed at least 2 months after the last induction cycle. The recommended mobilization schedule was intravenous cyclophosphamide 2 g/m2 on day 1 with 2-mercaptoethane sulfonate sodium (NA) (100% of the dose of cyclophosphamide intravenously) and subcutaneous lenograstim 150μg/m2/day for days 5-12. An alternative schedule for mobilization only for patients randomized to the autografting arm was Dexa-BEAM: oral dexamethasone 3 × 8 mg (days 1-10), intravenous carmustine 60 mg/m2 (day 2), intravenous etoposide 75 mg/m2 (days 4-7), intravenous cytarabine 100 mg/m2 every 12 hours (days 4-7), intravenous melphalan 20 mg/m2 (day 3), and subcutaneous lenograstim 150 μg/m2/day on day 11 until the last day of aphaeresis. PBSC collections were performed on 2 to 4 consecutive days after the hematopoietic recovery phase as soon as the leukocyte counts reached 2 × 109/L or as soon as the peripheral CD34+ counts exceeded 2 × 107/L. The aim was to achieve a harvest of more than 1 × 108/kg body weight–nucleated cells, CD34+ cells > 2 × 106/kg body weight, and/or CFU-GM > 5 × 104 /kg body weight. If these numbers were not met after 4 days, the patient was considered as a failure of mobilization and the mobilization was discontinued. For conditioning regimens, 2 standard schedules were recommended: (1) cyclophosphamide: 60 mg/kg body weight on days −5 to −4 (+ 2-mercaptoethane sulfonate sodium [NA]) plus total body irradiation 10 Grays on days −3 to −1 with lung shielding; (2) BEAM: intravenous carmustine 300 mg/m2 at day −6, intravenous cytarabine 200 mg/m2 every 12 hours days on −5 to −2, intravenous etoposide 100 mg/m2 every 12 hours on days −5 to −2, and melphalan 140 mg/m2 on day −1. The use of granulocyte-colony stimulating factor was allowed after transplantation according to local practice. The study design is shown in Figure 1.
Study end points and response criteria
The primary study end point was the event-free survival (EFS) at 5 years (from randomization). The secondary end points included overall survival (OS) at 5 years (from randomization), time to disease requiring therapy or death (whatever came first from time of randomization), feasibility of first-line versus second-line autograft, and for autografted patients, engraftment and mortality of the transplantation procedure. Treatment effects were monitored by physical examination, imaging techniques (chest x-ray and abdomen ultrasound or chest and abdomen computed tomography at the investigator's discretion), blood count evaluation, bone marrow aspiration, and biopsy. Response assessments were scheduled every 4 months from randomization during the first year and every 6 months thereafter during 4 years or until progression. Guidelines for response were those developed by the National Cancer Institute-Sponsored Working Group.1
EFS was defined as survival from randomization to disease progression or death. OS was measured from randomization to death from any cause. Disease progression was considered if at least one of the following occurred: an increase in the absolute lymphocyte count > 10 × 109/L, increase of 50% in new lymph nodes, increase of 50% in liver or spleen below the costal margin, the appearance of palpable hepatomegaly or splenomegaly, or the development of an aggressive lymphoma. Disease requiring therapy was defined by the presence of at least one of the following symptoms: B symptoms, symptomatic or progressive lymphadenopathy, symptomatic splenomegaly, progressive reduction of hemoglobin, and/or platelets, which, in the view of the clinician, required treatment.
Statistical analysis
EFS at 5 years after randomization was expected to be approximately 30% in the observation arm and approximately 50% in the autografting arm by intention to treat. To detect an absolute difference of 20% with a 2-sided significance level of 0.05 and a power of 0.90 required 134 patients in each arm. The target accrual was 270 patients.
The randomization was performed by telephone or fax,26 and it was stratified according to participation groups and minimized on Binet stage, disease status, and whether achieved after first- or second-line therapy. EFS and OS curves were plotted by use of the Kaplan-Meier method.27 The probabilities of EFS and OS were compared between groups by the log-rank test. A multivariate analysis of potential factors influencing EFS and OS was performed with a step-wise Cox regression. For each analysis, P < .05 was considered statistically significant.
Results
Patient characteristics
Between October 2001 and July 2007, 223 patients from 11 different European countries were enrolled onto the study (France: n = 99, United Kingdom: n = 63, Germany: n = 36, Switzerland: n = 10, other European countries: n = 15). A total of 112 patients were randomly assigned to autografting and 111 patients to observation. There were 161 men (72%) and 62 women with a median age of 54 years (range, 31-65 years) Thirty patients (13%) were in Binet stage A progressive, 148 (66%) in stage B, and 45 (20%) in stage C.
One hundred eighty-four (83%) patients were randomized after first-line treatment and 39 (17%) patients after second-line treatment. Disease status at randomization was 132 (59%) CR, 60 (27%) VGPR and 31 (14%) nPR. Induction treatment consisted of regimens containing fludarabine and alkylating agents administered concurrently or sequentially in the majority of patients (71%). However, only 9 patients (4%) received combinations of purine analogues and rituximab. Information on fluorescence in situ hybridization (FISH) karyotype was available only in less than one-half of the patients (Table 1).
Patient characteristics according to randomization arm
| Characteristic . | Watch-and-wait arm . | Auto-arm . |
|---|---|---|
| Number of patients | 111 | 112 |
| Age, y | ||
| Median | 53 | 54 |
| Range | 35-65 | 31-65 |
| Time diagnosis-randomization, mo | ||
| Median | 20.9 | 21.1 |
| Range | 3.7-252.7 | 2.7-203 |
| Sex | ||
| Male | 86 (77) | 75 (67) |
| Female | 25 (23) | 37 (33) |
| Binet stage | ||
| A progressive | 14 (13) | 16 (14) |
| B | 76 (68) | 72 (64) |
| C | 21 (19) | 24 (22) |
| Disease status at randomization | ||
| CR | 63 (57) | 69 (62) |
| VGPR | 31 (28) | 29 (26) |
| nPR | 17 (15) | 14 (12) |
| Patient randomization | ||
| After first-line therapy | 92 (83) | 92 (82) |
| After second-line therapy | 19 (17) | 20 (18) |
| First-line treatments | ||
| Fludarabine | 10 (9) | 9 (8) |
| CHOP then fludarabine | 50 (45) | 49 (44) |
| Fludarabine + cyclophosphamide | 20 (18) | 24 (21) |
| FCR | 3 (3) | 0 |
| CHOP ± rituximab | 10 (9) | 5 (4) |
| Rituximab-cladribine | 3 (3) | 3 (3) |
| Other associations | 11 (10) | 11 (10) |
| Unknown | 4 (3) | 11 (10) |
| Cytogenetics | ||
| Del 17p | ||
| Positive | 0 (0) | 2 (2) |
| Negative | 50 (45) | 51 (46) |
| Unknown | 61 (55) | 59 (53) |
| Del 11q | ||
| Positive | 15 (13) | 5 (4) |
| Negative | 42 (38) | 50 (45) |
| Unknown | 54 (49) | 57 (51) |
| Country | ||
| France | 50 (45) | 49 (44) |
| United Kingdom | 30 (27) | 33 (30) |
| Germany | 18 (16) | 18 (16) |
| Switzerland | 5 (5) | 5 (4) |
| EBMT centers | 8 (7) | 7 (6) |
| Characteristic . | Watch-and-wait arm . | Auto-arm . |
|---|---|---|
| Number of patients | 111 | 112 |
| Age, y | ||
| Median | 53 | 54 |
| Range | 35-65 | 31-65 |
| Time diagnosis-randomization, mo | ||
| Median | 20.9 | 21.1 |
| Range | 3.7-252.7 | 2.7-203 |
| Sex | ||
| Male | 86 (77) | 75 (67) |
| Female | 25 (23) | 37 (33) |
| Binet stage | ||
| A progressive | 14 (13) | 16 (14) |
| B | 76 (68) | 72 (64) |
| C | 21 (19) | 24 (22) |
| Disease status at randomization | ||
| CR | 63 (57) | 69 (62) |
| VGPR | 31 (28) | 29 (26) |
| nPR | 17 (15) | 14 (12) |
| Patient randomization | ||
| After first-line therapy | 92 (83) | 92 (82) |
| After second-line therapy | 19 (17) | 20 (18) |
| First-line treatments | ||
| Fludarabine | 10 (9) | 9 (8) |
| CHOP then fludarabine | 50 (45) | 49 (44) |
| Fludarabine + cyclophosphamide | 20 (18) | 24 (21) |
| FCR | 3 (3) | 0 |
| CHOP ± rituximab | 10 (9) | 5 (4) |
| Rituximab-cladribine | 3 (3) | 3 (3) |
| Other associations | 11 (10) | 11 (10) |
| Unknown | 4 (3) | 11 (10) |
| Cytogenetics | ||
| Del 17p | ||
| Positive | 0 (0) | 2 (2) |
| Negative | 50 (45) | 51 (46) |
| Unknown | 61 (55) | 59 (53) |
| Del 11q | ||
| Positive | 15 (13) | 5 (4) |
| Negative | 42 (38) | 50 (45) |
| Unknown | 54 (49) | 57 (51) |
| Country | ||
| France | 50 (45) | 49 (44) |
| United Kingdom | 30 (27) | 33 (30) |
| Germany | 18 (16) | 18 (16) |
| Switzerland | 5 (5) | 5 (4) |
| EBMT centers | 8 (7) | 7 (6) |
Values are n (%) unless otherwise noted.
CHOP indicates cyclophosphamide, hydroxydaunorubicin (doxorubicin), Oncovin (vincristine), and prednisone/prednisolone; CR, complete remission; EBMT, European Group for Blood and Marrow Transplantation; FCR, fludarabine, cyclophosphamide, and rituximab; nPR, nodular partial remission; and VGPR, very good partial remission.
Efficacy
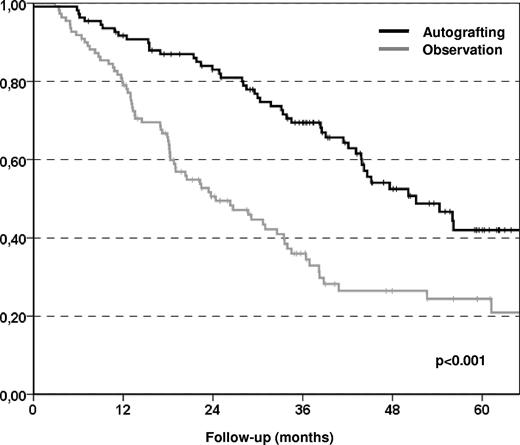
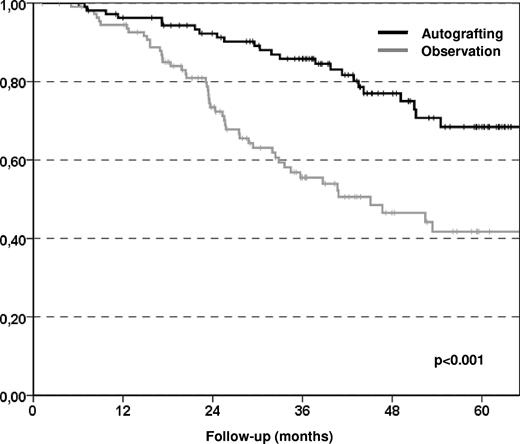
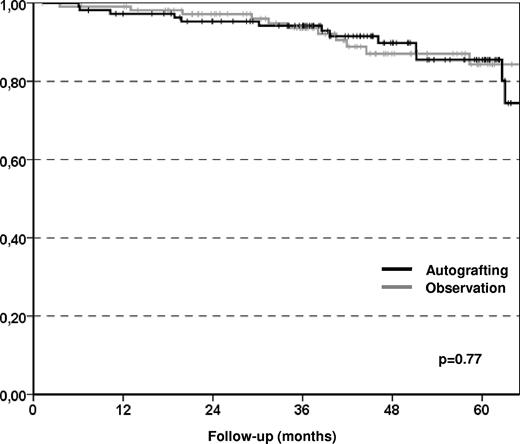
The primary end point of the study was the EFS at 5 years, which improved significantly in the autografting arm compared with the observation arm with 42% and 24%, respectively (P < .001; Figure 2), although only 80 (72%) of the patients assigned to the autografting arm actually underwent transplantation. The reasons for failing to proceed to autografting were 22 collection failures, 5 patient refusals, 2 progressive disease or secondary malignancy, and 2 postinduction complications. The median EFS was significantly longer in the autografting arm than in the observation arm (51 months vs 24 months) with a hazard ratio (HR) of 0.43 (95% confidence interval [95% CI] 0.29-0.65; P < .001). This finding was attributable to a significantly lower relapse incidence in the autografting arm at 5 years (54.2%) compared with observation arm (75.5%) with a HR of 0.39 (95% CI 0.26-0.59; P < .001). The median time to relapse requiring therapy or death (whatever came first) was 40 months (range, 25-56 months) in the observation arm, and 65 months (range, 59-71 months) in the autografting arm (P = .002; Figure 3). We did not observe any significant difference in terms of nonrelapse mortality between the 2 study arms (0% in the observation arm, 4% in the autografting arm, P = 033). MDS was seen in 3 patients in the autografting arm, and 1 case occurred in the observation arm. However, after a median follow-up of 43.7 months (range, 1.2-95.7 months), there was no statistically significant difference in survival between the 2 arms, the probability of OS at 5 years was 85.5% (range, 77%-94%) in the autografting arm and 84.3% (range, 75%-93%) in the observation arm (P = .77; Figure 4).
Kaplan-Meier plots of EFS. Patients receiving autologous transplantation had statistically better EFS comparing to patients in the observation arm.
Kaplan-Meier plots of EFS. Patients receiving autologous transplantation had statistically better EFS comparing to patients in the observation arm.
Kaplan-Meier plots of relapse requiring therapy or death (whatever came first). Patients receiving autologous transplantation had longer time to relapse requiring therapy or death comparing to patients in the observation arm.
Kaplan-Meier plots of relapse requiring therapy or death (whatever came first). Patients receiving autologous transplantation had longer time to relapse requiring therapy or death comparing to patients in the observation arm.
Kaplan-Meier plots of OS. No statistically significant difference was observed in term of overall survival between the two randomization arms.
Kaplan-Meier plots of OS. No statistically significant difference was observed in term of overall survival between the two randomization arms.
Univariate and multivariate analyses
In our univariate analysis we studied the impact of factors other than randomization arms, such as being randomized after first- or second-line treatment, the type of treatment, the disease status at randomization, the effect of country, and cytogenetics (FISH karyotype). The only significant factor impacting negatively on the EFS, OS, and relapse incidence was the FISH karyotype 17p− and/or 11q−.
Multivariate analysis with Cox modeling adjusting for the impact of previous factors in addition to randomization arm confirmed the positive impact of autografting (HR 0.44, 95% CI 0.30-0.65; P < .001), the negative impact of FISH karyotype 17p−/11q− on EFS (HR 3.60, 95% CI 2.05-6.31; P < .001), and the negative impact of disease status (VGPR) on OS (HR 3.80, 95% CI 1.55-9.30; P = .004). Significant results in multivariate analysis on EFS, OS, and relapse are shown in Table 2.
Significant factors in multivariate analysis for EFS, OS, and relapse
| Variable . | Impact on EFS . | Impact on OS . | Impact on relapse . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Randomization arm | |||||||||
| Observation | 1 | 1 | |||||||
| Autografting | 0.44 | 0.30-0.65 | < .001 | 0.40 | 0.27-0.59 | < .001 | |||
| Disease status | |||||||||
| CR | 1 | ||||||||
| VGPR | 3.80 | 1.55-9.30 | .004 | ||||||
| Del 17p and/or del 11q | |||||||||
| No | 1 | 1 | |||||||
| Yes | 3.60 | 2.05-6.31 | < .001 | 3.79 | 2.16-6.67 | < .001 | |||
| Unknown | 1.58 | 1.04-2.39 | .032 | 1.54 | 1.01-2.35 | .045 | |||
| Variable . | Impact on EFS . | Impact on OS . | Impact on relapse . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Randomization arm | |||||||||
| Observation | 1 | 1 | |||||||
| Autografting | 0.44 | 0.30-0.65 | < .001 | 0.40 | 0.27-0.59 | < .001 | |||
| Disease status | |||||||||
| CR | 1 | ||||||||
| VGPR | 3.80 | 1.55-9.30 | .004 | ||||||
| Del 17p and/or del 11q | |||||||||
| No | 1 | 1 | |||||||
| Yes | 3.60 | 2.05-6.31 | < .001 | 3.79 | 2.16-6.67 | < .001 | |||
| Unknown | 1.58 | 1.04-2.39 | .032 | 1.54 | 1.01-2.35 | .045 | |||
CI indicates confidence interval; CR, complete remission; EFS, event-free survival; HR, hazard ratio; nPR, nodular partial remission; and VGPR, very good partial remission.
Discussion
This is the first randomized trial of autografting in CLL. By halving the relapse risk, autografting prolonged median EFS from 2 years to more than 4 years, implying that the impact of autografting on EFS was even greater than was originally anticipated for this group of patients. The EFS benefit of autografting was stable more than all contributing groups. These data are in line with the results of 2 large prospective phase 2 trials on autologous PBSC transplantation as first-line consolidation in CLL, namely the Medical Research Council pilot trial12,14 and the German CLL Study Group CLL3 trial,13 where median EFS was approximately 5 years and 6.3 years, respectively.
However, in the present study autografting consolidation was added to a first-line therapy of relatively moderate activity. Although the induction regimens used in our trial contained a combination of fludarabine with alkylating agents in the majority of patients and therefore might be expected to be more effective than the monotherapy standard treatments used in the German matched-pair analysis,15 they can be hardly considered as state-of-the-art in the current era of rituximab-based combination regimens. None of the patients in this trial had alemtuzumab or rituximab as part of the standard induction therapy, although some received these antibodies as second-line therapy. Moreover, OS was not different between the 2 arms, implying that relapses in the observation arm could be successfully managed with effective salvage strategies. Only 9 patients randomized to the observation arm went on subsequently to receive an autologous transplantation, and thus the majority of patients who relapsed were salvaged with a nontransplantation treatment. Allogeneic transplantation was performed after relapse in 15 patients in the observation arm and in 4 patients in the auto-arm. However, with more than 50% and 85%, respectively, both EFS and OS of the auto arm are in the range of the figures reported for the fludarabine, cyclophosphamide, and rituximab (FCR) regimen, which is currently considered as the most effective front-line treatment for patients with CLL,28,29 although it has to be kept in mind that our patient population was restricted to those who had already achieved a CR, VGPR, or nPR.
Both fludarabine-alkylator combinations followed by autografting and fludarabine-antibody combinations provide very effective disease control when applied as first-line treatment, but still approximately one-half of all patients will relapse within 5 years after beginning treatment. Because the prognosis of patients who relapse after FCR or autografting is poor, in particular if it occurs early,30 there is some rationale to aim at improving remission duration further. This may be particularly important for patient subpopulations with poor-risk factors (eg, unmutated VH status) that retain their adverse impact after FCR and autografting, respectively.28,29 Therefore, it might be attractive to explore strategies that combine fludarabine-rituximab regimens with consolidating autografting to maximize disease control for those patients who have predefined high-risk features.
Not all the patients intended to receive the autograft underwent the process. The main reason for this was the inability to collect adequate stem cells; this can be potentially resolved by the introduction of the new mobilization agents.
Another way of improving the results of autografting in CLL might be the use of ex vivo or in vivo purging with monoclonal antibodies.12,31 However, evidence from prospective studies proving the benefit of purging in this setting is still lacking,32 and in studies that used vigorous ex vivo purging, the authors failed to prove a curative effect of autografting in CLL when used as part of first-line treatment.12,14
Recruitment into the trial was slower than anticipated, which was attributable to different factors. Publication of the U.K. and U.S. phase 2 series12,14 demonstrated no evidence of a plateau on the survival curve with early signs of molecular relapse predating clinical progression. These studies also signaled concerns about high levels of MDS in CLL patients undergoing autografting. To this end, the trial was safe with no significant excess of deaths in the autotransplantation arm. MDS did occur in the autotransplantation arm at relatively low levels, but the follow-up of the patients in this trial was limited to 5 years after randomization.
Further follow-up of the patients at risk will provide greater confidence concerning the risks of secondary MDS. In view of the sharply decreasing rate of accrual in the final 2 years of the trial, a decision was made by the Trial Management Group, in the absence of knowledge of the results, to close the trial before the target accrual was met. Although the original target was 270 patients, the greater-than-expected event rate (approximately 20% rather than 30% EFS) clearly increased the power, and a significant difference was found.
Although the results presented here exceeded the expectations at the time of trial design there remain questions about the role of autografting in CLL. Data on quality of life are awaited; however, the fact a similar outcome may be achieved with simpler treatments make it difficult to recommend autografting as a standard of care. It is of course feasible that the additional tumor kill achieved after an autograft may result in a superior outcome even after rituximab treatment but this will require a further trial to define.
Taken together, in patients with CLL in first or second remission after a fludarabine-containing regimen, consolidating autologous transplantation strongly reduces the risk of progression and prolongs time to retreatment but has no effect on overall survival. Given the size of the effect on disease control and the biologically different therapeutic principles, there may be a rational to study autografting as consolidation after rituximab-based treatment, at least in patients with defined high-risk CLL. Allogeneic transplantation offers the potential of cure for some patients but at increased risk. As greater knowledge is accrued over the safety and applicability of allogeneic transplantation in high-risk CLL,7 the potential role for autografting will be further challenged.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: All coauthors contributed to patients' enrollment in this study and approval of final report; R.B. performed statistical analysis; M.v.O. collected and verified data; and M.M., P.D., M.S., and D.M. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
For a complete list of study participants, please see the supplemental Appendix, available on the Blood Web site (see the Supplemental Materials link at the top of the online article).
Correspondence: Professor Mauricette Michallet, MD, PhD, Hematology, Edouard Herriot Hospital, 5, Place d'Arsonval, Lyon, Cedex 0369437, France; e-mail: mauricette.michallet@chu-lyon.fr.