Abstract
We describe outcomes after human leukocyte antigen-matched sibling bone marrow transplantation (BMT) for 179 patients with β-thalassemia major. The median age at transplantation was 7 years and the median follow-up was 6 years. The distribution of Pesaro risk class I, II, and III categories was 2%, 42%, and 36%, respectively. The day 30 cumulative incidence of neutrophil recovery and day 100 platelet recovery were 90% and 86%, respectively. Seventeen patients had graft failure, which was fatal in 11. Six of 9 patients with graft failure are alive after a second transplantation. The day 100 probability of acute graft-versus-host disease and 5-year probability of chronic graft-versus-host disease was 38% and 13%, respectively. The 5-year probabilities of overall- and disease-free survival were 91% and 88%, respectively, for patients with Pesaro risk class II, and 64% and 62%, respectively, for Pesaro risk class III. In multivariate analysis, mortality risks were higher in patients 7 years of age and older and those with hepatomegaly before BMT. The leading causes of death were interstitial pneumonitis (n = 7), hemorrhage (n = 8), and veno-occlusive disease (n = 6). Proceeding to BMT in children younger than 7 years before development of end-organ damage, particularly in the liver, should improve results after BMT for β-thalassemia major.
Introduction
β-Thalassemia major, a genetic defect that causes reduced or absent β-globin synthesis, results in an imbalanced accumulation of α-globin chains and ineffective erythropoiesis with hemolysis.1 The hallmarks of treatment include regular red blood cell (RBC) transfusions with iron chelation therapy for transfusion-related iron overload and supportive care to treat the complications of iron overload.2,3 However, the only curative therapy is replacement of the defective gene by hematopoietic cell transplantation.4-6
Over the past 2 decades, transplantation as a therapeutic option for thalassemia has undergone considerable investigation and refinement. The largest experience occurred in Pesaro, Italy where a standard pretransplantation risk assessment was developed. It divides patients into 3 risk classes based on liver size by physical examination, the presence or absence of fibrosis by liver biopsy, and adherence to regular iron chelation.4-6 In the initial reports, outcomes after transplantation were affected significantly by the pretransplantation risk status. More recently, modifications to the conditioning regimen have been used to reduce the risk of transplantation-related complications in high-risk recipients. As a result, outcomes after transplantation have become more similar across risk categories.7-11 The most recent results after human leukocyte antigen (HLA)-matched sibling bone marrow transplantation (BMT) for Pesaro class I or II and class III recipients show thalassemia-free survival probabilities of 87%, 85%, and 80%, respectively. Despite these improvements, pretransplantation transfusion exposures and organ damage from iron overload still appear to impact transplantation outcomes negatively. In the most recent update, the transplantation-related mortality in class I or II and class III patients was 3% and 10%, respectively, and 8% to 12% of pediatric recipients experienced graft rejection after BMT.6
Most patient series outside Italy have observed comparatively worse outcomes after HLA-matched sibling BMT for thalassemia major.12-16 Although the reasons for this disparity are unknown, there have been differences in recipient ethnicity, in the ability to assign a pretransplantation risk status, in how patients were prepared for transplantation, and in graft-versus-host disease (GVHD) prophylaxis, which might account for the disparate outcomes. There is also the possibility of a center effect, which might have contributed to an apparent poorer outcome in the smaller patient series outside Italy. To investigate these possibilities, we conducted a survey of β-thalassemia major transplantations recently in locations other than Italy treated by HLA-matched BMT and reported to the Center for International Blood and Marrow Transplant Research.
Methods
Data source
Data were obtained from the Center for International Blood and Marrow Transplant Research, a voluntary group of more than 450 transplantation centers worldwide that report data on consecutive transplantations to a Statistical Center at the Medical College of Wisconsin. Patients are followed longitudinally, with yearly follow-up. Computerized checks for errors and on-site audits of participating centers ensure the quality of data. This study included patients (age < 20 years) who had received an HLA-matched sibling BMT for β-thalassemia major between 1995 and 2001 and received myeloablative conditioning (busulfan + cyclophosphamide). During this period, 233 patients met the selection criteria. Thirty-one transplantation centers were contacted for participation/disease-specific information for their patients. Nineteen centers in 12 countries participated (n = 179 patients); 12 centers declined to participate (n = 54 patients). Approximately half of the transplantations were performed in India, 28% in Iran, and 12% in China. The remaining 10% of transplantations occurred in Australia, Belgium, Canada, Denmark, Germany, Israel, South Africa, Spain, and the United States. Information on busulfan administration was available for 111 of 179 patients; all except 2 patients received oral busulfan. In 43% of the cases, the conditioning regimen included antithymocyte globulin (ATG), and 85% of patients received cyclosporine and methotrexate for GVHD prophylaxis. Among survivors, the median follow-up was 73 months (range, 6-127 months). This study was approved by the Institutional Review Board, Medical College of Wisconsin.
Endpoints
Primary endpoints were acute GVHD (grade II-IV), chronic GVHD, and overall survival (OS). Other endpoints studied include hematopoietic recovery and disease-free survival (DFS). Neutrophil recovery was defined as first of 3 consecutive days with absolute neutrophil count more than or equal to 0.5 × 109/L and platelets, more than or equal to 20 × 109/L, unsupported for 7 days. Acute grade II-IV GVHD and chronic GVHD were graded using standard criteria.17,18 DFS was defined as survival without graft failure, a second transplantation, or death. For analyses of OS, failure was defined as death from any cause; surviving patients were censored at the date of last contact.
Statistical analyses
Univariate probabilities of OS and DFS were calculated by the Kaplan-Meier estimator; the log-rank test was used for univariate comparisons. Probabilities of hematopoietic recovery and GVHD were calculated using the cumulative incidence estimator. For hematopoietic recovery and acute or chronic GVHD, death without the event was the competing risk. Estimates of SE for the survival function were calculated by the Greenwood formula, and 95% confidence intervals (CI) were constructed using arcsine-transformed intervals. Potential patient-, disease-, and treatment-related prognostic factors for GVHD and OS were evaluated in multivariate analyses using Cox proportional hazards regression. Multivariate models were built using a stepwise forward selection with a significance level of .05. In each model, the assumption of proportional hazards was tested for each variable. The variables considered in the multivariate model included patient-related factors (age, sex, performance score, and pre-BMT cytomegalovirus serologic status); disease related factors (Pesaro risk class, liver size [ ≤ 2 cm or > 2 cm or unknown], presence or absence of hepatic fibrosis, serum ferritin [ < 2500 ng/mL, ≥ 2500 ng/mL, or unknown], alanine aminotransferase/aspartate aminotransferase [ < 2 times or ≥ 2 times the upper limit of normal or unknown], number of RBC transfusions [ < 20, 20-50, ≥ 50, or unknown]); and transplantation-related factors (donor-recipient gender match and year of transplantation [1995-1997 or 1998-2001]). All P values are 2-sided. All analyses were done with SAS, Version 9.0.
Results
Patients
The demographic data are summarized in Table 1. The median age at BMT was 7 years, and the median follow-up among survivors was 6 years. The transplantation regimens for conditioning and GVHD prophylaxis were fairly uniform. All patients received busulfan and cyclophosphamide for transplantation conditioning; busulfan (16 mg/kg) with cyclophosphamide (200 mg/kg) was the most frequent combination used. Almost all patients (98%) received cyclosporine-containing GVHD prophylaxis. There were significant transfusion exposures before BMT; in 85% of patients, more than 20 RBC units were administered and 62% of patients were transfused more than 50 RBC units before transplantation. Iron chelation therapy was deemed inadequate in 94% of the transplant recipients; inadequate chelation was defined as not initiating chelation therapy within 18 months of the first transfusion and administered subcutaneously for 8 to 10 hours per day at least 5 days per week. Inadequate iron chelation therapy in almost all patients and the presence of hepatomegaly and hepatic fibrosis in approximately 50% of patients imply that most transplant recipients were in a high-risk category with respect to transplantation-related morbidity and mortality. Of the 87% of recipients who had a liver biopsy performed before transplantation, 59% had histologic evidence of portal fibrosis. Although serum bilirubin was normal in 84% of patients, hepatic inflammation was common. The serum aspartate aminotransferase or alanine aminotransferase was greater than 2 times the upper limit of normal in more than 30% of patients. Only 9% of the patients had a documented pretransplantation history of viral hepatitis. Data about hepatic synthetic function were not collected.
Patient, disease, and transplantation characteristics
| Variable . | N (%) or median (range) . |
|---|---|
| No. of patients | 179 |
| Age at transplantation, years | |
| < 5 | 57 (32) |
| 6-9 | 55 (31) |
| 10-20 | 67 (37) |
| Male | 98 (55) |
| Karnofsky-Lansky score before transplantation | |
| < 90% | 7 (4) |
| ≥ 90% | 169 (94) |
| Not reported | 3 (2) |
| CMV serostatus | |
| Donor negative/recipient negative | 28 (16) |
| Donor negative/recipient positive | 16 (9) |
| Donor positive/recipient negative | 11 (6) |
| Donor positive/recipient positive | 100 (56) |
| Not reported | 24 (13) |
| Total serum bilirubin before conditioning, μM | |
| Median (range) | 14 (3-104) |
| < 35 | 151 (84) |
| 35-50 | 9 (5) |
| 50-100 | 6 (3) |
| ≥ 100 | 2 (1) |
| Not reported | 11 (6) |
| Presence of portal fibrosis on liver biopsy | |
| Absent | 63 (35) |
| Present | 92 (51) |
| Not reported | 24 (13) |
| Liver size | |
| > 2 cm below costal margin | 83 (46) |
| ≤ 2 cm below costal margin | 78 (44) |
| Not reported | 18 (10) |
| Iron chelation therapy | |
| Inadequate | 163 (94) |
| Adequate* | 10 (6) |
| Not reported | 6 (3) |
| Pesaro risk class | |
| I | 3 (2) |
| II | 75 (42) |
| III | 64 (36) |
| Not reported | 37 (21) |
| Serum ferritin level before conditioning, μg/L | |
| < 2500 | 73 (41) |
| ≥ 2500 | 63 (35) |
| Not reported | 43 (24) |
| ALT/AST before conditioning | |
| Normal (< 2 times upper limit of normal) | 101 (56) |
| Elevated (≥ 2 times upper limit of normal) | 45 (25) |
| Not reported | 33 (18) |
| No. of blood transfusions before transplantation | |
| ≤ 20 | 13 (7) |
| 20-50 | 41 (23) |
| > 50 | 111 (62) |
| Not reported | 14 (8) |
| Interval from diagnosis to transplantation | |
| ≤ 5 years | 73 (41) |
| > 5 years | 102 (57) |
| Not reported | 4 (2) |
| Conditioning regimen | |
| Busulfan + cyclophosphamide + ATG | 77 (43) |
| Busulfan + cyclophosphamide without ATG | 102 (57) |
| Donor-recipient gender match | |
| Male donor/male recipient | 42 (23) |
| Male donor/female recipient | 37 (21) |
| Female donor/male recipient | 56 (31) |
| Female donor/female recipient | 44 (25) |
| GVHD prophylaxis | |
| Cyclosporine + methotrexate | 152 (85) |
| Cyclosporine with or without other (not methotrexate) | 23 (13) |
| Methorexate with or without other (not cyclosporine) | 2 (1) |
| None | 2 (1) |
| Year of transplantation | |
| 1995-1997 | 101 (56) |
| 1998-2001 | 78 (44) |
| Median follow-up of survivors, months | 73 (6-127) |
| Variable . | N (%) or median (range) . |
|---|---|
| No. of patients | 179 |
| Age at transplantation, years | |
| < 5 | 57 (32) |
| 6-9 | 55 (31) |
| 10-20 | 67 (37) |
| Male | 98 (55) |
| Karnofsky-Lansky score before transplantation | |
| < 90% | 7 (4) |
| ≥ 90% | 169 (94) |
| Not reported | 3 (2) |
| CMV serostatus | |
| Donor negative/recipient negative | 28 (16) |
| Donor negative/recipient positive | 16 (9) |
| Donor positive/recipient negative | 11 (6) |
| Donor positive/recipient positive | 100 (56) |
| Not reported | 24 (13) |
| Total serum bilirubin before conditioning, μM | |
| Median (range) | 14 (3-104) |
| < 35 | 151 (84) |
| 35-50 | 9 (5) |
| 50-100 | 6 (3) |
| ≥ 100 | 2 (1) |
| Not reported | 11 (6) |
| Presence of portal fibrosis on liver biopsy | |
| Absent | 63 (35) |
| Present | 92 (51) |
| Not reported | 24 (13) |
| Liver size | |
| > 2 cm below costal margin | 83 (46) |
| ≤ 2 cm below costal margin | 78 (44) |
| Not reported | 18 (10) |
| Iron chelation therapy | |
| Inadequate | 163 (94) |
| Adequate* | 10 (6) |
| Not reported | 6 (3) |
| Pesaro risk class | |
| I | 3 (2) |
| II | 75 (42) |
| III | 64 (36) |
| Not reported | 37 (21) |
| Serum ferritin level before conditioning, μg/L | |
| < 2500 | 73 (41) |
| ≥ 2500 | 63 (35) |
| Not reported | 43 (24) |
| ALT/AST before conditioning | |
| Normal (< 2 times upper limit of normal) | 101 (56) |
| Elevated (≥ 2 times upper limit of normal) | 45 (25) |
| Not reported | 33 (18) |
| No. of blood transfusions before transplantation | |
| ≤ 20 | 13 (7) |
| 20-50 | 41 (23) |
| > 50 | 111 (62) |
| Not reported | 14 (8) |
| Interval from diagnosis to transplantation | |
| ≤ 5 years | 73 (41) |
| > 5 years | 102 (57) |
| Not reported | 4 (2) |
| Conditioning regimen | |
| Busulfan + cyclophosphamide + ATG | 77 (43) |
| Busulfan + cyclophosphamide without ATG | 102 (57) |
| Donor-recipient gender match | |
| Male donor/male recipient | 42 (23) |
| Male donor/female recipient | 37 (21) |
| Female donor/male recipient | 56 (31) |
| Female donor/female recipient | 44 (25) |
| GVHD prophylaxis | |
| Cyclosporine + methotrexate | 152 (85) |
| Cyclosporine with or without other (not methotrexate) | 23 (13) |
| Methorexate with or without other (not cyclosporine) | 2 (1) |
| None | 2 (1) |
| Year of transplantation | |
| 1995-1997 | 101 (56) |
| 1998-2001 | 78 (44) |
| Median follow-up of survivors, months | 73 (6-127) |
CMV indicates cytomegalovirus; and ALT/AST, alanine aminotransferase/aspartate aminotransferase.
The adequacy of iron chelation is defined as the chelation therapy was initiated within 18 months of the first transfusion and administered subcutaneously for 8 to 10 hours per day for at least 5 days/week; otherwise, the iron chelation is deemed inadequate.
Thus, among the 142 (79%) patients who were eligible for risk-group assignment, the assignment to risk categories was 2% to class I, 42% to class II, and 36% to class III. It was not possible to assign a risk category in 20% of the study population. As there were so few patients who had class I features, the findings presented herein pertain to patients with class II and III risk-group features.
Hematopoietic recovery and graft failure
By day 30 after transplantation, the cumulative incidence of neutrophil recovery was 90% (95% CI, 85%-94%) and platelet recovery, 51% (95% CI, 43%-58%). The cumulative incidence of platelet recovery by day 100 improved to 86% (95% CI, 80%-90%). We also examined for the effect of Pesaro risk group and of ATG on neutrophil recovery and did not observe significant differences. The day 30 cumulative incidence of neutrophil recovery for patients in Pesaro class II and class III was 92% (95% CI, 85%-97%) and 91% (95% CI, 83%-96%), respectively (P = .44). Corresponding cumulative incidence of neutrophil recovery for patients who received ATG and did not receive ATG was 89% (95% CI, 82%-94%) and 91% (95% CI, 84%-94%), respectively (P = .13). Seventeen patients (10%) had graft rejection that was fatal in 11 cases. Five of these 17 patients were assigned to Pesaro risk class II, 8 patients risk class III, and in the remaining 4 patients we could not assign a risk category. Five patients with graft rejection received ATG. Six of 9 patients with graft failure are alive and free of thalassemia after a successful second transplantation. The source of hematopoietic cells for the second transplantation was bone marrow in 5 patients, and 4 patients received mobilized peripheral blood stem cells.
GVHD
The day 100 probability of acute GVHD (grade II-IV) was 38% (95% CI, 31%-45%) and the 5-year probability of chronic GVHD was 13% (95% CI, 9%-18%). In multivariate analysis, the administration of more than 50 RBC transfusions before transplantation was associated with a lower risk of acute GVHD (relative risk [RR] = 0.52, 95% CI, 0.31-0.86, P = .010). No risk factors were identified for the development of chronic GVHD. The use of ATG had no apparent association with the risk of GVHD in this analysis. Of those who received ATG before transplantation, 29 of 77 (38%) developed acute GVHD compared with 44 of 99 (44%) in patients who did not receive ATG. There was also no effect of ATG on the incidence of chronic GVHD, in which 10 of 77 (13%) who received ATG and 16 of 99 (16%) who did not receive ATG developed chronic GVHD.
Overall and disease-free survival
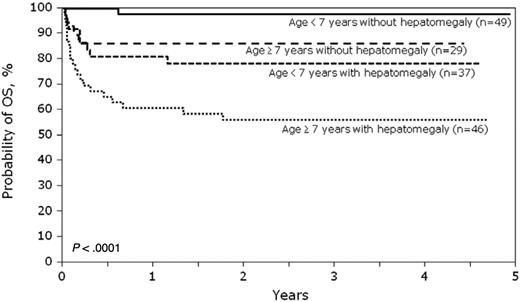
A total of 144 patients are alive at last follow-up. In multivariate analysis, the risk of mortality was higher in patients 7 years of age or older compared with patients younger than 7 years (RR = 2.72, 95% CI, 1.29-5.70; 69% vs 90%, respectively, P = .008) and in those with hepatomegaly defined as more than 2 cm below the coastal margin at BMT compared with those without hepatomegaly (RR = 5.17, 95% CI, 1.98-13.47; 66% vs 94%, respectively, P < .001). Age at transplantation and liver size are independent predictors of mortality after transplantation. The probabilities of OS and DFS are shown in Figures 1 and 2. In patients younger than 7 years and without hepatomegaly, the 5-year probabilities of OS and DFS were 98% (95% CI, 92%-100%) and 94% (95% CI, 85%-99%), respectively. The 5-year probabilities of OS and DFS in older patients (age ≥ 7 years) without hepatomegaly were 86% (95% CI, 72%-96%) and 83% (95% CI, 67%-94%), respectively.
Pesaro risk class had a significant association with survival in univariate analysis. The 5-year overall survival was 91% (95% CI, 83%–96%) and 64% (95% CI, 52%–75%) in patients with risk class II and class III, respectively (P = .0002; Figure 3). Pesaro risk class did not enter the final multivariate model because it is highly correlated with liver size; hepatomegaly entered the stepwise model-building process early and was retained in the final multivariate model. Given the relatively small numbers of patients in each risk group, it is difficult to examine the effect of liver size in patients assigned to risk group II. In subset analysis, mortality risks were higher in patients assigned to risk group II with hepatomegaly compared with those assigned risk group II without hepatomegaly; however, this difference was not statistically significant (RR = 3.63, 95% CI, 0.91-14.57, P = .07). Only 3 patients who were 4, 12, and 16 years of age were assigned to risk class I; one patient died and the 2 remaining patients were alive at last follow-up.
Thirty-five patients died, and their primary causes of death are shown in Tables 2 and 3. Most deaths (n = 25) occurred within the first 3 months after BMT. Veno-occlusive disease of the liver (VOD) occurred frequently after transplantation, particularly in patients with high-risk disease. Fifty-eight patients (32%) developed VOD; of these, 2 patients had risk class I, 22 patients had risk class II, and 33 patients had risk class III features. Approximately one-third of patients with risk class II and 50% of patients with risk class III features developed VOD. VOD occurred more frequently in patients with a serum ferritin more than or equal to 2500 μg/L compared with those with a serum ferritin less than 2500 μg/L (52% and 32%, respectively). Of the 58 patients who developed VOD, 21 patients are dead (n = 6 reported VOD as the primary cause of death and n = 3 reported VOD as a contributing cause of death).
Causes of death by Pesaro risk class
| Cause of death . | Class I . | Class II . | Class III . | Unknown . |
|---|---|---|---|---|
| Graft rejection | 0 | 0 | 2 | 0 |
| Infection | 0 | 0 | 4 | 1 |
| IPN | 1 | 0 | 6 | 0 |
| ARDS | 0 | 1 | 1 | 0 |
| GVHD | 0 | 1 | 1 | 1 |
| VOD | 0 | 2 | 3 | 1 |
| Cardiac failure | 0 | 0 | 1 | 0 |
| Hemorrhage | 0 | 3 | 5 | 0 |
| Other | 0 | 0 | 0 | 1 |
| Cause of death . | Class I . | Class II . | Class III . | Unknown . |
|---|---|---|---|---|
| Graft rejection | 0 | 0 | 2 | 0 |
| Infection | 0 | 0 | 4 | 1 |
| IPN | 1 | 0 | 6 | 0 |
| ARDS | 0 | 1 | 1 | 0 |
| GVHD | 0 | 1 | 1 | 1 |
| VOD | 0 | 2 | 3 | 1 |
| Cardiac failure | 0 | 0 | 1 | 0 |
| Hemorrhage | 0 | 3 | 5 | 0 |
| Other | 0 | 0 | 0 | 1 |
N values are given.
IPN indicates idiopathic interstitial pneumonitis; ARDS, adult respiratory distress syndrome, and VOD, veno-occlusive disease.
Causes of death by age group
| Cause of death . | < 7 years . | ≥ 7 years . |
|---|---|---|
| Graft rejection | 1 | 1 |
| Infection | 1 | 4 |
| IPN | 2 | 5 |
| ARDS | 0 | 2 |
| GVHD | 0 | 3 |
| VOD | 1 | 5 |
| Cardiac failure | 0 | 1 |
| Hemorrhage | 4 | 4 |
| Other | 1 | 0 |
| Cause of death . | < 7 years . | ≥ 7 years . |
|---|---|---|
| Graft rejection | 1 | 1 |
| Infection | 1 | 4 |
| IPN | 2 | 5 |
| ARDS | 0 | 2 |
| GVHD | 0 | 3 |
| VOD | 1 | 5 |
| Cardiac failure | 0 | 1 |
| Hemorrhage | 4 | 4 |
| Other | 1 | 0 |
N values are given.
IPN indicates idiopathic interstitial pneumonitis; ARDS, adult respiratory distress syndrome, and VOD, veno-occlusive disease.
Discussion
In this report, we have attempted to capture most of the most recent transplantation experience outside Italy that included children with β-thalassemia major who were treated with HLA-matched sibling BMT. Thus, this represents the largest contemporary series yet reported and generates new information about assigning transplantation risk, which should assist in optimizing outcomes and selecting suitable candidates for transplantation. These results must be interpreted while also considering recent advances in supportive therapy, particularly in iron chelation therapy, which have reduced the risks of morbidity and mortality in those who do not proceed to transplantation.2,16,19-21 Our results confirm that HLA-matched sibling BMT for β-thalassemia major is a suitable therapeutic option to consider, as we also observed DFS rates that exceeded 90% in children with good-risk features (Figure 1).4,9 However, as noted in the initial Pesaro experience, children with high-risk features fared much worse, with an event-free survival rate that approached 50%.4,22,23 These observations underscore the importance of carefully selecting good-risk patients for transplantation, but also if treating high-risk patients, the importance of using modifications to the transplantation regimen that might improve the safety of the procedure.6,7,11,24
We were able to identify 2 readily accessible prognostic indicators in a cohort of high-risk thalassemia recipients, namely, liver size and patient age, which other groups also have identified.19,20 The observed effects of age and liver size were independent of each other, although liver size is highly correlated with Pesaro risk group. By classifying this high-risk cohort by these criteria (ie, age and liver size), it was possible to refine and broaden the class II and III Pesaro risk groupings into distinct patterns of survival and event-free survival, which are represented in Figures 1 and 2. The 5-year survival probabilities, which ranged from 53% to 96%, are broader than the initial Pesaro experience.4,9,20 Although these criteria do not supplant the utility of a liver biopsy, which provides important information about liver histology and can also direct post-transplantation interventions to reduce iron overload, there may be situations where a liver biopsy and the ability to assess liver histology and iron concentration are not readily available. Using the risk features identified in the current analysis, it may be possible to assign a conditioning therapy that is better suited to those who are most in need of such modifications and in clinical settings where assigning a Pesaro risk classification is not possible.7,12,22 We speculate that, for the diverse cohort in the current analysis, it may be appropriate to dispense with the Pesaro classification in high-risk patients and rely on this facile and novel risk assignment.
It appears that much of the toxicity of transplantation observed in this series might have been related to impaired hepatic function and a propensity for developing interstitial pneumonitis and VOD, particularly in the older, high-risk patients. Previously, a high rate of regimen-related toxicity and graft rejection was noted in class II and III patients after standard busulfan and cyclophosphamide preparation, particularly in young adult patients with thalassemia, for whom the transplantation-related mortality was 35%.25 An early study of busulfan pharmacokinetics as a predictor of clinical outcome in thalassemia major failed to identify a relationship between the busulfan level and toxicity, although this analysis included transplantation regimens that differed in their cyclophosphamide dosing and in postgrafting immunosuppression.26 Other studies in children with hematologic malignancies have demonstrated the utility of busulfan pharmacokinetics in predicting the risk of toxicity and have generally endorsed the adoption of targeted busulfan dosing to avoid this toxicity.27-29 Moreover, a more recent clinical study defined the important contribution by cyclophosphamide and its metabolites to the toxicity of the busulfan and cyclophosphamide regimen, suggesting that the administration of busulfan before cyclophosphamide alters cyclophosphamide pharmacokinetics and thus might also exacerbate the risk of VOD in patients with underlying hepatic injury.30,31 To mitigate these risks, investigative teams in Italy have reduced the dosing of cyclophosphamide in high-risk patients.7 Alternatively, the replacement of cyclophosphamide with fludarabine might also mitigate the risk of significant transplantation-related toxicity.32 Intravenous busulfan in lieu of oral busulfan also appears to improve the safety profile of busulfan as does the practice of therapeutic drug monitoring.11,33-35 The observations made in this series thus highlight the importance of modifying the standard busulfan and cyclophosphamide regimen in very high-risk patients to avoid unacceptably high transplantation-related mortality.
In other patient series, graft rejection after HLA-matched sibling BMT is generally accompanied by thalassemia recurrence and recovery of autologous hematopoietic function.5,36,37 However, in this series graft failure, which occurred in nearly 10% of cases, was fatal in 11 cases and required rescue by second transplantation to restore normal hematopoiesis. The reasons for this are unclear. The use of ATG did not appear to be useful in reducing the risk of graft failure. In the future, it is possible that the development of alternative regimens that provide more intensive immune suppression before transplantation without myeloablation might reduce the elevated risk of graft failure we observed in high-risk patients with thalassemia.24,38
When defining the role of transplantation for β-thalassemia major, which is a chronic, nonmalignant condition, one must also consider the natural history of the disorder and the impact of improved supportive care measures on patient outcomes. In selected regions of the world, the outcomes are outstanding when there is ready access to expanded supportive care services, with very few persons dying of thalassemia or of complications related to its treatment through the third and fourth decades of life.12,20,21 In other areas of the world, however, supportive care measures are often difficult to access, which as predicted, has had a negative impact on survival.39,40 We speculate that many of the transplant recipients included in this series lacked ready access to vital supportive care measures, such as regular iron chelation therapy, and there may have been delays in proceeding to transplantation in cases where resources were limited. For these reasons, the results shown here reflect a variety of healthcare issues that have the potential to affect outcomes in children with β-thalassemia who reside outside North America and Europe. Considering the impact of these mitigating factors, we think that the transplantation outcomes reported should motivate families and their physicians to strongly consider the possibility of HLA-matched sibling BMT earlier in the course of their disease, particularly when there are limited resources for chronic thalassemia-centered medical care.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The Center for International Blood and Marrow Transplant Research is supported by the National Cancer Institute, the National Heart, Lung and Blood Institute, and the National Institute of Allergy and Infectious Diseases (Public Health Service Grant/Cooperative Agreement U24-CA76518); National Cancer Institute, the National Heart, Lung and Blood Institute (Grant/Cooperative Agreement 5U01HL069294); Health Resources and Services Administration (contract HHSH234200637015C); the Office of Naval Research (grants N00014–06-1–0704 and N00014–08-1–0058); and grants from the following: AABB; Allos, Inc; Amgen, Inc; anonymous donation to the Medical College of Wisconsin; Astellas Pharma US, Inc; Be the Match Foundation; Biogen IDEC; BioMarin Pharmaceutical, Inc; Biovitrum AB; BloodCenter of Wisconsin; Blue Cross and Blue Shield Association; Bone Marrow Foundation; Buchanan Family Foundation; CaridianBCT; Celgene Corporation; CellGenix, GmbH; ClinImmune Labs; CTI Clinical Trial and Consulting Services; Eisai, Inc; Genentech, Inc; Genzyme Corporation; Histogenetics, Inc; HKS Medical Information Systems; Hospira, Inc; Kirin Brewery Co, Ltd; Merck & Company; Medical College of Wisconsin; Millennium Pharmaceuticals, Inc; Miller Pharmacal Group; Milliman USA, Inc; Miltenyi Biotec, Inc; National Marrow Donor Program; Nature Publishing Group; Novartis Oncology; Oncology Nursing Society; Osiris Therapeutics, Inc; Otsuka America Pharmaceutical, Inc; Pall Life Sciences; Pfizer Inc; Schering Corporation; Sigma-Tau Pharmaceuticals; Soligenix, Inc; StemCyte, Inc; StemSoft Software, Inc; Sysmex America, Inc; THERAKOS, Inc; Vidacare Corporation; ViraCor Laboratories; ViroPharma, Inc; and Wellpoint, Inc.
The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, or any other agency of the U.S. Government.
National Institutes of Health
Authorship
Contribution: M.S., B.R.L., C.N.B., M.E., J.H., M.C.W., G.A.H., R.P.G., and S.M.I. participated in conception and design of the study; M.C., S.H., A.G., C.-K.L., and M.J.C. contributed patients; Z.W. and B.R.L. assembled the data and performed the statistical analysis; M.S. and M.C.W. had primary responsibility for manuscript preparation; and all authors participated in interpretation of data, manuscript preparation, and approval of the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mark C. Walters, Children's Hospital & Research Center, Oakland, Pediatric Hematology/Oncology, 747 52nd St, Oakland, CA, 94609; e-mail: mwalters@mail.cho.org.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal