Abstract
Over the past 2 decades considerable evidence has accumulated on the association between hepatitis C virus (HCV) and hepatitis B virus (HBV) and several hematologic malignancies, most notably B-cell non-Hodgkin lymphoma (NHL). In this review we summarize this evidence, address possible mechanisms whereby hepatitis viruses may contribute to lymphomagenesis, and discuss the therapeutic fallouts from this knowledge. Most of this evidence is on HCV, and this is the main focus of the review. Moreover, we mainly address the association with NHL, the most prevalent hematologic malignancy, and the most extensively investigated with regard to an association with hepatitis viruses. Available evidence on the association with other hematologic malignancies is also addressed briefly.
Hepatitis C virus and hepatitis B virus
Hepatitis C virus (HCV) is a positive, single-stranded RNA virus, member of the Flaviviridae family.1 During its replicative cycle it goes through a negative-stranded RNA, but not DNA, intermediate, so that integration of HCV nucleic acid sequences into the host genome seems unlikely. As such, it lacks a pivotal property of classical oncogenic retroviruses. The HCV genome produces a single polyprotein that is proteolytically processed by viral and cellular proteases to produce structural (nucleocapsid, E1, E2) and nonstructural (NS) proteins (NS2, NS3, NS4A, NS4B, NS5A, and NS5B).
Hepatitis B virus (HBV) is a small DNA virus, member of the Hepadnaviridae family.2 It presents the unusual feature, for a DNA virus, to replicate through a RNA intermediate and can integrate into the host genome. Moreover, during replication, it undergoes transformation into covalently closed circular DNA. Detection of closed circular DNA HBV-, or negative-stranded HCV-intermediates is important because it identifies genuine viral replication instead of more trivial events such as viral absorption. HBV is characterized by a genome consisting of 4 overlapping open-reading frames: the S gene, encoding envelope proteins; the core gene, encoding the core and “e” proteins; the P gene, encoding DNA polymerase; and the X gene, encoding a transcriptional transactivator.
Both viruses can induce acute and chronic hepatitis and are strongly associated with hepatocellular carcinoma (HCC).
Epidemiology of non-Hodgkin lymphoma
Non-Hodgkin lymphoma (NHL) is the hematologic malignancy with the highest prevalence worldwide. Incidence rates have grown fast up to the beginning of the new millennium, with an annual percentage increase of nearly 3%, which is faster than for most cancers. Thus, delay-adjusted Surveillance, Epidemiology and End Results incidence rate for all races was 11.06/100 000 in 1975, and 20.12/100 000 in 2002. Since then, incidence rates have started to level off, at least in some geographic areas, such as the United States (20.89 in 2007).3 NHL incidence rates are higher in developed countries such as those in western Europe, North America, and Australia and lower in South America and Asia, but the rise in incidence has been consistent across countries. The increasing incidence of NHL is largely unexplained. AIDS-related NHL accounts for some of the increase. NHL includes a wide range of subtypes of either B-cell or T-cell lymphomas, with B-cell lymphomas representing the majority. Unless otherwise stated, in this review the term NHL will always refer to B-cell NHL. Among the risk factors for NHL are primary and acquired immune deficiency, as well as several infectious agents, such as HIV (through induction of immune deficiency), Epstein-Barr virus (EBV; associated with organ transplant, acquired immune deficiency–related NHL, and Burkitt lymphoma), human T-cell lymphotropic virus-I (peripheral T-cell NHL), human herpes virus 8 (primary effusion lymphoma), and Helicobacter pylori (mucosa-associated lymphoid tissue [MALT] lymphoma).4
Epidemiology of the association between hepatitis viruses and NHL or other hematologic malignancies
Association of HCV and mixed cryoglobulinemia
The most frequently studied association between a hepatitis virus and NHL has been with HCV. The initial finding that resulted in the extensive investigation of this association was the very high prevalence (nearly 100%) of HCV infection in patients with mixed cryoglobulinemia (MC).5,6 Cryoglobulins are serum immunoglobulins (Igs) that precipitate at temperatures below 37°C. Mixed cryoglobulins are characterized by immune complexes consisting of a mixture of monoclonal and polyclonal Igs (type II MC) or polyclonal Igs (type III MC). The antigenic component of the immune complexes has been found to be highly enriched in HCV-RNA.6 The monoclonal component of type II MC is often an IgM with rheumatoid factor (RF) activity. Most of these monoclonal RFs express the Wa cross-reactive idiotype (CRI).7 The prevalence of mixed cryoglobulins presents great geographic heterogeneity, being more common in Southern Europe than in Northern Europe, North America, or Japan. Low levels of circulating mixed cryoglobulins can be detected in > 50% of HCV-infected persons, whereas overt cryoglobulinemic vasculitis develops in ≤ 5% of HCV-infected persons, with values presenting also in this case great geographic heterogeneity. The female-to-male ratio is 3:1.8 Symptoms range from mild clinical symptoms (purpura, arthralgia) to fulminant life-threatening complications (glomerulonephritis, widespread vasculitis). Importantly, the overall risk of NHL in HCV-infected patients with symptomatic MC is greatly increased compared with the general population (eg, ≤ 35 times in an Italian multicenter study).9 Overall, depending on the absence or presence of a monoclonal component, MCs can be considered benign. Its presence, however, may result in preneoplastic lymphoproliferative diseases and an increasing risk of irreversible neoplastic transformation.
Association of HCV and NHL
A positive association between HCV and NHL was first described by Ferri et al10 and Pozzato et al,11 a finding that has now been confirmed in a large number of studies (eg, Mele et al,12 Duberg et al,13 and Anderson et al14 ). In a meta-analysis15 that selected 15 studies, the pooled relative risk (RR) of all NHL among HCV-positive persons was found to be 2.5 (95% confidence interval [CI], 2.1-3.1) in case-control studies and 2.0 (95% CI, 1.8-2.2) in cohort studies. RRs were similarly increased for all major NHL subtypes and primary sites of presentation. Previous suggestions that the RRs for HCV differed by NHL subtype were not confirmed. In fact, only slightly higher RRs for extranodal compared with nodal NHL were observed for HCV, but this difference was largely due to the early studies. In addition, no clear differences emerged on the association between HCV and major histologic B-cell NHL subtypes (diffuse large B-cell, follicular, marginal zone, and chronic lymphocytic leukemia/small lymphocytic lymphoma).16 Early results that suggested a stronger association of HCV with certain subtypes, such as lymphoplasmacytic/Waldenström lymphomas, may have been because these studies were performed mainly in HCV-infected patients with type II MC,17 a subset of patients in which said subtype has been reported to be highly prevalent.18 However, the RR was significantly higher in geographic areas with high rather than with low HCV prevalence. In fact, in several early studies from countries with low HCV prevalence, the association between HCV and NHL could not be observed.19-21 This suggests the possibility that in areas where HCV prevalence among NHL-free subjects is low, the spread of the virus may be recent, thus not allowing the full consequences on NHL development to be seen.15 Moreover, studies from areas with low HCV prevalence may not have included sufficient numbers of patients to detect a significant association between HCV and NHL. In support of this possibility, a study that included the entire cohort of notified HCV patients in Sweden,13 a country with a very low prevalence of HCV infection, evidenced a significantly increased risk of NHL in HCV-positive persons. Overall, the causative fraction of NHL attributable to HCV varies greatly by country but may be upward of 10% in areas where HCV prevalence is high.15
Association of HBV and NHL
The association of NHL with HBV has been studied much less intensively than with HCV. This is somehow surprising because the first report of a positive association between HBV and NHL was published in the same year as the first reports on the HCV-NHL association.22 More recently, several case-control studies with large numbers of patients have been published.23,24 These studies have been recently reviewed in a meta-analysis25 that included 12 studies. The pooled odds ratio, which, given the rarity of the disorder, is a good estimation of the RR, was found to be consistent with a significant association (odds ratio, 2.56; 95% CI, 2.24-2.92). Data were not sufficient to assess an association between HBV and major NHL subtypes. Most recently, results of a very large cohort study of South Korean workers and their dependents enrolled during 1992-1995 have been published.26 In accordance with previous studies, risk of NHL was consistently raised in hepatitis B surface antigen (HBsAg)–positive participants throughout 14 years of follow-up (hazard ratio, 1.74; 95% CI, 1.45-2.09). HBsAg positivity was not associated with follicular or T-cell NHL, Hodgkin lymphoma, multiple myeloma, or various leukemias.
Available data may underestimate the real association between HBV and NHL, if one considers also persons with occult hepatitis infection. Occult HBV infections have been defined for patients who test negative for HBsAg but positive for HBV-DNA in serum or tissues or both.27 Because HBV-DNA can be present sometimes only in tissues, this implies that occult infection may occur even if HBV-DNA is not present in the serum. In fact, complete HBV eradication is now considered to be a rare event, if it occurs at all28,29 ; a replication-competent HBV-DNA probably persists in the liver or lymphocytes or both for many years or even life long. Recent reports examining HBV-DNA in serum have shown that occult HBV infection further adds to the associations of HBsAg-positive HBV infection with NHL and chronic lymphocytic leukemia.30-32 It is also possible that some persons can be positive for HBV-DNA in hepatocytes or lymphocytes or both but negative in serum, which would further affect the association between HBV infection and NHL.
Association of hepatitis viruses and NHL subtypes or other hematologic malignancies
It is difficult to draw firm conclusions pertaining to the association of HCV with rare NHL subtypes, such as those that do not originate from germinal center (GC) or post-GC B cells, such as mantle-cell NHL, Burkitt lymphoma, T-cell lymphoma, and Hodgkin lymphoma, or with other hematologic malignancies. This is because studies on the association of hepatitis viruses and these hematologic tumors have been often based on a relatively small number of patients (eg, Bianco et al33 ). Nevertheless, some tentative conclusions can be drawn. A meta-analysis referred to previously15 found similarly significant associations also for rare histologic NHL subtypes and T-cell NHL. Weaker associations were found also with Hodgkin lymphoma and multiple myeloma. These are important observations because these tumors cannot arise as a consequence of chronic antigenic stimulation of B cells, the most commonly evoked mechanism to explain HCV-driven lymphomagenesis (see “Evidence for an oncogenic role of HCV in NHL” for a discussion of this and other possible mechanisms of HCV-induced lymphomagenesis).
Given the dearth of related studies, nothing can be said about the association of HBV with these other hematologic malignancies.
Possible mechanisms for a causal relationship between hepatitis viruses and NHL
The association of the hepatitis viruses HCV and HBV with NHL appears to be firmly established. The question is whether these associations reflect causal relationships. There are 3 possibilities to explain these associations. First, the risk of viral infection or reactivation increases because of a direct immunosuppressive effect of the tumor itself. This possibility can be refuted because many studies have been conducted with patients with newly diagnosed disease, before any therapeutic intervention was carried out, and thus at a stage in which significant immune deficiency had not yet occurred. Second, another (unknown) virus with a mode of transmission similar to the hepatitis virus might represent the actual oncogenic stimulus. The lack of any evidence in favor of this hypothesis renders it merely theoretical. We are then left with the third possibility, ie, the existence of causal relationships between hepatitis viruses and NHL, with hepatitis viruses playing an oncogenic role in NHL development. In the next section we discuss the evidence in favor of this possibility and its possible mechanisms. It should, however, be pointed out that the association of HCV and HBV with NHL is not very strong, with a RR of infection in the 2–3 range. This is much less than the enormous increase in risk for the development of HCC brought about by infection with both HBV or HCV. The RR of HBV carriers for HCC, for example, approaches 200, one of the highest for a human malignancy.34 Any discussion on the mechanistic interpretation of the role of hepatitis viruses in lymphomagenesis must take this fact in consideration.
Evidence for an oncogenic role of HCV in NHL
Most studies on a lymphomagenic role of hepatitis viruses have focused on HCV. The most straightforward demonstration of an oncogenic role of HCV in NHL comes from trials showing that antiviral therapy, now based mostly on peginterferon and ribavirin, resulted in complete or partial remissions of lymphoma in HCV-positive but not HCV-negative NHL patients.35,36 A systematic review has shown that complete responses were achieved in 75% of the HCV-positive cases.37 These trials have been conducted in indolent lymphomas during a phase in which no other therapeutic intervention was implemented (“watch-and-wait” policy). Recent results suggest that antiviral therapy may be extended also to the treatment of aggressive lymphomas.38 In most patients, antiviral treatment led also to disappearance of Ig heavy chain (IgHC) and t(14;18) translocation.39 Importantly, a recent study has shown that HCV-infected patients who had received interferon therapy and had undergone a sustained virologic response had a hazard ratio of lymphomagenesis that was significantly lower than patients who had not received antiviral treatment.40 This shows that antiviral treatment is efficacious also in preventing lymphomagenesis in HCV-infected patients.
Results obtained with antiviral treatment of HCV-positive patients with NHL are similar to those obtained with antibiotic treatment of H pylori–positive extranodal marginal zone B-cell lymphoma of MALT type. Treatment aimed at eradicating H pylori infection results in lymphoma remissions in most patients.41,42 As such, HCV- and H pylori–associated lymphomas represent unique cases of anticancer treatment on the basis of the eradication of the causing factor.
Although clinical responses to antiviral therapy strongly support a role for HCV in lymphomagenesis, they do not inform about the mechanism(s) linking HCV to NHL induction. Evidence from experimental studies suggests that there are ≥ 3 different mechanisms whereby HCV might contribute to NHL induction.
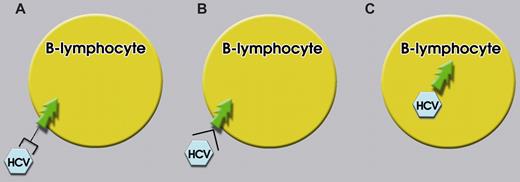
The first of these mechanisms is chronic antigenic stimulation (Figure 1A). A first observation that lends support to this mechanism is the histologic presentation of many, but not all, HCV-associated hematologic malignancies, which is typical of GC and post-GC B cells.43 This suggests that lymphomagenesis occurs when B cells proliferate in response to antigen. Further evidence comes from the antibody response and Ig variable (V) gene usage in patients with chronic HCV infection and HCV-associated NHL. The HCV-E2 protein is the primary target of antibody responses against HCV.44,45 Monoclonal antibodies derived from HCV-infected patients use a restricted IgV gene repertoire, with a strong bias for VH1-69 (also known as 51p1) and Vκ3-A27 (also known as κν325).46,47 In addition, the monoclonal IgM component of type II MC is often encoded by the same set of V region genes, VH1-69 and Vκ3-A27. In fact, many of the monoclonal RFs from patients with type II MC express the Wa CRI, which is often associated with light and heavy chain CRIs characteristic for V regions encoded by VH1-69 and Vκ3-A27.48 Eventually, these genes are expressed also by most HCV-associated NHLs.49 These data suggested that HCV-associated NHLs derive from B cells activated during HCV infection, with some of these B cells being specific for HCV-E2, and representing the malignant counterpart of type II MC.
Three pathways for HCV-induced lymphomagenesis. (A) Chronic antigenic stimulation of a B cell that interacts through its surface Igs with the cognate HCV antigen. (B) HCV-E2 protein engaging its high-affinity receptor CD81 expressed on B cells. (C) Direct infection of a B cell by HCV.
Three pathways for HCV-induced lymphomagenesis. (A) Chronic antigenic stimulation of a B cell that interacts through its surface Igs with the cognate HCV antigen. (B) HCV-E2 protein engaging its high-affinity receptor CD81 expressed on B cells. (C) Direct infection of a B cell by HCV.
Evidence in accordance with this possibility was brought by Quinn et al,50 who expressed as soluble Igs the B-cell receptors from 2 HCV-associated lymphomas. One of 2 lymphoma Ig test cases bound the HCV-E2 protein from multiple viral genotypes, suggesting reactivity with a conserved epitope. This finding confirmed that at least some HCV-associated lymphomas originated from B cells that were initially activated by the HCV-E2 protein. In addition, Ig V region genes expressed by HCV-associated lymphomas exhibited an ongoing process of somatic mutations, indicative of antigen selection.48 Altogether, these data indicate that lymphomagenesis in HCV-infected patients may occur when B cells undergo chronic, antigen-driven proliferation. The possibility that HCV-E2 could be one of the antigens responsible for this chronic antigenic stimulation is strongly suggested by several studies.
A second potentially lymphomagenic mechanism of HCV infection derives from the high-affinity interaction between HCV-E2 and one of its receptors, the tetraspanin CD8151 (Figure 1B). Multimeric engagement of CD81 on human B cells by a combination of HCV-E2 and anti-CD81 antibodies led to polyclonal activation of naive, CD27− B cells.52 In addition to direct effects on B cells, engagement of CD81 on T cells lowered the threshold for interleukin-2 production, resulting in strongly increased T-cell proliferation.53 This could lead to T-cell activation in response to suboptimal stimuli and bystander activation of B cells. Altogether, these results suggest that CD81 engagement on B and T cells may lead to direct or indirect activation and possibly chronic proliferation of B cells.
Chronic B-cell proliferation, in response to antigenic stimulation or polyclonal activation, may predispose to genetic aberrations such as translocation or overexpression or both of the antiapoptotic protein Bcl-2.54 Bcl-2 overexpression is especially frequent in follicular lymphomas.55 It has been found also in B cells of patients with MC56 and even in HCV-positive cases without cryoglobulins.57 Moreover, interaction between HCV-E2 and CD81 on B cells has been shown to trigger the enhanced expression of activation-induced cytidine deaminase and to induce double-strand DNA breaks in the IgVH gene locus, thereby further contributing to a mutator phenotype conducive to malignant transformation.58
The third tumorigenic mechanism of HCV that has been proposed is direct infection of B cells (Figure 1C). The conditio sine qua non for this mechanism to be acceptable is the demonstration that HCV is indeed able to infect and replicate in B cells. This basic issue, however, has not yet been definitively settled. Thus, although HCV has been detected in lymphocytes from HCV-infected patients59,60 and patients with MC,61 only in a minority of cases also RNA-negative strands, ie, the HCV replicative intermediates suggestive of viral replication, were detected in the cells.59 Detection of RNA-negative strands by polymerase chain reaction, however, may give rise to false-positive results.62,63 More recently, Marukian et al64 have shown that culture-grown HCV replicated in hepatoma cells, but no HCV replication was detected in B or T cells, monocytes, macrophages, or dendritic cells from healthy donors. Moreover, no blood cell subset tested expressed significant levels of claudin-1, a tight junction protein that acts as coreceptor and is required for HCV infection.65 The block in HCV entry could be bypassed by transfecting HCV-RNA into blood cell subsets. Transfected RNA, however, was not detectably translated and induced high levels of interferon. These results suggest that peripheral blood cells may neither be permissive for HCV entry nor for HCV replication. However, Stamataki et al66 have provided results showing that HCV is able to infect B cells, but B cells were not able to support virus replication. Overall, results on the capacity of HCV to infect and replicate in B cells and other blood mononuclear cells still appear highly conflicting. There are possibilities, however, to accommodate the discrepant results on this issue that have appeared in the literature over the years. First, HCV may infect and replicate only a relatively rare subset of B cells, such as CD5+ B cells. These cells have been shown to express high levels of CD81 and to expand in HCV-infected liver.67 Alternatively, B cells may need another event to become permissive for HCV infection or replication or both. This event could be coinfection with another virus. EBV, for example, is detected in 37% of the tissues of HCC and is especially frequent in cases with HCV. This led Sugawara et al68 to investigate the effect of EBV infection on the replication of HCV. They found that EBV-infected cell clones, but not their uninfected counterparts, promoted HCV replication. Moreover, HCV persistence has been shown in human hematopoietic cells from HCV-infected patients injected into immunodeficient mice.69 The results were compatible with a low rate of virus replication. Interestingly, many of the immunodeficient mice that had been inoculated with hematopoietic cells from HCV-positive subjects developed a lymphoblastic tumor, and all of these tumors were positive for EBV, again suggesting that EBV infection may be required for HCV infection or replication or both in B cells. Coinfection with EBV or another permissive event would explain also the observation of the generation of B-cell lymphoma lines expressing HCV-RNA and proteins and, in a few cases, also continuously producing HCV virions in cell culture.70
Although the mode of virus penetration and replication in B cells is still unclear, several pieces of evidence suggest that the presence of HCV virus or HCV proteins in these cells represents an oncogenic stimulus. Thus, interferon regulatory factor-1–null (irf-1−/−) mice with inducible and persistent expression of HCV structural proteins (irf-1/CN2 mice) have an extremely high incidence of lymphomas and lymphoproliferative disorders.71 Other evidence comes from transgenic mice expressing HCV core protein.72 Of the lines that were established, one developed malignant lymphoma of the follicular center cell type with a high frequency (80%) at > 20 months of age. Hepatocellular adenoma was also observed in this line of transgenic mouse. Expression of HCV core protein and mRNA was detected in the liver of these mice, and core mRNA was detected in the enlarged lymph nodes of lymphoma-bearing mice.
With regard to the molecular signals whereby HCV infection of B cells could contribute to lymphomagenesis, it has been shown73 that HCV-infected cells, including B cells, display a mutator phenotype through induction of error-prone DNA polymerase ζ, polymerase ι, and activation-induced cytidine deaminase, which contribute to a 5- to 10-fold increase of mutation frequency of Ig heavy chain, BCL-6, p53, and β-catenin genes. Mutated protooncogenes were amplified in HCV-associated lymphomas and HCCs.
A multifactorial model for lymphomagenesis induced by HCV and other hepatitis viruses
In the previous section we have reviewed potential lymphomagenic mechanisms of HCV. Here, we propose that these different mechanisms are not mutually exclusive, and we put forward a multicausal model74 to explain HCV-induced lymphomagenesis. Thus, we suggest that any one of the oncogenic signals that have been described under the previous heading has a low potential to be lymphomagenic on its own. We refer to these as subliminal oncogenic signals. We propose that integration of ≥ 2 oncogenic signals is required for lymphoma formation to occur. The probability that ≥ 2 signals are lymphomagenic may be still relatively small, but, nevertheless, they may now give rise to supraliminal oncogenic signals that result in a statistically greater incidence of lymphoma formation in HCV-infected than in noninfected persons. This would explain the relatively modest increase in risk of NHL in HCV-positive persons.
The ≥ 2 signals may derive from the virus itself. Thus, simultaneous engagement of CD81 and the HCV-E2–specific B-cell receptor (Figure 2A) has been reported to activate the c-jun N-terminal protein kinase pathway, an effect that was not observed on individual ligation.52 Previous work had shown that dual engagement of these 2 signaling complexes, CD81 and B-cell receptor, reduces the threshold of B-cell activation.75 It has also been hypothesized that immune-complexed HCV could induce expansion of RF-positive B cells by dual engagement of the B-cell receptor and toll-like receptor 7.76 In fact, simultaneous binding of immune complexes to B-cell receptor and toll-like receptors has been found in several instances to activate RF-expressing B cells.77,78 Thus, simultaneous ligation may yield synergistic effects and give rise to intracellular signals that are not generated on individual ligation of 2 different receptors on B cells.
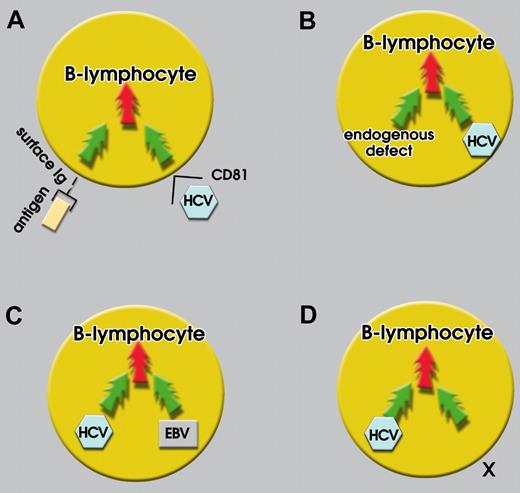
Integration of an HCV-derived oncogenic signal with an HCV-independent one. Individually, signals are subliminal and nononcogenic (green arrows); on integration, signals may become supraliminal and oncogenic (red arrows). Four examples of signal integration are shown. (A) HCV-E2 protein-CD81 interaction and specific antigen-surface Ig interaction. (B) An endogenous defect (eg irf-1−/− mice) and HCV infecting a B cell. (C) HCV and EBV coinfection. (D) HCV infection and signal X (eg, environmental factor or others).
Integration of an HCV-derived oncogenic signal with an HCV-independent one. Individually, signals are subliminal and nononcogenic (green arrows); on integration, signals may become supraliminal and oncogenic (red arrows). Four examples of signal integration are shown. (A) HCV-E2 protein-CD81 interaction and specific antigen-surface Ig interaction. (B) An endogenous defect (eg irf-1−/− mice) and HCV infecting a B cell. (C) HCV and EBV coinfection. (D) HCV infection and signal X (eg, environmental factor or others).
In addition, we propose also that an oncogenic signal from HCV may be integrated by another, HCV-independent signal, so as to generate a supraliminal composite signal of sufficient intensity or duration or both to become lymphomagenic. There is experimental evidence for this possibility. As mentioned before,71 irf-1−/− mice expressing HCV structural proteins have high incidence of lymphoma, reflecting an endogenous defect that integrates HCV in generating a supraliminal composite signal (Figure 2B). The defect present in these mice is artificially induced, but it could be equivalent to a susceptibility factor like those deriving from gene polymorphisms or translocations. Another possibility to generate a supraliminal oncogenic signal, which has already been mentioned, is coinfection with another virus, such as EBV68,69 (Figure 2C). Thus, in the study of Bronowicki et al,69 many of the immunodeficient mice that had been inoculated with hematopoietic cells from HCV-positive subjects developed a lymphoblastic tumor, and all of these tumors were positive for EBV.
We believe that a multicausal model such as the one herein proposed can also explain geographic discrepancies between incidence rates of NHL and prevalence rates of viral infection.79 In fact, a geographic area with a relatively low prevalence of HCV infection may have a high prevalence of a yet undefined environmental factor (X) that may integrate the HCV-derived signal(s) to give rise to a supraliminal composite signal (Figure 2D). However, in a geographic area with a low prevalence of this factor, but a high prevalence of HCV infection, a supraliminal composite signal may not be generated. We also propose that the effect of an individual signal on the composite signal varies, depending on the affected tissue. Thus, chronic antigenic stimulation may play a role of particular relevance in the pathogenesis of MALT lymphomas.80
We believe that this model of virus-induced lymphomagenesis can be applied also to HBV, despite the differences in structure and the infective behavior between these 2 viruses and despite that, as of today, lymphomagenic mechanisms of HBV have been much less studied than for HCV.
Conclusions
In this review we have summarized existing evidence on the association of HCV and HBV with NHL. We have also proposed a model that takes into account the possible mechanisms whereby hepatitis viruses contribute to lymphomagenesis, and that may be of help in explaining the modest RR of NHL that accompanies hepatitis virus infections. We propose that this model could be also relevant beyond NHL. Thus, it has been recently reported that HCV infection is positively associated with renal cell carcinoma, and that this association is expressed by a RR similar to that observed for NHL.81
We have already mentioned that it is difficult to draw conclusions with regard to the contribution of hepatitis viruses to the overall risk of NHL. The uncertain effect of occult HBV infections and the unknown, but probably long incubation period,13 are some of the reasons that make precise estimates difficult. Notwithstanding these difficulties, current knowledge on these associations might be the basis for therapeutic advances. One road, ie, antiviral therapy for HCV-positive patients, has already been taken. Another possibility of intervention is vaccination. Our multicausal model of lymphomagenesis assigns a necessary causative role to hepatitis virus infection. It thus stands to reason that vaccination against HCV or HBV should protect persons from lymphomagenesis as a result of exposure to that virus. Vaccines against HCV are not available, whereas vaccines against HBV have been available for several decades. As already mentioned, it appears likely that lymphomagenesis driven by a hepatitis virus, including HBV, requires long incubation times, eg, in the order of 15 years.13 Therefore, it is to assume that an equal number of years must elapse before the effect of vaccination on lymphomagenesis can be seen. In Italy, HBV vaccination has been mandatory for 19 years and even for a longer period for certain categories at risk. Thus, the effect of vaccination may already be, or may soon become, statistically detectable. It is therefore timely and important to evaluate whether HBV vaccination can reduce the risk of NHL and other hematologic malignancies.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Drs Moyses Szklo and Francesco Rosmini for critical reading of the manuscript.
This work was supported in part by the “Fondazione Banca Nazionale delle Comunicazioni (BNC)” (Rome, Italy).
The “Fondazione Banca Nazionale delle Comunicazioni (BNC)” had no role in the design and execution of the study, as well as in the preparation of the manuscript. All researchers participating to the study are independent from the supporter of the study.
Authorship
Contribution: F.M. designed the review content and wrote the review; and A.M. designed the review content.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Fabrizio Marcucci, Centro Nazionale di Epidemiologia, Sorveglianza e Promozione della Salute (CNESPS), Istituto Superiore di Sanità (ISS), Via Giano della Bella 34, 00162 Rome, Italy; e-mail: fabmarcu@tin.it.