The pathophysiology of chronic graft-versus-host disease (GVHD) in humans remains poorly understood. While donor T cells play a major role, B cells have emerged in the past few years as significant actors too. In this issue of Blood, 2 papers add important advances to our knowledge of how B-cell subsets are implicated in the protean forms of the disease and how these subsets are affected by treatment with Rituximab.
B cells are central players in the humoral immune response. They produce antibodies and provide a protective immune defense against bacterial and viral pathogens. Over the past decade this view of B cells solely acting in humoral immune responses has changed dramatically. Accumulating evidence suggests that apart from antibody production, B cells contribute to the immune response by antibody-independent mechanisms such as presentation of antigen, the production of cytokines and chemokines, as well as by acting as regulatory cells (reviewed in Shimabukuro-Vornhagen1 ).
The role of B cells in the pathogenesis of chronic GVHD has been underestimated for many years. However, early studies pointed out the clinical similarities, as a multi-organ systemic disease, between chronic GVHD and some autoimmune diseases such as systemic lupus and scleroderma. Indeed, clinicians reported that some patients developed auto-antibodies. However, these auto-antibodies are neither constantly found nor always of the same type (eg, anti-nuclear, -nucleolus, -double strand DNA). Then, studies from Storek et al2 and us3 described an association between low total B-cell counts with high infection rate and chronic GVHD. Also at that time a fascinating paper by Glas et al4 reported the relative lack of immunoglobulin (Ig)D negative peripheral B cells (considered as memory B cells for the time being), 1 year after grafting. In this study, neither healthy subject T cells nor transplant-recipient T cells were able to induce the accumulation of somatic mutation in stem cell transplant-recipient B cells, suggesting that B cells after transplantation had an intrinsic inability to be driven to accumulate somatic mutations.
In the 1990s multicolor flow cytometry became more easily available and knowledge of B-cell ontogeny improved, although mainly in the murine system. Several B-cell subsets were discovered (see figure) and their distortion in patients with autoimmune diseases reported. In particular, it was reported that CD21 negative B cells (immature/transitional cells) are increased in lupus and in primary immunodeficiency and that CD27 was a marker of memory B cells. Somewhat surprisingly, however, it was not until the late 2000s that B-cell reconstitution of these subsets after allogeneic stem cell transplantation was studied. Two groups were particularly involved, that of Ritz in Boston and Greinix in Vienna:
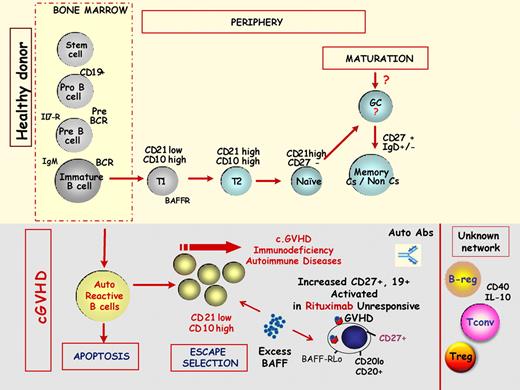
B-cell homeostasis in chronic GVHD. The top part of the figure schematizes the physiologic B-cell maturation from bone marrow to the peripheral blood of main B-cell subsets. BCR indicates B-cell receptor; Il7-R, interleukin 7 receptor; T1, transitional 1 B cell; T2, transitional 2 B cell; Cs, class switch; and GC, germinal center. The bottom part of the figure schematizes the current knowledge of these B-cell subsets in the pathophysiology of chronic GVHD. Breg indicates B regulatory cells; Treg, T regulatory cells; Tconv, conventional T cells; and BAFF, B cell activation factor.
B-cell homeostasis in chronic GVHD. The top part of the figure schematizes the physiologic B-cell maturation from bone marrow to the peripheral blood of main B-cell subsets. BCR indicates B-cell receptor; Il7-R, interleukin 7 receptor; T1, transitional 1 B cell; T2, transitional 2 B cell; Cs, class switch; and GC, germinal center. The bottom part of the figure schematizes the current knowledge of these B-cell subsets in the pathophysiology of chronic GVHD. Breg indicates B regulatory cells; Treg, T regulatory cells; Tconv, conventional T cells; and BAFF, B cell activation factor.
The Boston group reported that patients with chronic GVHD had increased B-cell activation factor (BAFF)/B cells ratio, delayed reconstitution of naive B cell and that BAFF serum level correlated with increased number of pre-germinal center B cells.5
The Vienna group reported that patients with active chronic GVHD had elevated numbers of CD21 negative transitional B cells and deficiency of memory CD27 positive B cells.6 The association of a deficiency in memory B-cell reconstitution in patients with chronic GVHD was recently confirmed by our group in a large number of patients followed longitudinally for more than 2 years from transplantation.7
Thus, chronic GVHD is associated with perturbed B-cell homeostasis. Patients who develop chronic GVHD have a relative reduction in naive B cells and relatively higher numbers of activated memory type. Elevated levels of BAFF have been correlated with the development and severity of chronic GVHD. High levels of BAFF in the presence of lower numbers of naive B cells might thus foster the survival of activated alloreactive and auto-reactive B cells, resulting in immune pathology (reviewed in Shimabukuro-Vornhagen1 ). It was thus a logical step to introduce treatment with Rituximab in the chronic GVHD therapeutic arsenal.8 Responses were reported in roughly 50% of patients failing first-line treatment with prednisone and cyclosporine.
In this issue of Blood, both Sarantopoulos et al9 and Kuzmina et al10 add significant pieces to the understanding of the role of B cells in chronic GVHD. Sarantopoulos et al9 studied 20 patients more than 2 years after treatment with Rituximab. The authors convincingly show that naive B-cell reconstitution and decreased BAFF/B-cell ratios were associated with clinical response after Rituximab whereas patients who failed to respond had persistent elevation of BAFF and a predominance of circulating B cells possessing an activated BAFF-R-low/CD20-low cell-surface phenotype. Kuzmina et al10 aimed to characterize B-cell subsets in relation to γ globulin levels, in patients with chronic GVHD. Seventy-six patients were studied a median of nearly 4 years after transplantation. Patients with hypogammaglobulinemia had elevated CD19+/CD21low (immature) and CD19+/CD21high/CD38high/IgM high (transitional) B cells. CD19+/CD10−/CD27−/CD21high naive B cells were highly elevated in all patients with chronic GVHD. Besides significantly higher BAFF/B-cell ratios many more patients with hyper-IgG had auto-antibodies compared with those with hypogammaglobulinemia. Both studies are of major importance in providing biologic markers of response to treatment with Rituximab in the former case, and relation of B-cell subsets with clinical and biologic patient presentation in the latter case. However, both illustrate inherent current problems in cellular subset definitions in humans (eg, B-cell subsets being defined differently at the phenotypic level).
Current knowledge of the potent involvement of B-cell subsets is summarized in the figure. However, it is my own bias that the role of B cells in GVHD and tolerance is only at its early stage of investigation. Among others we can summarize the following fields of current investigations: Is there any role for marginal zone B cells that have been implicated in T cell–independent response to polysaccharides of pneumococcal species in the setting of chronic GVHD? Is there a role of the B regulatory cells recently described in humans? And last but not least (because it is probably one of the key questions), how much is the T-cell–B-cell cross-talk (T-cell helper function) disturbed in the setting of chronic GVHD and to what degree does it contribute to its pathophysiology and its associated immune deficiency? New techniques such as high-throughput sequencing recently applied to B cells might help to answer such questions.11
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal