Abstract
Graft-versus-host disease (GVHD), a life-threatening complication after allogeneic hematopoietic stem cell transplantation, is caused by alloreactive donor T cells that trigger host tissue damage. The inflammatory environment inside recipients is critical for GVHD pathogenesis, but the underpinning mechanisms remain elusive. Using mouse model of human GVHD, we demonstrate osteopontin (OPN), a potent proinflammatory cytokine, plays an important role in regulating activation, migration, and survival of alloreactive T cells during GVHD. OPN was significantly elevated after irradiation and persisted throughout the course of GVHD. Blockade of OPN attenuated GVHD with reduced accumulation of donor T cells in recipient organs. Amelioration was the result of migration and survival suppression caused by anti-OPN treatment on donor-derived T cells for 2 reasons. First, OPN promoted the migration and infiltration of naive and alloreactive CD8+ T cells into host organs. Second, it also facilitated activation and viability of donor-derived CD8+ T cells via synergizing with T-cell receptor/CD3 signaling. Finally, anti-OPN treatment retained graft-versus-leukemia effect of alloreactive CD8+ T cells. This study demonstrates, to our knowledge for the first time, the critical effect of OPN in the initiation and persistence of CD8+ T cell-mediated GVHD and validates OPN as a potential target in GVHD prevention.
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) is an important curative therapy for hematologic malignancies, some metastatic solid tumors, and a variety of nonmalignant diseases. The most significant limitation to allogeneic HSCT is graft-versus-host disease (GVHD), the major cause of post-transplantation mortality and morbidity.1-3 GVHD occurs when donor T cells are transferred into hosts expressing histocompatibility differences. These T cells recognize major or minor histocompatibility antigens (miHAs) displayed by antigen-presenting cells and are rapidly activated to proliferate and differentiate into alloreactive effector T cells.4-9 These alloreactive T cells are then recruited into the liver, intestine, and skin, mediating host tissue damage and subsequently the disease.10,11 Although T-cell depletion of donor grafts has successfully reduced the incidence of acute GVHD, it has also resulted in increased rates of engraftment failure and relapse of chronic myelogenous leukemia.4-6 This is because the therapeutic potential of HSCT relies on a graft-versus-leukemia (GVL) effect, by which donor T cells eradicate residual tumor cells expressing host or tumor-associated antigens. Thus, comprehensive understanding of the mechanisms that lead to T cell-mediated GVHD is crucial for optimizing this therapeutic modality.
It is widely accepted that the inflammatory environment caused by preconditioning before transplantation is critical for the behavior of donor T cells during GVHD. Serial proinflammatory cytokines, including interleukin-1 (IL-1), IL-17, interferon-γ, tumor necrosis factor-α, and chemokines, contribute to different events during the pathogenesis of donor T cells, such as activation, polarization, and migration.5,11-13 However, how the inflammatory host environment influences the initiation, development, and persistence of GVHD remains elusive.
Recently, osteopontin (OPN), a multifunctional extracellular matrix protein secreted by a variety of tissues and cells,14 has been recognized as a potential proinflammatory cytokine associated with inflammatory responses.15,16 OPN is involved in both physiologic and pathologic processes in multiple organs and tissues, including biomineralization, cell movement and survival, cancer progression, and inflammation.14,17 High levels of OPN have been reported in rheumatoid arthritis, inflammatory lesions in multiple sclerosis, and coronary artery disease,18-21 implying association of OPN with immune-related diseases. However, OPN seems to have contradictory functions on the inflammatory pathogenesis. OPN is a major amplifier of Th1-immune responses and has been considered as a proinflammatory cytokine associated with local inflammation in some inflammatory diseases.18,22-24 However, it also shows little effect or even anti-inflammatory role in other pathologic responses, such as dextran sulfate sodium-induced experimental colitis and the K/BxN model of autoantibody-mediated arthritis.25,26 This suggests complex and wide-ranging roles of OPN in the pathogenesis of disease. Moreover, it is still unknown whether OPN participates in the course of GVHD; if so, its function, whether proinflammatory or anti-inflammatory, is also unclear.
Therefore, using a well-described allogeneic HSCT mouse model that is closely related to human GVHD directed against miHAs, we undertook a detailed analysis on the role of OPN in CD8+ T cell-mediated GVHD. We found that the expression of OPN inside recipients was significantly elevated during GVHD, and anti-OPN antibody (Ab) treatment significantly relieved the symptoms of GVHD with reduced donor CD8+ T-cell residence in host organs. On one hand, anti-OPN Ab hampered the migration of donor CD8+ T cells in recipients without impacting the expression of other chemokines and chemokine receptors. On the other hand, anti-OPN treatment suppressed the activation and viability of donor CD8+ T cells during GVHD because OPN could promote the activation and survival of T cells via synergizing with T-cell receptor (TCR)/CD3 signaling. Finally, anti-OPN Ab retained GVL effect of alloreactive CD8+ T cells. These novel findings demonstrate the facilitative role of OPN in the initiation and persistence of CD8+ T cell-mediated GVHD and suggest therapeutic potential of targeting OPN in clinical GVHD prevention.
Methods
Mice
B6/SJL (H-2Db, CD45.1+), C3H.SW (H-2Db, CD45.2+ and Ly9.1+), DBA/2 (H-2Dd), and BALB/c (H-2Dd) mice were purchased from Jackson ImmunoResearch Laboratories. Animals were housed in a specific pathogen-free facility at the Chinese Academy of Sciences. Animal protocols were approved by the Institutional Review Board of the Institute of Health Sciences, as previously described.27
Cytokines, Abs, and cell lines
Purified mouse OPN and mouse OPN immunoassay kit (enzyme-linked immunosorbent assay [ELISA]) were purchased from R&D Systems. ELISA was performed according to the manufacturer's instructions. Anti-OPN Ab was prepared as previously described.28,29 Microbead-conjugated Abs for magnetic activated cell sorting were purchased from Miltenyi Biotec. Abs for immunofluorescence staining were obtained from BD Biosciences PharMingen. Multiple-color flow cytometric analysis was performed using FACSAria (BD Biosciences). B6 mouse-derived EL-4 leukemic cells (H-2Db) were obtained from ATCC.
Cell preparation
T cell-depleted bone marrow cells (TCD BM) were prepared by depleting CD4+ and CD8+ T cells from BM using CD4 and CD8 microbeads, followed by magnetic activated cell sorting. As donor T cells, CD8+ T cells were isolated from spleens and lymph nodes of C3H.SW mice by magnetic activated cell sorting with CD8 microbeads (C3H.SW CD8+ T cells). The purity of sorted cells in this study was consistently more than 95%.
GVHD induction and treatment with anti-OPN Ab
Lethally irradiated mice underwent allogeneic BM transplantation, as previously described.27 Briefly, B6/SJL mice were irradiated with 9.5 Gy administered in 3 fractions from a 137Cs source. C3H.SW CD8+ T cells (2 × 106) plus C3H.SW TCD BM (5 × 106) were transplanted into the irradiated B6/SJL mice. These B6/SJL mice were then administered with anti-OPN Ab or control IgG (200 μg/mouse) via tail vein at days 0, 3, 6, 10, and 14 after transplantation. Recipient mice were monitored for survival and clinical signs of GVHD, which was first described by Cooke et al.30 According to animal protocols approved by the Institutional Review Board, mice were killed when the clinical score achieved 8.0.
Histology
Specimens of liver and intestine were provided for histopathologic assessment of GVHD in blinded fashion by 2 pathologists, using a semiquantitative scoring system for abnormalities known to be associated with GVHD.31 Seven parameters were scored for colon (crypt regeneration, crypt epithelial cell apoptosis, crypt loss, surface colonocyte vacuolization, surface colonocyte attenuation, lamina propria inflammatory cell infiltrate, and mucosal ulceration), and 10 parameters for liver (portal tract expansion by an inflammatory cell infiltrate, lymphocytic infiltrate of bile ducts, bile duct epithelial cell apoptosis, bile duct epithelial cell sloughing, vascular endothelialitis, parenchymal apoptosis, parenchymal microabscesses, parenchymal mitotic figures, hepatocellular cholestasis, and hepatocellular steatosis). The scoring system for each parameter was as follows: 0 indicates normal; 0.5, focal and rare; 1, focal and mild; 2, diffuse and mild; 3, diffuse and moderate; and 4, diffuse and severe.
Adoptive transfer model
Alloreactive donor-derived CD8+ T cells were isolated from the spleens and lymph nodes of irradiated B6/SJL mice receiving C3H.SW CD8+ T cells plus TCD BM at day 14 after transplantation. These T cells (2 × 106) were then transferred into lethally irradiated secondary B6/SJL mice accompanied by C3H.SW TCD BM (5 × 106).
CFSE labeling and analysis
Freshly isolated CD8+ T cells were labeled with 2.5μM 5-(and 6)-carboxyfluorescein diacetate succinimidyl ester (CFSE; Invitrogen) and then transplanted into irradiated B6/SJL mice in both GVHD model and the adoptive transfer model. The cells infused in recipient organs were recovered at indicated time after transplantation, and their CFSE fluorescence intensities were measured using flow cytometry by gating in donor CD8+ T cells.
Quantitative real-time PCR
Total RNA was extracted from recipient tissues or sorted donor CD8+ T cells at indicated times and was subsequently reverse-transcribed using AMV Reverse Transcription System (Promega). Quantitative real-time polymerase chain reaction (PCR) was performed using SYBR Green PCR mix on an ABI Prism 7900HT (Applied Biosystems). Thermocycler conditions included 2-minute incubation at 50°C, then 95°C for 10 minutes; this was followed by a 2-step PCR program, as follows: 95°C for 15 seconds and 60°C for 60 seconds for 40 cycles. β-Actin was used as an internal control to normalize for differences in the amount of total RNA in each sample. The primer sequences were as follows (in the 5′ to 3′ orientation): β-actin forward, TGTCCACCTTCCAGCAGATGT, β-actin reverse, AGCTCAGTAACAGT-CCGCCTAGA; OPN forward, TGGCAGTGATTTGCTTTTGC, OPN reverse, TGGGTGCAGGCTGTAAAGCT; Mig forward, GAACCCTAGTGATAAGGAATG-CA, Mig reverse, CTGTTTGAGGTCTTTGAGGGATT; IP-10 forward, GCCGTCA-TTTTCTGCCTCAT, IP-10 reverse, GGCCCGTCATCGATATGG; I-TAC forward, AGGAAGGTCACAGCCATAGC, I-TAC reverse, CGATCTCTGCCATTTTGACG; MIP-1α forward, ACCATGACACTCTGCAACCA, MIP-1α reverse, GTGGAATCT-TCCGGCTGTAG; MCP-1 forward, ATTGGGATCATCTTGCTGGT, MCP-1 reverse, CCTGCTGTTCACAGTTGCC; RANTES forward, CCACTTCTTCTCTGGGTTGG, RANTES reverse, GTGCCCACGTCAAGGAGTAT; E-cadherin forward, CTGTCA-CTGTGGACGTGGTAGAC, E-cadherin reverse, GATTTCCTGACCCACACCAAA; ICAM-1 forward, CGTGTGCCATGCCTTTAGCT, ICAM-1 reverse, GCACCAGA-ATGATTATAGTCCAGTTATT; VCAM-1 forward, TGACAAGTCCCCATCGTTGA, VCAM-1 reverse, ACCTCGCGACGGCATAATT; IL-2 forward, CCTTCAAATTTT-ACTTGCCCA, IL-2 reverse, TGAGTCAAATCCAGAACATGC; Bcl-xL forward, T-GACCACCTAGAGCCTTGGA, Bcl-xL reverse, TGTTCCCGTAGAGATCCACAA.
Chemotaxis assay
Chemotaxis analysis was performed using 96-well ChemoTx System (NeuroProbe) as previously described.27 Briefly, chemotaxis medium containing OPN protein (0-10 μg/mL) was added to the lower chamber, and CD8+ T cells with 10 μg/mL IgG or anti-OPN Ab were input into the upper chamber, separated from the lower by a membrane (8-μm pore size). Chemotaxis then proceeded at 37°C in 5% CO2 incubator. Three hours later, cells in the lower chamber were recovered and counted. The ratio of migrating cells was calculated by dividing the number of cells in the lower chamber by the total input cells.
Ex vivo stimulation of CD8+ T cells and proliferation assay
CD8+ T cells were magnetically separated from naive C3H.SW mice, cultured in complete medium containing 2% fetal bovine serum and then treated with different reagents. Cells were harvested at the indicated times for further assays, including real-time PCR, Western blot, and flow cytometry. In proliferation assay, cells were cultured for 72 hours, and [3H]thymidine (1 μCi/mL) was added 18 hours before the end of culture. Cells were then harvested onto glass fiber mats for measurement of [3H]thymidine incorporation.
Induction and assessment of GVL effect
To measure cytolytic ability, splenic donor-derived CD8+ T cells were isolated from IgG or anti-OPN–treated GVHD mice and incubated with EL-4 cells for 12 hours. The supernatant from each well was then collected for measurement of released lactate dehydrogenase using nonradioactive cytotoxicity detection kit (Promega). Leukemia was induced by injection of 5000 EL-4 leukemic cells into peritoneal cavities of lethally irradiated B6/SJL mice at day 7 after allogeneic BM transplantation as previously described.27
Statistical analysis
Log-rank test was used for survival analysis. Differences between 2 groups were compared using t test or Wilcoxon rank test. Analysis of variance or Kruskal-Wallis test was used for statistics among multigroups and followed by Student-Newman-Keuls or Nemeny test to compare difference between the 2 groups, respectively. χ2 test was used to analyze CFSE data. Values of P less than .05 were considered significant.
Results
OPN is required for alloreactive CD8+ T cell-mediated GVHD
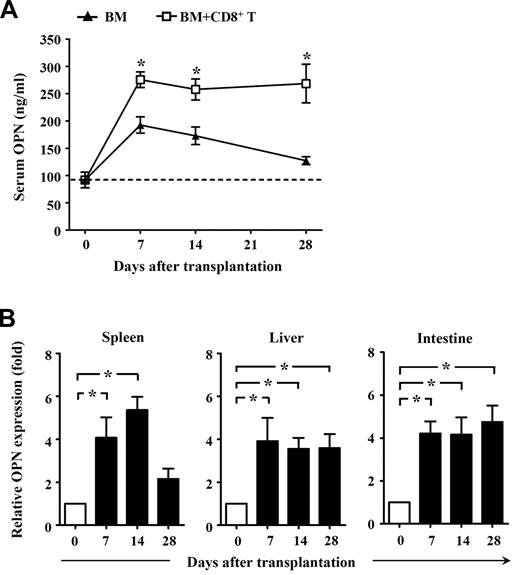
To determine whether OPN might contribute to GVHD, we first assessed the expression of OPN in GVHD mice. Donor CD8+ T cells derived from normal C3H.SW mice were transplanted together with TCD BM into lethally irradiated B6/SJL mice to induce GVHD, with TCD BM alone transplanted as a control. As expected, all B6/SJL mice receiving donor CD8+ T cells plus TCD BM developed severe GVHD, with approximately 80% dying of GVHD. By contrast, all mice that received TCD BM alone did not develop any clinical signs of GVHD. ELISA analysis showed that serum OPN was significantly increased in mice receiving TCD BM alone by day 7 after transplantation, and it gradually decreased thereafter (Figure 1A). This indicates that OPN expression can be induced by irradiation conditioning in BM transplantation. However, compared with mice receiving TCD BM alone, mice receiving TCD BM plus donor CD8+ T cells produced significantly higher levels of serum OPN that persisted throughout the course of GVHD (Figure 1A). These results suggest a positive association between OPN level and development of CD8+ T cell–mediated GVHD. Quantitative real-time PCR analyses also showed significantly elevated OPN mRNA in both lymphoid tissue and target tissues throughout GVHD (Figure 1B). Interestingly, in vivo administration of anti-OPN Ab significantly inhibited the development of GVHD in B6/SJL mice. Compared with GVHD mice treated with control IgG, mice treated with anti-OPN Ab showed significantly improved clinical score of GVHD signs, and approximately 70% of them survived more than 70 days after transplantation (Figure 2A). Histologic examination also showed that anti-OPN Ab treatment inhibited the infiltration of inflammatory cells in target tissues and tissue damage, including liver and intestine, compared with those T cell–replete transplant recipients treated with control IgG (Figure 2B). These results demonstrate that OPN participates in and promotes the course of CD8+ T cell-mediated GVHD, and blockade of OPN with neutralizing Ab can significantly attenuate the severity of GVHD. Similarly, in the CD4+ T cell-dependent GVHD model, blockade of OPN improved overall survival of BABL/c mice receiving DBA/2 splenocytes and significantly attenuated the symptom of proteinuria in recipient mice (Figure 2C).
Expression of OPN during GVHD. Lethally irradiated B6/SJL recipient mice (CD45.1+) were injected with C3H.SW CD8+ T cells (CD45.2+, 2 × 106) plus C3H.SW TCD BM (5 × 106) or C3H.SW TCD BM (5 × 106) alone. (A) Levels of serum OPN in recipients after transplantation were measured by ELISA. (B) Expression of OPN in spleen, liver, and intestine from GVHD recipients was measured by quantitative real-time PCR. Values for relative fold expression are normalized to gene expression in mice at day 0. Data are mean ± SEM of 6 mice analyzed. These results were representative of 3 independent experiments. *P < .05.
Expression of OPN during GVHD. Lethally irradiated B6/SJL recipient mice (CD45.1+) were injected with C3H.SW CD8+ T cells (CD45.2+, 2 × 106) plus C3H.SW TCD BM (5 × 106) or C3H.SW TCD BM (5 × 106) alone. (A) Levels of serum OPN in recipients after transplantation were measured by ELISA. (B) Expression of OPN in spleen, liver, and intestine from GVHD recipients was measured by quantitative real-time PCR. Values for relative fold expression are normalized to gene expression in mice at day 0. Data are mean ± SEM of 6 mice analyzed. These results were representative of 3 independent experiments. *P < .05.
Inhibition of CD8+ T cell-mediated GVHD by anti-OPN Ab. Lethally irradiated B6/SJL recipient mice were injected with C3H.SW CD8+ T cells plus C3H.SW TCD BM or C3H.SW TCD BM alone. These recipients were treated with anti-OPN Ab or control IgG (200 μg/mouse) at days 0, 3, 6, 10, and 14 after transplantation. (A) Survival and clinical GVHD signs were monitored over time after transplantation. Symbols are as follows: (▴), TCD BM alone; (■), TCD BM plus CD8+ T cells plus control IgG treatment; and (○), TCD BM plus CD8+ T cells plus anti-OPN Ab treatment; n = 12 for each group. (B) These murine livers and intestines were collected at day 35 after the aforementioned transplantation and were sectioned for histologic examination with hematoxylin and eosin and substantially semiquantitative histopathology scoring; original magnification: × 200; n = 6 for each group. Samples were visualized with a Nikon Eclipse 80i microscope equipped with Nikon 20×/0.75 PLAN Apo objective and Nikon DXM1200F digital camera. Nikon ACT-1 software Version 2.63 was used to acquire images and Adobe Photoshop 7.0 software was used to manipulate images. (C) Splenocytes (5 × 107) of DBA/2 mice were injected intravenously into sublethally irradiated (6.5 Gy) BALB/c recipient mice accompanied by DBA/2 TCD BM (1 × 107). These recipients were treated with anti-OPN Ab or control IgG (200 μg/mouse) at days 0, 3, 6, 10, and 14 after transplantation. The recipients were monitored for survival (n = 12 per group) and symptom of proteinuria (n = 6 per group). Symbols are as follows: ( × ), irradiation only; (▴), TCD BM alone; (■), TCD BM plus splenocytes plus control IgG treatment; and (○), TCD BM plus splenocytes plus anti-OPN treatment. Results were representative of 3 independent experiments. *P < .05.
Inhibition of CD8+ T cell-mediated GVHD by anti-OPN Ab. Lethally irradiated B6/SJL recipient mice were injected with C3H.SW CD8+ T cells plus C3H.SW TCD BM or C3H.SW TCD BM alone. These recipients were treated with anti-OPN Ab or control IgG (200 μg/mouse) at days 0, 3, 6, 10, and 14 after transplantation. (A) Survival and clinical GVHD signs were monitored over time after transplantation. Symbols are as follows: (▴), TCD BM alone; (■), TCD BM plus CD8+ T cells plus control IgG treatment; and (○), TCD BM plus CD8+ T cells plus anti-OPN Ab treatment; n = 12 for each group. (B) These murine livers and intestines were collected at day 35 after the aforementioned transplantation and were sectioned for histologic examination with hematoxylin and eosin and substantially semiquantitative histopathology scoring; original magnification: × 200; n = 6 for each group. Samples were visualized with a Nikon Eclipse 80i microscope equipped with Nikon 20×/0.75 PLAN Apo objective and Nikon DXM1200F digital camera. Nikon ACT-1 software Version 2.63 was used to acquire images and Adobe Photoshop 7.0 software was used to manipulate images. (C) Splenocytes (5 × 107) of DBA/2 mice were injected intravenously into sublethally irradiated (6.5 Gy) BALB/c recipient mice accompanied by DBA/2 TCD BM (1 × 107). These recipients were treated with anti-OPN Ab or control IgG (200 μg/mouse) at days 0, 3, 6, 10, and 14 after transplantation. The recipients were monitored for survival (n = 12 per group) and symptom of proteinuria (n = 6 per group). Symbols are as follows: ( × ), irradiation only; (▴), TCD BM alone; (■), TCD BM plus splenocytes plus control IgG treatment; and (○), TCD BM plus splenocytes plus anti-OPN treatment. Results were representative of 3 independent experiments. *P < .05.
OPN facilitates the tissue dominance of donor-derived CD8+ T cells by promoting cell migration
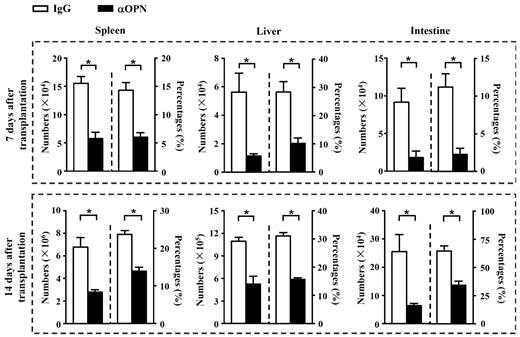
We next investigated the mechanisms by which OPN accelerated GVHD. Donor-derived CD8+ T cells were recovered at day 7 and day 14 after transplantation from B6/SJL mice receiving C3H.SW CD8+ T cells plus TCD BM. Flow cytometry analysis showed that anti-OPN Ab treatment significantly decreased the absolute numbers and percentages of donor-derived CD8+ T (CD45.2+CD8+) cells in spleen, liver, and intestine of B6/SJL recipients (Figure 3, P < .05). These results suggest that OPN was important for accumulation of alloreactive T cells in host organs during GVHD, and anti-OPN treatment was effective in reducing this dominance.
Anti-OPN treatment reduces persistence of donor T cells in recipient tissues. At day 7 and 14 after transplantation, splenic, hepatic, and intestinal intraepithelial lymphocytes were isolated from GVHD recipient mice treated with anti-OPN Ab or control IgG. Absolute numbers and percentages of donor-derived CD8+ T cells (CD45.2+CD8+) in these tissues were determined. Data are mean ± SEM of 6 mice for each group. These results were representative of 3 independent experiments. *P < .05.
Anti-OPN treatment reduces persistence of donor T cells in recipient tissues. At day 7 and 14 after transplantation, splenic, hepatic, and intestinal intraepithelial lymphocytes were isolated from GVHD recipient mice treated with anti-OPN Ab or control IgG. Absolute numbers and percentages of donor-derived CD8+ T cells (CD45.2+CD8+) in these tissues were determined. Data are mean ± SEM of 6 mice for each group. These results were representative of 3 independent experiments. *P < .05.
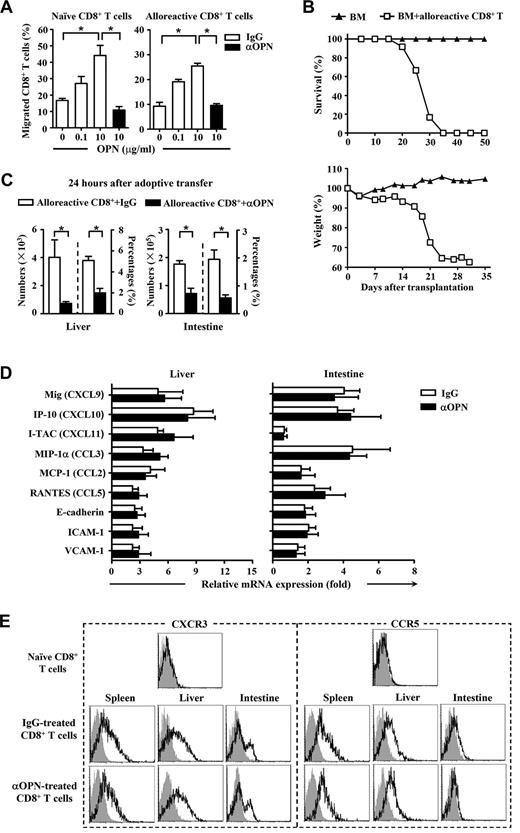
Because the ability of donor-derived T cells to migrate was critical for their accumulation and infiltration in recipient tissues, we tested the effect of OPN on cell motility. Chemotaxis assay demonstrated that OPN was able to trigger migration of both naive and alloreactive CD8+ T cells, and anti-OPN Ab was able to block the migration induced by OPN (Figure 4A). We next performed alloreactive donor T cell–adoptive transfer to confirm the promigratory role of OPN in vivo. Unlike naive donor T cells, activated donor T cells can rapidly transmigrate to the recipient organs without activation and amplification in lymphoid tissue and substantially cause severe GVHD in the secondary recipients (Figure 4B). Interestingly, anti-OPN treatment also reduced the absolute numbers and percentages of donor-derived CD8+ T cells in liver and intestine of the secondary recipients (Figure 4C), indicating a promigratory effect of OPN on naive and activated donor T cells in GVHD pathogenesis. However, it is also possible that the ability of OPN to promote cell migration is dependent on other chemotactic molecules. To test this possibility, changes in levels of chemokines, chemokine receptors, and adhesive molecules involved in GVHD pathogenesis were examined. Unexpectedly, there were no significant differences between control and anti-OPN treatment in the expression of critical chemokines and adhesion molecules in recipient livers and intestines (Figure 4D). Administration of anti-OPN Ab did not affect the expression of CCR5 and CXCR3 on donor-derived CD8+ T cells recovered from recipient spleen, liver, or intestine (Figure 4E). As CCR5 and CXCR3 are the most important chemokine receptors participating in GVHD pathogenesis,11,27,32-34 these results imply that the chemotactic role of OPN on donor T cells is independent. Taken together, OPN is chemotactic for both naive and alloreactive donor T cells during GVHD, and anti-OPN Ab can block this chemotactic migration without influencing other chemokines, chemokine receptors, or adhesion molecules.
Effect of OPN on migration of donor-derived CD8+ T cells. C3H.SW CD8+ T cells (CD45.2+CD8+) were injected into lethally irradiated B6/SJL recipient mice (CD45.1+) accompanied by C3H.SW TCD BM (CD45.2+) to induce GVHD. The alloreactive donor-derived CD8+ T cells (CD45.2+CD8+) were recovered from the spleens and lymph nodes of the GVHD recipients at day 14 after transplantation. (A) Mobility of naive donor CD8+ T cells and alloreactive donor CD8+ T cells in response to OPN protein were analyzed in the absence or presence of anti-OPN Ab. Percentages of migrated CD8+ T cells were determined. (B) The alloreactive donor CD8+ T cells (2 × 106) were adoptively transferred into lethally irradiated secondary B6/SJL recipient mice accompanied by C3H.SW TCD BM (5 × 106). Survival and weight loss of the secondary B6/SJL recipients were monitored (n = 12). Symbols are as follows: (▴), TCD BM alone; and (□), TCD BM plus alloreactive CD8+ T cells. (C) The adoptive transferred mice were treated with anti-OPN Ab or control IgG (200 μg/mouse) and were killed 24 hours after adoptive transfer. Absolute numbers and percentages of donor-derived CD8+ T cells (CD45.2+CD8+) in their livers and intestines were determined. (D) mRNAs of chemokines and adhesion molecules in livers and intestines of GVHD recipients treated with anti-OPN Ab or control IgG were measured by quantitative real-time PCR at day 7 after transplantation. Results were normalized to the gene expression in naive B6/SJL mice. (A,C-D) Data are mean ± SEM of 6 mice for each group, respectively. (E) At day 14 after transplantation, donor-derived CD8+ T cells (CD45.2+CD8+) were recovered from GVHD recipients treated with anti-OPN Ab or control IgG, and the levels of CXCR3 and CCR5 molecules on these T cells were measured with flow cytometry. Gray shadow and solid line indicated the immunofluorescence intensity of cells as a control and the test of Abs, respectively. The results are representative of 3 independent experiments. *P < .05.
Effect of OPN on migration of donor-derived CD8+ T cells. C3H.SW CD8+ T cells (CD45.2+CD8+) were injected into lethally irradiated B6/SJL recipient mice (CD45.1+) accompanied by C3H.SW TCD BM (CD45.2+) to induce GVHD. The alloreactive donor-derived CD8+ T cells (CD45.2+CD8+) were recovered from the spleens and lymph nodes of the GVHD recipients at day 14 after transplantation. (A) Mobility of naive donor CD8+ T cells and alloreactive donor CD8+ T cells in response to OPN protein were analyzed in the absence or presence of anti-OPN Ab. Percentages of migrated CD8+ T cells were determined. (B) The alloreactive donor CD8+ T cells (2 × 106) were adoptively transferred into lethally irradiated secondary B6/SJL recipient mice accompanied by C3H.SW TCD BM (5 × 106). Survival and weight loss of the secondary B6/SJL recipients were monitored (n = 12). Symbols are as follows: (▴), TCD BM alone; and (□), TCD BM plus alloreactive CD8+ T cells. (C) The adoptive transferred mice were treated with anti-OPN Ab or control IgG (200 μg/mouse) and were killed 24 hours after adoptive transfer. Absolute numbers and percentages of donor-derived CD8+ T cells (CD45.2+CD8+) in their livers and intestines were determined. (D) mRNAs of chemokines and adhesion molecules in livers and intestines of GVHD recipients treated with anti-OPN Ab or control IgG were measured by quantitative real-time PCR at day 7 after transplantation. Results were normalized to the gene expression in naive B6/SJL mice. (A,C-D) Data are mean ± SEM of 6 mice for each group, respectively. (E) At day 14 after transplantation, donor-derived CD8+ T cells (CD45.2+CD8+) were recovered from GVHD recipients treated with anti-OPN Ab or control IgG, and the levels of CXCR3 and CCR5 molecules on these T cells were measured with flow cytometry. Gray shadow and solid line indicated the immunofluorescence intensity of cells as a control and the test of Abs, respectively. The results are representative of 3 independent experiments. *P < .05.
OPN promotes viability of donor-derived CD8+ T cells during GVHD
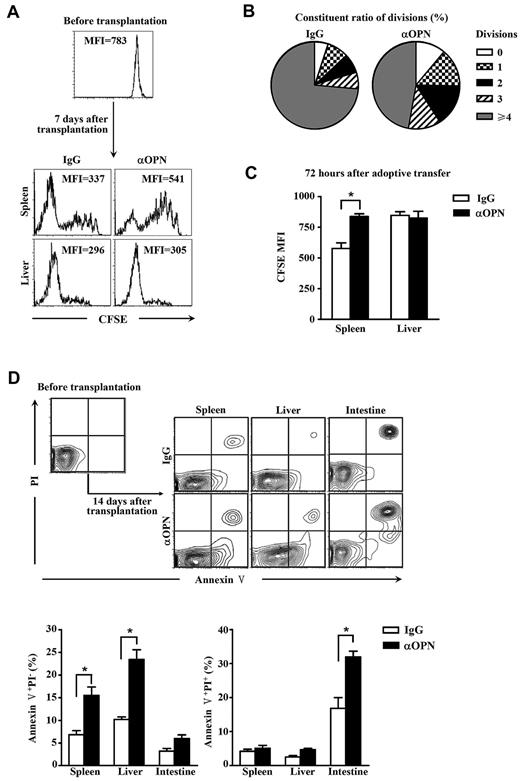
It is also possible that a change of lymphocyte persistence in tissues is associated with impaired cell proliferation and survival in organs. To test these possibilities, proliferation of alloantigen-activated T cells in host tissues was examined first. CFSE-labeled naive C3H.SW CD8+ T cells were transplanted into lethally irradiated allogeneic B6/SJL recipients treated with anti-OPN Ab and control IgG, and fluorescence intensity of CFSE on donor-derived CD8+ T cells recovered from recipients was measured. In spleen, Ab-treated donor-derived CD8+ T cells showed delayed division compared with those treated with control IgG (Figure 5A-B; P < .05). However, in liver, Ab-treated donor-derived CD8+ T cells showed low CFSE fluorescence intensity as well as those treated with control IgG (Figure 5A). It was known that, on infusion into allogeneic recipients, donor T cells are initially activated in the lymphoid organs, such as spleen to divide, which can be tracked by the dilution of CFSE. These donor T cells undergo expansion, acquire effector functions, and eventually egress from lymphoid tissue to target organs, such as the liver and intestine.11,35 Most of these donor T cells infiltrating in the nonlymphoid tissues may represent fully differentiated effector T cells that have undergone significantly more rounds of cell division than those donor T cells residing in the spleen and therefore lose CFSE. This may explain why all donor-derived T cells recovered from liver showed low fluorescence intensity of CFSE in Figure 5A. Similar results were observed in the model of alloreactive donor-derived CD8+ T cell-adoptive transfer (Figure 5C), suggesting that OPN was important for the expansion of both naive and activated T cells in lymphoid tissue. In addition, we examined the effects of anti-OPN Ab on the survival of donor T cells. Ab treatment resulted in more apoptotic death of donor-derived CD8+ T cells in spleen, liver, and intestine of recipients (Figure 5D), suggesting that OPN is required for donor T-cell survival in both lymphoid and target tissues. These results indicate that OPN can enhance the persistence of donor T cells in recipient tissues by promoting cell viability throughout the course of GVHD.
Anti-OPN Ab reduces donor T-cell survival. C3H.SW CD8+ T cells (CD45.2+CD8+) were labeled with CFSE and injected into lethally irradiated recipient mice accompanied by donor TCD BM. These recipients were treated with anti-OPN Ab or control IgG (200 μg/mouse) at days 0, 3, and 6 after transplantation. The donor-derived CD8+ T cells were recovered from liver and spleen of the recipients at day 7 after transplantation. Their CFSE dilution was analyzed by flow cytometry. Data were represented as CFSE histograms (A) and as the percentages of the cells from spleen with given number of divisions (B). P < .05. Alloreactive donor-derived CD8+ T cells were recovered from GVHD recipients at day 14 after transplantation, labeled with CFSE, and adoptively transferred into secondary recipients treated with anti-OPN Ab or control IgG. These cells were then recovered from secondary recipients 72 hours after adoptive transplantation, and fluorescence intensities of CFSE were analyzed (C). Donor-derived CD8+ T cells were recovered at day 14 after transplantation from GVHD recipients treated with anti-OPN Ab or control IgG. Apoptosis of those cells were observed by staining with annexin V and propidium iodide (PI) (D). MFI indicates mean fluorescence intensity. Results were representative of 5 mice for each group of 3 independent experiments. *P < .05.
Anti-OPN Ab reduces donor T-cell survival. C3H.SW CD8+ T cells (CD45.2+CD8+) were labeled with CFSE and injected into lethally irradiated recipient mice accompanied by donor TCD BM. These recipients were treated with anti-OPN Ab or control IgG (200 μg/mouse) at days 0, 3, and 6 after transplantation. The donor-derived CD8+ T cells were recovered from liver and spleen of the recipients at day 7 after transplantation. Their CFSE dilution was analyzed by flow cytometry. Data were represented as CFSE histograms (A) and as the percentages of the cells from spleen with given number of divisions (B). P < .05. Alloreactive donor-derived CD8+ T cells were recovered from GVHD recipients at day 14 after transplantation, labeled with CFSE, and adoptively transferred into secondary recipients treated with anti-OPN Ab or control IgG. These cells were then recovered from secondary recipients 72 hours after adoptive transplantation, and fluorescence intensities of CFSE were analyzed (C). Donor-derived CD8+ T cells were recovered at day 14 after transplantation from GVHD recipients treated with anti-OPN Ab or control IgG. Apoptosis of those cells were observed by staining with annexin V and propidium iodide (PI) (D). MFI indicates mean fluorescence intensity. Results were representative of 5 mice for each group of 3 independent experiments. *P < .05.
OPN synergizes with TCR/CD3 signal to activate CD8+ T cells
We next investigated how OPN promoted the viability of donor T cells during GVHD. Reduced proliferation and enhanced apoptosis observed in CD8+ T cells on anti-OPN Ab treatment suggested a possible role of OPN in T-cell activation in response to allogeneic antigens during GVHD. Compared with control IgG treatment, donor-derived CD8+ T cells from GVHD recipients treated with anti-OPN Ab showed decreased expression of CD69, CD25, and CD44 and increased expression of CD62L (Figure 6A). This indicates that OPN facilitates activation of donor T cells in response to alloantigen in vivo.
Effects of OPN on T-cell activation. C3H.SW CD8+ T cells (CD45.2+CD8+) were injected into lethally irradiated B6/SJL recipient mice accompanied by C3H.SW TCD BM. These recipients were treated with anti-OPN Ab or control IgG at days 0, 3, and 6 after transplantation. Donor-derived CD8+ T cells were recovered from spleen of the recipients at day 7 after transplantation, and their levels of CD69, CD25, CD44, and CD62L molecules were measured by flow cytometry (A). In ex vivo experiments, C3H.SW CD8+ T cells were stimulated with the indicated reagents. Dose as follows: plate-coated anti-CD3 Ab (10 μg/mL) or soluble anti-CD3 Ab (1 μg/mL), anti-CD28 Ab (2 μg/mL), OPN (10 μg/mL), LY294002 (LY, 20μM). Twenty-four hours after stimulation, expression of the CD69 molecule was measured by flow cytometry (B). Gray shadow and solid line indicate the immunofluorescence intensity of cells as a control and the test of Ab, respectively, and the levels of IL-2 and Bcl-xL were measured by quantitative real-time PCR (E). Results were normalized to the expression in naive CD8+ T cells and expressed as mean plus or minus SEM of 3 cultures. Seventy-two hours after the aforementioned stimulation, T-cell proliferation under the indicated treatments was measured by incorporation of [3H]thymidine (C). Data are mean plus or minus SEM of 4 cultures. Levels of cyclin A, p-Rb, and p27kip1 in activated CD8+ T cells 24 hours after stimulation were determined by Western blot (D). Levels of p-Akt in CD8+ T cells at the indicated time points after stimulation were tested by Western blot (F). Quantification was done by densitometry of Western blot bands. Densities were normalized to β-actin, and relative folds were normalized to expression levels in CD8+ T cells with control treatment. These results are representative of 3 independent experiments. *P < .05.
Effects of OPN on T-cell activation. C3H.SW CD8+ T cells (CD45.2+CD8+) were injected into lethally irradiated B6/SJL recipient mice accompanied by C3H.SW TCD BM. These recipients were treated with anti-OPN Ab or control IgG at days 0, 3, and 6 after transplantation. Donor-derived CD8+ T cells were recovered from spleen of the recipients at day 7 after transplantation, and their levels of CD69, CD25, CD44, and CD62L molecules were measured by flow cytometry (A). In ex vivo experiments, C3H.SW CD8+ T cells were stimulated with the indicated reagents. Dose as follows: plate-coated anti-CD3 Ab (10 μg/mL) or soluble anti-CD3 Ab (1 μg/mL), anti-CD28 Ab (2 μg/mL), OPN (10 μg/mL), LY294002 (LY, 20μM). Twenty-four hours after stimulation, expression of the CD69 molecule was measured by flow cytometry (B). Gray shadow and solid line indicate the immunofluorescence intensity of cells as a control and the test of Ab, respectively, and the levels of IL-2 and Bcl-xL were measured by quantitative real-time PCR (E). Results were normalized to the expression in naive CD8+ T cells and expressed as mean plus or minus SEM of 3 cultures. Seventy-two hours after the aforementioned stimulation, T-cell proliferation under the indicated treatments was measured by incorporation of [3H]thymidine (C). Data are mean plus or minus SEM of 4 cultures. Levels of cyclin A, p-Rb, and p27kip1 in activated CD8+ T cells 24 hours after stimulation were determined by Western blot (D). Levels of p-Akt in CD8+ T cells at the indicated time points after stimulation were tested by Western blot (F). Quantification was done by densitometry of Western blot bands. Densities were normalized to β-actin, and relative folds were normalized to expression levels in CD8+ T cells with control treatment. These results are representative of 3 independent experiments. *P < .05.
To explore the mechanisms, ex vivo stimulation experiments were performed. Unexpectedly, neither OPN alone nor anti-CD3 Ab alone could efficiently activate CD8+ T cells. By contrast, combined treatment with anti-CD3 Ab and OPN showed much higher potential to activate CD8+ T cells sufficiently (Figure 6B), indicating a synergetic role of OPN in T-cell activation primed by TCR/CD3 signal. Cooperation of OPN and anti-CD3 Ab was further demonstrated to promote cell proliferation by facilitating the cell cycle (Figure 6C-D). OPN plus anti-CD3 Ab elevated both cyclin A, which was required for S phase progression, and the phosphorylation of rentinoblastoma protein (Rb), which was required for the G1-S transition. In addition, OPN reduced the level of the cyclin-dependent kinase inhibitor p27kip1. The facilitation of T-cell activation and proliferation supported by OPN may explain why anti-OPN Ab hampered the division of donor T cells in the spleen as mentioned. As well, treatment with OPN plus anti-CD3 Ab also elevated mRNA levels of IL-2 and Bcl-xL (Figure 6E), the most important downstream target genes of costimulatory signal transduction that confer resistance to apoptosis. This suggests that OPN may provide prosurvival signals similar to the costimulation signaling in donor CD8+ T cells and protect donor CD8+ T cells from apoptosis as mentioned.
Previous studies showed that OPN could activate the intracellular phosphoinositide 3-kinase (PI3K)/Akt signal pathway.36 Here, we also found that OPN plus anti-CD3 Ab together resulted in robust phosphorylation of Akt in CD8+ T cells (Figure 6F). Treatment with the PI3K inhibitor LY294002 hampered the synergetic effect on cell activation, proliferation, and expression of IL-2 and Bcl-xL (Figure 6B-C,E). These observations suggest that PI3K/Akt signaling is important for the synergistic effect of OPN. Together, these data suggest that OPN promotes activation and persistence of donor T cells during GVHD by synergizing with TCR/CD3 signaling.
Anti-OPN treatment retains GVL effect
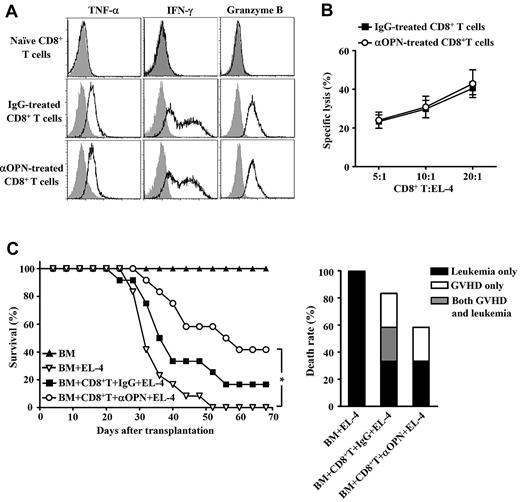
Finally, we examined whether anti-OPN treatment influenced CD8+ T cell-mediated GVL effect. Donor-derived CD8+ T cells were recovered from the spleen of recipients treated with anti-OPN Ab. These cells showed robust potency to produce interferon-γ, tumor necrosis factor-α, and granzyme B (Figure 7A), indicating that they maintained cytotoxic capability. Cytolytic assay also demonstrated that administration of anti-OPN Ab did not affect the frequency of cytolytic T cells against EL-4 leukemic cells (Figure 7B). Moreover, anti-OPN treatment did not reduce survival of B6/SJL mice that were both challenged with EL-4 cells and received donor CD8+ T cells plus TCD BM, compared with control IgG treatment (Figure 7C). Analysis of the cause of death showed that Ab treatment balanced the GVHD inhibition and leukemia prevention (Figure 7C). Thus, anti-OPN treatment can prevent GVHD while retaining CD8+ T cell–mediated GVL effect. This suggests significant therapeutic potential of targeting OPN in clinical GVHD prevention.
Maintenance of GVL on anti-OPN treatment. Lethally irradiated B6/SJL recipient mice were injected with C3H.SW CD8+ T cells plus C3H.SW TCD BM. These recipients were treated with anti-OPN Ab or control IgG at days 0, 3, 6, 10, and 14 after transplantation. (A) Splenic donor-derived CD8+ T cells were recovered from anti-OPN Ab or control IgG treated recipients at day 14 after transplantation, and the levels of inflammatory cytokine production were assessed by intracellular staining and flow cytometry. Gray shadow and solid line indicate the immunofluorescence intensity of cells as a control and the test of Abs, respectively. (B) Abilities of the recovered CD8+ T cells to lyse EL-4 cells were examined. Data of the percentages of specific lysis are mean plus or minus SEM of 6 mice per group. (C) The recipient mice were in vivo challenged with EL-4 cells at day 7 after transplantation. Survival and causes of death were monitored (n = 12 per group). Symbols are as follows: (▴), TCD BM alone; (▿), TCD BM plus EL-4 cells; (■), TCD BM plus CD8+ T cells plus EL-4 cells plus control IgG treatment; and (○), TCD BM plus CD8+ T cells plus EL-4 cells plus anti-OPN Ab treatment. These results were representative of 3 independent experiments. *P < .05.
Maintenance of GVL on anti-OPN treatment. Lethally irradiated B6/SJL recipient mice were injected with C3H.SW CD8+ T cells plus C3H.SW TCD BM. These recipients were treated with anti-OPN Ab or control IgG at days 0, 3, 6, 10, and 14 after transplantation. (A) Splenic donor-derived CD8+ T cells were recovered from anti-OPN Ab or control IgG treated recipients at day 14 after transplantation, and the levels of inflammatory cytokine production were assessed by intracellular staining and flow cytometry. Gray shadow and solid line indicate the immunofluorescence intensity of cells as a control and the test of Abs, respectively. (B) Abilities of the recovered CD8+ T cells to lyse EL-4 cells were examined. Data of the percentages of specific lysis are mean plus or minus SEM of 6 mice per group. (C) The recipient mice were in vivo challenged with EL-4 cells at day 7 after transplantation. Survival and causes of death were monitored (n = 12 per group). Symbols are as follows: (▴), TCD BM alone; (▿), TCD BM plus EL-4 cells; (■), TCD BM plus CD8+ T cells plus EL-4 cells plus control IgG treatment; and (○), TCD BM plus CD8+ T cells plus EL-4 cells plus anti-OPN Ab treatment. These results were representative of 3 independent experiments. *P < .05.
Discussion
It is imperative to identify the molecules involved in the pathogenesis of GVHD, both for use as markers in monitoring disease outcome and as targets for future therapies. Our studies demonstrate, for the first time, that OPN promotes GVHD. We find that anti-OPN treatment can ameliorate miHA-mismatched GVHD with decreased persistence of donor-derived CD8+ T cells in host target tissues. The tissue residence of T cells is widely and closely associated with many events in T-cell life cycle, including proliferation, migration, and apoptosis. Our work shows that OPN has extensive effects on promoting malgenic T-cell activation, migration, and survival. The promigratory effect of OPN is independent of other chemokines and chemokine receptors. By contrast, its ability to facilitate activation and survival could result from synergism with TCR/CD3 signaling, implying that OPN is required for GVHD initiation and persistent tissue damage. Finally, anti-OPN treatment maintains a CD8+ T cell-mediated GVL effect, suggesting an application for prevention of clinical GVHD.
The development of GVHD in HSCT involves at least 3 phases5-9,11 : (1) afferent phase: conditioning (irradiation or chemotherapy) induced initial host cell damage and inflammatory cytokine cascade; (2) induction and expansion phase: host antigen-presenting cell-dependent donor T-cell activation, proliferation, and differentiation; and (3) effector phase: recruitment of cytolytic T cells to target tissue and subsequently the host tissue damage by cytolytic effector mechanisms. Our results show that OPN, induced by irradiation conditioning, participates in each phase of GVHD development, providing multilevel regulation and control in the pathogenesis.
OPN is produced by various cell types, including epithelial tissues, smooth muscle cells, osteoblasts, immune cells, and tumor cells.17,37 However, the regulation of OPN expression is incompletely understood. To our knowledge at present, expression of OPN can be enhanced by toxicants and certain proinflammatory cytokines in almost every injured organ.38-42 Here we found that, after irradiation, serum OPN was elevated, which might be produced by osteoblasts and epithelial cells on radiation stress. However, participation of CD8+ T cells significantly increases and maintains serum OPN level long after irradiation. This suggests that activity of CD8+ T cells may provide a positive feedback mechanism for OPN expression, with the possibility that activated CD8+ T cells can produce OPN themselves or indirectly elevate OPN expression by producing other proinflammatory cytokines.
Previous studies have already demonstrated numerous functions of OPN in multiple biologic processes. OPN generally functions as both an extracellular matrix component and a cytokine signaling through binding cell surface integrins and CD44, contributing to cell motility.14 Our studies provide a direct evidence to confirm that OPN participates in recruitment of CD8+ T cells in the inflammatory environment. Leukocyte migration and infiltration directed by chemokines and their cognate chemokine receptors in inflammation are well understood in various pathologic processes. In GVHD, chemokine receptors CCR5 and CXCR3 and their chemokine ligands have been characterized as the most critical molecules mediating activated donor T-cell trafficking into GVHD target organs.11,27,32-34 However, the relationships between OPN and those chemotactic molecules are obscure. Previous studies showed that host inflammatory environment could regulate the expression of chemokine receptors on donor-derived T cells and subsequently contribute to the T-cell migration and tissue inflammation,27 and OPN could also induce expression of chemokines MCP-1 and MIP-1β in rheumatoid arthritis.43 We thus examined whether OPN regulated migration by affecting expression of those chemotactic molecules during GVHD. However, we have not observed any changes in the expression of important chemokines and adhesion molecules in recipients or critical chemokine receptors on donor-derived T cells. These results imply that, in GVHD, OPN can facilitate recruitment of donor T cells directly and independently.
Besides cell motility, OPN promotes cell survival, especially during cancer progression. OPN contributes to malignancies through both inhibition of apoptosis and activation of various matrix-degrading proteases, leading to tumor growth and metastasis.14,24 In signaling of cell survival, the PI3K/Akt pathway is a central regulator.14 The interaction of OPN with CD44 can induce the activation of phospholipase C-γ/PKC/PI3K pathways, promoting cell survival, and binding of OPN to αV integrin can also induce PI3K/Akt-dependent nuclear factor-κB activation in cancer cells.14 PI3K signaling is one of the most important pathways participating in immune responses. PI3K can be activated by the costimulatory signaling from CD28 or other costimulatory receptors. Costimulatory signaling is essential for the sufficient activation of T cells, and the absence of costimulatory signaling results in anergy and apoptosis. Although OPN had been found associated with T-cell activation and named as Eta-1 (early T lymphocyte activation 1) previously,15,16,37 the mechanism remains elusive. We found that OPN alone had limited capability to induce cell activation, but OPN could supply costimulatory-like signals to TCR/CD3 stimulation to facilitate sufficient T-cell activation, cell cycle progression, and proliferation. Notably, combined OPN and anti-CD3 stimulation augmented not only the production of IL-2, which acted as an extrinsic survival factor for T cells, but also the expression of Bcl-xL, which was a member of Bcl-2 protein family and provides intrinsic ability of T cells to resist apoptosis. In addition, p-Rb, which was also elevated by the synergism, was also able to protect cells from apoptosis.44 These results are consistent with the observations that anti-OPN treatment increases the apoptosis in donor-derived T cells in vivo. Thus, these data not only confirm the activation and prosurvival effects of OPN in allogeneic transplantation model but also elucidate the mechanisms.
Besides motility and survival, OPN also regulates cell polarization. Especially, OPN is shown to influence IL-17 production and Th17 response not only in the extracellular space as a secretary protein but also in cytosol as a signaling component.45-48 These are important in the pathogenesis of inflammatory diseases, including rheumatoid arthritis and multiple sclerosis.47,48 However, the relationship between IL-17/Th17 and GVHD is still elusive. Both in vitro-differentiated Th17 cells and augmented Th17 differentiation in vivo exacerbated tissue damage during GVHD,49 but the absence of IL-17 resulted in augmented GVHD with up-regulated Th1 differentiation and infiltration.13 Here, we did not detect any IL-17 in serum or tissues in our GVHD model (C3H.SW→B6/SJL), suggesting that IL-17 may not be involved in this particular model, but the matter could be different in other GVHD models. Therefore, whether OPN impacts GVHD through IL-17/Th17 pathway needs further investigation.
Our research raises important questions about how inflammatory host environments impact the behavior or functions of donor-derived lymphocytes. We and others have found that host antigen-presenting cells are essential to initiate CD8+ T cell–mediated GVHD, and host dendritic cells and IL-2 are responsible for the dynamic expression of chemokine receptor on alloreactive CD8+ T cells during GVHD, which contributes to the T-cell migration and subsequent tissue inflammation.7,8,27 Here, we identify OPN as a multipotent proinflammatory cytokine and a critical accelerator of GVHD via its regulatory effects on donor-derived pathogenic T cells. We also identify OPN as a new target for GVHD therapy. Neutralizing anti-OPN Ab has been applied in treatments of experimental inflammatory diseases.28,50 In our studies, anti-OPN Ab showed promise for both inhibiting GVHD and maintaining GVL, which can facilitate immune reconstitution and prevent leukemia relapse after HSCT. Two reasons may account for the maintenance of the GVL effect in anti-OPN–treated BM transplant recipients. First, although anti-OPN treatment suppressed activation of naive CD8+ T cells, it did not hamper the cytolytic function of allo-activated CD8+ T cells. Second, OPN also plays a critical role in tumor progression through survival promotion and metastasis facilitation.14 Therefore, blockade of OPN in vivo should be inhibitive for tumor progression. Thus, optimizing the treatment by targeting OPN will benefit strategies for GVHD prevention.
In conclusion, using miHA-mismatched allogeneic HSCT mouse model, we demonstrate that OPN is critical for initiating and maintaining of CD8+ T cell-mediated GVHD. The mechanisms involve promoting donor T-cell activation in lymphoid tissue, triggering donor T-cell migration toward target tissues, and protecting pathogenic T cells from apoptosis in both lymphoid and peripheral tissues. Finally, anti-OPN provides a new approach to balance GVHD inhibition and leukemia prevention.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs Qiang Liu and Min Yao (Renji Hospital, Shanghai, China) for histopathologic assessment, Dr Hongmei Li (Soochow University, Suzhou, China) for statistical analysis, and Drs Yi Zhang (University of Michigan) and Gary Brewer (University of Medicine and Dentistry of New Jersey) for critical review of the manuscript.
This work was supported by the Ministry of Science and Technology of China (2011CB966200, 2010CB945600), Knowledge Innovation Project of the Chinese Academy of Sciences (KSCX1-YW-22), Innovation Fund of New Drug (2009ZX09503–024), National Natural Science Foundation of China (30670911, 30901317), and Leading Academic Discipline Project of Shanghai Municipal Education Commission (J50207).
Authorship
Contribution: F.Z. designed and performed research, analyzed data, and wrote the manuscript; Yi Zhang performed research, contributed ideas, and helped write the manuscript; H.W. prepared anti-OPN Ab; M.J. and S.H. performed research; Y.S. provided guidance and revised the manuscript; Y.G. prepared anti-OPN Ab and provided overall guidance; and Yanyun Zhang designed experiments, provided overall guidance, and helped write the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yanyun Zhang, Key Laboratory of Stem Cell Biology, Institute of Health Sciences, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences and Shanghai Jiao Tong University School of Medicine, and Shanghai Institute of Immunology, Institutes of Medical Sciences, Shanghai Jiao Tong University School of Medicine, 225 South Chongqing Road, Shanghai 200025, China; e-mail: yyzhang@sibs.ac.cn; and Yajun Guo, International Joint Cancer Institute, Second Military Medical University, 800 Xiangyin Road, Shanghai 200433, China; e-mail: yjguo@smmu.edu.cn.
References
Author notes
Y.G. and Yanyun Zhang contributed equally to this study.


![Figure 6. Effects of OPN on T-cell activation. C3H.SW CD8+ T cells (CD45.2+CD8+) were injected into lethally irradiated B6/SJL recipient mice accompanied by C3H.SW TCD BM. These recipients were treated with anti-OPN Ab or control IgG at days 0, 3, and 6 after transplantation. Donor-derived CD8+ T cells were recovered from spleen of the recipients at day 7 after transplantation, and their levels of CD69, CD25, CD44, and CD62L molecules were measured by flow cytometry (A). In ex vivo experiments, C3H.SW CD8+ T cells were stimulated with the indicated reagents. Dose as follows: plate-coated anti-CD3 Ab (10 μg/mL) or soluble anti-CD3 Ab (1 μg/mL), anti-CD28 Ab (2 μg/mL), OPN (10 μg/mL), LY294002 (LY, 20μM). Twenty-four hours after stimulation, expression of the CD69 molecule was measured by flow cytometry (B). Gray shadow and solid line indicate the immunofluorescence intensity of cells as a control and the test of Ab, respectively, and the levels of IL-2 and Bcl-xL were measured by quantitative real-time PCR (E). Results were normalized to the expression in naive CD8+ T cells and expressed as mean plus or minus SEM of 3 cultures. Seventy-two hours after the aforementioned stimulation, T-cell proliferation under the indicated treatments was measured by incorporation of [3H]thymidine (C). Data are mean plus or minus SEM of 4 cultures. Levels of cyclin A, p-Rb, and p27kip1 in activated CD8+ T cells 24 hours after stimulation were determined by Western blot (D). Levels of p-Akt in CD8+ T cells at the indicated time points after stimulation were tested by Western blot (F). Quantification was done by densitometry of Western blot bands. Densities were normalized to β-actin, and relative folds were normalized to expression levels in CD8+ T cells with control treatment. These results are representative of 3 independent experiments. *P < .05.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/5/10.1182_blood-2010-04-281659/4/m_zh89991065790006.jpeg?Expires=1770161360&Signature=oYQN3POtsOgqtUa7wHSM4u90J--oTwRpELjFINPzF8Ni0~4lT1avysvAct9SmSMJXg6ruIpF-eRDQqA-EW3ydRa5WxQ7U0Tm-creC6IEbMSrjWhbkpNvpHQOOQJd16qxl3b6Jbm7F79xKem8yLtv1U28ZUhdddReTjykUNx2PxMMSeHkArxEloblay3KcLxucqnuzOGD53OGDT60pzSSijM6nxC~THrO3pT2FhYf9FFwILoqD1gL5Zr6pgHE~qDYCu81xvjhGYb-YItHiJVIiihSlqVKhUkS2Z75nf1fUeb3Ju0YTiMvG774yNV8CpegvkklcogemsIvc-vyTHJHyQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal