Abstract
Older patients with acute myeloid leukemia (AML) have limited treatment options and a poor prognosis, thereby warranting novel therapeutic strategies. We evaluated the efficacy of lenalidomide as front-line therapy for older AML patients. In this phase 2 study, patients 60 years of age or older with untreated AML received high-dose (HD) lenalidomide at 50 mg daily for up to 2 28-day cycles. If patients achieved a complete remission (CR)/CR with incomplete blood count recovery (CRi) or did not progress after 2 cycles of HD lenalidomide, they received low-dose lenalidomide (10 mg daily) until disease progression, an unacceptable adverse event, or completion of 12 cycles. Thirty-three AML patients (median age, 71 years) were enrolled with intermediate (55%), unfavorable (39%), or unknown (6%) cytogenetic risk. Overall CR/CRi rate was 30%, and 53% in patients completing HD lenalidomide. The CR/CRi rate was significantly higher in patients presenting with a low (< 1000/μL) circulating blast count (50%, P = .01). The median time to CR/CRi was 30 days, and duration of CR/CRi was 10 months (range, 1- ≥ 17 months). The most common grades ≥ 3 toxicities were thrombocytopenia, anemia, infection, and neutropenia. HD lenalidomide has evidence of clinical activity as initial therapy for older AML patients, and further study of lenalidomide in AML and MDS is warranted. This study is registered at www.clinicaltrials.gov as #NCT00546897.
Introduction
Advances in the therapy of acute myeloid leukemia (AML), including risk stratification, combination chemotherapy, and stem cell transplantation, have improved outcomes for patients younger than 60 years.1-3 However, AML is primarily a disease of older adults with an annual incidence of approximately 15 in 100 000 in those 60 years of age or older, which represents more than two-thirds of all AML cases.4 In this older AML patient age group, the prognosis is poor with a median survival of less than 1 year.5-7 These suboptimal outcomes in older AML patients are related to a combination of unfavorable biologic characteristics of the leukemia, as well as comorbidities, poor performance status, or organ dysfunction that limit aggressive treatment options. In addition, AML in older patients frequently arises from an antecedent myelodysplastic syndrome (MDS), overexpresses chemotherapy efflux genes (MDR1), and has a greater representation of poor-risk cytogenetics. Collectively, these factors contribute to the lower CR rates and higher induction-related mortality when these patients are treated with an anthracycline plus cytarabine (7 + 3), compared with younger AML patients.5,7 Thus, therapeutic options that are both better tolerated and efficacious are needed for older AML patients.8
Currently, lenalidomide is approved at a dose of 10 mg/day for treatment of patients with lower-risk MDS associated with the 5q− cytogenetic abnormality and transfusion-dependent anemia. Lenalidomide is known to cause myelosuppression; in the trials that led to Food and Drug Administration approval of lenalidomide for this indication, patients with severe neutropenia and/or thrombocytopenia were excluded and doses were reduced for cytopenias.9,10 Using this approach in MDS, primarily erythroid responses were observed in MDS patients, and many patients were further dose reduced to less than 5 mg/day for hematologic adverse events. Notably, several responses were observed at the cytogenetic level and in patients with excess myeloblasts, suggesting that lenalidomide may eliminate the abnormal MDS clone.9-11 AML and MDS share multiple clinical and pathologic features, including poor-risk cytogenetic abnormalities, and MDS frequently evolves to AML. We therefore hypothesized that higher-dose lenalidomide, which typically causes drug-related myelosuppression, may be necessary to induce CR in AML patients. Our study evaluated the activity and toxicity of high-dose (HD) lenalidomide as front-line therapy for older patients with AML without dose reductions for hematologic adverse events. We performed an initial pilot study of HD (50 mg/day) lenalidomide in untreated older AML patients who included a 30-day drug rest period between the 2 short (14- and 21-day) cycles, selecting 50 mg/day based on phase I studies that demonstrated this dose was well tolerated, except myelosuppression.12,13 After an initial reduction in bone marrow (BM) myeloblasts, a large fraction of patients were removed from this pilot study because of progressive disease that occurred during the treatment gap between the 2 HD cycles.14,15 Based on the experience in this pilot cohort, we designed and report here the results of a prospective phase 2 study of untreated older AML patients with up to 2 sequential 28-day HD (50 mg/day) cycles of lenalidomide followed by low-dose (LD) lenalidomide therapy.
Methods
Patient eligibility
Patients 60 years of age or older with a World Health Organization diagnosis of AML (de novo, treatment-related, or transformed from MDS) and intermediate- or poor-risk cytogenetics,16 without isolated 5q abnormalities, who had not received prior AML therapy were eligible for this study. Additional inclusion criteria included: Eastern Cooperative Oncology Group performance status of 0 to 2, and adequate renal (serum creatinine < 1.5 × upper limit of normal) and hepatic function (bilirubin < 2.0 mg/dL and aspartate aminotransferase/alanine transaminase < 5 × upper limit of normal). Patients with acute promyelocytic leukemia, central nervous system leukemia, or prior use of lenalidomide were excluded. All older AML patients were considered eligible regardless of whether they were considered candidates for 7 + 3 by their treating physician. The study (#NCT00546897) was conducted in accordance with the Declaration of Helsinki, approved by the Washington University Institutional Review Board, and all patients provided written informed consent.
Study design and treatment
This was an open-label, prospective, single-arm phase 2 study of HD oral lenalidomide (50 mg/day in a single daily dose) on days 1 to 28 of a 28-day cycle for up to 2 cycles, followed by LD lenalidomide therapy (10 mg/day in 28-day cycles). Lenalidomide was provided by the manufacturer (Celgene). Patients who obtained a CR/CRi after HD cycle 1, or any patient without progressive disease after HD cycle 2, continued with LD therapy for a maximum of 12 cycles. HD therapy was not interrupted nor were doses modified for expected hematologic toxicities, but therapy could be interrupted for febrile neutropenia or grade ≥ 3 nonhematologic toxicities. During LD lenalidomide cycles, doses were delayed or reduced for hematologic toxicities following the guidelines established for MDS.17 Patients continued to receive lenalidomide therapy until an unacceptable adverse event, disease progression, or completion of 12 cycles. Growth factors were not administered routinely but were allowed as clinically indicated. Response was assessed by BM biopsy and complete blood count at day 15 of HD cycle 1, after completion of HD cycles 1 and 2, and as clinically indicated during LD therapy. BM biopsies were evaluated by Washington University School of Medicine hematopathologists. All adverse events were evaluated and coded using the National Cancer Institute Common Terminology Criteria for Adverse Events, Version 3.0.
Response criteria and statistical analysis
Responses were assessed using the International Working Group critera.18 The primary endpoint was morphologic CR and CRi. Secondary endpoints included overall response rate, duration of remission, progression-free survival (PFS), and overall survival (OS). The Southwest Oncology Group cytogenetic risk categories were used as described.16 The sample size (n = 27 evaluable patients) was based on an α of 0.05 and a power of 0.8 to detect the target CR/CRi rate of 25% over an unacceptable CR/CRi rate of 5%. Six patients did not receive at least one cycle of HD lenalidomide resulting from adverse events; therefore, additional patients were accrued for a total of 33 patients. The primary efficacy analysis was performed using all patients who received at least one dose of lenalidomide (N = 33). CR/CRi rate was calculated along with 95% confidence intervals, and Kaplan-Meier analysis was used to estimate PFS and OS. The assessment of baseline clinical characteristics on disease response was assessed by comparison of proportions using the Fisher exact test (categorical variables) or the Wilcoxon 2-sample test (continuous variables).
Results
Patient characteristics
Thirty-three patients were enrolled in this study, with a median age of 71 years (range, 60-88 years; Table 1). Thirty percent of patients had secondary AML arising from MDS (24%) or prior chemotherapy (6%). Of the 8 patients with prior MDS, 3 had received DNA hypomethylator therapy. Baseline cytogenetic categories were intermediate (55%), unfavorable (39%), or unknown (6%) risk. The median white blood cell count (WBC) at presentation was 4200/μL (range, 600-44 000/μL), with 76% of patients having detectable peripheral blood (PB) blasts (median, 140/μL; range, 0-22 770/μL). The median BM blast percentage was 53% (range, 21%-97%), and 70% of patients presented with more than 30% BM blasts. Two patients had an indeterminate BM myeloblast percentage: one acute erythroid leukemia and one patient with a hemodilute aspirate and 75% circulating myeloblasts (14 110 blasts/μL). The median time from diagnosis of AML to enrollment on study was 13 days (range, 4-40 days).
Patient baseline characteristics (N = 33)
| Characteristic . | No. (%) of patients . | Value . |
|---|---|---|
| Sex | ||
| Male | 21 (64) | |
| Female | 12 (36) | |
| Age, y | ||
| Median | 71 | |
| Range | 60-88 | |
| 60-64 | 6 (18) | |
| 65-69 | 8 (24) | |
| ≥ 70 | 19 (58) | |
| ECOG performance status | ||
| 0 | 16 (49) | |
| 1 | 13 (39) | |
| 2 | 4 (12) | |
| AML type | ||
| De novo | 23 (70) | |
| Prior MDS | 8 (24) | |
| Treatment-related | 2 (6) | |
| Presenting WBC count (per microliter) | ||
| Median | 4200 | |
| Range | 600-44 000 | |
| < 10 000 | 24 (73) | |
| ≥ 10 000 | 9 (27) | |
| Presenting BM blast, % | ||
| Median | 53% | |
| Range | 21%-97% | |
| Undefined | 2 (6) | |
| 20-30 | 8 (24) | |
| 31-50 | 7 (21) | |
| > 50 | 16 (49) | |
| Presenting PB blast count (per microliter) | ||
| Median | 140 | |
| Range | 0-22 770 | |
| < 1000 | 20 (61) | |
| 1000-10 000 | 9 (27) | |
| > 10 000 | 4 (12) | |
| Cytogenetic risk category | ||
| Intermediate | 18 (55) | |
| Unfavorable | 13 (39) | |
| Unknown | 2 (6) |
| Characteristic . | No. (%) of patients . | Value . |
|---|---|---|
| Sex | ||
| Male | 21 (64) | |
| Female | 12 (36) | |
| Age, y | ||
| Median | 71 | |
| Range | 60-88 | |
| 60-64 | 6 (18) | |
| 65-69 | 8 (24) | |
| ≥ 70 | 19 (58) | |
| ECOG performance status | ||
| 0 | 16 (49) | |
| 1 | 13 (39) | |
| 2 | 4 (12) | |
| AML type | ||
| De novo | 23 (70) | |
| Prior MDS | 8 (24) | |
| Treatment-related | 2 (6) | |
| Presenting WBC count (per microliter) | ||
| Median | 4200 | |
| Range | 600-44 000 | |
| < 10 000 | 24 (73) | |
| ≥ 10 000 | 9 (27) | |
| Presenting BM blast, % | ||
| Median | 53% | |
| Range | 21%-97% | |
| Undefined | 2 (6) | |
| 20-30 | 8 (24) | |
| 31-50 | 7 (21) | |
| > 50 | 16 (49) | |
| Presenting PB blast count (per microliter) | ||
| Median | 140 | |
| Range | 0-22 770 | |
| < 1000 | 20 (61) | |
| 1000-10 000 | 9 (27) | |
| > 10 000 | 4 (12) | |
| Cytogenetic risk category | ||
| Intermediate | 18 (55) | |
| Unfavorable | 13 (39) | |
| Unknown | 2 (6) |
ECOG indicates Eastern Cooperative Oncology Group.
Treatment with lenalidomide
The median duration of therapy was 65 days (range, 3-413 days); 70% completed HD cycle 1, 57% HD cycle 2, and 30% initiated LD lenalidomide (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). For the 39 complete HD cycles administered, 12 (31%) were delayed for a median of 5 days (range, 1-13 days). For 10 patients who received LD therapy, the median number of LD cycles administered was 6.5 (range, 1-12), and 3 of 70 (4%) cycles were delayed for infection (2 days) and cytopenias (7 and 12 days). LD therapy was reduced in 1 of 70 LD cycles for cytopenias. Patients discontinued LD therapy for unacceptable adverse events (n = 2), progressive disease (n = 4), or completion of 12 cycles (n = 4). Median follow-up from time of enrollment was 129 days (range, 6-518 days).
Response to lenalidomide
The primary endpoint of the study, CR/CRi rate, was 30% (95% confidence interval [CI], 16%-49%) and included 3 cytogenetic CRs (CRc), 3 morphologic CR, and 4 CRi (Tables 2,3). The CR/CRi rate was 53% (95% CI, 29%-76%) for the 19 patients completing induction with up to 2 cycles of HD lenalidomide, although this does not account for patients with rapid progressive disease during induction therapy. Responses were observed across all categories of AML (de novo, transformed from MDS, and secondary to prior therapy) and in patients with intermediate, unfavorable, and unknown risk cytogenetics (Table 2). Two patients had loss of chromosome 5 by routine cytogenetics in the setting of a complex karyotype, and neither patient obtained a CR/CRi. One of 3 patients who had failed a prior DNA-hypomethylating agent for MDS obtained a CR. Compared with higher-intensity induction chemotherapy (eg, 7 + 3), no patient developed BM aplasia (cellularity < 10%) during lenalidomide therapy; however, BM cellularity did decrease while on study (Table 4). CR/CRi were observed only in patients with a low presenting WBC count (< 10 000/μL) and PB blast count (< 1000/μL). Responses were rapid with a median time to CR of 30 days (13-72 days), and CR/CRi occurred at cycle 1 day 15 (n = 2), cycle 1 day 28 (n = 4), or cycle 2 (n = 4). Responses did not appear to have a predilection for a specific karyotype; and of the 5 patients with a clonal cytogenetic abnormality at diagnosis, 4 (80%) had resolution of the cytogenetic abnormality at the time of CR/CRi (Table 3). The median CR/CRi duration was 10 months (range, 1- ≥ 17 months), with 4 patients remaining in CR/CRi after ≥ 13, ≥ 15, ≥ 15, and ≥ 17 months. These 4 patients completed 12 cycles of LD therapy, and 2 patients are receiving postprotocol compassionate-use lenalidomide.
Response to treatment
| Variable . | No. of patients . | Patients with CR/CRi . | P* . | |
|---|---|---|---|---|
| No. . | % . | |||
| Overall (at least 1 dose of lenalidomide) | 33 | 10 | 30 | |
| Patients completing HD therapy | 19 | 10 | 53 | |
| ECOG PS | .43 | |||
| 0 | 15 | 6 | 40 | |
| 1 | 12 | 3 | 25 | |
| 2 | 4 | 1 | 25 | |
| AML diagnosis | .43 | |||
| De novo | 23 | 6 | 26 | |
| Prior MDS | 8 | 3 | 38 | |
| Treatment-related | 2 | 1 | 50 | |
| Cytogenetic risk category | .13 | |||
| Intermediate | 18 | 5 | 28 | |
| Unfavorable | 13 | 3 | 23 | |
| Unknown | 2 | 2 | 100 | |
| Age, y | .39 | |||
| 60-64 | 6 | 3 | 50 | |
| 65-69 | 8 | 3 | 38 | |
| > 70 | 19 | 4 | 21 | |
| Presenting WBC count (per microliter) | ||||
| < 10 000 | 24 | 10 | 42 | .044 |
| ≥ 10 000 | 9 | 0 | 0 | |
| Presenting BM blast, % | ||||
| Undefined | 2 | 0 | 0 | .003 |
| 20-30 | 8 | 5 | 63 | |
| 31-50 | 7 | 4 | 57 | |
| > 50 | 16 | 1 | 6 | |
| Presenting PB blast count (per microliter) | ||||
| < 1000 | 20 | 10 | 50 | .01 |
| 1000-10 000 | 9 | 0 | 0 | |
| > 10 000 | 4 | 0 | 0 | |
| Variable . | No. of patients . | Patients with CR/CRi . | P* . | |
|---|---|---|---|---|
| No. . | % . | |||
| Overall (at least 1 dose of lenalidomide) | 33 | 10 | 30 | |
| Patients completing HD therapy | 19 | 10 | 53 | |
| ECOG PS | .43 | |||
| 0 | 15 | 6 | 40 | |
| 1 | 12 | 3 | 25 | |
| 2 | 4 | 1 | 25 | |
| AML diagnosis | .43 | |||
| De novo | 23 | 6 | 26 | |
| Prior MDS | 8 | 3 | 38 | |
| Treatment-related | 2 | 1 | 50 | |
| Cytogenetic risk category | .13 | |||
| Intermediate | 18 | 5 | 28 | |
| Unfavorable | 13 | 3 | 23 | |
| Unknown | 2 | 2 | 100 | |
| Age, y | .39 | |||
| 60-64 | 6 | 3 | 50 | |
| 65-69 | 8 | 3 | 38 | |
| > 70 | 19 | 4 | 21 | |
| Presenting WBC count (per microliter) | ||||
| < 10 000 | 24 | 10 | 42 | .044 |
| ≥ 10 000 | 9 | 0 | 0 | |
| Presenting BM blast, % | ||||
| Undefined | 2 | 0 | 0 | .003 |
| 20-30 | 8 | 5 | 63 | |
| 31-50 | 7 | 4 | 57 | |
| > 50 | 16 | 1 | 6 | |
| Presenting PB blast count (per microliter) | ||||
| < 1000 | 20 | 10 | 50 | .01 |
| 1000-10 000 | 9 | 0 | 0 | |
| > 10 000 | 4 | 0 | 0 | |
ECOG indicates Eastern Cooperative Oncology Group; and PS, performance status.
P values were calculated using Fisher exact test.
Summary of CR/CRi patient characteristics
| Patient no. . | WHO diagnosis . | Cytogenetics . | Baseline WBC* . | Baseline blast count . | Response . | Cytogenetic response? . | Day of therapy at CR/CRi . | Duration of CR/Cri, mo . | PFS, mo . | OS, mo . |
|---|---|---|---|---|---|---|---|---|---|---|
| 17 | Therapy-related AML | Complex | 600 | 0 | CRc | Yes | C1D28 | 1 | 2 | 5 |
| 21 | AML with MDS-related features | Complex | 1200 | 20 | CRi | No | C2 | 6 | 8 | 11 |
| 23 | AML with MDS-related features† | Normal | 1600 | 0 | CR | NA | C1D28 | 6 | 7 | 16 |
| 25 | AML without maturation | t(2;14) | 1600 | 60 | CRc | Yes | C2 | 13 | 15 | 16 |
| 26 | Monoblastic AML (M5a) | Normal | 1700 | 0 | CRi | NA | C1D15 | 2 | 2 | 6 |
| 32 | AML with maturation | Normal | 4300 | 220 | CR | NA | C2 | ≥ 15 | ≥ 17 | ≥ 17 |
| 34 | AML with minimal differentiation | t(1;3) | 4200 | 710 | CRi | Yes | C1D15 | ≥ 17 | ≥ 17 | ≥ 17 |
| 39 | Monocytic AML (M5b) | MLL abnormal | 1600 | 0 | CRc | Yes | C1D28 | ≥ 15 | ≥ 16 | ≥ 16 |
| 44 | AML with MDS-related features | Normal | 2800 | 30 | CRi | NA | C1D28 | 1 | 2 | 3 |
| 45 | AML with maturation | Normal | 1300 | 0 | CR | NA | C2 | ≥ 13 | ≥ 14 | ≥ 14 |
| Patient no. . | WHO diagnosis . | Cytogenetics . | Baseline WBC* . | Baseline blast count . | Response . | Cytogenetic response? . | Day of therapy at CR/CRi . | Duration of CR/Cri, mo . | PFS, mo . | OS, mo . |
|---|---|---|---|---|---|---|---|---|---|---|
| 17 | Therapy-related AML | Complex | 600 | 0 | CRc | Yes | C1D28 | 1 | 2 | 5 |
| 21 | AML with MDS-related features | Complex | 1200 | 20 | CRi | No | C2 | 6 | 8 | 11 |
| 23 | AML with MDS-related features† | Normal | 1600 | 0 | CR | NA | C1D28 | 6 | 7 | 16 |
| 25 | AML without maturation | t(2;14) | 1600 | 60 | CRc | Yes | C2 | 13 | 15 | 16 |
| 26 | Monoblastic AML (M5a) | Normal | 1700 | 0 | CRi | NA | C1D15 | 2 | 2 | 6 |
| 32 | AML with maturation | Normal | 4300 | 220 | CR | NA | C2 | ≥ 15 | ≥ 17 | ≥ 17 |
| 34 | AML with minimal differentiation | t(1;3) | 4200 | 710 | CRi | Yes | C1D15 | ≥ 17 | ≥ 17 | ≥ 17 |
| 39 | Monocytic AML (M5b) | MLL abnormal | 1600 | 0 | CRc | Yes | C1D28 | ≥ 15 | ≥ 16 | ≥ 16 |
| 44 | AML with MDS-related features | Normal | 2800 | 30 | CRi | NA | C1D28 | 1 | 2 | 3 |
| 45 | AML with maturation | Normal | 1300 | 0 | CR | NA | C2 | ≥ 13 | ≥ 14 | ≥ 14 |
MLL indicates mixed lineage leukemia; C, cycle; D, day; and NA, not applicable.
WBC and blast count are shown per microliter of PB.
Patient 23 received 7 cycles of azacitidine for MDS ending 1 year before enrollment in the current study.
BM cellularity changes on study
| Study time point . | N . | Median BM cellularity, % (range) . |
|---|---|---|
| Study entry | 33 | 80 (10-100) |
| C1D15 | 25 | 60 (10-90) |
| C1D30 | 19 | 45 (15-100) |
| C2D30 | 9 | 60 (10-90) |
| Low-dose C2 | 6 | 30 (10-40) |
| Low-dose C6 | 5 | 20 (10-50) |
| Study time point . | N . | Median BM cellularity, % (range) . |
|---|---|---|
| Study entry | 33 | 80 (10-100) |
| C1D15 | 25 | 60 (10-90) |
| C1D30 | 19 | 45 (15-100) |
| C2D30 | 9 | 60 (10-90) |
| Low-dose C2 | 6 | 30 (10-40) |
| Low-dose C6 | 5 | 20 (10-50) |
C indicates cycle; and D, day.
The effects of baseline clinical characteristics on achievement of CR/CRi were explored (Fisher exact test), which showed that patients with a lower presenting WBC count (CR/CRi rate, 42%; P = .044), PB blast count (CR/CRi rate, 50%; P = .01), and BM blast percentage (P = .003) at diagnosis were more likely to achieve a CR/CRi with lenalidomide therapy (Table 2). In addition, when these parameters were analyzed as continuous variables (Wilcoxon 2-sample test), presenting WBC count (P = .0014), BM blast percentage (P = .013), and PB blast count (P = .0078) were all significantly associated with response. In contrast, the age of the patient was not significant either as a categorical (P = .39) or continuous variable (P = .25).
Survival
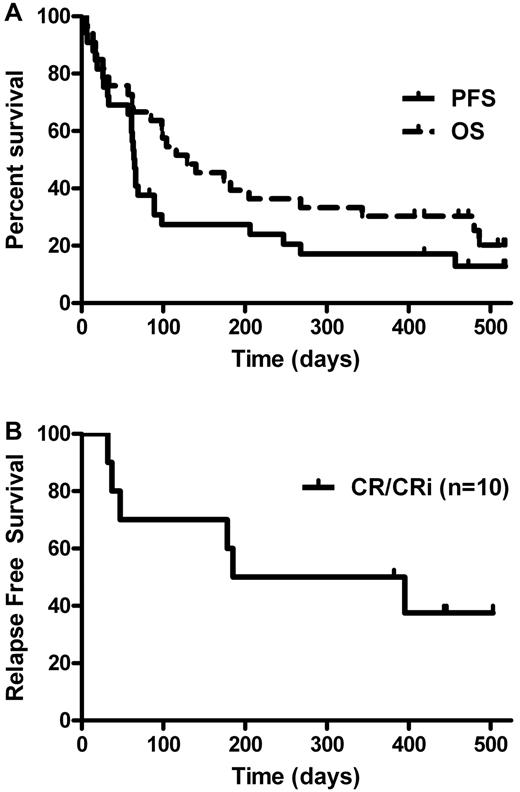
For the N = 33 patients who received at least one dose of lenalidomide, the median OS from study enrollment was 4 months (95% CI, 3-9 months), and median PFS was 2 months (95% CI, 2-3 months) (Figure 1). For the 19 patients who completed HD lenalidomide therapy, the median OS was significantly longer at 11 months (95% CI, 4-not estimated [NE] months, P < .001), and median PFS was 3 months (95% CI, 2-8 months). For the 10 patients who achieved a CR/CRi, the median OS was 16 months (95% CI, 6-NE months) and median PFS was 12 months (95% CI, 2-NE months). Median disease-free survival for the CR/CRi patients was 10 months (95% CI, 2-NE) and shown in Figure 1B. The effects of baseline clinical characteristics on OS was also explored, and patients with lower presenting WBC count (P = .043) and PB blast count (P = .009) at diagnosis had significantly longer survival. In this study, there was no significant difference in OS (P = .37) or relapse-free survival (P = .43) in CR versus CRi patients, although the comparison is limited by the number of patients analyzed.
Kaplan-Meier survival curves. (A) Kaplan-Meier curves for OS and PFS in all patients who received at least one dose of lenalidomide (N = 33). Median OS estimated at 4 months (95% CI, 3-9 months), and median PFS estimated at 2 months (95% CI, 2-3 months). (B) Kaplan-Meier curves for relapse-free survival in patients who obtained a CR/CRi (n = 10). Median relapse-free survival for the CR/CRi patients was 10 months (95% CI, 2-NE)
Kaplan-Meier survival curves. (A) Kaplan-Meier curves for OS and PFS in all patients who received at least one dose of lenalidomide (N = 33). Median OS estimated at 4 months (95% CI, 3-9 months), and median PFS estimated at 2 months (95% CI, 2-3 months). (B) Kaplan-Meier curves for relapse-free survival in patients who obtained a CR/CRi (n = 10). Median relapse-free survival for the CR/CRi patients was 10 months (95% CI, 2-NE)
Toxicity
The majority (91%) of patients experienced a grade ≥ 3 adverse events attributed to lenalidomide, and 24% of patients discontinued therapy resulting from an adverse event, including rash (n = 3), pneumonia (n = 2), mucositis (n = 1), elevated transaminases/bilirubin (n = 1), or central nervous system bleed (n = 1). As expected in this patient population, the most common grade more than or equal to 3 adverse events were thrombocytopenia, anemia, leukopenia, and neutropenia with infection or fever (Table 5). In addition to adverse events, reasons for lenalidomide treatment discontinuation included progressive disease (61%), completion of protocol therapy (12%), or patient preference (3%) (supplemental Table 2). The median days of hospitalization while on study for all patients was 6 days (range, 0-40 days), for non-CR/CRi patients (n = 23) was 6 days (range, 0-40 days), and for CR/CRi patients (n = 10) was 3 days (range, 0-14 days). The 30-day mortality was 24% (8 of 33) and the 60-day mortality encompassing the 60-day induction therapy period was 27% (9 of 33), with 7 of 9 (78%) deaths resulting from disease progression and 2 of 9 (22%) resulting from infection (supplemental Table 3). Notably, 7 of 9 patients who died early had a presenting PB blast count more than 1000/μL, 6 of 9 had a WBC count more than 10 000/μL, and 7 of 9 were more than 70 years old.
Most frequent grade 3 or higher adverse events
| Event . | No. (%) of patients . |
|---|---|
| Thrombocytopenia | 22 (67) |
| Anemia | 18 (55) |
| Leukopenia | 14 (42) |
| Neutropenic infection | 14 (42) |
| Neutropenia | 11 (33) |
| Neutropenic fever | 9 (33) |
| Fatigue | 5 (15) |
| Hypoxia | 5 (15) |
| Hyponatremia | 4 (12) |
| Event . | No. (%) of patients . |
|---|---|
| Thrombocytopenia | 22 (67) |
| Anemia | 18 (55) |
| Leukopenia | 14 (42) |
| Neutropenic infection | 14 (42) |
| Neutropenia | 11 (33) |
| Neutropenic fever | 9 (33) |
| Fatigue | 5 (15) |
| Hypoxia | 5 (15) |
| Hyponatremia | 4 (12) |
The most frequent adverse events occurred in > 10% of patients, regardless of attribution to lenalidomide.
Discussion
The optimal therapeutic approach for older (age ≥ 60 years) AML patients is currently unknown, and controversy exists about how to best treat this patient population with heterogeneous clinical outcomes and disease pathogenesis. As a whole, older AML patients have a poor prognosis with median OS rates of less than 1 year, and many do not receive antileukemic therapy.5-7,19 Thus, novel therapeutic agents are needed for older AML patients, especially those with an improved tolerability and distinct mechanism of action compared with 7 + 3. Here, we identify that HD lenalidomide (50 mg/day), administered as an oral medication in up to 2 sequential 28-day cycles, induced CR/CRi in 30% of untreated, older AML patients. These remissions were obtained rapidly (within 15-56 days of starting therapy) without BM aplasia. In some patients, AML-related cytopenias resolved concurrent with disappearance of leukemic blasts, suggesting that BM aplasia is not required for remission induction. CR/CRi after HD lenalidomide therapy was observed at higher rates in patients with low presenting WBC counts, peripheral blast counts, and BM blast percentages, identifying potential laboratory biomarkers that predict lenalidomide responders. CR/CRi was observed in all age groups, intermediate and unfavorable risk cytogenetics, and normal and diverse karyotypes. Thus, HD lenalidomide has clinical activity in older, untreated AML patients.
Currently used AML therapies have been characterized as “high,” “intermediate,” and “low” intensity based on their tolerability by older AML patients.3,5,7,8 High-intensity approaches are exemplified by 7 + 3, which result in 40% to 50% CR rates, induction-related mortality of 20% to 30%, and median OS of 7 to 12 months.20-23 Recently described “intermediate” intensity therapies include clofarabine24 and laromustine25 and yield CR/CRi rates of 32% to 46%, with induction-period mortality of 10% to 14%, and median OS of 3.2 to 4.5 months. In contrast, several “low” intensity therapies have been evaluated in older AML patients who include LD cytarabine,26 tipifarnib,27 azacitidine,28 and decitabine.29,30 CR rates with decitabine, azacitidine, LD cytarabine, and tipifarnib were 25% to 47%, 18%, 15%, and 8%, respectively, with median OS of 3.6 to 25 months and induction-period mortality of 10% to 25%.
Patients receiving HD lenalidomide experienced expected hematologic toxicities and infections; however, this did not result in substantial mucositis or other nonhematologic toxicities. The adverse event profile, outpatient oral administration, and median 6 days of hospitalization while on study support classifying HD lenalidomide as “low” intensity therapy. The CR/CRi rate observed with HD lenalidomide (30%) appears promising compared with other low-intensity agents. CR and CRi were combined as the primary endpoint of this study because of the known ability of lenalidomide to induce neutropenia and thrombocytopenia in combination with the prolonged duration of therapy, thereby preventing normal recovery of the platelet and absolute neutrophil counts required for a CR. Although the numbers are small, there was no significant difference in OS or relapse-free survival in CR versus CRi patients. In addition, lenalidomide induced CR/CRi rapidly (median, 30 days) compared with DNA-hypomethylating agents (median, 48-126 days). In this study, we observed a median OS of 4 months and a 27% 60-day induction period mortality, which appear lower than several of the studies described above in this section. It is important to emphasize that survival within older AML patients is heterogeneous, and in the context of clinical trials may be influenced by biases associated with single-arm phase 2 studies. Notably, of the 9 patients who died within the first 60 days on study, 7 died of progressive disease. In addition, 7 of 9 had a presenting PB blast count more than 1000/μL and 6 of 9 had a WBC count more than 10 000/μL (supplemental Table 2). The lack of response in rapidly progressing, hyperproliferative AML patients appears similar to the experience with DNA-hypomethylating agents, with no CRs in patients presenting with a high WBC count, and probably contributes to the relatively short OS in this study.29 Encouragingly, the median OS for patients who achieved CR/CRi with HD lenalidomide was significantly longer (16 months) than the entire patient cohort, suggesting that rational AML patient selection (eg, < 1000/μL circulating blasts) may improve survival in future studies. Definitive OS comparisons between different therapeutic approaches will require randomized phase III studies.
What is the optimal dose of lenalidomide for older patients with AML and MDS? Chandler et al have preliminarily reported a phase 1 dose escalation study in relapsed/refractory AML starting at 25 mg/day on days 1 to 21 of a 28-day schedule.31 Dose escalation identified 50 mg/day as the maximum tolerated dose in this trial, with higher doses resulting in dose-limiting fatigue. Overall, the CR rate was 5 of 31 (16%) in relapsed/refractory AML, responses were observed after 2 or 3 cycles of therapy, and most responses (3 of 5) were observed at 50 mg/day, suggesting that lower doses may not be as effective. The optimal duration of HD lenalidomide therapy remains unclear. In our pilot study, a 14-day HD lenalidomide cycle (50 mg/day) followed by a 30-day rest period resulted in patients coming off study resulting from progressive disease, and only one of 15 patients achieved a CR.14,15,32 This suggests that longer, continuous courses of HD lenalidomide are probably more effective than shorter courses. The current study design transitioned CR/CRi patients after 28 days of HD lenalidomide to LD therapy. In future trials, it may be preferable to administer 2 cycles of HD lenalidomide therapy regardless of CR/CRi status, or consider consolidation therapy, in an effort to further improve the duration of CR. In addition, clinical trials using combinations of low-intensity agents, especially those agents with disparate mechanisms of action, may optimize their use in older AML patients. Follow-up studies are already underway combining HD lenalidomide with azacitidine.
The same paradigm of HD lenalidomide may be applicable to MDS. Although LD (10 mg/day) lenalidomide clearly results in erythroid responses in MDS (especially 5q−) patients, fixed HD lenalidomide induction may better eliminate the MDS clone regardless of cytogenetics and provide trilineage responses, as observed in the current study. In this regard, it is intriguing that one of 3 patients who had failed a prior DNA-hypomethylating agent for antecedent MDS obtained a CR with HD lenalidomide. Prospective studies evaluating single-agent HD lenalidomide are planned for MDS patients. Sekeres et al noted that lenalidomide-induced neutropenia and thrombocytopenia correlated with lenalidomide red blood cell response in lower-risk MDs patients.33 We found no positive correlation for the development of neutropenia or thrombocytopenia and CR/CRi response in older AML patients on this study. In contrast, we detected a significant negative correlation (Fisher exact test, P = .03) between worsening (> 50% decrease in absolute neutrophil count) neutropenia and CR/CRi response, probably reflecting absolute neutrophil count improvement in those patients with CR/CRi, compared with no CR/CRi.
How lenalidomide induces CR in AML remains unclear. Lenalidomide has a wide spectrum of potential mechanisms of action, including direct effects on the viability, growth, or survival of malignant cells, immunomodulation of T and NK cells, alteration of cytokine production, blockade of angiogenesis, and additional effects on the tumor microenvironment.34 An initial case report of 2 independent lenalidomide-induced cytogenetic CRs in AML with trisomy 13 suggested that lenalidomide has activity in this uncommon, recurrent cytogenetic AML subset with a poor prognosis.32 Our larger study now demonstrates CRs in cytogenetically normal AML, as well as multiple AML karyotypes. Future studies are needed to evaluate the correlation between potential mechanisms of action in AML and clinical outcomes. An improved understanding of how lenalidomide induces CR in AML will aid in the development of rational combinations with other agents.
In conclusion, initial treatment of older AML patients with HD lenalidomide at 50 mg/day represents a novel therapeutic option in this patient population manifest by a CR/CRi rate of 30%, a rapid time to CR/CRi, and a short duration of hospitalization. Future studies will focus on the mechanism of action of lenalidomide in AML, defining predictors of lenalidomide response, refining the dosing regimen, and developing rational combinations with other novel agents in older patients with MDS and AML.
An Inside Blood analysis of this article appears at the end of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
T.A.F. is the recipient of the ASCO Foundation's Young Investigator Award that partially supported this clinical study. G.L.U. is supported by K23CA140707 and UL1 RR024992.
Authorship
Contribution: T.A.F. and R.V. conceived and designed the study; T.A.F., G.L.U., C.N.A., A.F.C., K.E.S.-G., P.W., J.F.D., and R.V. provided study materials or patients; T.A.F., K.T., A.D.N., J.D., and R.V. collected and assembled the data; T.A.F., K.T., and R.V. analyzed and interpreted data; T.A.F. and R.V. wrote the manuscript; and all authors had access to the primary clinical trial data and gave final approval of the manuscript.
Conflict-of-interest disclosure: T.A.F. and R.V. have received research funding from Celgene for this clinical study. R.V. and P.W. have received honoraria from Celgene. R.V. has performed consulting for Celgene. The remaining authors declare no competing financial interests.
Correspondence: Ravi Vij, 660 South Euclid Ave, Campus Box 8007, St Louis, MO 63110; e-mail: rvij@dom.wustl.edu.