Abstract
Pregnancy represents a major challenge to immunologic tolerance. How the fetal “semiallograft” evades maternal immune attack is unknown. Pregnancy success may involve alteration of both central (thymic) and peripheral tolerance mechanisms. HIV infection is characterized by CD4+ T-cell depletion, chronic immune activation, and altered lymphocyte subsets. We studied immunologic consequences of pregnancy in 20 HIV-infected women receiving highly active antiretroviral therapy (HAART), and for comparison in 16 HIV-negative women. Lymphocyte subsets, thymic output, and cytokine profiles were measured prospectively during pregnancy and postpartum. A significant expansion of CD4+CD25+CD127lowFoxP3+ regulatory T cells indicating alteration of peripheral tolerance was seen during second trimester, but only in HIV-negative women. HIV-infected women had lower CD4 counts, lower thymic output and Th-2 cytokines, and more immune activation at all time points compared with controls. Immune activation was decreased in HIV-infected patients during pregnancy. In contrast, CD4 counts were increased in both groups. In conclusion, the study does not indicate that pregnancy adversely affects the immunologic course of HIV infection. However, despite HAART during pregnancy, HIV-infected women display different immunologic profiles from HIV-negative women, which may have importance for the induction of fetal-maternal tolerance and in part explain the increased risk of abortion in HIV-infected women.
Introduction
During pregnancy, the maternal immune system is exposed to a major challenge. The fetus expresses paternal alloantigens, yet it is not rejected.1 The association between HY-restricting human leukocyte antigen (HLA) class II alleles and recurrent miscarriage subsequent to a firstborn boy indicates a CD4+ T cell-mediated mechanism in these cases.2 How the fetus normally evades a maternal immune response is not fully known, but development of fetal-maternal tolerance possibly relies on alterations of both central (thymic) tolerance3-6 and peripheral tolerance mediated by regulatory T cells (Tregs).7-11 The thymus is reduced in size and changed in structure during pregnancy, and in mice a substantial loss of thymocyte proliferation occurs from early pregnancy.5,6,12 This may promote survival of the fetus by reducing production of new potentially fetus-reactive T cells. Both thymic size and function return to normal postpartum.6 Likewise, peripheral tolerance is altered during pregnancy; levels of Tregs are expanded during both murine and human pregnancy.7-11 This expansion is crucial to fetal survival, and lack of mobilization of Tregs may terminate the pregnancy in abortion.7,9 A delicate balance of Th1-Th2 cytokines directed toward a Th2-dominant pattern is also considered a very important mechanism in favor of pregnancy success.13-15
Knowledge of immunologic changes during pregnancy in HIV-infected women is limited. An increased risk of spontaneous abortion has been reported in HIV-infected women, as has stillbirth, low birth weight, and preterm delivery.16 With the introduction of highly active antiretroviral therapy (HAART), the future prospects for HIV-infected patients have improved, and the risk of mother-to-child HIV transmission has been dramatically reduced to less than 1% in countries where resources have allowed for prophylactic interventions.17-19 In light of this achievement, an increasing number of HIV-infected women in the industrialized countries decide to become pregnant and have children. HIV infection is characterized by progressive depletion of CD4+ cells. Furthermore, HIV infects the thymus, leading to reduced thymic output of naive T cells and T cells containing T-cell rearrangement DNA excision circles (TRECs).20,21 In contrast, levels of Tregs seem to be elevated in HIV-infected patients, most probably reflecting their role in HIV pathogenesis as suppressors of HIV-induced chronic immune activation.22-27
It remains unclear whether or not pregnancy in HIV-infected women represents an extra challenge to the impaired immune system.28,29 The present prospective study was designed to investigate immunologic changes in HIV-infected and HIV-negative pregnant women, as measured by changes in thymic output, lymphocyte subpopulations including naive cells, immune activation, and Tregs as well as cytokine profiles during and after pregnancy.
Methods
Patients and study design
This prospective study was conducted during the period from September 2005 until September 2008 at Copenhagen University Hospital, Hvidovre, Denmark. Twenty HIV-infected pregnant women were included from the Department of Infectious Diseases. As control subjects, 16 healthy HIV-negative pregnant women matched for age and ethnicity were included. The study was approved by the local ethics committee of Copenhagen and Frederiksborg Kommuner (registration number KF 01-264902), and informed consent was obtained from all participants in accordance with the Declaration of Helsinki.
Blood samples were drawn from patients and controls at 4 time points during and after pregnancy (in the first trimester, 8-14 weeks, n = 32; in the second trimester, 20-28 weeks, n = 36; and in the third trimester, 34-40 weeks, n = 32; and once at 2-6 months postpartum, n = 33). All HIV-infected women were treated with HAART during pregnancy, 8 women were naive to HAART before pregnancy and were treated from gestation week 14 according to national Danish guidelines, and 12 women were on HAART already before pregnancy. Five of the 20 HIV-infected women were diagnosed with HIV in relation to current pregnancy. Most HIV-infected women delivered by cesarean section. In cases of low HIV-RNA, vaginal delivery was offered. One HIV-infected woman experienced progressive hypertension during the second trimester, and the pregnancy ended up in a stillborn child at gestational week 27. Characteristics of the included pregnant women are shown in Tables 1 and 2.
Clinical characteristics of HIV-infected pregnant women
| Patient ID . | Years since HIV diagnosis . | Nadir CD4 count, cells/μL . | HIV-related illness . | CD4 count 6 mo before pregnancy, cells/mL . | Gestational age at introduction of HAART, wk . | HAART regimen during current pregnancy . | Years of HAART before current pregnancy . |
|---|---|---|---|---|---|---|---|
| 1 | 6 | 152 | NA | 570 | 0 | AZT, 3TC, ABC | 6 |
| 2 | 14 | 252 | NA | 1341 | 0 | 3TC, ABC, DDI, RTV, SQV | 6 |
| 3 | 6 | 462 | NA | NA | 14 | AZT, 3TC, LPV, RTV | 0 |
| 4 | 0 | 263 | NA | NA | 14 | AZT, 3TC, LPV, RTV | 0 |
| 5 | 11 | 140 | NA | 710 | 0 | AZT, 3TC, LPV, RTV | 10 |
| 6 | 0 | 23 | Esophageal candida | NA | 14 | AZT, 3TC, LPV, RTV | 0 |
| 7 | 0 | 180 | NA | NA | 14 | AZT, 3TC, LPV, RTV | 0 |
| 8 | 6 | 259 | NA | 823 | 0 | 3TC, DDI, RTV, ATV | 6 |
| 9 | 5 | 266 | NA | 400 | 0 | AZT, 3TC, LPV, RTV | 5 |
| 10 | 11 | 257 | NA | 408 | 0 | 3TC, ABC, LPV, RTV | 2 |
| 11 | 8 | 286 | NA | 293 | 14 | FTC, TFV, RTV, ATV | 0 |
| 12 | 0 | 264 | NA | NA | 14 | 3TC, ABC, LPV, RTV | 0 |
| 13 | 0 | 158 | NA | NA | 14 | 3TC, ABC, LPV, RTV | 0 |
| 14 | 2 | 344 | NA | 740 | 14 | AZT, 3TC, LPV, RTV | 0 |
| 15 | 8 | NA | NA | 535 | 0 | FTC, TFV, RTV, ATV | 1 |
| 16 | 19 | 40 | Esophageal candida | 166 | 0 | DDI, TFV, FTC, LPV, RTV | 9 |
| 17 | 6 | 143 | NA | 567 | 0 | AZT, 3TC, LPV, RTV | 6 |
| 18 | 4 | 13 | Mycobacterium avium, herpes zoster | 390 | 0 | 3TC, ABC, NEV | 4 |
| 19 | 9 | 118 | Tuberculosis | 410 | 0 | ABC, TFV, RTV, ATV | 9 |
| 20 | 6 | 264 | NA | 734 | 0 | AZT, 3TC, LPV, RTV | 5 |
| Patient ID . | Years since HIV diagnosis . | Nadir CD4 count, cells/μL . | HIV-related illness . | CD4 count 6 mo before pregnancy, cells/mL . | Gestational age at introduction of HAART, wk . | HAART regimen during current pregnancy . | Years of HAART before current pregnancy . |
|---|---|---|---|---|---|---|---|
| 1 | 6 | 152 | NA | 570 | 0 | AZT, 3TC, ABC | 6 |
| 2 | 14 | 252 | NA | 1341 | 0 | 3TC, ABC, DDI, RTV, SQV | 6 |
| 3 | 6 | 462 | NA | NA | 14 | AZT, 3TC, LPV, RTV | 0 |
| 4 | 0 | 263 | NA | NA | 14 | AZT, 3TC, LPV, RTV | 0 |
| 5 | 11 | 140 | NA | 710 | 0 | AZT, 3TC, LPV, RTV | 10 |
| 6 | 0 | 23 | Esophageal candida | NA | 14 | AZT, 3TC, LPV, RTV | 0 |
| 7 | 0 | 180 | NA | NA | 14 | AZT, 3TC, LPV, RTV | 0 |
| 8 | 6 | 259 | NA | 823 | 0 | 3TC, DDI, RTV, ATV | 6 |
| 9 | 5 | 266 | NA | 400 | 0 | AZT, 3TC, LPV, RTV | 5 |
| 10 | 11 | 257 | NA | 408 | 0 | 3TC, ABC, LPV, RTV | 2 |
| 11 | 8 | 286 | NA | 293 | 14 | FTC, TFV, RTV, ATV | 0 |
| 12 | 0 | 264 | NA | NA | 14 | 3TC, ABC, LPV, RTV | 0 |
| 13 | 0 | 158 | NA | NA | 14 | 3TC, ABC, LPV, RTV | 0 |
| 14 | 2 | 344 | NA | 740 | 14 | AZT, 3TC, LPV, RTV | 0 |
| 15 | 8 | NA | NA | 535 | 0 | FTC, TFV, RTV, ATV | 1 |
| 16 | 19 | 40 | Esophageal candida | 166 | 0 | DDI, TFV, FTC, LPV, RTV | 9 |
| 17 | 6 | 143 | NA | 567 | 0 | AZT, 3TC, LPV, RTV | 6 |
| 18 | 4 | 13 | Mycobacterium avium, herpes zoster | 390 | 0 | 3TC, ABC, NEV | 4 |
| 19 | 9 | 118 | Tuberculosis | 410 | 0 | ABC, TFV, RTV, ATV | 9 |
| 20 | 6 | 264 | NA | 734 | 0 | AZT, 3TC, LPV, RTV | 5 |
NA indicates not available; AZT, Zidovudin; 3TC, Lamivudin; ABC, Abacavir; DDI, Didanosin; RTV, Ritonavir; LPV, Lopinavir; ATV, Atazanavir; FTC, Emtricitabine; TFV, Tenofovir; and NEV, Nevirapine.
Clinical characteristics of HIV-infected and HIV-negative pregnant women
| Characteristic . | HIV-infected women . | HIV-negative women . |
|---|---|---|
| No. included | 20 | 16 |
| Age, y (mean ± SEM) | 32.8 ± 1.3 | 32.5 ± 1.4 |
| Ethnicity (black/Asian/white) | 9/4/7 | 5/4/7 |
| Parity (primi/multi) | 13/7 | 8/8 |
| Mode of delivery (vaginal/caesarean section) | 4/16 | 12/4 |
| Characteristic . | HIV-infected women . | HIV-negative women . |
|---|---|---|
| No. included | 20 | 16 |
| Age, y (mean ± SEM) | 32.8 ± 1.3 | 32.5 ± 1.4 |
| Ethnicity (black/Asian/white) | 9/4/7 | 5/4/7 |
| Parity (primi/multi) | 13/7 | 8/8 |
| Mode of delivery (vaginal/caesarean section) | 4/16 | 12/4 |
Blood samples collected in tubes containing ethylenediaminetetraacetic acid were used to obtain a full blood count and for flow cytometry. Plasma was used to determine cytokine levels. Blood samples drawn into tubes containing heparin were used to obtain peripheral blood mononuclear cells and for enrichment of CD4+ cells and determination of TRECs. Remaining enriched CD4+ cells were cryopreserved. Thawed enriched CD4+ cell samples were used for flow cytometric analysis of Tregs. Plasma HIV-RNA levels were measured with a real-time polymerase chain reaction (PCR) kit (COBAS AmpliPrep/COBAS TaqMan; Roche Diagnostics) with a detection threshold of 40 copies/mL. Hemoglobin, leukocyte, and lymphocyte counts were performed at the Department of Clinical Biochemistry.
Flow cytometry
Determination of total and naive CD4+ and CD8+ cells and activated lymphocyte subsets was done on full blood as described previously.30 Naive and activated cells were defined as CD4+ or CD8+ cells coexpressing CD45RA+CD62L+CD27+ and CD38+HLA-DR+, respectively. Samples were analyzed using a 4-color FACSCalibur (BD Biosciences) equipped with a 488-nm argon-ion laser and a 635-nm red diode laser. Data were processed using CellQuestPro software Version 5.2 (BD Biosciences). Monoclonal antibodies used were isotype control γ1-fluorescein isothiocyanate (FITC)/γ1-phycoerythrin (PE)/γ1-peridinin chlorophyll protein (PerCP)/γ1-allophycocyanin (APC), CD3-APC, CD4-PerCP, CD8-PerCP, CD27-FITC, CD38-PE, CD45RA-PE, CD62L-APC, and HLA-DR-FITC, all purchased from BD Biosciences. To obtain the absolute number of a lymphocyte population, the fraction of cells in a lymphocyte gate expressing lymphocyte markers was multiplied by the lymphocyte count.
For quantification of percentages of Tregs, cryopreserved CD4+ cells were thawed, washed, and incubated overnight at 37°C in 5% CO2. Tregs were defined as CD3+CD4+CD25+CD127lowFOXP3+ cells, and naive and memory Tregs as Tregs coexpressing CD45RA+CD27+ and CD45RA−CD27−, respectively. CD4+ cells were immunostained with anti-CD3 PerCP (BD Biosciences), anti-CD4 APC-AlexaFluor-750 (eBioscience), anti-CD25 PE (Miltenyi Biotec), anti-CD127-FITC (eBioscience), anti-CD45RA PECy7 (eBioscience), anti-CD27 AlexaFluor-700 (eBioscience), and antiFoxP3 APC (eBioscience) antibodies. FoxP3 staining buffer set (eBioscience) was used for the intracellular staining of FoxP3, according to the manufacturer's instructions. Briefly, peripheral blood mononuclear cells were stained for surface markers, followed by fixation, permeabilization, and labeling of FoxP3. Stained cells were fixed using BD Stabilizing Fixative (BD Biosciences) and stored at 4°C until flow recording (within 24 hours).
Data on Tregs were acquired on a BD LSRII instrument, using BD FACSDiva software Version 4.0 (BD Biosciences) and analyzed with FlowJo software Version 8.8.6 (TreeStar).
Determination of TRECs
CD4+ cells were enriched from peripheral blood mononuclear cells using a magnetic cell separator (MACS; Miltenyi Biotec) as previously described.30 The purity of sorted populations was determined by flow cytometry and was always more than 80%. DNA was extracted from CD4+ cells using a salting out procedure, and quantification of signal-joint TREC was done by real-time quantitative PCR with the 5′-nuclease (TaqMan) assay as previously described.30 Samples were analyzed in triplicates that never varied by more than 10%, and the results were averaged. The CD4-TREC frequency (CD4-TREC%) was determined as the mean signal-joint TREC value/mean mannan-binding lectin coding sequence value. The CD4-TREC% was multiplied by the CD4 count to obtain total CD4-TRECs per milliliter blood.
Luminex
Cytokine analysis (interleukin-1b [IL-1b], IL-4, IL-6, IL-10, interferon-γ [IFN-gamma], transforming growth factor-β [TGF-β]) was performed with Luminex technology using an in-house assay as described.31 In brief, 50 μL plasma or standard diluted 1:10 in assay buffer (phosphate-buffered saline containing 0.5% Tween 20 and 1% bovine serum albumin) was incubated 1.5 hours with a 50-μL suspension of capture-antibody-conjugated beads, 1500 beads per analysis per sample diluted in assay buffer. The beads were washed twice with 200 μL washing buffer (phosphate-buffered saline containing 0.5% Tween) per well and incubated for 1.5 hours with a mixture (50 μL) of biotinylated detection antibodies each diluted 1:600 in assay buffer. A total of 50 μL of streptavidin-PE 20 μg/mL (Invitrogen) was added to the wells and the incubation continued for additional 30 minutes. The beads were washed twice and resuspended in 100 μL washing buffer. After 15 minutes of shaking, samples were analyzed on Luminex 100 (Luminex) according to the manufacturer's instructions. The results are given in median fluorescence intensities.
Statistical analysis
Data are presented as mean (± SEM) or median (25%; 75% quartiles). Differences in the development of investigated endpoints between groups during the course of the study were evaluated using a repeated measurements analysis of variance (the PROC MIXED model in the SAS software package). Group effects, time effects (effect of pregnancy), and the interaction between time and group effects (time by group) were included in the model. Differences in immunologic measurements between HIV-infected women already on HAART before pregnancy and HIV-infected women beginning HAART during pregnancy were tested, and they only differed with regard to viral load. All HIV-infected women were then analyzed together in further analysis. The correlation between measurements for the same person was taken into account by including an ante-dependent correlation structure in the analysis. Some variables were log-transformed before the analysis. The assumptions for using an analysis of variance were evaluated by visual inspection of histograms and residual plots for evaluating equal variances. Within the mixed repeated model, a significant time by group effect was analyzed at the 4 different time points, and the development of the variable was evaluated in HIV-infected and HIV-negative women separately. Left-censured data (HIV-RNA and cytokine measurements) were analyzed across time using Tobit regression with sandwich estimators to compensate for dependence within women over time.32 A 5% significance level was used. Analyses were performed using SAS, Version 9.1.3; and the R software, Version 2.10.1.33
Results
Significant increase in CD4 and CD8 counts postpartum in both HIV-infected and HIV-negative women
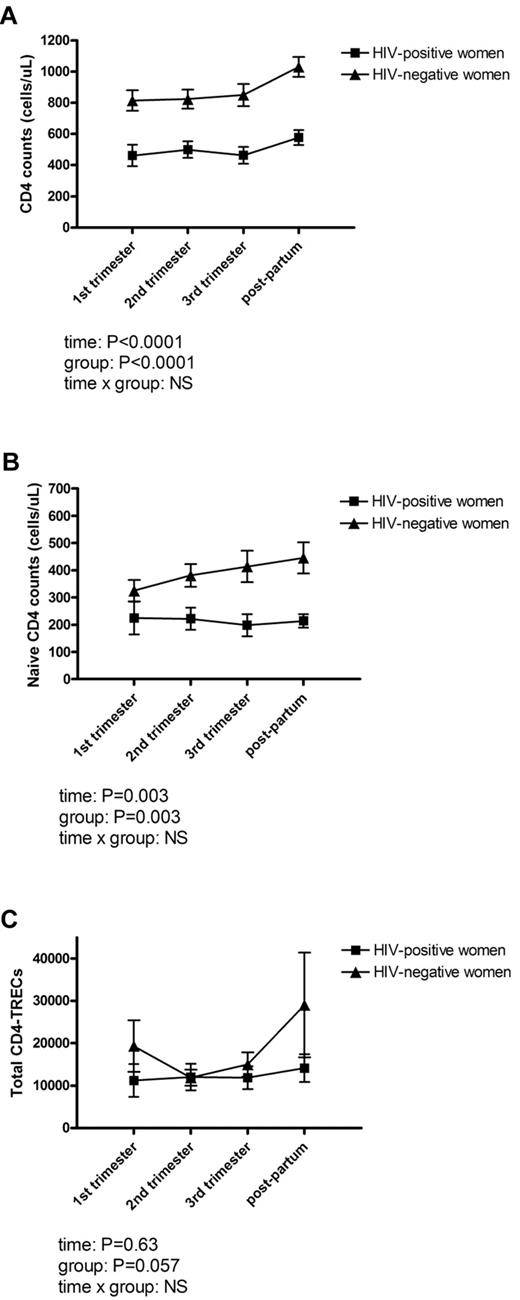
To evaluate the impact of pregnancy on hematologic and immunologic variables, hemoglobin, leukocyte counts, and lymphocyte counts were measured in all women at 4 time points during and after pregnancy (in the first, second, and third trimester and once at 2-6 months postpartum). No difference was found between HIV-infected women and controls in hemoglobin (P = .07), leukocyte counts (P = .25), or total lymphocyte counts (P = .75) at any time point. Total CD4 counts were significantly lower in HIV-infected women at all time points (P < .0001; Figure 1A), and so were percentages of CD4+ cells (P < .0001). In contrast, percentages of and total CD8 counts were higher in HIV-infected women at all time points. There was a significant effect of pregnancy on both CD4 counts (P < .0001) and CD8 counts (P < .0001). Thus, CD4 counts in both HIV-infected women and controls were significantly increased postpartum (from 461 ± 69 cells/μL in first trimester to 577 ± 48 cells/μL postpartum in HIV-infected women vs 814 ± 65 cells/μL in first trimester to 1029 ± 64 cells/μL postpartum in controls). There was no difference in CD4 counts between HIV-infected women who started HAART during current pregnancy and HIV-infected women on HAART before pregnancy. HIV-RNA was 3440 (1197; 52 600) in the first trimester in women who started HAART during current pregnancy. After initiation of HAART, HIV-RNA was significantly reduced to 40 (40; 71) in the second trimester and remained low during pregnancy. In the group of HIV-infected women already on HAART, HIV-RNA was 40 (40; 40) at all time points.
Thymic output during and after pregnancy. The effect of pregnancy on CD4 counts (A), naive CD4 counts (B), and total CD4-TRECs (C) in 20 HIV-infected and 16 HIV-negative women. Measurements were performed at 3 time points during pregnancy (in the first, second, and third trimesters) and 2 to 6 months postpartum. (A) CD4 counts were at all time points significantly lower in HIV-infected women, and there was a significant effect of pregnancy (P < .0001) with increased CD4 counts postpartum. (B) Naive CD4 counts were significantly lower at all time points in HIV-infected women compared with controls (P = .0030), and there was a significant effect of pregnancy (P = .003). (C) Total CD4-TRECs tended to be lower in HIV-infected women (P = .057). No significant effect of pregnancy on total CD4-TRECs was demonstrated. Data are mean (± SEM). P values for time, group, and time × group effect in the mixed repeated models are shown. NS indicates not significant.
Thymic output during and after pregnancy. The effect of pregnancy on CD4 counts (A), naive CD4 counts (B), and total CD4-TRECs (C) in 20 HIV-infected and 16 HIV-negative women. Measurements were performed at 3 time points during pregnancy (in the first, second, and third trimesters) and 2 to 6 months postpartum. (A) CD4 counts were at all time points significantly lower in HIV-infected women, and there was a significant effect of pregnancy (P < .0001) with increased CD4 counts postpartum. (B) Naive CD4 counts were significantly lower at all time points in HIV-infected women compared with controls (P = .0030), and there was a significant effect of pregnancy (P = .003). (C) Total CD4-TRECs tended to be lower in HIV-infected women (P = .057). No significant effect of pregnancy on total CD4-TRECs was demonstrated. Data are mean (± SEM). P values for time, group, and time × group effect in the mixed repeated models are shown. NS indicates not significant.
The effect of pregnancy on thymic output in HIV-infected and HIV-negative pregnant women
Naive CD4 and CD8 counts were measured in HIV-infected and HIV-negative pregnant women; and to further estimate thymic output, TREC measurements were done on purified CD4+ cells. Total naive CD4 counts were significantly lower at all time points in HIV-infected women compared with controls (P = .003; Figure 1B), whereas percentages of naive CD4+ cells were comparable between HIV-positive and HIV-negative women (P = .28). Likewise, naive CD8 counts were comparable between HIV-positive and HIV-negative women (P = .27). There was a significant effect of pregnancy on naive CD4 counts (P = .003; Figure 1B). The CD4-TREC% was comparable in HIV-infected and HIV-negative women (P = .23), whereas total CD4-TRECs tended to be lower in HIV-infected women (P = .057). However, we were not able to demonstrate a significant effect of pregnancy on either CD4-TREC% or total CD4-TRECs (Figure 1C).
Expansion of Tregs during second trimester in HIV-negative but not HIV-infected women
Levels of Tregs (CD3+CD4+CD25+CD127lowFOXP3 phenotype) and subpopulations of naive (CD45RA+CD27+ Tregs) and memory Tregs (CD45RA-CD27- Tregs) were determined in all women during and after pregnancy. Statistical analysis of %Tregs showed a significant interaction with time, indicating that Tregs changed differently during pregnancy in the 2 groups. In HIV-negative women, %Tregs were significantly expanded during the second trimester (from 1.3% ± 0.2% in the first trimester to 1.9% ± 0.1% in the second trimester, P = .007; followed by a decrease in the third trimester to 1.3% ± 0.1%, P = .002). In contrast, %Tregs in HIV-infected women were unchanged from first to second trimester. Thus, in the second trimester, %Tregs were significantly higher in controls (P = .014). After the second trimester, %Tregs increased significantly in HIV-infected women (from 1.5% ± 0.2% to 2.0% ± 0.3% postpartum, P = .032; Figure 2A).
Peripheral tolerance during and after pregnancy. The effect of pregnancy on percentages of CD4+CD25+CD127lowFOXP3+ Tregs (A), and CD4+CD38+HLA-DR+ cells (B), and on plasma level of TGF-β (C) in 20 HIV-infected and 16 HIV-negative women. Measurements were performed at 3 time points during pregnancy (in the first, second, and third trimesters) and 2 to 6 months postpartum. (A) Statistical analysis of percentage Tregs showed a significant interaction with time. In HIV-negative women, percentage Tregs were significantly expanded during the second trimester to levels significantly higher than in HIV-negative women (P = .02). (B) Percentages of CD4+CD38+HLA-DR+ cells were significantly higher in HIV-infected women than in controls (P = .0024) and decreased significantly during pregnancy (P = .032). (C) TGF-β was significantly lower in HIV-infected women at all time points, and a significant interaction with time was demonstrated (P = .035). Data are mean (± SEM). P values for time, group, and time × group effect in the mixed repeated models are shown. NS indicates not significant.
Peripheral tolerance during and after pregnancy. The effect of pregnancy on percentages of CD4+CD25+CD127lowFOXP3+ Tregs (A), and CD4+CD38+HLA-DR+ cells (B), and on plasma level of TGF-β (C) in 20 HIV-infected and 16 HIV-negative women. Measurements were performed at 3 time points during pregnancy (in the first, second, and third trimesters) and 2 to 6 months postpartum. (A) Statistical analysis of percentage Tregs showed a significant interaction with time. In HIV-negative women, percentage Tregs were significantly expanded during the second trimester to levels significantly higher than in HIV-negative women (P = .02). (B) Percentages of CD4+CD38+HLA-DR+ cells were significantly higher in HIV-infected women than in controls (P = .0024) and decreased significantly during pregnancy (P = .032). (C) TGF-β was significantly lower in HIV-infected women at all time points, and a significant interaction with time was demonstrated (P = .035). Data are mean (± SEM). P values for time, group, and time × group effect in the mixed repeated models are shown. NS indicates not significant.
We found no significant difference in naive Tregs between HIV-infected and HIV-negative women. To examine whether naive Tregs represent recent thymic emigrant Tregs, the association between CD4-TREC% and naive Tregs was investigated. In a mixed model, the percentage of naive Tregs was found to depend significantly on the CD4-TREC% in both HIV-infected women (P = .0004) and HIV-negative women (P = .017).
Immune activation during pregnancy
Immune activation was measured in all women during and after pregnancy. Percentages of CD4+CD38+HLA-DR+ and CD8+CD38+HLA-DR+ cells were significantly higher in HIV-infected women than in controls (P = .0024 and P = .0069, respectively). No overall effect of pregnancy was seen on either activated CD4+ or CD8+ cells. However, in HIV-infected women, activated CD4+ cells decreased significantly during pregnancy (P = .032; Figure 2B) both in HIV-infected women who started HAART during pregnancy and in HIV-infected women already on HAART before pregnancy. The association between immune activation and %Tregs was investigated in the mixed model, and the percentage of CD4+CD38+HLA-DR+ cells was found to depend significantly on %Tregs in HIV-infected women (P = .034). The same association was not found regarding CD8+CD38+HLA-DR+ cells (P = .36).
Significantly lower levels of IL-4, IL-10, and TGF-β in HIV-infected pregnant women
Plasma levels of IL-1b, IL-4, IL-6, IL-10, IFN-γ, and TGF-β were measured in all women at all time points. Levels of IL-4, IL-10, and TGF-β were significantly lower in HIV-infected women at all time points, and there was a significant interaction with time with regard to TGF-β (Figure 2C), indicating that TGF-β changed differently during pregnancy in the 2 groups. There was a statistically significant effect of pregnancy on IL-10 and TGF-β levels (P = .022 and P = .0001, respectively). No difference was found in levels of IL-1b, IL-6, and IFN-γ between HIV-infected women and controls. We found no association between percentages of Tregs and levels of TGF-β and IL-10, respectively.
Discussion
Pregnancy represents a major challenge to immunologic tolerance, and the present study was designed to investigate immunologic consequences of pregnancy in HIV-infected women during the HAART era. Lymphocyte subpopulations, thymic output, and cytokine levels were measured prospectively once during each trimester of pregnancy and once postpartum. For comparison, HIV-negative pregnant women were included. A significant expansion of Tregs indicating alteration of peripheral tolerance was seen during the second trimester, but only so in HIV-negative women. Significant alterations of central tolerance as estimated by TREC measurements were not found. HIV-infected women had lower total and naive CD4 counts, lower Th-2 cytokine levels, and more immune activation at all time points compared with HIV-negative women. CD4 counts were significantly increased during pregnancy in both HIV-infected women and controls. In contrast, immune activation was decreased in HIV-infected patients during pregnancy. Thus, despite the use of HAART during pregnancy, HIV-infected women display a different immunologic profile from HIV-negative pregnant women, and these differences may have importance for the induction of fetal-maternal tolerance.
How the immunologic paradox of pregnancy defying the rules of rejection develops has not been fully clarified. One hypothesis is that systemic regulatory processes are altered during pregnancy.1 Studies in mice have shown that Tregs are expanded from early pregnancy by paternal antigens, and the absence of Tregs can lead to pregnancy failure.7,11 Furthermore, mice undergoing abortion have a diminished number of Tregs compared with normal pregnant mice.34 Likewise, studies in humans have demonstrated an expansion of Tregs during pregnancy,8,10,11 and levels of human decidual Tregs are significantly lower in women undergoing spontaneous compared with induced abortion,9 supporting the important role of Tregs in determining pregnancy success. Inadequate numbers of Tregs have also been linked with infertility35 and pre-eclampsia36 and so has an imbalance in the ratio of FOXP3+ Tregs to IL-17-expressing CD4+ cells.37 In contrast, an increase in Tregs during pregnancy may explain why several autoimmune conditions tend to remit during pregnancy.38
We examined levels of CD4+CD25+CD127lowFOXP3+ Tregs in HIV-infected and HIV-negative women during and after pregnancy. In line with other studies, we found an expansion of Tregs with a peak during the second trimester and a drop during the third trimester, indicative of establishment of fetal-maternal tolerance, but only in HIV-negative women. Interestingly, Tregs in HIV-infected women were not mobilized. Levels of Tregs are generally increased in HIV-infected patients, although this finding has been debated.22-27 Accurate phenotypic identification of Tregs is difficult; and as a result, inconsistent phenotypes have been used to identify Tregs. Consequently, comparison of results between published studies is complicated. In this study, we used a multicolor flow assay and chose a stringent phenotypic method when identifying Tregs,27 combining the markers CD25, CD127, and FOXP3 to better distinguish Tregs from activated cells. However, newly activated T cells can transiently express FOXP3, yet it cannot be ruled out that the phenotypic analysis of Tregs in HIV-infected pregnant women with more immune activation is not as accurate as in uninfected women. This is the first study to report on levels of Tregs in HIV-infected pregnant women. Equal levels of Tregs were found in HIV-infected and HIV-negative women during the first trimester. A measurement before pregnancy would have been of interest because the expansion of Tregs in HIV-negative women may have begun from early gestation. Thus, the fact that the Treg level measured postpartum in HIV-negative women decreased to values below first trimester values may reflect that the levels we measured during first trimester were already elevated compared with prepregnancy levels. As we have no prepregnancy measurements, unfortunately, we cannot investigate this further; however, it is very well supported by others.10,11 In HIV-infected women, Tregs were not mobilized during the second trimester. Previous studies have reported on an increased risk of spontaneous abortion in HIV-infected women,16 and it cannot be ruled out that lack of mobilization of Tregs plays a role in this phenomenon. Moreover, Tregs seem to accumulate in lymphoid tissues in HIV infection,39 and the dynamics of this cell population may differ from that of healthy persons. It is thought that there exists an interaction and mutual regulation between Tregs and TGF-β levels.40 Interestingly, we saw a decrease in TGF-β levels in HIV-infected women during the second trimester. We did not, however, find an association between Tregs and TGF-β. In the third trimester and postpartum, our measurements of Tregs were higher in HIV-infected than in HIV-negative women, even if this did not reach statistical significance. At present, our group is recruiting patients in another study. From that, cohort data on CD4+CD25+CD127lowFOXP3+ Tregs on 8 HIV-infected nonpregnant women of childbearing age (mean age, 38 years) have been analyzed. The median level of Tregs in these nonpregnant HIV-infected women was 4.4%, thus higher than levels in HIV-negative women (even when increased during the second trimester) and higher than levels in HIV-positive women during pregnancy (J.C.G., personal communication, December 2010). These results support previous findings by us as well as others of increased levels of Tregs in HIV-infected patients compared with HIV-negative controls,22,24,27 most probably reflecting the role of Tregs in HIV-pathogenesis as suppressors of HIV-induced chronic immune activation. Furthermore, these additional data support the changes we describe in the present study with progressive increase in %Treg in HIV-infected women during the course of pregnancy and postpartum.
Another hypothesis explaining maternal acceptance of fetal-paternal antigens is that it relies on modulation of thymic function by deletion of new potentially fetus-reactive T cells centrally in the thymus in a way similar to deletion of self-reactive T cells during the process of negative selection.3 Thus, the thymus is reduced in size during pregnancy in various mammalian species,4,5 and in mice this reduction is accompanied by a substantial loss of thymocyte proliferation and decreased thymic output.6 In the present study, we did not expose the pregnant women to a computed tomography scan; therefore, thymic size was not evaluated. We measured thymic output as naive CD4+ cells and TREC-containing CD4+ cells. As expected, HIV-infected women had lower naive CD4 counts and tended to have fewer total CD4-TRECs. The CD4-TREC%, however, was not lower in HIV-infected women. Significant reductions in naive CD4 counts and TREC measurements were not found during pregnancy. TREC measurements are regarded as a more reliable measure of thymic output.20 Although not significantly reduced during pregnancy, visually there was a trend showing the expected decrease in both CD4-TREC% and total CD4-TRECs during the second trimester, but only in HIV-negative women. If thymic output is reduced in HIV-negative women during pregnancy, it may be reduced already from early in the first trimester and not be normalized until cessation of lactation.12 Thus, we might, as expected, have found lower CD4-TREC% and higher levels of Tregs in HIV-infected compared with HIV-negative women if additional measurements had been done before pregnancy and later postpartum.
We and other groups have previously addressed the question of whether Tregs with naive phenotype are recent thymic emigrant Tregs.24,41 Data from the present study support this hypothesis. Thus, the percentage of naive Tregs was found to depend significantly on the CD4-TREC%. Naive Tregs are thought to have unique self-generating capacities and their level to be critical for the suppressive function of the entire pool of Tregs.42,43 Thus, in the context of pregnancy, they may be important.
Up-regulation of Th-2 activity has been demonstrated during normal pregnancy, and Th-2 type cytokines, such as IL-4 and IL-10, may favor the maintenance of pregnancy and have been named embryo-protective factors. In contrast, Th-1 cytokines can induce trophoblast cell lysis and consequently fetal abortion.3,13-15,44 We found higher IL-4 and IL-10 levels in HIV-negative women compared with HIV-infected women throughout the study period. IFN-γ levels did not differ between the 2 groups. The higher levels of Th-2 cytokines in HIV-negative women may be related to the expansion of Tregs that was also seen only in HIV-negative women, or may be hormone-induced.44 It remains unclear, however, if a Th-2 dominant cytokine pattern is essential to pregnancy success because normal pregnancies have been observed in mice lacking Th-2 cytokines.45
Whether or not pregnancy represents an extra challenge to the already disturbed immune system in HIV-infected women is relevant, especially when counseling HIV-infected women about pregnancy. Studies conducted before the introduction of HAART have shown that pregnancy either slightly increased HIV disease progression defined as an AIDS-defining event or death, or had no effect.46,47 In contrast, studies conducted in the HAART era have demonstrated a protective effect of pregnancy on disease progression.48-50 Furthermore, there seems to be a survival advantage in women with 2 pregnancies compared with one pregnancy.49 This may be explained by the fact that healthier HIV-infected women are more likely to become pregnant or may be because of a possible beneficial interaction between pregnancy and HAART. Regarding CD4 counts and HIV viral loads, no negative pregnancy-induced effect has been demonstrated in studies on HIV-infected women conducted during the HAART era.48,50 In the present study, we found increased CD4 counts postpartum in both HIV-infected and HIV-negative pregnant women. HIV viral load remained low in HIV-infected women already on HAART before pregnancy and was significantly reduced in those women starting HAART at gestation week 14. Furthermore, immune activation was reduced during pregnancy. Even though the number of women included was small, our study supports the mounting data demonstrating that pregnancy does not alter the clinical, virologic, and immunologic course of HIV infection.
In conclusion, this study investigated immunologic features during HIV-positive pregnancy, including fetal-maternal tolerance. The study does not raise particular concern that pregnancy in HIV-infected women challenges the impaired immune system leading to lower CD4 counts and more immune activation. However, despite the use of HAART during pregnancy, HIV-infected women continue to display a different immunologic profile from HIV-negative pregnant women. Our results are suggestive of alterations in the immune balance during HIV-positive pregnancy, possibly interfering with the prevention of fetal rejection and partly accounting for the increased risk of abortion in HIV-infected women.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the women who made this study possible, May Olofsson and the midwives at the Department of Obstetrics for help including the pregnant women, and Marianne Lauemøller for invaluable help in the laboratory.
This work was supported by the Research Foundation of Copenhagen University Hospital, Hvidovre.
Authorship
Contribution: L.K. and S.D.N. designed the study and analyzed and interpreted data; L.K. and J.C.G. performed inclusion of patients; L.K., A.L.S., J.C.G., I.K., K.S., and L.P.R. performed experiments; L.K. and S.L. performed statistical analysis; L.K. wrote the manuscript; and all authors revised the manuscript and approved the final version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lilian Kolte, Department of Infectious Diseases, 144, Copenhagen University Hospital, Hvidovre, 2650 Hvidovre, Denmark; e-mail: lilian@kolte.dk.