Abstract
We assessed the predictive factors for outcome and response in 123 patients with chronic myeloid leukemia in chronic phase treated with second-generation tyrosine kinase inhibitors (TKIs) after imatinib failure. Better event-free survival rates with second-generation TKI therapy were associated with a previous cytogenetic response to imatinib (P < .001) and a performance status of 0 (P = .001). Patients with 0, 1, or 2 adverse factors had 2-year event-free survival rates of 78%, 49%, and 20% (P < .001), respectively; 2-year overall survival rates of 95%, 85%, and 40%, (P = .002), respectively; and a 12-month probability of achieving a major cytogenetic response of 64%, 36%, and 20% (P = .007), respectively. In conclusion, patients with poor performance status and no previous cytogenetic response to imatinib therapy have a low likelihood of responding to second-generation TKI with poor event-free survival and therefore should be offered additional treatment options. This scoring system could serve to advise patients of their prognosis and treatment options, as well as to evaluate the benefit of newer alternate options.
Introduction
The successful introduction of the tyrosine kinase inhibitors (TKIs), which suppress the molecular processes driving chronic myeloid leukemia (CML), has revolutionized the management and outlook in CML.1 Imatinib mesylate therapy induced high rates of complete cytogenetic and major molecular responses, and improved survival in CML.2-5 After imatinib treatment, more than 90% of patients obtain complete hematologic response, and more than 80% achieve a complete cytogenetic response. With 8 years of follow-up, the results are still very favorable, resulting in a major change in the natural history of the disease.6
Despite the benefit of imatinib over prior treatments, some patients may develop resistance,7 with a reported annual resistance rate of 2% to 4% in newly diagnosed patients in chronic phase, the incidence decreasing over time.8 Novel more potent TKIs, such as dasatinib, nilotinib, and bosutinib, have been developed to overcome imatinib resistance.9-11 These agents have shown significant activity after failure of imatinib therapy, with high rates of hematologic and cytogenetic responses.
The aims of the study were to assess the predictive factors for outcome and response in patients with chronic-phase CML treated with second-generation TKIs after imatinib failure.
Methods
A total of 123 patients with CML in chronic phase after imatinib failure were treated with second-generation TKIs in phase 2 pivotal trials. Entry criteria were similar for both trials. A total of 78 (63%) patients were treated with dasatinib and 45 (37%) with nilotinib. Chronic-phase CML was defined as the presence of blasts less than 15%, basophils less than 20%, blasts and promyelocytes less than 30%, and platelets more than 100 × 109/L.12 The definitions of imatinib failure have been previously described13,14 and are generally aligned with those later proposed by the European LeukemiaNet.15
Patients were treated with M. D. Anderson Cancer Center Institutional Review Board–approved protocols. Informed consent was obtained in accordance with the Declaration of Helsinki. Response criteria were as previously described.2 A complete hematologic response (CHR) was defined as a white blood cell count of less than 10 × 109/L, a platelet count of less than 450 × 109/L, no immature cells (blasts, promyelocytes, myelocytes) in the peripheral blood, and disappearance of all signs and symptoms related to leukemia (including palpable splenomegaly). This was further categorized by the best cytogenetic remission as complete (0% Philadelphia chromosome–positive metaphases, [Ph+]), partial (1%-35% Ph+), minor (36%-65% Ph+), and minimal (66%-95% Ph+). A major cytogenetic response (MCyR) included complete plus partial cytogenetic responses (ie, ≤ 35% Ph+). Response rates were calculated based on intention to treat.
Event-free survival (EFS) was measured from the start of treatment to the date of any of the following events while on therapy: death from any cause, loss of complete hematologic response, loss of complete cytogenetic response, discontinuation of therapy for toxicity or lack of efficacy, or progression to accelerated or blast phases. Survival probabilities were estimated by the Kaplan-Meier method and compared by the log-rank test.
Mutation analysis was performed as previously described.16 The published 50% inhibitory concentration (IC50) values for each drug for in vitro inhibition (in cell lines) of kinase activity of particular mutated BCR-ABL17-23 were used to classify mutations into high, intermediate, and low sensitivity to dasatinib (IC50 values ≤ 3nM, 3-60nM, and > 60nM, respectively) and nilotinib (IC50 values ≤ 50nM, 50-500nM, and > 500nM, respectively). Whenever a discrepancy in reported IC50 values was identified between different reports, the worst case scenario was adopted (ie, the highest IC50 to the corresponding TKI). Patients with multiple mutations were classified based on the mutation with the highest IC50.
Differences among variables were evaluated by the χ2 test and Mann-Whitney U test for categorical and continuous variables, respectively. Univariate and multivariate analyses were performed to identify potential prognostic factors associated with MCyR and survival. Factors retaining significance in the multivariate model were interpreted as being independently predictive of MCyR. Multivariate analysis of response used logistic regression model and survival used the Cox proportional hazard model.24-26
Results
Patients
A total of 123 patients with chronic-phase CML after imatinib failure treated with dasatinib (n = 78) or nilotinib (n = 45) were analyzed. Their characteristics are summarized in Table 1. Their median age was 56 years (range, 21-83 years). The median duration of chronic phase (CML diagnosis to start of second-generation TKI) was 67 months (range, 2-268 months). Their best response to imatinib was CHR only in 24% and cytogenetic response in 63% (28% complete, 17% partial, and 18% minor). Among the others, 2 patients were refractory to imatinib, 6 had unknown response, and 8 were intolerant. Twenty-nine patients (24%) had a performance status more than 0; only 6 of them were found to be possibly related to imatinib adverse events, and the rest of them were the result of disease progression, comorbidities, and older age. There was no difference in the patients' characteristics between patients treated with dasatinib and those treated with nilotinib (data not shown).
Patient characteristics (n = 123)
| Variable . | Category . | Median (range) or no. (%) . |
|---|---|---|
| Age, y | 56 (21-83) | |
| Sex | Female | 68 (55) |
| CML duration, months | 67 (2-268) | |
| Performance Status | > 1 | 29/118 (24) |
| Splenomegaly | Yes | 7 (6) |
| Prior interferon therapy | Yes | 70 (57) |
| Peripheral blood | Leukocytes, × 109/L | 11.5 (1.8-160.8) |
| Hemoglobin, g/dL | 12.3 (7.7-16.6) | |
| Platelets, × 109/L | 306 (103-1436) | |
| Blasts, % | 0 (0-14) | |
| Basophils, % | 2 (0-17) | |
| Bone marrow | Blasts, % | 2 (0-12) |
| Basophils, % | 2 (0-14) | |
| Best response to imatinib | Cytogentic response | 77 (63) |
| Complete | 34 (28) | |
| Partial | 21 (17) | |
| Minor | 22 (18) | |
| Complete hematologic response only | 30 (24) | |
| Intolerant | 8 (7) | |
| Resistant/unknown | 2 (2)/6 (5) | |
| Second TKI therapy | Dasatinib | 78 (63) |
| Nilotinib | 45 (37) | |
| Active disease at the start of second TKI | Yes | 84 (68) |
| Clonal evolution | Yes | 28 (23) |
| > 90% Philadelphia positivity | Yes | 82/117 (70) |
| Mutation | None | 42 (34) |
| Low IC50 | 23 (19) | |
| Intermediate IC50 | 12 (10) | |
| Not done | 43 (35) |
| Variable . | Category . | Median (range) or no. (%) . |
|---|---|---|
| Age, y | 56 (21-83) | |
| Sex | Female | 68 (55) |
| CML duration, months | 67 (2-268) | |
| Performance Status | > 1 | 29/118 (24) |
| Splenomegaly | Yes | 7 (6) |
| Prior interferon therapy | Yes | 70 (57) |
| Peripheral blood | Leukocytes, × 109/L | 11.5 (1.8-160.8) |
| Hemoglobin, g/dL | 12.3 (7.7-16.6) | |
| Platelets, × 109/L | 306 (103-1436) | |
| Blasts, % | 0 (0-14) | |
| Basophils, % | 2 (0-17) | |
| Bone marrow | Blasts, % | 2 (0-12) |
| Basophils, % | 2 (0-14) | |
| Best response to imatinib | Cytogentic response | 77 (63) |
| Complete | 34 (28) | |
| Partial | 21 (17) | |
| Minor | 22 (18) | |
| Complete hematologic response only | 30 (24) | |
| Intolerant | 8 (7) | |
| Resistant/unknown | 2 (2)/6 (5) | |
| Second TKI therapy | Dasatinib | 78 (63) |
| Nilotinib | 45 (37) | |
| Active disease at the start of second TKI | Yes | 84 (68) |
| Clonal evolution | Yes | 28 (23) |
| > 90% Philadelphia positivity | Yes | 82/117 (70) |
| Mutation | None | 42 (34) |
| Low IC50 | 23 (19) | |
| Intermediate IC50 | 12 (10) | |
| Not done | 43 (35) |
At the start of second-generation TKI, 84 (68%) patients had active disease (ie, not in CHR). A total of 23% had clonal evolution, and 70% had more than 90% Ph+ metaphases. Kinase domain sequencing was performed before the start of therapy in 80 (65%) patients. Mutations were detected in 48% of these patients: 19% were harboring sensitive mutations to dasatinib and nilotinib defined by in vitro IC50 values less than or equal to 3nM and 50nM, respectively; and 10% harbored intermediate sensitivity mutations to dasatinib and nilotinib defined by IC50 values between 3 and 60nM and 50 and 500nM, respectively16 ; patients with the T315I mutation were excluded from this analysis. The most common mutations observed were G250E (n = 8), M351T (n = 6), and F317L (n = 5).
The median follow-up time was 38 months (range, 13-67 months) from the start of the second-generation TKI. At the time of last follow-up, 94 of the 123 patients (76%) were alive, 46 (37%) remained in chronic-phase on study receiving second-generation TKI therapy. Seventy-seven patients (63%) were taken off therapy for the following reasons: 40 (32%) disease progression, 15 (12%) for toxicity, lack of compliance in 4 (3%), 10 (8%) were lost to follow-up, 3 (2%) (2 in complete, one in partial cytogenetic response) received an allogeneic stem cell transplantation, 4 (3%) died on treatment from non–CML-related causes, and one the result of other significant clinical event (altered mental status and recurrent urinary tract infections).
Response to second-generation TKI
Responses to second-generation TKI are shown in Table 2. The CHR rates were 87% and 84% in patients treated with dasatinib and nilotinib, respectively (P = .75). The MCyR rates were 64% and 62% (P = .85), and the complete cytogenetic response rates were 60% and 56% (P = .70). The rates of cytogenetic response at 6 and 12 months were 63% and 55% (P = .44) and 69% and 51% (P = .07) in patients treated with dasatinib and nilotinib, respectively, and the rates of complete cytogenetic response at these same time points were 41% and 36% (P = .7) and 48% and 35% (P = .18), respectively. The 3-year EFS and overall survival rates were 53% and 84%, respectively.
Response to second-generation TKIs (analyzed by intention to treat)
| Parameter . | % response . | P . | ||
|---|---|---|---|---|
| Overall (n = 123) . | Dasatinib (n = 78) . | Nilotinib (n = 45) . | ||
| CHR | 86 | 87 | 84 | .75 |
| Cytogenetic response | 74 | 76 | 71 | .67 |
| Major | 63 | 64 | 62 | .85 |
| Complete | 59 | 60 | 56 | .70 |
| Any CyR at 6 months | 60 | 63 | 55 | .44 |
| Any CyR at 12 months | 63 | 69 | 51 | .07 |
| CCyR at 6 months | 39 | 41 | 36 | .70 |
| CCyR at 12 months | 43 | 48 | 35 | .18 |
| Parameter . | % response . | P . | ||
|---|---|---|---|---|
| Overall (n = 123) . | Dasatinib (n = 78) . | Nilotinib (n = 45) . | ||
| CHR | 86 | 87 | 84 | .75 |
| Cytogenetic response | 74 | 76 | 71 | .67 |
| Major | 63 | 64 | 62 | .85 |
| Complete | 59 | 60 | 56 | .70 |
| Any CyR at 6 months | 60 | 63 | 55 | .44 |
| Any CyR at 12 months | 63 | 69 | 51 | .07 |
| CCyR at 6 months | 39 | 41 | 36 | .70 |
| CCyR at 12 months | 43 | 48 | 35 | .18 |
CyR indicates cytogenetic response; and CCyR, complete cytogenetic response.
Predictive factors for survival after treatment with second-generation TKI
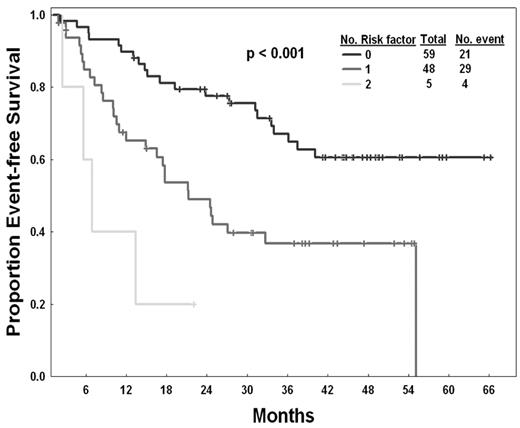
Table 3 summarizes the association between patient and disease characteristics and outcome. In the univariate analysis for EFS, factors associated with poor EFS were older age (> 55 years), lack of any cytogenetic response to previous imatinib therapy, an Eastern Cooperative Oncology Group performance status more than or equal to 1 at the start of second-generation TKI therapy, and more than or equal to 90% Ph+ metaphases at the start of second-generation TKI therapy. In a multivariate analysis, the lack of any cytogenetic response to previous imatinib therapy (P < .001) and an Eastern Cooperative Oncology Group performance status more than or equal to 1 at the start of second-generation TKI therapy (P = .007) were selected as independent factors associated with poor EFS. The relative impact of each of these 2 factors on EFS was similar. Thus, we assigned an arbitrary value of 1 to each of them. Patients with 0, 1, or 2 adverse factors had an estimated 24-month EFS with second-generation TKI therapy of 78%, 49%, and 20%, respectively (Figure 1).
Patients and disease characteristics associated with MCyR at 1-year and 3-year survival outcomes
| Parameter . | Category . | No. . | % 1-year . | % 3-year . | ||||
|---|---|---|---|---|---|---|---|---|
| MCyR . | P . | EFS . | P . | OS . | P . | |||
| Age, y | < 55 | 62 | 64 | .01 | 63 | .02 | 87 | .01 |
| > 55 | 61 | 40 | 43 | 81 | ||||
| Splenomegaly | No | 114 | 55 | .18 | 54 | .09 | 85 | .09 |
| Yes | 7 | 20 | 0 | 46 | ||||
| Hemoglobin, g/dL | < 10 | 15 | 29 | .007 | 36 | .22 | 59 | .08 |
| 10-11 | 41 | 43 | 50 | 83 | ||||
| ≥ 12 | 67 | 68 | 58 | 89 | ||||
| WBCs, × 109/L | < 10 | 56 | 59 | .35 | 52 | .88 | 87 | .48 |
| ≥ 10 | 67 | 50 | 53 | 80 | ||||
| Platelets, × 109/L | ≤ 450 | 82 | 57 | .33 | 55 | .38 | 81 | .62 |
| > 450 | 41 | 47 | 47 | 88 | ||||
| Peripheral basophils, % | < 2 | 57 | 53 | .97 | 54 | .32 | 86 | .92 |
| 2-6 | 43 | 54 | 49 | 82 | ||||
| ≥ 7 | 23 | 57 | 54 | 82 | ||||
| Peripheral blasts, % | 0 | 95 | 57 | .1 | 56 | .11 | 86 | .01 |
| 1-2 | 19 | 56 | 48 | 84 | ||||
| ≥ 3 | 9 | 14 | 15 | 56 | ||||
| Marrow basophils, % | < 2 | 55 | 57 | .67 | 55 | .64 | 89 | .23 |
| 2-4 | 43 | 48 | 49 | 74 | ||||
| > 4 | 21 | 50 | 45 | 85 | ||||
| Marrow blasts, % | 0 | 18 | 75 | .05 | 55 | .67 | 83 | .02 |
| 1-2 | 55 | 48 | 49 | 96 | ||||
| 3-4 | 31 | 59 | 55 | 71 | ||||
| ≥ 5 | 15 | 25 | 46 | 60 | ||||
| Clonal evolution | No | 94 | 53 | >.999 | 54 | .46 | 87 | .22 |
| Yes | 28 | 54 | 48 | 71 | ||||
| CML duration, years | 0-3 | 27 | 67 | .42 | 41 | .56 | 78 | .89 |
| 4-5 | 41 | 52 | 56 | 83 | ||||
| ≥ 6 | 55 | 51 | 55 | 87 | ||||
| CHR at the start of second TKI | No | 39 | 60 | .42 | 53 | .63 | 90 | .40 |
| Yes | 84 | 51 | 52 | 81 | ||||
| Best response to imatinib | Intolerant | 8 | 83 | > .999 | 50 | < .001 | 100 | .02 |
| MCyR | 55 | 75 | 67 | 87 | ||||
| mCyR | 22 | 45 | 52 | 81 | ||||
| No CyR | 32 | 20 | 30 | 81 | ||||
| No data | 6 | 40 | 40 | 50 | ||||
| Performance status | 0 | 89 | 51 | .82 | 56 | .02 | 91 | < .001 |
| ≥ 1 | 29 | 54 | 38 | 59 | ||||
| % Ph at the start | ≥ 90 | 35 | 75 | .04 | 66 | .03 | 91 | .41 |
| > 90 | 82 | 45 | 45 | 80 | ||||
| Prior IFN-α | No | 53 | 64 | .08 | 54 | .76 | 79 | .85 |
| Yes | 70 | 47 | 51 | 87 | ||||
| Mutation status | None | 42 | 50 | .08 | 55 | .22 | 81 | .09 |
| Low IC50 | 23 | 65 | 45 | 87 | ||||
| Intermediate IC50 | 12 | 25 | 25 | 50 | ||||
| Not done | 43 | NA | NA | NA | ||||
| Parameter . | Category . | No. . | % 1-year . | % 3-year . | ||||
|---|---|---|---|---|---|---|---|---|
| MCyR . | P . | EFS . | P . | OS . | P . | |||
| Age, y | < 55 | 62 | 64 | .01 | 63 | .02 | 87 | .01 |
| > 55 | 61 | 40 | 43 | 81 | ||||
| Splenomegaly | No | 114 | 55 | .18 | 54 | .09 | 85 | .09 |
| Yes | 7 | 20 | 0 | 46 | ||||
| Hemoglobin, g/dL | < 10 | 15 | 29 | .007 | 36 | .22 | 59 | .08 |
| 10-11 | 41 | 43 | 50 | 83 | ||||
| ≥ 12 | 67 | 68 | 58 | 89 | ||||
| WBCs, × 109/L | < 10 | 56 | 59 | .35 | 52 | .88 | 87 | .48 |
| ≥ 10 | 67 | 50 | 53 | 80 | ||||
| Platelets, × 109/L | ≤ 450 | 82 | 57 | .33 | 55 | .38 | 81 | .62 |
| > 450 | 41 | 47 | 47 | 88 | ||||
| Peripheral basophils, % | < 2 | 57 | 53 | .97 | 54 | .32 | 86 | .92 |
| 2-6 | 43 | 54 | 49 | 82 | ||||
| ≥ 7 | 23 | 57 | 54 | 82 | ||||
| Peripheral blasts, % | 0 | 95 | 57 | .1 | 56 | .11 | 86 | .01 |
| 1-2 | 19 | 56 | 48 | 84 | ||||
| ≥ 3 | 9 | 14 | 15 | 56 | ||||
| Marrow basophils, % | < 2 | 55 | 57 | .67 | 55 | .64 | 89 | .23 |
| 2-4 | 43 | 48 | 49 | 74 | ||||
| > 4 | 21 | 50 | 45 | 85 | ||||
| Marrow blasts, % | 0 | 18 | 75 | .05 | 55 | .67 | 83 | .02 |
| 1-2 | 55 | 48 | 49 | 96 | ||||
| 3-4 | 31 | 59 | 55 | 71 | ||||
| ≥ 5 | 15 | 25 | 46 | 60 | ||||
| Clonal evolution | No | 94 | 53 | >.999 | 54 | .46 | 87 | .22 |
| Yes | 28 | 54 | 48 | 71 | ||||
| CML duration, years | 0-3 | 27 | 67 | .42 | 41 | .56 | 78 | .89 |
| 4-5 | 41 | 52 | 56 | 83 | ||||
| ≥ 6 | 55 | 51 | 55 | 87 | ||||
| CHR at the start of second TKI | No | 39 | 60 | .42 | 53 | .63 | 90 | .40 |
| Yes | 84 | 51 | 52 | 81 | ||||
| Best response to imatinib | Intolerant | 8 | 83 | > .999 | 50 | < .001 | 100 | .02 |
| MCyR | 55 | 75 | 67 | 87 | ||||
| mCyR | 22 | 45 | 52 | 81 | ||||
| No CyR | 32 | 20 | 30 | 81 | ||||
| No data | 6 | 40 | 40 | 50 | ||||
| Performance status | 0 | 89 | 51 | .82 | 56 | .02 | 91 | < .001 |
| ≥ 1 | 29 | 54 | 38 | 59 | ||||
| % Ph at the start | ≥ 90 | 35 | 75 | .04 | 66 | .03 | 91 | .41 |
| > 90 | 82 | 45 | 45 | 80 | ||||
| Prior IFN-α | No | 53 | 64 | .08 | 54 | .76 | 79 | .85 |
| Yes | 70 | 47 | 51 | 87 | ||||
| Mutation status | None | 42 | 50 | .08 | 55 | .22 | 81 | .09 |
| Low IC50 | 23 | 65 | 45 | 87 | ||||
| Intermediate IC50 | 12 | 25 | 25 | 50 | ||||
| Not done | 43 | NA | NA | NA | ||||
OS indicates overall survival; WBCs, white blood cells; mCyR, minor cytogenetic response; CyR, cytogenetic response; IFN-α, interferon-α; and NA, not applicable.
EFS by risk score. The top curve represents patients with no risk factor, followed by patients with one risk factor, then those with 2 risk factors at the start of second-generation TKI.
EFS by risk score. The top curve represents patients with no risk factor, followed by patients with one risk factor, then those with 2 risk factors at the start of second-generation TKI.
Factors associated with poor overall survival in the univariate analysis were older age (> 55 years), increasing blood and marrow blasts, lack of any cytogenetic response to previous imatinib therapy, and an Eastern Cooperative Oncology Group performance status more than or equal to 1 at the start of second-generation TKI therapy. In a multivariate analysis, only a performance status more than or equal to 1 (P < .001) was independently associated with a lower probability of survival.
Impact of the achievement of an early cytogenetic response
Achieving an MCyR is a known major determinant of outcome in previous generations of therapy, including interferon-α and imatinib. In a previous report from our institution, patients who achieved a MCyR by 12 months from start of therapy with second-generation TKI had an improved EFS.27
We thus conducted a repeat multivariate analysis using a 12-month landmark. Adding the 12-month MCyR to the multivariate analysis revealed that lack of a 12-month MCyR to therapy constituted the only independent adverse factor for EFS (P < .001; hazard ratio = 2.3), displacing all other factors. In contrast, a performance status more than or equal to 1 (P = .02; hazard ratio = 2.4) constituted the only independent adverse factor for survival.
Therefore, we analyzed factors that were associated with the 12-month achievement of a MCyR. In the univariate analysis, younger age, high hemoglobin level, previous cytogenetic response to imatinib therapy, and less than or equal to 90% Ph+ metaphases were associated with the achievement of a MCyR at 12 months of therapy with second-generation TKI. In the subsequent multivariate analysis for response, the lack of any cytogenetic response to imatinib therapy (P = .01) was the sole independent predictive factor for 12-month MCyR (Table 3). Sokal score at the start of second-generation TKI therapy did not impact survival or the achievement of a 12-month MCyR.
Predictive model for outcome
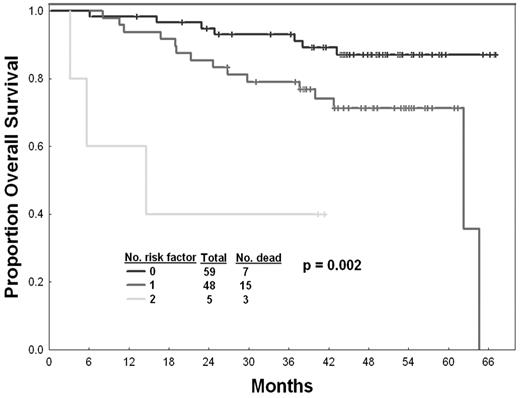
Based on these findings, we applied the risk factors identified as independent predictors for EFS to determine whether they can predict for response and overall survival after therapy with second-generation TKI (Table 4). These 2 factors (performance status and response to imatinib) had similar effect on the EFS according to their efficient obtained from the model. Three prognostic risk groups are thus proposed according to the number of risk factors: (1) low risk (no adverse factors; 48% of patients), with an estimated 2-year EFS of 78% (median not reached); (2) intermediate risk (one adverse factor; 39% of patients), with an estimated 2-year EFS of 49% (a median of 21 months); and (3) high risk (2 adverse factors; 4% of patients), with an estimated 2-year EFS of 20% (a median of 7 months) (Figure 1). As shown in Table 4, this model also predicts for the 12-month probability of achieving an MCyR and for overall survival. The 12-month probabilities of achieving an MCyR for patients with low, intermediate, and high risk were 64%, 36%, and 20%, respectively (P = .007). The estimated 2-year overall survival rates were 95%, 85%, and 40%, respectively (P = .002) (Figure 2).
Simplified risk model
| Risk factor . | N (%) . | 24 mo, % . | 12 mo, % . | |
|---|---|---|---|---|
| EFS . | OS . | MCyR . | ||
| 0 | 59 (48) | 78 | 95 | 64 |
| 1 | 48 (39) | 49 | 85 | 36 |
| 2 | 5 (4) | 20 | 40 | 20 |
| P | .001 | .002 | .007 | |
| Risk factor . | N (%) . | 24 mo, % . | 12 mo, % . | |
|---|---|---|---|---|
| EFS . | OS . | MCyR . | ||
| 0 | 59 (48) | 78 | 95 | 64 |
| 1 | 48 (39) | 49 | 85 | 36 |
| 2 | 5 (4) | 20 | 40 | 20 |
| P | .001 | .002 | .007 | |
OS indicates overall survival.
Overall survival by risk score. The top curve represents patients with no risk factor, followed by patients with one risk factor, then those with 2 risk factors at the start of second-generation TKI.
Overall survival by risk score. The top curve represents patients with no risk factor, followed by patients with one risk factor, then those with 2 risk factors at the start of second-generation TKI.
Discussion
The availability of second-generation TKIs has provided new therapeutic options for patients with CML after imatinib failure. Our study of patients with chronic-phase CML after imatinib failure is a large-scale, single-institution analysis evaluating the long-term results of patients treated with second-generation TKIs. It assessed pretreatment factors that might be associated with response and outcome after second-generation TKI therapy, including some factors that were not included in the analysis of the multinational studies (eg, the performance status at the start of second TKI therapy, the percentage of Ph+ metaphases, the percentage of blood and marrow basophils and blasts),13,14 as well as other treatment-associated variables.
The longer-term follow-up results in this study of patients with chronic-phase CML after imatinib failure treated with second-generation TKI were encouraging. Overall, 86% of patients achieved CHR, and 74% had a cytogenetic response, which was major in 63% and complete in 59%. The estimated 36-month EFS and overall survival rates were 53% and 84%, respectively. Thus, the results with second-generation TKI therapy continue to be positive with longer follow-up.
Better survival rates were observed in patients who had experienced cytogenetic relapse or had been intolerant to imatinib therapy than in those treated for hematologic relapse or resistance. Similarly, better rates were noted in patients with a good performance status. Better MCyR rates to second-generation TKI therapy were also observed in patients who had experienced previous cytogenetic response to imatinib therapy. Achievement of MCyR has been associated with improved long-term survival in CML.12 Complete cytogenetic responses after interferon-α therapy have been associated with 10-year survival rates as high as 80%,12 and 97% at 5 years after imatinib therapy.6 In this study, the multivariate analysis identified a lack of any cytogenetic response on imatinib therapy (P < .001) and a poor performance status (P = .007) as independent poor predictive factors of outcome. These findings are consistent with previous experience with imatinib therapy after interferon failure, where disease characteristics associated with a more aggressive form of disease (eg, high basophil percentage, lack of CHR at the start of therapy, and high percentage of Ph+ metaphases) were associated with poor response to imatinib therapy, suggesting the presence of aggressive clones with intrinsic resistance to therapy.12
The multivariate analysis for EFS identified 2 simple predictive factors that could estimate patient prognosis by the presence or absence of 0 (low-risk), 1 (intermediate-risk), or 2 (high-risk) factors: the 2-year EFS rates were 78%, 49%, and 20% (P < .001), respectively; the 3-year overall survival rates were 95%, 85%, and 40% (P = .002), respectively; and the 12-month probability of achieving an MCyR was 64%, 36%, and 20% (P = .007), respectively.
Recently, the Hammersmith group identified 4 factors as independently associated with the achievement of complete cytogenetic response on second-generation TKI therapy: low Sokal risk score at diagnosis, best cytogenetic response obtained on imatinib, occurrence of neutropenia at any time during imatinib therapy that required imatinib dose reduction below 400 mg/day despite growth factor support, and time from detection of imatinib failure to start of second TKI.28 In our study, 61% of the patients were referred to us after imatinib failure; therefore, we could not obtain accurate information regarding the Sokal score at diagnosis, the occurrence of neutropenia during imatinib therapy, and the exact time of imatinib failure. The time from which the patient was taken off imatinib to second-generation TKI, however, was not identified as a significant factor for EFS. The best cytogenetic response on imatinib therapy was, in contrast, identified as an independent factor for the outcome by both the M. D. Anderson Cancer Center and the Hammersmith group. A validation of our scoring system on an independent cohort of patients is needed.
Our model is a simple scoring system that is easily applicable in the clinic provided patients were adequately monitored during imatinib therapy, allowing assessment of their response to it. This scoring system could serve to advise patients of their prognosis and treatment options. Patients in the high-risk category clearly need alternative options. How this information is applied clearly depends also on other variables, such as the age of the patient and the alternatives available. For example, a younger patient with a sibling donor who has 2 adverse risk features would be well advised to pursue a stem cell transplantation at the time of imatinib failure. A trial of second-generation TKI could be done while preparing for a transplantation, but in view of the poor long-term prognosis, transplantation would be recommended when available. In contrast, an older patient or one with no donor might still find treatment with second-generation TKI their best option. However, close monitoring is required, and alternative options, such as new TKI (eg, ponatinib, DCC2036, XL228), or other new agents (eg, omacetaxine), could be considered, particularly if response is proving to be suboptimal at early time points after the start of therapy.
Achieving a 12-month MCyR is a known major determinant of outcome in previous generations of therapy, including interferon-α5 and imatinib,5 and in patients treated with second-generation TKI.27 Our 12-month landmark analysis revealed that a 12-month MCyR is the sole independent predictive factor for EFS after the start of therapy; and therefore, the achievement of an MCyR by 12 months may compensate for the presence of unfavorable baseline factors. This is in line with the results of the pivotal trial where the 2-year progression-free survival rates were 94% and 79% in patients with and without a 12-month MCyR, respectively.29
In conclusion, the outcome of patients after imatinib failure treated with second-generation TKIs is mainly dependent on whether patients had achieved a cytogenetic response to imatinib and on performance status at the start of therapy. Patients with poor performance status and no previous cytogenetic response to imatinib therapy have a low probability of responding to second-generation TKI with poor EFS and could therefore be offered additional treatment options.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: E.J. and J.C. designed the concept, analyzed data, and wrote and approved the manuscript; H.K. designed the concept and wrote and approved the manuscript; S.O.B., G.G.-M., W.W., F.R., and G.B. provided materials and approved the manuscript; and J.S. and M.B.R. analyzed the data and approved the manuscript.
Conflict-of-interest disclosure: E.J. received honoraria from BMS and Novartis. H.K. and J.C. received research grants from Novartis and BMS. The remaining authors declare no competing financial interests.
Correspondence: Elias Jabbour, Department of Leukemia, University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Blvd, Box 428, Houston, TX 77030; e-mail: ejabbour@mdanderson.org.