Abstract
Signaling through the receptor tyrosine kinase kit controls proliferation and differentiation of hematopoietic precursor cells and mast cells. Somatic point mutations of the receptor that constitutively activate kit signaling are associated with mastocytosis and various hematopoietic malignancies. We generated a Cre/loxP-based bacterial artificial chromosome transgenic mouse model that allows conditional expression of a kit gene carrying the kitD814V mutation (the murine homolog of the most common mutation in human mastocytosis, kitD816V) driven by the kit promoter. Expression of the mutant kit in cells of adult mice, including hematopoietic precursors, caused severe mastocytosis with 100% penetrance at young age frequently associated with additional hematopoietic (mostly B lineage–derived) neoplasms and focal colitis. Restriction of transgene expression to mature mast cells resulted in a similar mast cell disease developing with slower kinetics. Embryonic expression led to a hyperproliferative dysregulation of the erythroid lineage with a high rate of perinatal lethality. In addition, most adult animals developed colitis associated with mucosal mast cell accumulation. Our findings demonstrate that the effects of constitutive kit signaling critically depend on the developmental stage and the state of differentiation of the cell hit by the gain-of-function mutation.
Introduction
Mast cells are best known for their effector function in type I allergic responses1,2 but were recently shown to be important initiators and effectors also of innate immunity as well as modulators of adaptive immune responses.3,4 Differentiation and homeostasis of mast cells are dependent on signaling through the transmembrane receptor tyrosine kinase kit, the receptor for stem cell factor (SCF).5,6 In addition, mast cell migration and several proinflammatory mast cell functions are regulated by SCF-kit signaling.7 The kit gene is a proto-oncogene that was originally discovered as the cellular homolog (c-kit) of the feline sarcoma virus oncogene v-kit.8 Binding of SCF to the kit receptor activates various signaling cascades, including the phosphoinositide 3-kinases, protein kinase C, mitogen-activated protein kinase, and janus kinase/signal transducers and activators of transcription pathways to control hematopoiesis, lymphopoiesis, and gametogenesis.9
The human disease mastocytosis is characterized by abnormal numbers of mast cells accumulating in skin and/or various other organs. The patients may have inadequate release of mast cell mediators causing anaphylactoid symptoms with potentially fatal outcome. The condition is associated with gain-of-function mutations of kit, which result in uncontrolled autophosphorylation of the kit receptor with ligand-independent, constitutive signaling.10-13 The majority of mastocytosis patients carry one particular activating point mutation in exon 17 (D816V).10,14 The kitD816V protein was shown to enhance the proliferation of human cells in vitro and to transform murine cells in vivo under certain conditions that allow proper processing of the protein despite the cross-species situation.15
Clinical manifestations of mastocytosis associated with the kitD816V gain-of-function mutation can differ dramatically from patient to patient.11,14,16-18 In a disease subset designated “cutaneous mastocytosis,” the abnormal mast cell accumulation is restricted to the skin. Cutaneous mastocytosis with onset in childhood can resolve spontaneously,17,19,20 whereas manifestation of mastocytosis in adults usually persists for life and in most cases affects other organs and is therefore designated “systemic mastocytosis” (SM). Although mast cell numbers remain stable in “indolent” SM, progressively more mast cells accumulate and cause organ dysfunction in “aggressive” SM, often with unfavorable prognosis. In approximately one-third of cases, SM is accompanied by additional hematopoietic neoplasms that originate from nonmast cell lineages (SM with associated nonmast cell clonal hematologic disease), usually myeloproliferative or myelodysplastic syndromes or myeloid leukemia.11,12
Although several other less common activating kit mutations have been described as germline mutations causing familial disease,21,22 kitD816V is a somatic mutation, inheritance of which has never been unambiguously demonstrated,13 suggesting that germline kitD816V mutations may result in embryonic lethality. The somatic cell that is hit by the oncogenic mutation seems to be a committed mast cell precursor or even a mature mast cell in mild cutaneous forms in contrast to an undifferentiated hematopoietic precursor cell in the severe systemic forms of the disease.23,24
Recently, Zappulla et al described a transgenic mouse model expressing a human kitD816V cDNA in mature mast cells.25 A fraction of these mice (30%) developed a mild form of mastocytosis at old age. The low incidence and severity of disease in these animals may be explained by the fact that processing and intracellular trafficking of human kitD816V protein are abnormal in murine cells largely precluding signaling of the mutant receptor in this cross-species situation.15
In the present work, we use the Cre/loxP recombination system to express a mutant, constitutively active kit in different cellular compartments or at different stages of ontogeny.
Methods
Mice
The construction of kitD814Vflox bacterial artificial chromosome transgenic mice (Figure 1) is described in detail in Supplemental data (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). kitD814Vflox animals were bred to deleter-Cre,26 Mx1-Cre,27 Vav-Cre,28 or A-Mcpt5-Cre29 for conditional deletion of the stop element. Cre-mediated excision of the loxP-flanked fragment was detected by polymerase chain reaction (primers intron1–2 forward 5′-GAAAGAGCGGCAGACAAG-3′, intron1–2 reverse 5′-TGAGGTCTCTCAGCTCAGGTG-3′) or by Southern blot analysis of NotI and BamHI double-digested DNA and an intron1–2-specific probe (primers: forward, 5′-TCTTTGTGCACAGTGCTGGGGA-3′; reverse, 5′-CCTGCGATTACAAGTCGCATACA-3′). Mice were kept in a specific pathogen-free barrier facility. All animal experimentation was performed in accordance with institutional guidelines and was approved by the Landesamt für Natur, Umwelt und Verbraucherschutz North-Rhine Westphalia.
Flow cytometry
Blood from newborn mice was collected into heparinized glass capillaries after decapitation, whereas blood from adult mice was obtained by tail vein incision or by retro-orbital bleeding. Spleen, lymph node, fetal liver, as well as mast cell tumors were homogenized by mashing the tissue through a 70-μm nylon mesh followed by filtration through a 40-μm mesh. Cell suspensions were incubated with red blood cell lysis solution for 10 minutes before washing and staining for 30 minutes in phosphate-buffered saline containing 0.5% bovine serum albumin and 0.05% NaN3 using the monoclonal antibodies listed in the Supplemental data. Nucleated cells were identified by staining with Draq5 (Biostatus). Absolute numbers of erythrocytes, nucleated Ter119+ cells, and CD45 expressing cells were determined flow cytometrically by mixing fetal whole blood with a defined number of Calibrite PerCP beads (BD Biosciences), followed by staining for Ter119, CD45, and Draq5.
Bone marrow transfer
Bone marrow from donor mice (CD45.2) was sorted for kit+, lineage-negative (lin−, CD3, CD11b,CD19, CD45R, Gr-1, Nk1.1, Ter119) cells. Congenic recipient mice (B6.SJL-Ptprca Pepcb/BoyJ, CD45.1) were lethally irradiated with a dose of 8 Gy, and purified donor cells were injected retro-orbitally. Bone marrow chimerism was determined at various time points (4 weeks to 6 month after transfer) by CD45 allele expression of polymorphic mononuclear cells (CD11b+/Gr1hi cells) in peripheral blood.
Results
Conditional expression of a constitutively active kit in vivo
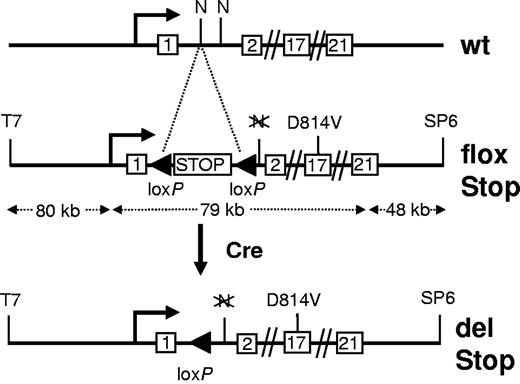
To control expression of the mutant kit receptor in vivo, we followed a transgenic approach (Figure 1) in which the mutant kit is expressed only on Cre/loxP-mediated deletion of a transcriptional stop element.30 The activating point mutation D814V, the murine homolog of the most common human gain-of-function mutation D816V, was introduced into exon 17, and the loxP-flanked transcriptional stop element was inserted into intron1-2 of the murine kit gene contained in a bacterial artificial chromosome vector. In this experimental setup, cells that express the wild-type kit loci and have deleted the stop element of the kit transgene by Cre-mediated recombination will express the mutant kit driven by the kit promoter elements present in the transgene. Three kitD814Vflox transgenic founder lines were analyzed, 2 of which carried a full-length integration of the transgene (Table 1). kitD814Vflox animals displayed no macroscopic abnormality and had normal mast cell numbers in skin and peritoneal cavity.
Overview of the kitD814Vflox transgenic construct. The transgene is based on bacterial artificial chromosome clone RPCI 23–274L11 containing the entire kit genomic sequence along with abundant flanking DNA. The activating point mutation (D814V) was introduced into exon 17, and a loxP-flanked transcriptional/translational stop cassette (STOP) was recombined into intron1–2. The wild-type (wt) kit locus and the transgenic kit allele before (flox Stop) and after Cre-mediated deletion of the stop element (del Stop) are shown. T7 and SP6 indicate promoter fragments at the ends of the vector backbone; and N, NotI restriction site. Sizes are in kilobases (kb), not drawn to scale.
Overview of the kitD814Vflox transgenic construct. The transgene is based on bacterial artificial chromosome clone RPCI 23–274L11 containing the entire kit genomic sequence along with abundant flanking DNA. The activating point mutation (D814V) was introduced into exon 17, and a loxP-flanked transcriptional/translational stop cassette (STOP) was recombined into intron1–2. The wild-type (wt) kit locus and the transgenic kit allele before (flox Stop) and after Cre-mediated deletion of the stop element (del Stop) are shown. T7 and SP6 indicate promoter fragments at the ends of the vector backbone; and N, NotI restriction site. Sizes are in kilobases (kb), not drawn to scale.
Overview of kitD814Vflox transgenic lines
| Transgene founder line . | Transgene copy number . | End sequences present . |
|---|---|---|
| 1 | 5-6 | T7, SP6 |
| 2 | > 10 | T7, SP6 |
| 3 | 2 | T7 |
| Transgene founder line . | Transgene copy number . | End sequences present . |
|---|---|---|
| 1 | 5-6 | T7, SP6 |
| 2 | > 10 | T7, SP6 |
| 3 | 2 | T7 |
kitD814Vflox founder lines were analyzed for transgene copy number by Southern blot analysis (not shown). Transgene integrity was determined by detection of short residual vector backbone sequences (T7 and SP6; Figure 1) by polymerase chain reaction.
Induced expression of constitutively active kit results in mast cell hyperplasia
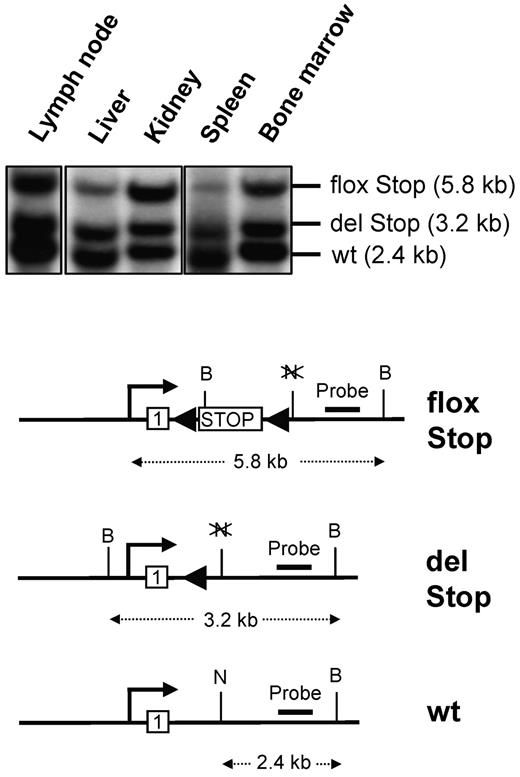
To experimentally mimic the somatic mutation events that are associated with human mastocytosis, we bred kitD814Vflox mice to the Mx1-Cre line, which expresses Cre recombinase under the control of the type I interferon-inducible Mx1 promoter.27 Cre expression was induced by 3 injections of 250 μg polyinosine-polycytidylic acid (pI:C) every second day to activate expression of the transgenic kitD814V in 4- to 10-week-old kitD814Vflox Mx1-Cre mice. Extensive deletion of the stop element was detected in genomic DNA extracted from various tissues by Southern blot analysis (Figure 2).
Southern blot analysis of Cre-mediated deletion of the stop element in induced kitD814Vflox Mx1-Cre animals. Southern blot analysis of genomic DNA extracted from lymph node, liver, kidney, spleen, and bone marrow from kitD814Vflox Mx1-Cre mice induced by intraperitoneal injections of pI:C. The Southern blot strategy based on a NotI (N) and BamHI (B) double digestion and an intron1–2-specific probe discriminates between the repressed transgene (flox Stop), kitD814V after Cre-mediated deletion of the stop cassette (del Stop), and the genomic wild-type kit allele (wt). Not drawn to scale.
Southern blot analysis of Cre-mediated deletion of the stop element in induced kitD814Vflox Mx1-Cre animals. Southern blot analysis of genomic DNA extracted from lymph node, liver, kidney, spleen, and bone marrow from kitD814Vflox Mx1-Cre mice induced by intraperitoneal injections of pI:C. The Southern blot strategy based on a NotI (N) and BamHI (B) double digestion and an intron1–2-specific probe discriminates between the repressed transgene (flox Stop), kitD814V after Cre-mediated deletion of the stop cassette (del Stop), and the genomic wild-type kit allele (wt). Not drawn to scale.
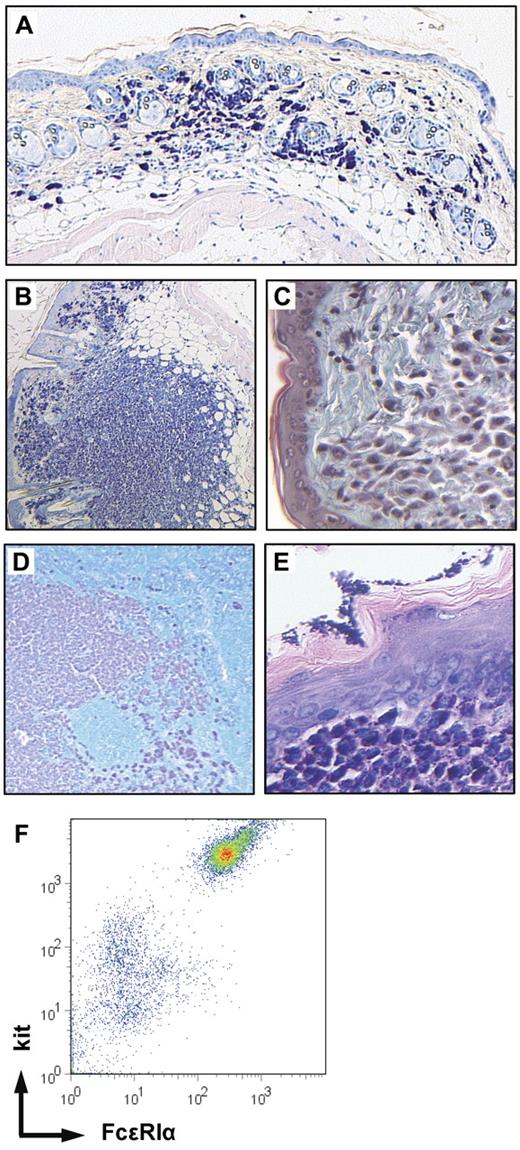
Thirteen of the 23 pI:C-induced animals were killed between 13 and 38 weeks after induction in the absence of gross health problems (supplemental Table 2). Ten mice were killed 3 to 14 weeks after induction for obvious severe disease (“Additional hematopoietic neoplasia in mice with induced expression of constitutively active kit”). Histologic examination of Giemsa-stained skin sections revealed that all animals displayed a variable increase in cutaneous mast cell numbers. This diffuse mastocytosis ranged from small patches of increased mast cell density (∼ 4-fold higher than in controls) in few animals to a substantial general increase of cutaneous mast cell numbers (up to 15-fold compared with controls) in most mice (Figure 3A). Most of these mast cells displayed a round morphology with some degree of hypergranulation with only few spindle-shaped cells (supplemental Table 5). In addition to this diffuse mastocytosis, 16 of the 23 mice analyzed showed large numbers of dermal mast cell tumors of variable size (Figure 3B, tumors arbitrarily defined as accumulations of densely packed round-shaped mast cells of > 100 μm in diameter.) In few mice, these tumors were visible macroscopically, reaching diameters of 5 mm. Flow cytometric analysis of a single-cell suspension prepared from one of the larger tumors demonstrated coexpression of kit and FcϵRIα by 80% of the tumor cells providing further evidence for the mast cell nature of the tumor (n = 2, Figure 3F). The tumors destroyed the dermal collagen (as demonstrated by trichrome-Masson staining, Figure 3C) but usually respected the panniculus carnosus (ie, the muscle layer of murine skin). In some animals (7 of 23), the skin covering mast cell tumors showed inflammatory changes, including mononuclear cell infiltration, acanthosis, and erosions (not shown). In most mice, diffuse infiltrations of skin-draining lymph nodes with mast cells as well as intranodal mast cell tumors were observed (Figure 3D). Some solitary mast cells were detected in the spleens of most mutant mice, whereas mast cells were detected in very low numbers or were completely absent in control spleens (not shown). Two mutant animals displayed a more intense infiltration of the spleen (supplemental Table 2). Mast cell infiltrates were detected in the forestomach of several mice (Figure 3E) and were also found in the intestinal mucosa (see “Constitutive kit activation causes intestinal inflammation” below). Two animals showed additional massive infiltration of the liver (supplemental Table 2). We did not observe mast cell infiltrates in the bone marrow, which was investigated by staining femoral sections for kit or by flow cytometric analysis of bone marrow suspensions stained for kit and FcϵRIα.
Mastocytosis in kitD814Vflox Mx1-Cre animals. Giemsa-stained skin sections (mast cells dark purple, 5×/0.15 NA objective) revealed increased numbers of mast cells (A) in all mice and the presence of multiple mast cell skin tumors (B, 5×/0.15 NA objective) in 24 of 39 animals. (C) The mast cell tumors destroyed the dermal collagen as demonstrated by trichrome-Masson staining (green represents collagenous fibers; and pink, mast cells, 20×/0.7 NA objective). (D) Mast cells infiltrating a skin draining lymph node (Giemsa staining: dark purple represents mast cells; and turquoise, nonmast cell hematopoietic cells, 5×/0.15 NA objective) and (E) mast cell infiltrate in the submucosa of the forestomach (Giemsa, 20×/0.7 NA objective). (F) Flow cytometric analysis of a dermal mast cell tumor. A single-cell suspension prepared from a skin mast cell tumor was stained for kit and the high-affinity IgE receptor-α chain (FcϵRIα) and analyzed by flow cytometry.
Mastocytosis in kitD814Vflox Mx1-Cre animals. Giemsa-stained skin sections (mast cells dark purple, 5×/0.15 NA objective) revealed increased numbers of mast cells (A) in all mice and the presence of multiple mast cell skin tumors (B, 5×/0.15 NA objective) in 24 of 39 animals. (C) The mast cell tumors destroyed the dermal collagen as demonstrated by trichrome-Masson staining (green represents collagenous fibers; and pink, mast cells, 20×/0.7 NA objective). (D) Mast cells infiltrating a skin draining lymph node (Giemsa staining: dark purple represents mast cells; and turquoise, nonmast cell hematopoietic cells, 5×/0.15 NA objective) and (E) mast cell infiltrate in the submucosa of the forestomach (Giemsa, 20×/0.7 NA objective). (F) Flow cytometric analysis of a dermal mast cell tumor. A single-cell suspension prepared from a skin mast cell tumor was stained for kit and the high-affinity IgE receptor-α chain (FcϵRIα) and analyzed by flow cytometry.
KitD814Vflox Mx1-Cre mice (n = 16, supplemental Table 2) not injected with the inducer pI:C, developed similar phenotypes as described for induced animals, which was most likely the result of the background recombination that was described earlier for the Mx1-Cre transgenic line.27,31 We did not observe mast cell-related phenotypes in Cre-negative kitD814Vflox controls. Southern blot analysis of DNA from uninduced kitD814Vflox Mx1-Cre mice demonstrated that the stop element was deleted in most bone marrow cells but only in a minor fraction of nonhematopoietic cells in 2 animals at the age of 8 and 11 months (not shown).
Additional hematopoietic neoplasia in mice with induced expression of constitutively active kit
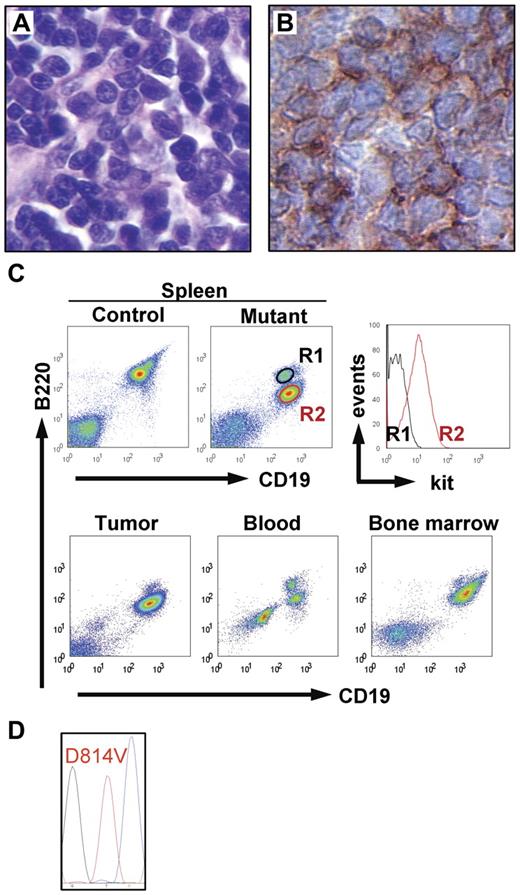
In addition to mastocytosis, 16 of the 39 kitD814Vflox Mx1-Cre animals (10 induced and 6 uninduced) had an additional malignant nonmast cell hematopoietic disease causing hind limb paralysis in most (15 of 16) of the animals. These 15 mice uniformly displayed an abnormal population of lymphoid blastic cells (Figure 4A) that could be detected (by histology or FACS analysis) in blood, spleen, liver, bone marrow, and skin. In 5 of the animals, the neoplastic cells formed large solid tumors. The spleen was enlarged in most mice (spleen weight, 221 ± 102 mg in leukemic mice, n = 12; vs 80 ± 15 mg in controls, n = 21). Histopathologically and cytologically, the condition resembled B-lymphoblastic leukemia and was characterized by immunoreactivity for the B-lineage marker B220 (Figure 4B) and Ki67 (not shown). Blood (n = 5), bone marrow (n = 4), spleen (n = 6), and tumor cell suspensions (n = 3) were analyzed by flow cytometry, confirming the expression of the B-lineage markers CD19 and B220 by the neoplastic cells, which could be easily differentiated from the normal B cells by their slightly lower expression of B220, up-regulation of kit (Figure 4C), and absence of surface immunoglobolin (not shown). Sequence analysis of kit reverse-transcribed polymerase chain reaction products from tumor tissue (n = 3) demonstrated that the abnormal B-cell population transcribed only the transgenic but not the wt kit alleles (Figure 4D). Collectively, these mice had a leukemic disease derived from an immature B-cell precursor. A single mouse was characterized by a condition resembling chronic myelogenous leukemia (supplemental Figure 1).
Hematopoietic neoplasia in kitD814Vflox Mx1-Cre animals. Fifteen of 39 kitD814Vflox Mx1-Cre animals developed B-cell lineage malignancy in addition to mastocytosis. (A) Histology of a solid lymphoid tumor (hematoxylin and eosin staining, 40×/0.75 NA objective). (B) Tumor cells showed immunoreactivity for the B-lineage marker B220 (40×/0.75 NA objective). (C) Top panel: Flow cytometric analysis of spleen cell suspensions from a control mouse and a kitD814Vflox Mx1-Cre animal that displayed leukemic disease with large nodular tumors. In addition to the normal splenic B-cell population (R1), a population of CD19+ B220 intermediate cells (R2) expressing kit (right panel) appears in the mutant mouse. Bottom panel: The same populations of normal B cells and neoplastic cells were identified in tumor cell suspensions, blood, and bone marrow (neoplastic B220 intermediate cells dominate in tumor and bone marrow). (D) Sequence analysis of reverse-transcribed polymerase chain reaction product obtained from neoplastic B-lineage cells detected only kitD814V, but not wt kit transcript.
Hematopoietic neoplasia in kitD814Vflox Mx1-Cre animals. Fifteen of 39 kitD814Vflox Mx1-Cre animals developed B-cell lineage malignancy in addition to mastocytosis. (A) Histology of a solid lymphoid tumor (hematoxylin and eosin staining, 40×/0.75 NA objective). (B) Tumor cells showed immunoreactivity for the B-lineage marker B220 (40×/0.75 NA objective). (C) Top panel: Flow cytometric analysis of spleen cell suspensions from a control mouse and a kitD814Vflox Mx1-Cre animal that displayed leukemic disease with large nodular tumors. In addition to the normal splenic B-cell population (R1), a population of CD19+ B220 intermediate cells (R2) expressing kit (right panel) appears in the mutant mouse. Bottom panel: The same populations of normal B cells and neoplastic cells were identified in tumor cell suspensions, blood, and bone marrow (neoplastic B220 intermediate cells dominate in tumor and bone marrow). (D) Sequence analysis of reverse-transcribed polymerase chain reaction product obtained from neoplastic B-lineage cells detected only kitD814V, but not wt kit transcript.
Constitutive kit activation causes intestinal inflammation
Unexpectedly, kitD814Vflox Mx1-Cre animals developed intestinal inflammation. This disease occurred in all mice derived from the transgenic founder lines 1 and 2 (regardless of pI:C induction) but was rare in animals originating from founder line 3. Macroscopically, diseased mice displayed weight loss and/or diarrhea with bloody feces and a prolapse of the rectal mucosa (which is a sign of severe intestinal inflammation in mice). Histologic analysis revealed that all kitD814Vflox Mx1-Cre animals (except those originating from transgenic founder line 3) displayed intestinal disease of variable intensity (Figure 5A-B, median disease score of 2.5 vs a score of 0 in controls; supplemental data, disease score). Severe inflammation was more frequent among older mice. The inflammatory changes affected sharply demarcated patches of the mucosa (Figure 5B). In few mice, one or several foci of inflammatory infiltration were found, whereas in the majority of the animals longer stretches of the mucosa were affected. Most lesions were encountered in the cecum and the ascending colon. The lesions were characterized by a massive infiltration of T cells, B cells, macrophages (as demonstrated by immunostaining for CD3ϵ, B220, or F4/80, supplemental Figure 2A-D), and neutrophils. In most cases, high numbers of intraepithelial mast cells, detected by chloroacetate esterase staining, were found in the crypt epithelium surrounding the lesions, whereas mast cells were encountered only rarely within the inflammatory infiltrate (Figure 5C). Mucosal mast cell numbers showed a positive correlation with disease severity. Colonic mucosa of mice derived from transgenic founder line 3 displayed normal numbers of mucosal mast cells, and the majority was devoid of inflammatory changes. The inflammatory process resulted in an extensive destruction of the mucosa with crypts separated from each other and from the lamina muscularis propria by the infiltrating cells. There were numerous neutrophilic crypt abscesses, an inflammatory edema and, in several mice, significant erosions of the mucosal surface. In the lesions of the most severely affected animals, the mucosa was completely replaced by a dense inflammatory infiltrate that massively increased the thickness of the colonic wall, and the only remaining epithelial structure was the single layered epithelium that covered the infiltrate toward the lumen. This surface epithelium showed erosions in several mice. The nonlesional colonic mucosa appeared normal, except for increased numbers of mast cells. In few animals (5 of 11 animals analyzed), also the small intestine showed mild inflammatory changes. To investigate a role of tumor necrosis factor-α (TNF-α) in the pathogenesis of the intestinal disease, kitD814Vflox Mx1-Cre mice were crossed to the TNFRI p55−/− strain, which lacks TNF-α signaling.32 However, kitD814Vflox Mx1-Cre p55−/− (n = 4) animals did not show reduced incidence or severity of intestinal inflammation (supplemental Figure 2E), indicating that TNF-α is dispensable for the inflammatory bowel disease of these mice.
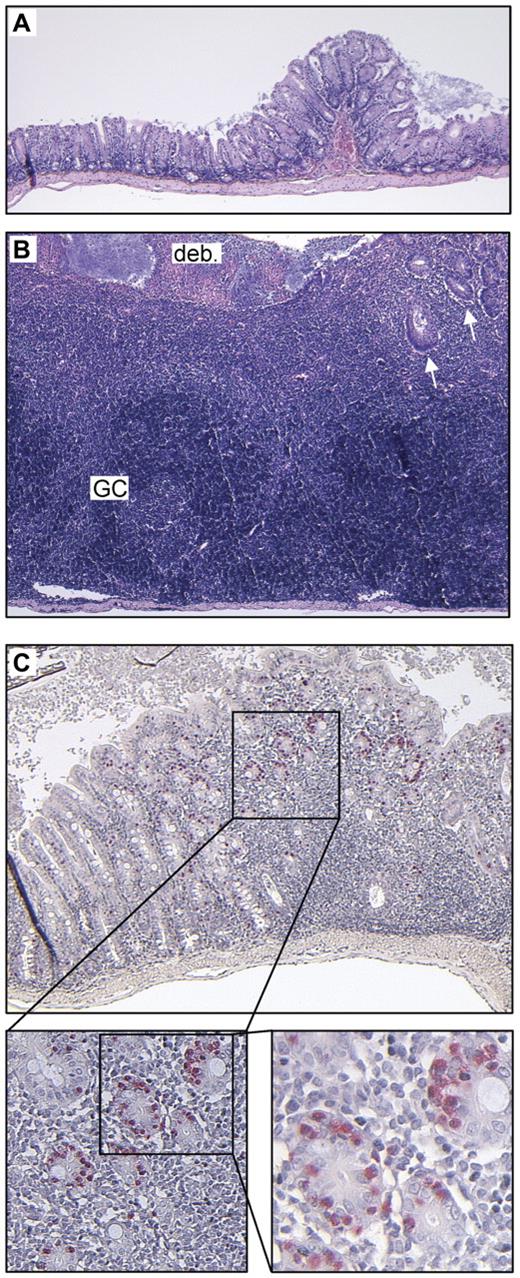
Intestinal inflammation in kitD814Vflox Mx1- Cre animals. Constitutive kit activation causes intestinal inflammation. Unaffected (A) and inflamed (B) colonic wall of the identical kitD814Vflox Mx1-Cre animal at the same magnification (hematoxylin and eosin staining, 5×/0.15 NA objective). Note the massive inflammatory infiltrate with almost complete destruction of epithelial structures and erosion of the luminal surface epithelium covered with fibrin and cellular debris (deb.). Some residual crypts can be found in the upper right quadrant (arrows). The inflammatory process has also partly destroyed the lamina muscularis propria (reduced thickness compared with the unaffected colonic wall). The inflammatory infiltrate contains (dark blue) areas densely populated by B and T lymphocytes (as demonstrated by immunostaining; supplemental Figure 2), which also form germinal centers (GC). (C) Mildly inflamed colonic wall of a kitD814Vflox Mx1-Cre mouse stained for chloroacetate esterase. Note the accumulation of high numbers of intraepithelial mucosal mast cells (stained red, 5×/0.15 NA, 10×/0.4, 20×/0.7 NA objectives, respectively) within the crypt epithelium surrounding the inflammatory lesion. The inflammatory infiltrate itself contains only few mast cells.
Intestinal inflammation in kitD814Vflox Mx1- Cre animals. Constitutive kit activation causes intestinal inflammation. Unaffected (A) and inflamed (B) colonic wall of the identical kitD814Vflox Mx1-Cre animal at the same magnification (hematoxylin and eosin staining, 5×/0.15 NA objective). Note the massive inflammatory infiltrate with almost complete destruction of epithelial structures and erosion of the luminal surface epithelium covered with fibrin and cellular debris (deb.). Some residual crypts can be found in the upper right quadrant (arrows). The inflammatory process has also partly destroyed the lamina muscularis propria (reduced thickness compared with the unaffected colonic wall). The inflammatory infiltrate contains (dark blue) areas densely populated by B and T lymphocytes (as demonstrated by immunostaining; supplemental Figure 2), which also form germinal centers (GC). (C) Mildly inflamed colonic wall of a kitD814Vflox Mx1-Cre mouse stained for chloroacetate esterase. Note the accumulation of high numbers of intraepithelial mucosal mast cells (stained red, 5×/0.15 NA, 10×/0.4, 20×/0.7 NA objectives, respectively) within the crypt epithelium surrounding the inflammatory lesion. The inflammatory infiltrate itself contains only few mast cells.
Mice with selective expression of the kitD814V transgene in mature mast cells develop mastocytosis with slow kinetics
To discriminate pathogenic effects of deregulated kit signaling in progenitor cells versus mature mast cells, we crossed kitD814Vflox mice to our A-Mcpt5-Cre line, which deletes loxP-flanked DNA selectively in mature mast cells.29 Bitransgenic animals developed mastocytosis and colitis similar to the phenotype of kitD814Vflox Mx1-Cre mice; however, the disease occurred significantly later and progressed slower. Although no increase in mast cell numbers was observed until the age of 28 weeks, all mice showed significantly increased mast cell numbers by the age of 36 to 52 weeks (n = 8, supplemental Table 3). In addition, half of the animals displayed multiple mast cell tumors and erosive skin lesions at this age. All mice, except kitD814Vflox A-Mcpt5-Cre animals derived from transgenic founder 3, developed severe colitis (n = 5, median disease score 4).
Attenuation of the transforming capacity of kitD814V in mature mast cells by low transgene expression
After the generation of our kitD814Vflox transgenic line, Berrozpe et al33 reported that an enhancer element located 154 kb upstream of the transcription start site of the murine kit gene was essential for expression of kit in mature mast cells. Because this element is not contained in the 80 kb of upstream flanking DNA of our transgene (Figure 1), we reasoned that down-regulation of transgene expression in maturing mast cells may attenuate the phenotype in our model. To address this question, bone marrow-derived mast cells (BMMCs, supplemental Figure 3C left panel) were generated from mice with a general deletion of the stop element in the early embryo (kitD814Vflox deleter-Cre mice, “Limited transforming effect of the constitutively active kit on hematopoietic stem/precursor cells”) and kit mRNA from these cells was reverse transcribed, amplified, and sequenced. Predominantly, the wt sequence was found at codon 814, whereas the point mutation of the transgenic transcript was barely detectable, indicating very low levels of transgene transcription in mature mast cells (supplemental Figure 3A-C middle panel). Western blot analysis of lysates of the BMMCs, however, revealed a detectable level of constitutive kit phosphorylation that was not seen in wt BMMCs (supplemental Figure 4). The low constitutive kit phosphorylation resulted in only slight activation of downstream signaling pathways (supplemental Figure 4). In accordance with these findings, BMMCs from kitD814Vflox deleter-Cre or kitD814Vflox Mx1-Cre did not show enhanced proliferation in vitro (in the presence or absence of SCF (supplemental Figure 3B-C right panel). Collectively, these data show that transgene expression is down-regulated to low levels in maturing mast cells. These low transcription levels, however, were sufficient to cause the slowly developing mastocytosis of kitD814Vflox A-Mcpt5-Cre mice.
Limited transforming effect of the constitutively active kit on hematopoietic stem/precursor cells
To investigate the transforming capacity of our kitD814V transgene in kit expressing hematopoietic stem/precursor cells, we attempted to transplant kit+ lin− cells from pI:C-induced KitD814Vflox Mx1-Cre or pI:C-injected littermate control mice purified from bone marrow by flow cytometry into nonirradiated or lethally irradiated CD45 congenic recipients. Although the bone marrow from both donor groups, as expected, rescued and repopulated irradiated recipients (supplemental Figure 5), KitD814Vflox Mx1-Cre bone marrow cells were not able to engraft in nonirradiated recipient mice. Recipients were analyzed for donor-derived neutrophilic granulocytes in peripheral blood at multiple time points starting 4 weeks up to 6 months after transfer. However, donor-derived (CD45.2+) cells could not be detected at any time point in the circulation of nonirradiated animals transplanted with mutant or control cells. Likewise, donor-derived mast cells were not found in the peritoneal lavage of nonirradiated recipients (not shown).
Embryonic expression of kitD814V results in perinatal lethality
The kitD816V mutation in human mastocytosis was never demonstrated to be inherited, whereas pedigrees of germline inheritance have been described for several other less common activating kit mutations.21,22 To generate an experimental situation equivalent to germline inheritance of the mutation, kitD814Vflox animals were bred to a hCMV deleter-Cre line,26 which deletes loxP-flanked DNA in the entire embryo before implantation. In kitD814Vflox deleter-Cre double-transgenic offspring with early and general deletion of the stop element, we therefore expect expression of the constitutively active kit in cells expressing the wild-type kit gene.
The kitD814Vflox deleter-Cre+ genotype resulted in rapid postnatal lethality in a majority (∼ 75%) of neonatal mice. All neonatal animals analyzed within 24 hours after birth (live or shortly after spontaneous death) displayed a dramatic dysregulation of hematopoiesis characterized by excessive numbers of nucleated cells in the peripheral blood as determined by counting in a hemocytometer (transgene line 1: 3.21 ± 2.71 × 105/μL n = 19; transgene line 2: 2.13 ± 0.29 × 105/μL, n = 5; transgene line 3: 0.32 ± 0.31 × 105/μL, n = 7, vs 5 ± 1.7 × 103/μL in controls, n = 65). This increase in nucleated cells was primarily the result of a population of small blastic cells with sparse cytoplasm as demonstrated by blood smears (Figure 6C). In addition, blood smear examination showed a high number of nuclear shadows (Figure 6C), suggesting the presence of cells with increased mechanical fragility. FACS analysis revealed the appearance of an abnormal population of small, nongranulated cells (Figure 6A), which accounted for approximately 5% to 10% of total cells in unlysed blood. The abnormal cells expressed Ter119 and CD71 (transferrin receptor; Figure 6B) but not CD45, CD3, CD19, B220, or CD11b, suggesting that they were of erythroid origin. Approximately 50% of these cells were dying or dead as indicated by uptake of propidium iodide (Figure 6A lower panel) providing a probable explanation for the nuclear shadows detected on the blood smears. Immunohistochemical staining of whole-mount sections of newborn mice showed that most circulating nucleated cells expressed the proliferation marker Ki67 (Figure 6D-E) In addition, the diseased animals displayed increased erythrocyte numbers (7.44 ± 1.29 × 106/μL, n = 5, vs 5.12 ± 0.28 × 106/μL in controls, n = 8) and hematocrit (54.9% ± 1%, n = 7, vs 36.8% ± 3.8% in controls, n = 6), most probably reflecting maturation of a fraction of the abnormal cells (Figure 6J). In contrast to the dysregulation of the erythroid lineage, the bitransgenic mice did not show increased numbers of CD45+ leukocytes (4.46 ± 2.07 × 103 CD45+Ter119− cells/μL, n = 5, vs 4.61 ± 1.59 × 103/μL in controls, n = 8). Histologic analysis demonstrated a pronounced infiltration of the liver by the Ter119+ cells replacing most of the liver parenchyma (Figure 6F-G), which was also reflected on flow cytometry of liver cell suspensions (Figure 6H-I). Mast cell numbers in the skin of neonatal bitransgenic mice (n = 3) were not increased compared with littermate controls. The same lethal phenotype was observed in mice with early and general expression of the mutant kit limited to hematopoietic cells from breedings of kitD814Vflox animals to Vav-Cre mice,28 which formally proves that the reason for lethality is within the hematopoietic system (n = 1, not shown). Collectively, these data suggest that most mice with early general constitutive activation of kit signaling die of a hyperproliferative dysregulation of erythropoiesis.
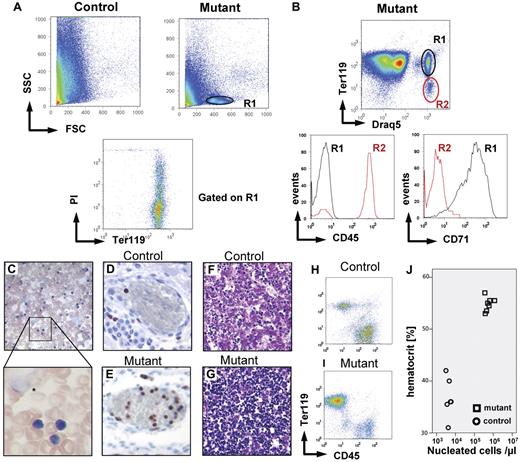
Dysregulated erythopoiesis in kitD814Vflox deleter-Cre neonatal mice. The kitD814Vflox deleter-Cre genotype, which results in early and general deletion of the stop element and expression of kitD814V in all cells expressing wt kit, causes perinatal lethality in approximately 75% of newborn mice and is associated with a hyperproliferative dysregulation of the erythroid lineage. (A) FACS analysis of peripheral blood from newborn mutant animals displays an abnormal population of small, nongranulated cells (region R1). Gating on this population (lower panel) reveals that these cells express the erythroid marker Ter119 and that approximately 50% of the cells take up the dye propidium iodide and are therefore dying or dead. (B) Positive staining with the cell-permeant dye Draq5 provides additional evidence that this population (R1) does not represent normal erythrocytes but indeed nucleated cells. These cells also express the transferrin receptor (CD71) but not CD45 (bottom panel). Blood smears (C, 40×/0.75 objective and 63×1.3 NA oil objective) show large numbers of small cells with dense nuclei and sparse basophilic cytoplasm resembling erythroblasts, but also numerous nuclear shadows (*) represent remnants of cells, indicating the presence of a cell population with increased mechanical fragility. Whole-mount sections of bitransgenic newborns demonstrate drastically increased numbers of nucleated Ki67 cells in blood vessels (Ki67 immunostaining: D, control; E, mutant, 20×/0.7 NA objective). The liver of the bitransgenic mice (F, mutant; G, control, 20×/0.7 NA objective) contained a massively increased number of hematopoietic cells (dense, dark blue nuclei, hematoxylin and eosin staining). Flow cytometry of fetal liver homogenates for the markers CD45 and Ter119 gated on DNA containing cells reveals an increase of nucleated Ter119+ cells within the liver of kitD814Vflox deleter-Cre mutants (I) versus control (H). Dramatic increase in hematocrit (J) in mutants (☐) versus control animals (○).
Dysregulated erythopoiesis in kitD814Vflox deleter-Cre neonatal mice. The kitD814Vflox deleter-Cre genotype, which results in early and general deletion of the stop element and expression of kitD814V in all cells expressing wt kit, causes perinatal lethality in approximately 75% of newborn mice and is associated with a hyperproliferative dysregulation of the erythroid lineage. (A) FACS analysis of peripheral blood from newborn mutant animals displays an abnormal population of small, nongranulated cells (region R1). Gating on this population (lower panel) reveals that these cells express the erythroid marker Ter119 and that approximately 50% of the cells take up the dye propidium iodide and are therefore dying or dead. (B) Positive staining with the cell-permeant dye Draq5 provides additional evidence that this population (R1) does not represent normal erythrocytes but indeed nucleated cells. These cells also express the transferrin receptor (CD71) but not CD45 (bottom panel). Blood smears (C, 40×/0.75 objective and 63×1.3 NA oil objective) show large numbers of small cells with dense nuclei and sparse basophilic cytoplasm resembling erythroblasts, but also numerous nuclear shadows (*) represent remnants of cells, indicating the presence of a cell population with increased mechanical fragility. Whole-mount sections of bitransgenic newborns demonstrate drastically increased numbers of nucleated Ki67 cells in blood vessels (Ki67 immunostaining: D, control; E, mutant, 20×/0.7 NA objective). The liver of the bitransgenic mice (F, mutant; G, control, 20×/0.7 NA objective) contained a massively increased number of hematopoietic cells (dense, dark blue nuclei, hematoxylin and eosin staining). Flow cytometry of fetal liver homogenates for the markers CD45 and Ter119 gated on DNA containing cells reveals an increase of nucleated Ter119+ cells within the liver of kitD814Vflox deleter-Cre mutants (I) versus control (H). Dramatic increase in hematocrit (J) in mutants (☐) versus control animals (○).
Few kitD814Vflox deleter-Cre animals survive and develop mastocytosis and colitis
In contrast to the lethal disease in the majority of kitD814Vflox deleter-Cre animals, approximately 25% of these mice grew up without macroscopically evident hematopoietic abnormality and were killed at the age of 2.5 to 14 months (supplemental Table 4). One animal died spontaneously (age 1.5 months). Histologic analysis, blood smears, or FACS analysis of peripheral blood did not reveal any sign of the hyperproliferative dysregulation of the erythroid lineage that we had observed in all neonatal kitD814Vflox deleter-Cre animals analyzed. These findings represent indirect evidence for a spontaneous regression of the hyperproliferation of red cell precursors in the surviving mice (incomplete deletion of the stop element in the surviving mice was ruled out by Southern blot analysis; not shown). Histologic examination of skin samples on necropsy demonstrated that, except for the one animal that died spontaneously, all surviving kitD814Vflox deleter-Cre mice (n = 20) developed mastocytosis of skin and various organs as well as intestinal inflammation similar to the phenotype described for kitD814Vflox Mx1-Cre animals, except that kitD814Vflox deleter-Cre from transgene line 3 do not develop intestinal inflammation (supplemental Table 4). We did not find evidence for additional hematopoietic neoplasia in any of the surviving kitD814Vflox deleter-Cre mice analyzed.
Discussion
Our novel mouse model for diseases associated with activating kit mutations is based on transgenic expression of a mutant, constitutively active kit receptor driven by the kit promoter. Expression can occur only after a loxP-flanked stop element is excised by Cre recombinase. KitD814Vflox transgenic mice were bred to the Mx1-Cre line for inducible expression of Cre in somatic cells.27 Induced bitransgenic offspring developed marked mastocytosis with infiltrates of mast cells in the skin and other organs, most frequently lymph nodes and colon. The intensity of the mast cell disease that occurred with 100% penetrance in these animals was variable; however, most mice showed a more than 5-fold increase in skin mast cells and, in addition, the majority of the animals developed numerous mast cell tumors. This phenotype reproduced some features of aggressive SM in humans, a subentity of SM defined by progressive tissue infiltration by neoplastic mast cells with subsequent impairment of organ functions.11,34 In humans with aggressive SM, mast cells most commonly infiltrate bone marrow, liver, spleen, and the intestinal tract.11 The disease is often associated with additional clonal hematologic nonmast cell lineage disease, approximately 80% to 90% of which are myeloid disorders, including myelodysplastic syndromes, myeloproliferative neoplasms, and acute myelogenous leukemia,35 whereas 10% to 20% represent lymphatic malignancies, most frequently plasma cell myelomas. Approximately half (16 of 39) of our kitD814Vflox Mx1-Cre animals developed nonmast cell lineage hematopoietic neoplasia, which was in most cases derived from the B-cell lineage. Similar results had earlier been obtained by Kitayama et al36 in mouse models in which a kitD814V transgene was expressed under the control of an H-2L promoter. The finding of B-cell leukemia in only half of the our mice suggests the requirement for further oncogenic events in addition to kitD814V for full transformation. The likelihood for such additional mutations is particularly high in the B-cell lineage with high proliferative activity and genomic rearrangement of Ig loci during B-cell development. The occurrence of B lineage neoplasms in mice expressing the mutant murine kit versus myeloid disorders in kitD816V+ human patients and MPD-like phenotypes in a mouse model driven by mutant human kit may be explained by a missing putative phosphotyrosine interaction domain in human kit protein.15
Even without induction of the IFN-sensitive Mx1-promoter by injection of pI:C, kitD814Vflox Mx1-Cre animals developed mast cell disease of comparable severity and kinetics compared with the induced group. Low-level baseline activity of the Mx1-Cre transgene has earlier been reported.27,31 Sporadic Cre-mediated deletion events occurring in few hematopoietic progenitor cells, which induce expression of the constitutively active kit, may represent a growth advantage for these cells resulting in overgrowth of the hematopoietic system by kitD814V expressing cells. Our finding that most hematopoietic cells, but only few cells outside the hematopoietic system, had deleted the stop element (as demonstrated by Southern blot in 2 mice; not shown) is in accordance with this concept. A growth advantage for precursor cells expressing mutant kit may also play an important role in the pathogenesis of human mastocytosis, which represents a monoclonal disease originating from a single mutation event. Our model differs from this situation in that numerous independent mutant clones arise as a result of sporadic or induced Mx1-Cre activity.
Our transgenic mice expressing the mutant, constitutively active kit receptor (with or without pI:C induction) displayed severe spontaneous inflammation of the colonic wall. Circumscribed patches of massive, lymphocyte-dominated inflammatory infiltration and severe, in many cases complete, destruction of the epithelial structures were encountered primarily in the cecum and ascending colon. A massive increase in mucosal mast cell numbers was found within the epithelial crypt structures at the border of the lesions. These findings may reflect a pathogenic role of mucosal mast cells in the intestinal inflammation of our mice. TNF-α plays a prominent role in the pathogenesis of human inflammatory bowel disease as well as in some mouse models of intestinal inflammation.37 Because mast cells have been shown to produce TNF-α in human intestinal mucosa,38,39 we tested whether the intestinal inflammation could be rescued by a cross of our kitD814Vflox Mx1-Cre mice onto a TNFRI-deficient background. TNFRI p55-deficient kitD814Vflox Mx1-Cre mice developed intestinal inflammation of similar severity compared with p55+/− littermate control animals (supplemental Figure 2E). This result as well as the finding of normal TNF-α serum levels in diseased mice (not shown) argue against an important pathogenic role of TNF-α in the bowel disease of our mice. To test the dependency of the intestinal inflammation on the microbial flora, we treated 4 adult mice with ciprofloxacin and metronidazole for 14 weeks but did not observe amelioration of the disease.
Numerous reports describe either elevated mucosal mast cell numbers39-45 or exaggerated mast cell mediator release46 in human inflammatory bowel disease, providing suggestive evidence for a pathogenic role of mast cells in inflammatory bowel disease. We cannot yet formally rule out that the deregulated kit signaling results in alterations of immune cells other than mast cells, which may contribute to the pathogenesis of colitis in our model. However, the finding that also KitD814Vflox Mcpt5-Cre animals, in which transgene expression is limited to the mast cell lineage, develop colitis suggests that alterations, which primarily affect the mast cell can contribute to intestinal inflammation under certain conditions. Yamada et al reported another mouse model of deregulated tyrosin kinase receptor activity (platelet-derived growth factor receptor-α) with increased numbers of mucosal mast cells in the small intestine, which, however, did not result in intestinal inflammation.47
Although other activating kit mutations were described to be inherited through the germline,21,22 inheritance of the D816V mutation in human mastocytosis seems to be an extremely rare event or may not occur at all. In our mouse model, early and general activation of kitD814V expression, a situation equivalent to a germline mutation, resulted in a high rate of perinatal lethality associated with a hyperproliferative dysregulation of the erythroid lineage, which was characterized by high numbers of abnormal nucleated erythroid cells circulating in the peripheral blood and a substantial increase in erythrocyte numbers and hematocrit. The liver seemed to be a major site of exaggerated erythropoiesis because the normal liver tissue was found largely replaced by erythroid cells. Lethality in the mutants may be caused by altered hemodynamic properties resulting from the increased hematocrit and the high numbers of nucleated cells. We hypothesize that the reason for absence of familial mastocytosis associated with the kitD816V mutation may be that human embryos carrying a germline D816V mutation are not viable because of a similar severe dysregulation of hematopoiesis.
Whereas 100% of neonatal kitD814Vflox deleter-Cre animals killed for analysis displayed the hyperproliferative dysregulation of the erythroid lineage, a fraction of kitD814Vflox deleter-Cre mice survived into adulthood. When killed at the age of several months, these mice did not show hematopoietic abnormalities, except for severe mastocytosis. This is indirect evidence that the disease observed in neonatal animals spontaneously regresses in some animals and suggests that this condition represents a hyperproliferative dysregulation but not genuine malignant growth. Kit signaling has a strong synergistic effect with various growth factors, including erythropoietin48 ; therefore, one could speculate that the hyperproliferative dysregulation occurs only during a certain developmental window and regresses later, if the animal survives this phase. The incomplete penetrance of the lethal phenotype may be explained by environmental factors, differences in transgene expression levels, or in slight differences in the timing of transgene expression.
Zappulla et al described Bchm kitD816V transgenic mice,25 which express a human kitD816V receptor under the control of a baboon chymase promoter, develop moderate mast cell hyperplasia in one-third of the animals older than 12 months. Thus, the mastocytosis phenotype of these animals is of considerably lower incidence and severity compared with the findings in our transgenic model. In addition to the results of Zappulla et al,25 Mayerhofer et al49 also described a low transforming potential of the human mutant kitD816V protein in the murine system. Xiang et al15 provided a probable explanation for these observations by demonstrating that intracellular processing/trafficking of the human kitD816V protein is abnormal in murine cells resulting from cross-species problems. Most human kit protein accumulates in the endoplasmic reticulum of mouse cells where it cannot signal. Therefore, human kitD816V is of low oncogenicity in murine cells. The human kitD816V intracellular domain, however, displays transforming capacity if intracellular processing is restored by either deletion of the human extracellular domain or by its replacement by the murine extracellular portion. This work clearly demonstrates that low transforming potential is not an intrinsic property that discriminates human kitD816V from its murine analog kitD814V. Our model avoids the cross-species problems by expression of the murine protein in the mouse. To determine the transforming potential of kitD814V in murine hematopoietic stem and progenitor cells in vivo, kit+ lin− bone marrow cells were transplanted into irradiated or nonirradiated recipient mice. The kitD814V bone marrow cells rescued lethally irradiated recipients but did not engraft in unirradiated mice, demonstrating that the kitD814V transgene does not sufficiently transform hematopoietic cells to allow displacement of recipient cells from their niche.
Interestingly, the severity of mast cell disease was lower in our Mcpt5-Cre model with kitD814V expression in more mature cells only compared with the Mx1-Cre and the deleter-Cre models, in which the mutant kit is expressed already in undifferentiated hematopoietic progenitors. The latter animals uniformly developed severe mast cell disease at a young age. This is in accordance with the finding in mastocytosis patients in whom mutations occurring in undifferentiated progenitors resulting in multilineage involvement cause severe systemic disease, whereas activating kit mutations that hit committed mast cell precursors or even mature mast cells result in milder forms of the disease.23 Most cases of kitD816V+ mastocytosis belong to the group of indolent systemic mastocytosis, characterized by little or no progression of mast cell disease, whereas our animals show a slow progression of mast cell numbers. A direct comparison of the 2 conditions, however, is difficult, taking into account, for example, the difference in life span between mouse and humans.
In conclusion, our kitD814Vflox line represents a mouse model for neoplastic mast cell disease, which reproduces some features of systemic mastocytosis with or without associated nonmast cell clonal hematologic disease. Although the model does not reproduce all aspects of the human condition, it could be the basis for development of new therapeutic approaches in mast cell disorders.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Carmen Berns and Silke Kummer for excellent technical assistance; Margot Junker for preparing the histologic sections; Wibke Johannis, Department of Clinical Chemistry, and Karl-Anton Kreuzer, Department of Internal Medicine I, University of Cologne for helpful discussions; and Wilhelm Bloch, German Sport University, for help with histology of neonatal mice.
This work was supported by the Fritz Thyssen Foundation (research grant 10.05.2.171 and 10.07.2.142; K.H., A.R.) and German Research Council (Deutsche Forschungsgemeinschaft; research grant RO 2133/2; A.R., K.H.).
Authorship
Contribution: A.G. designed and performed experiments, interpreted data, and wrote the manuscript; C. Wickenhauser and H.-P.H. evaluated histologic sections; J.S., K.P., and S.D. performed experiments; R.N. performed the pronucleus injection of the transgenic construct; W.M., T. Kamradt and C. Waskow designed experiments; T. Krieg revised the manuscript; and K.H. and A.R. designed and supervised experiments and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Axel Roers, Institute for Immunology, Medical Faculty Carl Gustav Carus, Dresden Technical University, Fetscherstrasse 74, 01307 Dresden, Germany; e-mail: axel.roers@tu-dresden.de; and Karin Hartmann, Department of Dermatology, University of Cologne, Kerpener Str 62, 50937 Cologne, Germany; e-mail: karin.Hartmann@uni-koeln.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal