Abstract
During innate immune responses, the inflammatory CC chemokine receptors CCR1, CCR2, and CCR5 mediate the recruitment of blood monocytes to infected tissues by promoting cell migration in response to chemokines CCL2-5. Toll-like receptors also play an essential role, allowing pathogen recognition by the recruited monocytes. Here, we demonstrate that Toll-like receptor 2 (TLR2) stimulation by lipoteichoic acid (LTA) from Staphylococcus aureus leads to gradual down-modulation of CCR1, CCR2, and CCR5 from the plasma membrane of human blood-isolated monocytes and inhibits chemotaxis. Interestingly, LTA does not promote rapid desensitization of chemokine-mediated calcium responses. We found that the TLR2 crosstalk with chemokine receptors is not dependent on the Toll/interleukin-1 receptor domain-containing adaptor protein, but instead involves phospholipase C, the small G protein Rac1, and is phorbol ester sensitive. Activation of this pathway by LTA lead to β-arrestin–mediated endocytosis of Ser349-phosphorylated CCR5 into recycling endosomes, as does CCL5 treatment. However, LTA-induced internalization of CCR5 is a slower process associated with phospholipase C–mediated and phorbol ester–sensitive phosphorylation. Overall, our data indicate that TLR2 negatively regulates CCR1, CCR2, and CCR5 on human blood monocytes by activating the machinery used to support chemokine-dependent down-modulation and provide a molecular mechanism for inhibiting monocyte migration after pathogen recognition.
Introduction
Chemokine receptors are 7-transmembrane G protein–coupled receptors (GPCRs) expressed on hematopoietic cells and involved in the development, maintenance, and host defense activities of the immune system. Some chemokine receptors have a homeostatic role, whereas others are considered as inflammatory and implicated in immune responses. Chemokine receptors are particularly important for the function of leukocytes because they regulate their traffic within the immune system and their positioning in different tissues of the body.1 This is the case for the 3 inflammatory CC chemokine receptors CCR1, CCR2, and CCR5 found on the surface of leukocytes and responsible for the recruitment of these cells to sites of infection or injury.2
The fundamental role of chemokine receptors is to transmit information about the extracellular environment to the interior of a cell. Chemokine binding and activation of their receptors on the surface of target cells initiates a cascade of intracellular events that culminate in biologic effects.2,3 Binding of the CC chemokine ligand 3 (CCL3)/macrophage-inflammatory protein-1α and CCL5/regulated on activation normal T-cell expressed and secreted or CCL2/monocyte chemotactic protein-1 activates the receptors CCR1 and CCR5 or CCR2, respectively, and triggers directed-leukocyte movement (chemotaxis). Leukocytes expressing all 3 receptors are CD14+ monocytes that contribute to the innate immune defense against pathogens, and they represent 95% of human circulating blood monocytes.4,5 During infection, these monocytes transmigrate from blood vessels into tissues attracted by CCL2 secreted from endothelial cells and macrophages, before moving toward the site of infection in response to CCL3 and CCL5 released by activated tissue macrophages.6
The recognition of pathogens by recruited monocytes is mediated by pattern recognition receptors. In the case of Gram-positive bacteria such as Staphylococcus aureus one of these pattern recognition receptors, the Toll-like receptor 2 (TLR2), is responsible for the recognition of bacterial cell-wall components and is essential for protective innate immune responses.7 Pathogen recognition triggers TLR2 activation, which can induce diverse cell responses through different signaling pathways. There is evidence of distinct proinflammatory and proadhesive responses occurring by Toll/interleukin-1 receptor domain–containing adaptor molecules (TIRAP) and MyD88-dependent or -independent signaling pathways.8-11 Interestingly, a recent study has shown that a TLR2 ligand (Pam3CSK4) suppressed the migration of mouse leukocytes, including monocytes, by down-regulation of CCR1, CCR2, and CCR5 gene expression.12 Another study that used the bacterial wall component lipoteichoic acid (LTA) showed that this TLR2 ligand blocked the chemotactic activity of mouse neutrophils by inhibiting cell-surface expression of another chemokine receptor, CXC chemokine receptor-2 (CXCR2).13 These findings suggest a general mechanism of cross-regulation between TLR2 and chemokine receptors that could be important to retain migrated cells at sites of infection. However, the molecular mechanisms and intracellular pathways involved have not yet been elucidated.
The regulation of chemokine receptor expression on cells can occur by 2 means: one slow, known as downregulation (within hours), with long-term effects on gene expression and mRNA stability; the other rapid and termed down-modulation (within minutes), affecting temporarily the level of active receptors present at the plasma membrane.14,15 Down-modulation of chemokine receptors is part of the process of desensitization, which allows cells to fine-tune their response to chemokine stimulation. Classically, desensitization aims to rapidly inactivate chemokine-stimulated receptors and involves phosphorylation of serine residues in the receptor cytoplasmic tail by a G protein–coupled receptor kinase (GRK). This is followed by β-arrestin binding to the phosphorylated receptor, which uncouples receptors from G proteins, preventing further downstream signaling events, and removal of these receptors from the cell-surface by endocytosis.16,17 However, chemokine-independent or heterologous desensitization also occurs. It is because of crosstalk with other surface receptor pathways, which can involve either direct receptor interactions, communication at the G protein level, or cross-talk from downstream signaling cascades.18
Here, we delineate a pathway of heterologous down-modulation for CC chemokine receptors on human blood monocytes triggered by the TLR2-specific ligand LTA and regulating chemotaxis in a TLR2-dependent manner. This is a Ras-related C3 botulinum toxin substrate 1 (Rac1)– and phospholipase C (PLC)–mediated but TIRAP-independent response that, in the case of CCR5, triggers β-arrestin–mediated endocytosis of phosphorylated receptors. Our results indicate that the TLR2 activation pathway feeds into the normal chemokine-mediated pathway of down-modulation to desensitize CC chemokine receptors in human blood monocytes.
Methods
Reagents and antibodies
Tissue culture reagents and secondary antibodies were purchased from Invitrogen; other reagents were from Sigma-Aldrich, unless stated. Purified LTA from S aureus and ultra pure lipopolysaccharide (LPS; Escherichia coli 0111:B4) were purchased from Invivogen. NSC23766 and U73122 inhibitors were from TOCRIS; the synthetic cell-permeable TIRAP inhibitor peptide (138-151) and all other signaling inhibitors were from Calbiochem. Antibodies were from BD PharMingen, with the following exceptions: the mouse anti-CCR5 (MC-5) was provided by Prof M. Mack (Department of Internal Medicine II, University of Regensburg, Germany)19 ; rabbit anti–human lysosomal-associated membrane protein 1 (LAMP1) was from Dr Ashley Toye (Department of Biochemistry, University of Bristol, United Kingdom),20 and anti-TLR2 clone T2.5 was purchased from eBioscience. Purified unlabeled or phycoerythrin (PE)–conjugated clone E11/19, a phospho-specific CCR5 antibody binding phosphoserine 349 in the cytoplasmic tail of CCR5 was from Biolegend.21 Anti–human CCR1 or CCR2 antibodies and recombinant chemokines were purchased from R&D Systems.
Primary cell isolation and culture
Peripheral blood mononuclear cells were isolated from buffy coats of healthy donors (supplied by the National Blood Service, United Kingdom) by density centrifugation gradient with the use of lymphoprep (Axis-Shield). Monocytes were separated from lymphocytes by adherence22 and cultured in RPMI containing 20mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), 10% fetal bovine serum (FBS; PAA), 100 U/mL penicillin, 0.1 mg/mL streptomycin, and 2mM l-glutamine. Monocyte purity and CCR5 expression were assessed by flow cytometric analysis 12 hours after isolation, and experiments were performed 24 hours later. Macrophages were generated from monocytes by 10 days of culture in media containing 50 ng/mL macrophage colony-stimulating factor (PeproTech). Activated lymphoblasts were established from lymphocytes by 3 days of culture in 5 μg/mL phytohemagglutinin followed by 10 days of culture in media containing 10 U/mL interleukin-2 (PeproTech).
Chemotaxis
Microchemotaxis assays were performed in 48-well chemotaxis chambers (Neuro Probe Inc) according to the manufacturer's protocol with the use of a 5-μm polycarbonate filter. Monocytes incubated for 1 hour in buffer alone or with LTA were resuspended in RPMI with 0.1% bovine serum albumin at 1.6 × 106 cells/mL and applied to the upper chamber. After 3 hours at 37°C in a 5% CO2 incubator, transmigrated cells were counted with the use of a hemocytometer. When applicable, monocytes were preincubated for 30 minutes at 37°C with 30 μg/mL anti-TLR2 monoclonal antibody or an equal concentration of an isotype-matched control antibody.
Calcium flux
Monocytes were loaded with 2.5μM Fluo-8 AM (Stratech) before experiments were performed largely following the protocol described by Schepers et al23 (see supplemental material, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) on a CyAn flow cytometer (Beckman Coulter).
Down-modulation and endocytosis assays
Down-modulation assays were performed in binding media (BM: RPMI at pH 7.05 containing 2% bovine serum albumin). Cells were resuspended at 2 × 106 cells/mL and incubated in BM alone or containing the indicated chemokine or bacterial compound for 1 hour at 37°C, transferred to ice, and labeled as described in “Cell labeling for flow cytometry.” For endocytosis assays, monocytes were cooled to ice to prelabel cell-surface CCR5 receptors with the use of MC-5–biotin (5 μg/mL) in BM for 90 minutes at 4°C, before being treated or not with CCL5 or LTA at 37°C for 1 hour and subsequently labeled with secondary antibody, as described below.
Cell labeling for flow cytometry
Treated cells were resuspended in ice-cold BM containing 25 μg/mL human immunoglobulin G (IgG) to saturate Fc receptors before adding the relevant primary antibody (5 μg/mL) and incubated for a further 2 hours at 4°C. Samples were washed, fixed in 3% formaldehyde (Polysciences Inc), and quenched in 50mM NH4Cl as previously described.24 Cells were stained for 1 hour at room temperature (RT) with anti–mouse IgG biotin-conjugated in fluorescence-activated cell sorting (FACS) buffer (phosphate-buffered saline [PBS] containing 1% FBS and 0.05% NaN3 and 5 μg/mL human IgG), then 1 hour with streptavidin-PE (1/500; BD PharMingen). Cell-associated fluorescence was measured by flow cytometry with the use of a FACSArray flow cytometer (BD Biosciences). Data were analyzed with FlowJo Version 8.8.6 software (TreeStar Inc). For down-modulation experiments, percentage of receptor down-modulation was calculated from the mean fluorescence intensity (MFI) background and calculated as follows: (1 − [MFI treated/MFI medium]) × 100.
Immunofluorescence staining for microscopy
Treated monocytes were fixed, quenched, and adhered onto poly-D-lysine–coated coverslips. Cells were permeabilized, and Fc receptors were blocked in PBS containing 0.05% saponin (PBS/Sapo), 1% FBS, and 5 μg/mL human IgG. MC-5 (4 μg/mL) was then added for 1 hour incubation at RT. Samples washed in PBS/Sapo were labeled with a biotin-conjugated goat anti–mouse secondary and stained with streptavidin–Alexa Fluor 594 or 647. For colocalization experiments, cells were labeled with MC-5 (IgG2a) and colabeled with anti-TLR2 (T2.5; IgG1, 5 μg/mL), rabbit anti-LAMP1 (1/1000), or anti–transferrin receptor (H68.4; IgG1, 5 μg/mL). Cells were then stained with Alexa Fluor 594–conjugated anti–mouse IgG2a or IgGs plus Alexa Fluor 488–conjugated anti–mouse IgG1 or anti–rabbit (4 μg/mL). After washes in PBS/Sapo, coverslips were stained with DAPI (4′-6′-diamidine-2-phenylindole) and mounted in mowiol. Samples were examined with the use of a Zeiss LSM 510 confocal microscope with an Axiocam HRC camera; images were taken with a 63×/1.4 NA Plan-apochromat oil objective and analyzed with Axiovision software Version 4.8 (Carl Zeiss Ltd) and assembled with Adobe Photoshop CS2.
Detection of CCR5 phosphorylation with the use of a phospho site–specific antibody
Monocytes were treated with CCL5 or LTA at 37°C, before being transferred to ice and fixed in 3% formaldehyde. For flow cytometric experiments, cells were permeabilized in ice-cold 90% methanol for 30 minutes on ice, washed in FACS buffer containing phosphatase inhibitors (Roche) for 10 minutes before staining for 1 hour with PE-conjugated anti–phospho-CCR5 E11/19.25 For microscopy, fixed cells were stained with unlabeled E11/19 (5 μg/mL) in the presence of phosphatase inhibitors, as previously described.21
Western blotting
Monocytes were resuspended in BM at a density of 2 × 107 cells/mL, and 500 μL of cell suspension was used for each sample. Cells were treated in BM alone or including CCL5 or LTA for 5 minutes at 37°C, before cell lysates were prepared, and nonreduced nonboiled samples were run on a 10% sodium dodecyl sulfate–polyacrylamide electrophoresis gel. After electrophoresis, proteins were transferred on nitrocellulose membranes, and Westerns were blotted with MC-5 (5 μg/mL) and a goat anti–mouse horseradish peroxidase, as previously described.26
Data and statistical analyses
Data are expressed as the mean ± SE of the stated number of experiments and were analyzed with GraphPad PRISM v5.03 with the use of analysis of variance or a Student paired t test, where appropriate.
Results
LTA blocks monocyte migration to CCL2 and CCL5 in a TLR2-dependent manner
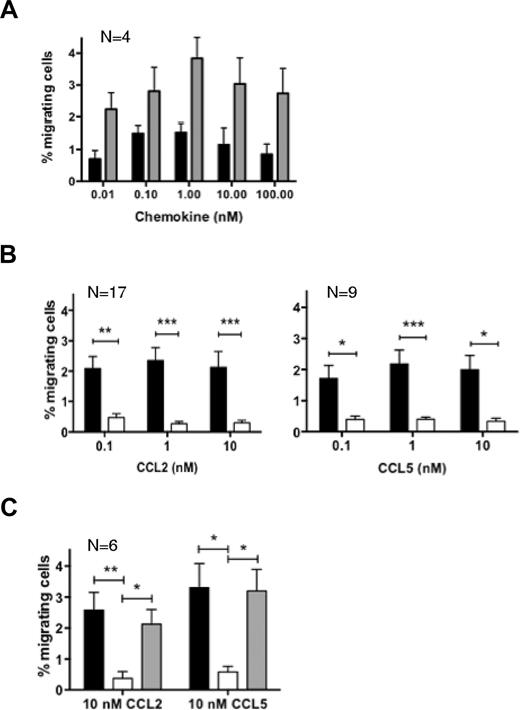
To evaluate whether LTA could modulate the responsiveness of human blood monocytes to inflammatory chemokines, we performed chemotaxis assays. We assessed the dose-dependent chemotactic response of purified blood monocytes to the CCR2 ligand CCL2, or CCL5, a shared ligand for CCR1 and CCR5 (Figure 1A). The optimal chemotactic doses of 0.1-1 or 10nM were used for future experiments. We examined the effect of the microbial compound by preincubating or not monocytes with purified LTA before exposure to chemokines. Monocytes treated with LTA failed to migrate to CCL2 and CCL5 irrespective of the dose of chemokine they were exposed to (Figure 1B). We confirmed that our purified LTA from S aureus was a specific ligand for TLR2 by demonstrating its ability to trigger IL-8 secretion in HEK293 cells transfected with TLR2 but not TLR4 (supplemental Figure 1). We demonstrated that the LTA-dependent block of migration involved TLR2 by repeating chemotaxis assays in the presence of a neutralizing anti-TLR2 monoclonal antibody (Figure 1C). TLR2 neutralization completely reversed the LTA effect for both CCL2 and CCL5, whereas the isotype control antibody did not have any effect. Our findings indicate that purified LTA from S aureus inhibits the migratory response of monocytes to CC chemokines by stimulation of its receptor TLR2.
The TLR2 ligand LTA inhibits human blood monocyte chemotactic response to CCL2 and CCL5. (A) Human blood-isolated monocytes were tested for migration toward increasing concentrations of CCL2 (■) or CCL5 (▩) with the use of a multiwell microchemotaxis chamber. (B) Migration was assessed with (□) or without (■) pretreatment with 10 μg/mL LTA. (C) Similar assays were carried out on monocytes exposed to 10nM CCL2 or CCL5 (■), pretreated with LTA in the presence of a neutralizing anti-TLR2 monoclonal antibody (▩) or an isotype control antibody (□). Assays were performed in duplicate; each graph represents the average result from N independent experiments. *P < .05, **P < .01, and ***P < .005.
The TLR2 ligand LTA inhibits human blood monocyte chemotactic response to CCL2 and CCL5. (A) Human blood-isolated monocytes were tested for migration toward increasing concentrations of CCL2 (■) or CCL5 (▩) with the use of a multiwell microchemotaxis chamber. (B) Migration was assessed with (□) or without (■) pretreatment with 10 μg/mL LTA. (C) Similar assays were carried out on monocytes exposed to 10nM CCL2 or CCL5 (■), pretreated with LTA in the presence of a neutralizing anti-TLR2 monoclonal antibody (▩) or an isotype control antibody (□). Assays were performed in duplicate; each graph represents the average result from N independent experiments. *P < .05, **P < .01, and ***P < .005.
LTA induces TLR2-dependent down-modulation of CCR1, CCR2, and CCR5
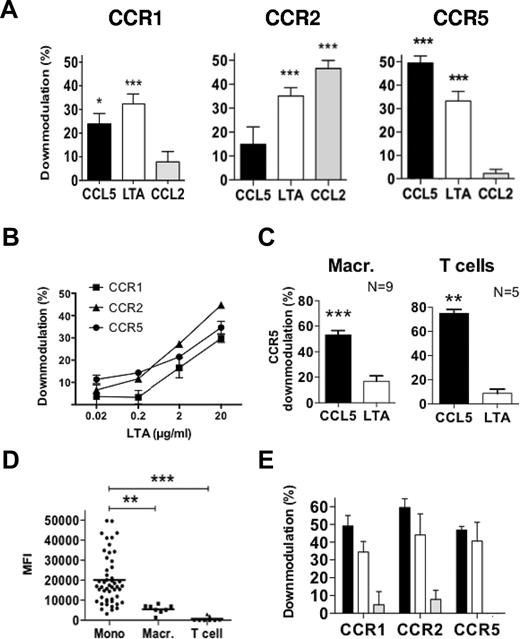
The inhibition described above could be explained by a simple functional inactivation of surface chemokine receptors or by their down-modulation. We first assessed changes in cell-surface expression of individual receptors by flow cytometry, comparing untreated to chemokine- or LTA-treated cells (Figure 2). Figure 2A shows that each receptor was down-modulated after treatment with chemokine. As expected, CCL5, which acts as an agonist for CCR1 and CCR5, affected both receptors, whereas CCL2, which specifically activates CCR2, only affected this receptor. Importantly, we found that LTA treatment induced down-modulation of all 3 receptors, with levels comparable to chemokine-treated samples. Titration experiments showed that the LTA effect is concentration dependent (Figure 2B), and the extent of down-modulation appears to correlate with the level of TLR2 expression. Indeed, down-modulation experiments performed on monocyte-derived macrophages or T lymphoblasts (Figure 2C) showed only minor or no effect of LTA on CCR5 surface expression for these 2 cell types that have very low or near undetectable levels of surface TLR2, respectively (Figure 2D).
LTA induces TLR2-dependent down-modulation of CCR1, CCR2, and CCR5 from the surface of monocytes. Monocytes (A-B), macrophages, or T-cell blasts (C) derived from human peripheral blood mononuclear cells were incubated in BM alone or treated either with the indicated chemokine at 100nM and 10 μg/mL LTA (A,C) or with increasing concentration of LTA (B) for 1 hour at 37°C, before samples were immunolabeled for cell-surface CCR1, CCR2, and/or CCR5. Results are expressed as the percentage of down-modulation relative to cells in medium alone, and graphs represent the average values from 7 (A), 3 (B), or N (C) independent triplicate experiments. (D) The surface expression level for TLR2 on the different cell types was assessed by flow cytometry. (E) Monocytes were incubated for 1 hour at 37°C with 10 μg/mL LTA (■) or LPS (▩) or pretreated with LPS before adding LTA (□), and samples were stained for cell-surface CCR1, CCR2, and CCR5; (n = 2); *P < .05, **P < .01, and ***P < .005.
LTA induces TLR2-dependent down-modulation of CCR1, CCR2, and CCR5 from the surface of monocytes. Monocytes (A-B), macrophages, or T-cell blasts (C) derived from human peripheral blood mononuclear cells were incubated in BM alone or treated either with the indicated chemokine at 100nM and 10 μg/mL LTA (A,C) or with increasing concentration of LTA (B) for 1 hour at 37°C, before samples were immunolabeled for cell-surface CCR1, CCR2, and/or CCR5. Results are expressed as the percentage of down-modulation relative to cells in medium alone, and graphs represent the average values from 7 (A), 3 (B), or N (C) independent triplicate experiments. (D) The surface expression level for TLR2 on the different cell types was assessed by flow cytometry. (E) Monocytes were incubated for 1 hour at 37°C with 10 μg/mL LTA (■) or LPS (▩) or pretreated with LPS before adding LTA (□), and samples were stained for cell-surface CCR1, CCR2, and CCR5; (n = 2); *P < .05, **P < .01, and ***P < .005.
Because CD14 and CD36 can function as coreceptors for TLR2-mediated signaling in response to LTA on human monocytes,27 we investigated whether these molecules could contribute to chemokine receptor down-modulation. CD14 is an essential coreceptor for TLR4-mediated signaling in response to E coli LPS, and we used this property to test its participation in the LTA effect. We carried out a down-modulation assay on cells incubated with purified LTA or LPS for 1 hour or treated with LPS alone for 15 minutes before adding LTA to the cell suspension (Figure 2E). LPS did not affect the down-modulation of chemokine receptors observed in response to LTA, suggesting that CD14 is not involved in the LTA effect. Furthermore, these experiments show that our purified LPS, which specifically activated TLR4 signaling (supplemental Figure 1), was unable to trigger the down-modulation of CCR1, CCR2, and CCR5. Cell pretreatment with a CD36 neutralizing antibody did not interfere with LTA-induced down-modulation of the chemokine receptors (data not shown). Altogether, these experiments indicate that LTA does not need a coreceptor to exert its effect by TLR2.
LTA induces PLC-dependent calcium flux with no effect on chemokine receptor signaling
To address whether TLR2 stimulation could trigger functional inactivation of cell-surface chemokine receptors before down-modulation, we performed calcium flux assays. LTA has been shown to activate calcium-dependent responses by TLR2, including in blood monocytes.10,11 Indeed, we found that LTA induced a concentration-dependent calcium flux (supplemental Figure 2). We confirmed that the sustained increase in cytosolic calcium was dependent on PLC and required mobilization of intracellular stores plus entry of extracellular calcium, because the membrane impermeable chelator EGTA (ethylene glycol tetraacetic acid) shortened the duration of the calcium response (Figure 3A). We then investigated the effect of LTA-induced calcium flux on the signaling activity of chemokine receptors. Figure 3B shows that CCL5 and CCL2 trigger very transient calcium flux in monocytes. Pretreatment of cells with LTA may increase the level of cytosolic calcium but does not prevent calcium mobilization in response to chemokines (Figure 3C). Only a long 1 hour of exposure to LTA, which we show down-modulates CCR1, CCR2, and CCR5 from the plasma membrane, strongly inhibited the chemokine-mediated calcium signal (Figure 3D). These experiments show that LTA does not act by inhibition of CCR1, CCR2, or CCR5 signaling activity.
LTA-triggered rapid increase in intracellular calcium does not block chemokine-induced CCR2- and CCR5-mediated calcium flux. Monocytes loaded with Fluo-8 AM were stimulated with LTA alone, in the presence of 2mM EGTA, or treated with 10μM U73122, and changes in the intracellular calcium concentration were determined by analysis of the fluorescence of the cells on a CyAn flow cytometer with the use of an argon laser at a wavelength of 488 nm (A). Similar experiments were carried out for cells stimulated with chemokines alone (B) or after short-term (C) or long-term (D) preexposure to 10 μg/mL LTA. Data are expressed as changes in the mean fluorescence intensity (MFI) of Fluo-8–loaded cells over time and are representative of 3 (A) to 5 (B-D) independent experiments.
LTA-triggered rapid increase in intracellular calcium does not block chemokine-induced CCR2- and CCR5-mediated calcium flux. Monocytes loaded with Fluo-8 AM were stimulated with LTA alone, in the presence of 2mM EGTA, or treated with 10μM U73122, and changes in the intracellular calcium concentration were determined by analysis of the fluorescence of the cells on a CyAn flow cytometer with the use of an argon laser at a wavelength of 488 nm (A). Similar experiments were carried out for cells stimulated with chemokines alone (B) or after short-term (C) or long-term (D) preexposure to 10 μg/mL LTA. Data are expressed as changes in the mean fluorescence intensity (MFI) of Fluo-8–loaded cells over time and are representative of 3 (A) to 5 (B-D) independent experiments.
Role of signaling pathways downstream of TLR2 in LTA-mediated down-modulation
To identify the link between TLR2 activation and chemokine receptor down-modulation, we initially focused on the established TLR2 MyD88-mediated pathway. MyD88 is a signaling adaptor protein that, in the case of TLR2, requires the contribution of another adaptor, TIRAP also called MAL, to interact with its cytoplasmic tail.28 We used a cell-permeable TIRAP inhibitor peptide known to interfere with this signaling pathway in mouse and human cells.29,30 We confirmed the inhibitory activity of this peptide on monocytes by showing its ability to block the production of IL-6 in response to LPS stimulation, as previously described30 (supplemental Figure 3). Down-modulation experiments were then performed on cells incubated in BM alone or containing the TIRAP inhibitor for an hour before addition of LTA. Figure 4A shows that the inhibitory peptide was unable to prevent LTA-induced down-modulation of CCR1, CCR2, or CCR5, suggesting that the TIRAP/MyD88 pathway may not mediate crosstalk with these chemokine receptors. The small guanosine triphosphatase (GTPase) Rac1 has been involved in TLR2-mediated signaling,31 in cooperation with or independently of MyD88.8,32-34 Indeed, transient activation of Rac was detectable in LTA-treated monocytes (supplemental Figure 4A-B). To test a possible role of Rac1 in LTA-mediated chemokine receptor down-modulation we used a small molecule inhibitor (NSC23766) targeting Rac1 activation by a Rac1-specific guanine nucleotide exchange factor.35 For these experiments, monocytes were cultured overnight in the presence of the cell permeable inhibitor NSC23766 or left untreated, as previously published.36 NSC23766 treatment did not affect the cell-surface expression of CCR1, CCR2, and CCR5 (supplemental Figure 4C) or their down-modulation on chemokine stimulation but significantly reduced the effect of LTA (Figure 4B). These findings suggest specific participation of Rac1 in the crosstalk between LTA-stimulated TLR2 and chemokine receptors.
LTA-induced chemokine receptor down-modulation is sensitive to PMA, inhibition of Rac1 and PLC, but not to inhibition of TIRAP or PKC. Receptor down-modulation was assessed by flow cytometry after 1 hour of exposure to 100nM chemokine (CCL2 for CCR2; CCL5 for CCR1 and CCR5) or 10 μg/mL LTA, without (■) or with (□) inhibitor (A-B,D) or as specified (C). Conditions are as follow: (A) 20μM TIRAP inhibitor peptide; (B) 100μM Rac1 inhibitor NSC23766; (C) 5μM PKC inhibitor GF109203X and 100nM PMA; (D) 10μM PLC inhibitor U73122. The results are expressed as the percentage of down-modulation and represent the average values from ≥ 4 independent experiments. *P < .05 and **P < .01.
LTA-induced chemokine receptor down-modulation is sensitive to PMA, inhibition of Rac1 and PLC, but not to inhibition of TIRAP or PKC. Receptor down-modulation was assessed by flow cytometry after 1 hour of exposure to 100nM chemokine (CCL2 for CCR2; CCL5 for CCR1 and CCR5) or 10 μg/mL LTA, without (■) or with (□) inhibitor (A-B,D) or as specified (C). Conditions are as follow: (A) 20μM TIRAP inhibitor peptide; (B) 100μM Rac1 inhibitor NSC23766; (C) 5μM PKC inhibitor GF109203X and 100nM PMA; (D) 10μM PLC inhibitor U73122. The results are expressed as the percentage of down-modulation and represent the average values from ≥ 4 independent experiments. *P < .05 and **P < .01.
To further assess the involvement of these pathways, we performed experiments on samples treated with inhibitors targeting kinases involved downstream of Rac1 and/or the TLR adaptor proteins,37-39 but the use of phosphatidylinositol-3 kinase, mitogen-activated protein/extracellular signal-related kinase kinase, p38 mitogen-activated protein kinase, c-Jun N-terminal kinase (JNK), or protein kinase C (PKC) inhibitors did not significantly interfere with either chemokine- or LTA-induced down-modulation (Table 1; Figure 4C). On the contrary, p38 and JNK inhibition selectively increased the LTA effect. Interestingly, we found that phorbol 12-myristate 13-acetate (PMA), an activator of PKC and non-PKC phorbol ester–binding proteins,40 specifically inhibited the effect of LTA on CCR2 and CCR5 (Table 1; Figure 4C). Finally, we investigated whether the calcium-dependent signaling pathway activated by TLR2 could be involved by performing assays in the presence of a phosphatidylinositol–PLC inhibitor and found that U73122 treatment considerably reduced LTA-mediated down-modulation of CCR2 and CCR5 and had some effect on chemokine-induced CCR5 but not CCR2 down-modulation (Figure 4D; Table 1).
Effect of kinase- or lipase-specific modulating compounds on CCR2 and CCR5 down-modulation
| Compound (μM) . | Primary target . | Percentage of CCR2 downmod to CCL2 ± SD (n) . | Percentage of CCR2 downmod to LTA ± SD (n) . | Percentage of CCR5 downmod to CCL5 ± SD (n) . | Percentage of CCR5 downmod to LTA ± SD (n) . |
|---|---|---|---|---|---|
| None | N/A | 40.25 ± 16.68 (10) | 35.52 ± 10.08 (10) | 34.35 ± 13.32 (10) | 28.14 ± 11.91 (10) |
| LY294002 [1.0] | PI3K inhibitor | 47.18 ± 15.12 (4) | 32.93 ± 8.52 (4) | 33.50 ± 14.41 (5) | 39.70 ± 19.12 (5) |
| Wortmannin [0.1] | PI3K inhibitor | 55.53 ± 13.23 (4) | 25.15 ± 1.97 (4) | 43.26 ± 19.18 (5) | 25.02 ± 17.69 (5) |
| PD98059 [20.0] | MAPKK (MEK) inhibitor | 52.35 ± 13.68 (4) | 28.58 ± 10.04 (4) | 35.44 ± 15.66 (5) | 36.18 ± 19.48 (5) |
| SB203580 [10.0] | p38 MAPK inhibitor | 40.86 ± 19.61 (2) | 44.54 ± 16.97 (2) | 53.94 ± 0.67 (2) | 58.04 ± 3.41 (2) |
| SP600125 [10.0] | JNK inhibitor | 51.37 ± 11.72 (2) | 61.42 ± 4.76 (2) | 56.19 ± 0.89 (2) | 58.81 ± 0.54 (2) |
| GF109203X [5.0] | PKC inhibitor | 33.73 ± 25.24 (4) | 24.50 ± 8.83 (4) | 34.40 ± 16.08 (4) | 35.32 ± 10.70 (5) |
| PMA [0.1] | PKC activator | 43.73 ± 28.33 (6) | 7.63 ± 3.19 (4) | 49.45 ± 19.87 (7) | -4.44 ± 14.06 (5) |
| U73122 [10.0] | PI-PLC inhibitor | 32.90 ± 12.01 (4) | 8.72 ± 10.89 (4) | 7.45 ± 5.27 (4) | 2.15 ± 1.81 (4) |
| Compound (μM) . | Primary target . | Percentage of CCR2 downmod to CCL2 ± SD (n) . | Percentage of CCR2 downmod to LTA ± SD (n) . | Percentage of CCR5 downmod to CCL5 ± SD (n) . | Percentage of CCR5 downmod to LTA ± SD (n) . |
|---|---|---|---|---|---|
| None | N/A | 40.25 ± 16.68 (10) | 35.52 ± 10.08 (10) | 34.35 ± 13.32 (10) | 28.14 ± 11.91 (10) |
| LY294002 [1.0] | PI3K inhibitor | 47.18 ± 15.12 (4) | 32.93 ± 8.52 (4) | 33.50 ± 14.41 (5) | 39.70 ± 19.12 (5) |
| Wortmannin [0.1] | PI3K inhibitor | 55.53 ± 13.23 (4) | 25.15 ± 1.97 (4) | 43.26 ± 19.18 (5) | 25.02 ± 17.69 (5) |
| PD98059 [20.0] | MAPKK (MEK) inhibitor | 52.35 ± 13.68 (4) | 28.58 ± 10.04 (4) | 35.44 ± 15.66 (5) | 36.18 ± 19.48 (5) |
| SB203580 [10.0] | p38 MAPK inhibitor | 40.86 ± 19.61 (2) | 44.54 ± 16.97 (2) | 53.94 ± 0.67 (2) | 58.04 ± 3.41 (2) |
| SP600125 [10.0] | JNK inhibitor | 51.37 ± 11.72 (2) | 61.42 ± 4.76 (2) | 56.19 ± 0.89 (2) | 58.81 ± 0.54 (2) |
| GF109203X [5.0] | PKC inhibitor | 33.73 ± 25.24 (4) | 24.50 ± 8.83 (4) | 34.40 ± 16.08 (4) | 35.32 ± 10.70 (5) |
| PMA [0.1] | PKC activator | 43.73 ± 28.33 (6) | 7.63 ± 3.19 (4) | 49.45 ± 19.87 (7) | -4.44 ± 14.06 (5) |
| U73122 [10.0] | PI-PLC inhibitor | 32.90 ± 12.01 (4) | 8.72 ± 10.89 (4) | 7.45 ± 5.27 (4) | 2.15 ± 1.81 (4) |
Monocytes were pretreated with vehicle (DMSO or H2O) or signalling modulator for 30 minutes at 37°C before addition of 100nM CCL2, CCL5, 10μg/mL LTA or BM alone and a further incubation of 1 hour. Cell-surface levels of CCR2 or CCR5 were determined by flow cytometry as described in “Methods.” Results from N independent experiments performed in triplicate are expressed as the percentage of downmodulation ± SD.
Downmod indicates downmodulation; PI3K, phosphatidyl inositol-3 kinase; MAPKK, mitogen-activated protein kinase kinase; MEK, mitogen-activated protein/extracellular signal-related kinase kinase; and PI, phosphatidylinositol.
LTA induces internalization of CCR5 but not TLR2
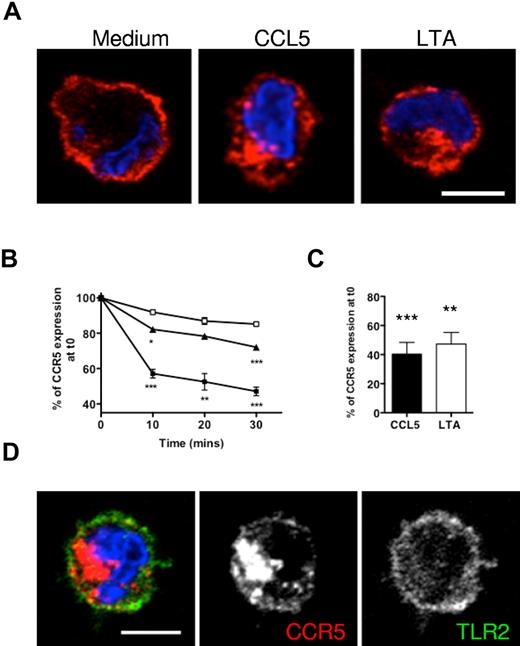
Because LTA appeared similarly competent to chemokine in triggering receptor down-modulation, we asked whether the 2 processes involved the same mechanisms. We used an anti-CCR5 antibody (MC-5), known not to impede CCR5 trafficking,26 to analyze the behavior of MC-5–bound receptors during CCL5 or LTA treatment. Immunofluorescence experiments indicated that CCR5 receptors labeled on the plasma membrane before either CCL5 or LTA treatment relocated to an internal compartment in the perinuclear area of the cells (Figure 5A). We then carried out endocytosis assays on MC-5 prelabeled cells analyzed by flow cytometry.24 We found that the rate of internalization was slower for LTA- than for CCL5-treated cells (Figure 5B). On average, 18% and 29% of MC-5 surface staining was lost with LTA after 10 and 30 minutes, respectively, compared with 43% and 53% with CCL5. However, by 60 minutes the difference between LTA- and CCL5-treated cells was negligible (Figure 5C). As previously published studies have described internalization of TLR2 into early endosomes,27 we checked whether co-internalization of CCR5 with TLR2 occurred, but TLR2 appeared exclusively located on the cell surface and its distribution did not change on LTA treatment (Figure 5D).
LTA induces slow internalization of cell-surface CCR5 molecules. Monocytes were preincubated on ice with MC-5 to label cell-surface CCR5 molecules before being transferred to 37°C for internalization assays. (A) The distribution of MC-5–labeled receptors after 1 hour of treatment was assessed by microscopy on fixed and permeabilized cells stained with a fluorescent secondary antibody (red) and DAPI (blue). (B-C) Internalization of labeled receptors was assessed by flow cytometry by measurement of cell-surface MC-5 fluorescence intensity on monocytes incubated for ≤ 30 minutes (B) in medium alone (□), with 100nM CCL5 (■), or 10 μg/mL LTA (▴), or an hour (C). Average CCR5 internalization values were determined from the results of 3 independent duplicate experiments; *P < .05, **P < .01, and ***P < .005. (D) MC-5–prelabeled LTA-treated cells were costained for TLR2. Single confocal sections are shown (scale bar = 5 μm).
LTA induces slow internalization of cell-surface CCR5 molecules. Monocytes were preincubated on ice with MC-5 to label cell-surface CCR5 molecules before being transferred to 37°C for internalization assays. (A) The distribution of MC-5–labeled receptors after 1 hour of treatment was assessed by microscopy on fixed and permeabilized cells stained with a fluorescent secondary antibody (red) and DAPI (blue). (B-C) Internalization of labeled receptors was assessed by flow cytometry by measurement of cell-surface MC-5 fluorescence intensity on monocytes incubated for ≤ 30 minutes (B) in medium alone (□), with 100nM CCL5 (■), or 10 μg/mL LTA (▴), or an hour (C). Average CCR5 internalization values were determined from the results of 3 independent duplicate experiments; *P < .05, **P < .01, and ***P < .005. (D) MC-5–prelabeled LTA-treated cells were costained for TLR2. Single confocal sections are shown (scale bar = 5 μm).
CCR5 molecules internalized on LTA treatment follow the pathway of chemokine-treated receptors
To determine whether the route of transport and fate of internalized receptors might differ in cells treated with CCL5 or LTA, we performed immunofluorescence experiments to colocalize CCR5 with markers of the endocytic pathway as previously described.19,26 We examined the subcellular distribution of internalized CCR5 molecules in CCL5- and LTA-treated monocytes (Figure 6A; supplemental Figure 5). MC-5–labeled monocytes were costained after treatment for markers of early endosomes (EEA1), early and recycling endosomes (transferrin receptor, TfR), or lysosomes (LAMP1). With CCL5-treated monocytes, CCR5 appeared in EEA1 containing early endosomes by 15 minutes, whereas in LTA-treated cells CCR5 only overlapped with EEA1 after 60 minutes (Figure 6A). Similar observations were made for the TfR (Figure 6A). In both conditions, CCR5 was mainly distributed in early endosomes containing the TfR, detected as peripheral vesicles near the plasma membrane or as a dense perinuclear compartment corresponding to the recycling endosomes. In contrast, there was no colocalization of CCR5 with the lysosomal marker LAMP1, indicating that neither chemokine treatment nor LTA-dependent activation of TLR2 target CCR5 for degradation (Figure 6A; supplemental Figure 5).
Trafficking of CCR5 molecules internalized on chemokine or LTA treatment. Immunofluorescence analysis of CCR5 internalization and colocalization with endocytic molecules: monocytes preincubated on ice with MC-5 to label cell-surface CCR5 molecules were left in medium alone or treated with 100nM CCL5 or 10 μg/mL LTA for the indicated time to allow endocytosis. Fixed and permeabilized cells were costained for CCR5 (red), nucleus (blue), and endosomal markers [(A; green); early endosomes: EEA-1; early/recycling endosomes: TfR; late endosome/lysosomes: LAMP1], or the GPCR-specific adaptors for internalization β-arrestins (B; green). Single confocal sections are shown (scale bar = 5 μm).
Trafficking of CCR5 molecules internalized on chemokine or LTA treatment. Immunofluorescence analysis of CCR5 internalization and colocalization with endocytic molecules: monocytes preincubated on ice with MC-5 to label cell-surface CCR5 molecules were left in medium alone or treated with 100nM CCL5 or 10 μg/mL LTA for the indicated time to allow endocytosis. Fixed and permeabilized cells were costained for CCR5 (red), nucleus (blue), and endosomal markers [(A; green); early endosomes: EEA-1; early/recycling endosomes: TfR; late endosome/lysosomes: LAMP1], or the GPCR-specific adaptors for internalization β-arrestins (B; green). Single confocal sections are shown (scale bar = 5 μm).
β-Arrestin 1 and 2 act as clathrin adaptors for endocytosis of GPCRs and are required for internalization of phosphorylated CCR5 after chemokine binding.41 Figure 6B shows that β-arrestin 1 and 2 are located in the cytoplasm of untreated cells and become membrane associated on CCL5 or LTA treatment. An overlap between CCR5 and β-arrestin is seen with CCL5-stimulated monocytes from 3 minutes after the addition of the chemokine; the 2 molecules colocalize mainly in peripheral vesicles just under the plasma membrane (Figure 6B). By 60 minutes, endocytized CCR5 remained colocalized with β-arrestins but in the perinuclear area of the cell corresponding to the recycling compartment. However, at 3 minutes LTA-treated cells still displayed cell-surface MC-5 staining and no overlap with β-arrestins (Figure 6B). Only after 60 minutes of LTA stimulation did CCR5 and β-arrestins colocalize in peripheral and perinuclear structures. This, together with our earlier findings, suggests that TLR2 stimulation recruits the machinery used for ligand-dependent down-modulation of CCR5 and highlight a shared pathway of endocytosis between ligand-treated and TLR2-desensitized CCR5, albeit with different kinetics.
Phosphorylation of CCR5 molecules internalized after LTA stimulation
CCR5 internalization in response to chemokine stimulation requires GRK phosphorylation of a serine residue (S349) in the cytoplasmic tail of the receptor.21,42 We have previously reported that phosphorylation of chemokine-treated CCR5 is visible by Western blotting as a decrease in the electrophoretic mobility of CCR5 in sodium dodecyl sulfate–polyacrylamide gel electrophoresis.26 Figure 7A shows a representative Western blot of total lysates from monocytes incubated for 5 minutes at 37°C in BM alone, with CCL5 or LTA. In all experiments, a slight molecular shift was observed between untreated and CCL5-treated samples, but this was not seen with LTA, suggesting that activation of TLR2 may not trigger CCR5 phosphorylation. To investigate this further, we assessed CCR5 phosphorylation by flow cytometry with the use of a phospho-specific antibody targeted against Serine 349 (p-S349).21 At the early time point of 15 minutes after adding the stimulant, only the CCL5-treated samples stained positively for p-S349 (Figure 7B). By 60 minutes, CCL5 and LTA samples were both labeled for p-S349 but with fewer positive cells than CCL5-treated samples at 15 minutes (Figure 7C). We then used the same antibody by immunofluorescence to localize phosphorylated CCR5 in permeabilized cells (Figure 7D). As with flow experiments, only CCL5-treated cells show staining above background after 15 minutes, whereas CCL5 and LTA-treated cells were stained for p-S349 CCR5 after 60 minutes. In both cases, phospho-CCR5 was distributed to intracellular organelles located in the peripheral cytoplasm close to the plasma membrane and in a perinuclear cluster, confirming internalization of CCR5 receptors phosphorylated on the GRK-specific site S349 (Figure 7D). These results show that CCR5 down-modulation in response to TLR2 activation by LTA occurs by slow internalization of phosphorylated CCR5 molecules, compared with ligand-treated cells.
LTA induces slow PMA and PLC inhibition-sensitive phosphorylation of CCR5 cytoplasmic tail. (A) Phosphorylated CCR5 band-shift observed by Western blot on total cell lysates from blood monocytes treated for 5 minutes with CCL5 (C), compared with medium (M) and LTA (L). (B) Detection of phosphorylated CCR5 in monocytes by flow cytometry on permeabilized cells with the use of a CCR5 phosphospecific (p-S349) monoclonal antibody after 15 minutes of stimulation with 100nM CCL5 or 10 μg/mL LTA [percentage of p-S349–positive cells]. SSC indicates side scatter. (C) Quantification of the average number of p-S349 CCR5-positive monocytes after 15 and 60 minutes of CCL5 (■) or LTA (□) treatment compared with untreated cells (dashed line) from ≥ 7 independent experiments; ***P < .005; ns, non significant. (D) Single slide views of Z stack confocal compressions showing untreated or CCL5- or LTA-treated monocytes (15 and 60 minutes) stained permeabilized for p-S349 CCR5. (E) Single confocal sections showing untreated or CCL5- or LTA-treated monocytes (60 minutes) incubated in the absence (Medium) or presence of 10μM PLC inhibitor (U73122) or 0.1μM phorbol esters (PMA) and stained for p-S349 CCR5 (scale bars = 10 μm).
LTA induces slow PMA and PLC inhibition-sensitive phosphorylation of CCR5 cytoplasmic tail. (A) Phosphorylated CCR5 band-shift observed by Western blot on total cell lysates from blood monocytes treated for 5 minutes with CCL5 (C), compared with medium (M) and LTA (L). (B) Detection of phosphorylated CCR5 in monocytes by flow cytometry on permeabilized cells with the use of a CCR5 phosphospecific (p-S349) monoclonal antibody after 15 minutes of stimulation with 100nM CCL5 or 10 μg/mL LTA [percentage of p-S349–positive cells]. SSC indicates side scatter. (C) Quantification of the average number of p-S349 CCR5-positive monocytes after 15 and 60 minutes of CCL5 (■) or LTA (□) treatment compared with untreated cells (dashed line) from ≥ 7 independent experiments; ***P < .005; ns, non significant. (D) Single slide views of Z stack confocal compressions showing untreated or CCL5- or LTA-treated monocytes (15 and 60 minutes) stained permeabilized for p-S349 CCR5. (E) Single confocal sections showing untreated or CCL5- or LTA-treated monocytes (60 minutes) incubated in the absence (Medium) or presence of 10μM PLC inhibitor (U73122) or 0.1μM phorbol esters (PMA) and stained for p-S349 CCR5 (scale bars = 10 μm).
Because we found that PLC inhibition and phorbol ester treatment interfered with LTA-induced CCR5 down-modulation, we assessed whether U73122 and PMA acted directly on the receptor by staining for phospho-CCR5 in the presence of these drugs (Figure 7E). Cells labeled after 60 minutes of LTA treatment with either of the drugs were poorly labeled with the p-S349 CCR5-specific antibody compared with CCL5-treated cells, indicating that PLC and PMA specifically affect CCR5 phosphorylation in response to LTA.
Discussion
There is increasing evidence for crosstalk between TLR and chemokine receptors on cells from the innate immune system, suggesting a coordinated action of these receptors for microbial recognition. Whether such communication is part of a normal protective immune response or reflects the ability of microbes to hijack the system remains controversial and may depend on the microbial component considered and the receptors involved. For example, it has been suggested that CXCR4/TLR2 crosstalk impairs host defense against Porphyromonas gingivalis and TLR2/CXCR2 cross-regulation may have a detrimental role in polymicrobial sepsis, whereas a TLR2 ligand has been shown to repress inflammation by modulating CCR1, CCR2, and CCR5 expression in vivo.12,13,43 Most of these observations were made several hours after mice infection or systemic treatment with a TLR2 ligand and were due to chemokine receptor down-regulation. This is a slow process referring to a decrease in total number of chemokine receptors because of a halt in receptor gene expression plus degradation of existing receptor molecules.44
Here, we demonstrate in human monocytes that crosstalk between TLR2 and chemokine receptor pathways can occur within minutes of TLR2 stimulation by LTA from S aureus. LTA does not act by functionally inactivating CCR1, CCR2, and CCR5 at the cell surface but by inducing progressive down-modulation of these receptors. Our flow cytometric experiments, chemotaxis assays, and morphologic analyses indicate that the TLR2-dependent cross-regulation of the chemokine receptors involves PLC- and Rac1-mediated events, is inhibited by phorbol esters and leads, in the case of CCR5, to activation of the intracellular machinery used to support chemokine-dependent receptor endocytosis. To our knowledge, this is the first pathway of heterologous desensitization identified for chemokine receptors in primary human cells with a clear functional significance. Blood circulating monocytes contribute to antimicrobial defense by migrating to sites of infection in response to inflammatory chemokines, where they respond to microbial stimuli by secreting cytokines and killing phagocytosed pathogens.5 Therefore, inhibition of monocyte migration by microbial components seems logical to retain infiltrating monocytes at the site of infection. Interestingly, our experiments show a correlation between the level of TLR2 expression and the degree of LTA-induced down-modulation on motile monocytes and largely immobile monocyte-derived macrophages. It is possible that the cross-modulation of inflammatory chemokine receptors we describe might be part of a complex process leading to monocyte arrest in response to pathogen recognition. Note that a TLR2-mediated TIRAP/MyD88-independent pathway involving Rac1 as well as a calcium-dependent pathway involving PLC-promoting monocyte adhesion and arrest have been described.8,45
Recent studies have suggested the importance of coreceptors in TLR2 activity, including CD14 contributing to the binding of LTA and CD36 being required for phagocytosis of S aureus.7,46,47 However, in our study cell treatment with LPS or a neutralizing CD36 antibody did not alter the effect of LTA, indicating that CD14 or CD36 are unlikely to be involved in TLR2-mediated cross-regulation of CCR1, CCR2, and CCR5.
As for other GPCRs, heterologous desensitization and down-modulation of chemokine receptors is thought to occur either through direct physical association with other cell-surface receptors and sometimes co-internalization, or indirectly by signaling crosstalk acting on heterotrimeric G proteins or molecules regulating GPCR surface expression.18,48 Here, we show that TLR2 does not co-internalize with CCR5 on LTA treatment and have evidence that LTA treatment does not affect the level of TLR2 expression at the cell surface (data not shown), suggesting an indirect connection between the 2 types of receptors. Our experiments after calcium responses to chemokines indicate that LTA does not trigger rapid G protein uncoupling, but experiments that used specific inhibitors highlight a crosstalk through downstream signaling events involving the small GTPase Rac1, PLC, and a phorbol ester binding protein. Although we were able to show that PLC and PMA acted on CCR5 phosphorylation, the mechanism by which Rac1 contributes to cross–down-modulation remains unclear. While PMA is a PKC-activating reagent, it also affects non–PKC-mediated events through other kinases or nonkinase molecules containing C1 domains that our study cannot tell apart.49 In fact, PMA mimics the action of the second messenger diacylglycerol generated by PLC and can activate any diacylglycerol-binding protein, including protein kinase D, diacylglycerol kinase, and lipid-regulated Rac GTPase-activating proteins called chimaerins.49 Interestingly, the β2 isoform of chimaerins has been shown to be activated by PMA and to act in cells by limiting Rac1 signaling.50,51 This may explain the PMA effect observed in our study and confirm the importance of Rac activation in the crosstalk, although further investigations will be needed to ascertain this hypothesis. The lack of inhibition seen on TIRAP, mitogen-activated protein/extracellular signal-related kinase kinase, p38 mitogen-activated protein kinase, and JNK blockade support the idea that the standard TIRAP/MyD88 pathway is not involved. This is not unique; recent publications have described cases of TLR2-mediated but TIRAP/MyD88-independent responses to bacteria.8,52 Our findings would rather point toward a role of the TLR2 calcium-activated pathway in driving the crosstalk, but further studies will be required to elucidate how.
Our investigations into the mechanisms mediating CCR5 down-modulation show that CCL5 and LTA trigger CCR5 phosphorylation on a known GRK-specific site,21 followed by internalization of cell-surface receptors into the early endocytic pathway with accumulation of β-arrestin–bound receptors in recycling endosomes. This indicates that TLR2 stimulation switches on the machinery responsible for agonist-mediated internalization, but with reduced efficacy because all the LTA-triggered events occur at a slower rate. Importantly, these experiments provide the first demonstration that CCR5 remains associated with β-arrestins during its transport along the endocytic pathway and for the first time identify CCR5 as a class B GPCR.53 Although there may be several explanations for the delay in CCR5 phosphorylation and internalization, we can exclude 2 mechanisms: (1) the need of new protein synthesis because brefeldin A, which inhibits transport from the Golgi, did not block LTA effects (supplemental Figure 6C); (2) a down-regulation of the GPCR machinery as for mouse neutrophils,54 because protein levels of GRK2/3 and β-arrestins remained unchanged after LTA stimulation (data not shown). We also excluded an autocrine effect by chemokines produced in response to TLR2 activation, because CCL5 secretion was undetected and only picomolar concentrations of CCL2 and CCL3 were measured after an hour of treatment, not enough to trigger down-modulation (supplemental Figure 6A-B). In addition, blocking secretion with brefeldin A, or transferring the supernatant of LTA-treated monocytes to fresh cells when TLR2 activation was prevented by neutralizing antibodies, did not trigger down-modulation (supplemental Figure 6C-D). Our aim is now to uncover which component(s) of the intracellular machinery mediating agonist-dependent endocytosis might be the target(s) of TLR2/Rac1/PLC signals.
The discovery of an endogenous desensitization pathway from TLR2 toward inflammatory chemokine receptors modulating the chemotactic activity of human monocytes underpins the physiologic relevance of chemokine receptor cross-regulation. It certainly would be a useful process for monocytes to rapidly tune their responsiveness to chemokines. If blood monocytes move along chemotactic gradients to reach sites of infection, they may have to pause on sensing components of the infectious agent; inducing chemokine receptor down-modulation could be the way to achieve this. Unlike the long-term stimulation of TLR2 described by McKimmie et al,12 which triggers a permanent change in monocytes by inducing loss of CCR1, CCR2, and CCR5, a short stimulation as described here could bring a temporary change in monocyte behavior by only affecting cell-surface receptor expression and being reversible. These observations highlight the importance of time and concentration thresholds in the way monocytes modulate chemokine receptor activity in response to bacterial stimuli.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank colleagues from the CII, the Department of Biology at the University of York, and beyond who have contributed reagents and ideas to this work. In particular, Dr Marika Kullberg for supplying HEK293 cells expressing TLRs, Prof Matthias Mack and Dr Ashley Toye for providing antibodies. We thank Profs Paul Kaye and Debbie Smith from the CII and Dr Gareth Evans from the Department of Biology for constructive discussions and critically reading the manuscript.
This work was supported by grants from the Biotechnology and Biological Sciences Research Council and The Royal Society (London, United Kingdom).
Authorship
Contribution: J.M.F. performed most of the experiments presented in the paper and their analysis; E.L. was responsible for setting up the endocytosis assay by flow cytometry, C.J.O. was responsible for testing the specificity of LPS and LTA; J.M.F contributed to the interpretation of the data and helped with the writing of the manuscript; and N.S. performed experiments in addition to being responsible for the design and supervision of the study, much of the data interpretation, and the writing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Nathalie Signoret, Centre for Immunology and Infection, Department of Biology and Hull York Medical School, University of York, York YO10 5DD, United Kingdom; e-mail nathalie.signoret@york.ac.uk.



![Figure 6. Trafficking of CCR5 molecules internalized on chemokine or LTA treatment. Immunofluorescence analysis of CCR5 internalization and colocalization with endocytic molecules: monocytes preincubated on ice with MC-5 to label cell-surface CCR5 molecules were left in medium alone or treated with 100nM CCL5 or 10 μg/mL LTA for the indicated time to allow endocytosis. Fixed and permeabilized cells were costained for CCR5 (red), nucleus (blue), and endosomal markers [(A; green); early endosomes: EEA-1; early/recycling endosomes: TfR; late endosome/lysosomes: LAMP1], or the GPCR-specific adaptors for internalization β-arrestins (B; green). Single confocal sections are shown (scale bar = 5 μm).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/6/10.1182_blood-2010-05-287474/4/m_zh89991066310006.jpeg?Expires=1769161139&Signature=3ODl6-8HLgbxIhUHenkruDexcvrcaBG~WveSBhpLhI5PJMikZgMNCCUXHq7xqpUy2R5saZXvZM1TlWaVCurPdNxsZ7Rs1h19J0~qhmXQbTGAkaYLyCymI5gxUtQO3AOs3xQIkBl2xxAdMneqnGQSpDv2oYiicf1TPRcLwwEhc0PIL7lhfbEdNGK37LDUb60i61HdjS13OjEJ53c6suB1l2iilCUlGn1TSgSbSQuDtOUekP~iWxy~pmBobKICZvj-2MQrNtVWXgWlDDU3otAaosqRTMkbpevOOb0lJV6PaQkf9lDFT4rNwql1IUgmom9Xm2-rFtx03qQGpfyeeKEP9A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 7. LTA induces slow PMA and PLC inhibition-sensitive phosphorylation of CCR5 cytoplasmic tail. (A) Phosphorylated CCR5 band-shift observed by Western blot on total cell lysates from blood monocytes treated for 5 minutes with CCL5 (C), compared with medium (M) and LTA (L). (B) Detection of phosphorylated CCR5 in monocytes by flow cytometry on permeabilized cells with the use of a CCR5 phosphospecific (p-S349) monoclonal antibody after 15 minutes of stimulation with 100nM CCL5 or 10 μg/mL LTA [percentage of p-S349–positive cells]. SSC indicates side scatter. (C) Quantification of the average number of p-S349 CCR5-positive monocytes after 15 and 60 minutes of CCL5 (■) or LTA (□) treatment compared with untreated cells (dashed line) from ≥ 7 independent experiments; ***P < .005; ns, non significant. (D) Single slide views of Z stack confocal compressions showing untreated or CCL5- or LTA-treated monocytes (15 and 60 minutes) stained permeabilized for p-S349 CCR5. (E) Single confocal sections showing untreated or CCL5- or LTA-treated monocytes (60 minutes) incubated in the absence (Medium) or presence of 10μM PLC inhibitor (U73122) or 0.1μM phorbol esters (PMA) and stained for p-S349 CCR5 (scale bars = 10 μm).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/6/10.1182_blood-2010-05-287474/4/m_zh89991066310007.jpeg?Expires=1769161139&Signature=BhyR6EBQ4IwEb0ScBRkAcyIJo9MU5aJgUFJ73ma6qmAuIHxgfhxFvfvSRZAFnqdtZvzx8cGbrvOJddT-n9v9~l7GJcD6wxwTdR6PMVrS2Uvv8keDRJkW4wAhG12b9fmxbKe11jpRxyuP4Eix3oCnVDOubEkt9RRZJZ-hvP~LeE7Q8MIWl87U6VCL70xsoidem9gXhERH-e-hgWkCyBzXrvRT87fLN8Zah5AQYt1EE7Rk~xBp~1k6ARRpZhBQ6WW9yBcj1bNilZqTuAkHYNE1EDWJ0jlPYs66Ws8pitiTx2vY~r3~8bIDWE~56R1wzZJxafORVUV4S9bfvOpQVtlACg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal