Factor IX (FIX)–activating catalytic antibodies in acquired hemophilia patients reported in this issue of Blood by Wootla et al1 reveal the potential of the immune system to influence life processes in unexpected ways.
Catalytic antibodies were originally described as pathogenic mediators in autoimmune disease,2 analogous to the traditional role of reversibly binding autoantibodies as mediators of Ehrlich's horror autotoxicus theory of autoimmunity. The FIX-activating antibodies, in contrast, are proposed to exert a beneficial procoagulant effect that compensates for the anticoagulant effect of conventional autoantibodies to Factor VIII (FVIII) found in acquired hemophilia patients. The authors present biochemical evidence for generation of activated FIX and improved blood coagulation occurring upon the enzyme-like cleavage of FIX by catalytic antibodies. Despite the heterogeneous clinical histories of the patients, the presence of FIX catalytic antibodies was widespread, and the authors claim a tendency toward improved survival. Only low-level FIX binding activity was detected. Because of the ability of a single catalytic antibody molecule to turnover multiple FIX molecules, catalysts can be detected more readily than traditional antibodies that bind autoantigens stoichiometrically by noncovalent means. For the same reason, catalysts exert more potent functional effects (see figure). The ability to activate the FIX zymogen by a cleavage reaction adds a new dimension, as binding of the target autoantigen by traditional antibodies only suppresses target autoantigen function. A previously published example of this type of zymogen activation is the generation of thrombin-like fragments on cleavage of prothrombin by autoantibodies from lupus patients.3,4
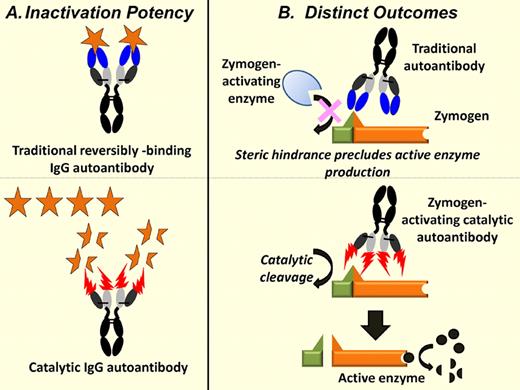
Functional outcomes because of production of traditional and catalytic autoantibody. (A) The traditional IgG autoantibody binds 2 molecules of the autoantigen reversibly (top), whereas the catalytic autoantibody turns over repeatedly and can cleave thousands of autoantigens molecules over its lifetime in the blood (bottom). The inactivation of several autoantigens by catalytic autoantibodies has been described. (B) Reversibly binding autoantibodies can sterically mask the cleavage site in a zymogen, inhibiting its fragmentation by the zymogen-activating enzyme (top). In contrast, the catalytic autoantibodies described by Wootla et al themselves serve as the FIX zymogen-activating enzymes, resulting in the generation of the enzymatically active FIX fragment that in turn catalyzes FX activation (bottom).
Functional outcomes because of production of traditional and catalytic autoantibody. (A) The traditional IgG autoantibody binds 2 molecules of the autoantigen reversibly (top), whereas the catalytic autoantibody turns over repeatedly and can cleave thousands of autoantigens molecules over its lifetime in the blood (bottom). The inactivation of several autoantigens by catalytic autoantibodies has been described. (B) Reversibly binding autoantibodies can sterically mask the cleavage site in a zymogen, inhibiting its fragmentation by the zymogen-activating enzyme (top). In contrast, the catalytic autoantibodies described by Wootla et al themselves serve as the FIX zymogen-activating enzymes, resulting in the generation of the enzymatically active FIX fragment that in turn catalyzes FX activation (bottom).
The immune genesis of catalytic antibodies is also distinctive. Mature antibodies are generated from approximately 100 variable region germ line genes along with the diversity, joining and constant region genes. Structural adaptation of the variable domains expands the repertoire, resulting in the capability of producing a very large number of antibodies with distinct antigen-binding specificity. Antibodies produced by healthy humans and animals display a promiscuous catalytic activity that cleaves peptide bonds by a nucleophilic mechanism, akin to the proteolytic pathway used by the serine protease family enzymes. Catalysis, therefore, appears to be a homeostatic function of the innate immune system expressed without a requirement for antigen-driven adaptive maturation.5 Indeed, decreased survival of septic shock patients is associated with reduced expression of promiscuous catalytic antibody activity,6 and patients with autoimmune disease also display reduced promiscuous catalytic antibodies.5
Unlike the noncovalent binding function, stimulation of the immune system by exogenous antigens generally fails to induce rapid improvement of catalysis by immunoglobulin G (IgG) antibodies. The terminal step of the catalytic cycle is release of antigen fragments. Adaptive improvement of catalysis, therefore, militates against the B-cell clonal selection theory, which holds that continued antigen binding of antibody expressed as the B-cell receptor (BCR) drives immune selection. Autoantibodies, on the other hand, can frequently express antigen-specific catalytic activity, including IgGs that hydrolyze nucleic acids and polypeptide autoantigens such as vasoactive intestinal peptide, thyroglobulin, FVIII, myelin basic protein, amyloid β peptide, and now FIX. While the antigenic stimulus driving the formation of FIX-activating catalytic antibodies is not known, acquired hemophilia is generally idiopathic or arises in association with autoimmune disease, malignancy, and pregnancy, and treatment of this disorder with immunosuppressive drugs would not induce the autoantibodies. The FIX catalytic antibodies, therefore, are likely to be true autoantibodies, and the frequent expression of catalysis by autoantibodies demands an explanation.
The answer may lie in electrophilic autoantigens that bind B cells and induce adaptive improvement of the germline-encoded catalytic function. Engineered antigens containing artificial electrophiles are capable of inducing proteolytic antibodies by binding covalently to nucleophilic BCR sites,7 thereby rendering the nucleophilic reactivity underlying catalysis immunologically selectable over the course of B-cell differentiation. Autoimmune disease is associated with increased posttranslational generation of autoantigen adducts of lipid peroxidation metabolites and advanced glycation end-products.8 Such adducts contain reactive electrophiles capable of stimulating adaptive immune selection of catalytic antibody nucleophilicity. Yet another possibility is that under the abnormal B-cell regulatory pathways found in autoimmune disease, peptide bond cleavage by catalytic BCRs is itself a selectable event. The cleavage reaction liberates a large amount of energy, and dysfunctional cellular signal transduction might permit productive use of the energy to drive B-cell division. In view of accumulating evidence for autoantigen-specific catalytic autoantibodies, further study of such nontraditional B-cell stimulatory mechanisms is warranted.
The functional outcome of catalytic autoantibody production depends on the biologic role of the target autoantigen. For instance, accumulation of amyloid β peptide aggregates in the brain is the hallmark of Alzheimer disease, and overproduction of amyloid β peptide has no known physiologic utility. Catalytic autoantibodies that hydrolyze amyloid β peptide are proposed to be beneficial by virtue of their autoantigen clearance function.9 In acquired hemophilia, production of autoantibodies to FVIII is thought to underlie pathogenesis of the bleeding disorder, including a catalytic autoantibody subset that cleaves FVIII and renders it incapable of fulfilling its cofactor role in blood coagulation.10 The FIX-activating catalytic antibodies found in acquired hemophilia are hypothesized by Wootla et al1 to fulfill a beneficial compensatory role by accelerating the next step in coagulation, activated FIX-catalyzed FXa generation. The existence of diverse autoantibody specificities combined with distinctive B-cell regulatory pressures render credible the conception of catalytic autoimmune reactions as a competing set of functionally harmful and beneficial interactions.
Conflict-of-interest disclosure: The author owns an interest in catalytic antibody patents and equity in a privately held company that intends to commercialize catalytic antibodies. ■
Acknowledgments
Drs Yasuhiro Nishiyama and Stephanie Planque contributed to developing the views expressed in this commentary.
National Institutes of Health

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal