Abstract
Use of unrelated donor cord blood (CB) as an alternative stem cell source is increasing, and yet there is little information to guide transplant centers in the unique aspects of the search and selection of CB grafts. There is no mechanism to easily access the global inventory of CB units, nor is the product information provided by all banks standardized. To address these challenges, this manuscript reviews the logistics of the search, selection process, and acquisition of CB grafts as practiced by our center. Topics include who should be considered for a CB search, how to access the global CB inventory, and how to balance total nucleated cell dose and human leukocyte antigen match in unit selection. We discuss aspects of unit quality and other graft characteristics (processing methods, unit age, availability of attached segments, infectious disease, and hemoglobinopathy screening) to be considered. We incorporate these considerations into a unit selection algorithm, including how to select double-unit grafts. We also describe how we plan for unit shipment and the role of backup grafts. This review aims to provide a framework for CB unit selection and help transplantation centers perform efficient CB searches.
Introduction
Unrelated donor cord blood transplantation (CBT) has become a widely accepted treatment for lethal hematologic diseases. Both the number of CB transplantations1 and the global inventory of CB units (estimated at 400 000)2 are growing rapidly. Therefore, it is critically important that transplantation centers (TCs) have a thorough understanding of how to perform a CB search as well as the challenges encountered in the selection and acquisition of CB grafts. This article is a practical guide for the TCs, especially those new to the field of CBT, and is based on our daily experience of searching the global CB inventory, our knowledge of CB banking and CB testing standards, and evaluation of recently published data. Thus, we outline our search practices at Memorial Sloan-Kettering Cancer Center (MSKCC).
Who should get a formal CB search with confirmatory HLA typing of CB units
There is a progressive decrease in post-transplantation survival with each human leukocyte antigen (HLA) or allele mismatch at HLA-A, -B, -C, -DRB1 loci of adult unrelated donor (URD) grafts, with donor-recipient HLA-DQ disparity being detrimental when present with other mismatches.3 Therefore, TCs must decide what level of HLA disparity will be tolerated with a URD before alternative hematopoietic stem cell sources such as CB are sought. In addition, as CB grafts are available faster than URD,4 transplantation urgency may be an additional reason to use CB. Prolonged URD searches are unlikely to result in acquisition of a suitably matched URD if one is not identified early in the search.5,6 Knowledge of the patient's ancestry is critical given that patients from racial and ethnic minorities (including all those with non-European ancestry) frequently do not have suitably matched URDs,7 a result of extensive HLA polymorphisms and limited volunteer donor availability.
At MSKCC, 10 of 10 HLA-A, -B, -C, -DRB1, -DQ allele-matched URD grafts are currently our first choice for patients without HLA-identical sibling donors if time permits. However, simultaneous CB searches are frequently performed, especially if the transplantation is urgent. This approach is based on published data comparing the outcomes of pediatric patients with hematologic malignancies transplanted with single-unit CB grafts or URD bone marrow (BM) grafts after myeloablation.8 In this study, patients who received 6 of 6 HLA-A, -B antigen, -DRB1 allele-matched CB units demonstrated higher survival, and recipients of 4 or 5 of 6 HLA-matched units had survival comparable survival to recipients of 8 of 8 HLA-allele matched BM grafts.8 In addition, promising survival has been reported for adult CBT recipients.9-14 Recently, Eapen et al have reported comparable 2-year leukemia-free survival after single-unit CBT and 7 or 8 of 8 HLA allele-matched URD peripheral blood or BM transplantation in adults.15 Moreover, Brunstein et al have found comparable 5-year leukemia-free survival after double-unit CBT, HLA-matched related donor, and HLA-allele matched or 1-antigen mismatched URD transplantation.14 Thus, a simultaneous URD and CB search is appropriate and optimizes the timely acquisition of a graft.
Within the first days to weeks of the search our coordinators determine the likelihood of obtaining a suitably matched URD. If fully matched URDs are unlikely in the required time period based on the patient's ancestry, the preliminary search results, and review of the patient's HLA typing (taking into account the National Marrow Donor Program [NMDP] Haplogic prediction,1 and/or the transplantation is urgent), we proceed with confirmatory HLA typing (CT) of CB units of interest. Alternatively, we may delay typing CB units if multiple 10 of 10 HLA-A, -B, -C, -DRB1, -DQ allele-matched URDs are probable, or the transplantation is not urgent. Further, if a URD collection is delayed because of problems with donor health or availability, a prompt decision is made whether to abandon the URD search in favor of CB.
How to access the global CB inventory
To maximize the chance of identifying optimal CB unit(s), we conduct a global search while being aware that there is no global regulatory oversight of CB banking standards. It is important to know which banks are included in the NMDP consortium of banks, in Netcord, and in the NMDP Cooperative Registries.16 To obtain a rapid overview of potential units of interest, our institutional strategy is to search the Registries of the Bone Marrow Donors Worldwide and the NMDP. We give equal consideration to domestic and international units as the primary units of the graft, whereas we prefer domestic units for backup. Notably, the US Food and Drug Administration (FDA) intends to regulate CB banking by requiring Biologic License Applications and/or Investigational New Drug applications by October 2011 for any bank that will supply units to patients in the United States. How this change will impact the daily practice of acquiring CB units is unclear at this time.
The primary unit selection criteria: TNC, HLA match, and bank of origin
Regardless of whether a TC is selecting a single- or double-unit graft, unit selection should be based primarily on the cryopreserved total nucleated cell dose/kilogram recipient weight (TNC/kg), the 4 to 6 of 6 HLA-A, -B antigen and -DRB1 allele match, and the bank of origin, although availability of attached segments for unit identity testing and infectious disease testing results must also be considered (Table 1). Single-unit CBT is considered standard by many TCs, and comparable survival has recently been published in both pediatric and adult recipients of single-unit CBT and URD hematopoietic stem cell transplantation.8,15 However, retrospective studies have suggested that double-unit grafts improve the likelihood of engraftment and reduce transplantation-related mortality (TRM) in adults and larger children compared with single-unit CBT controls,10,12 and that they are associated with a reduced risk of relapse.11,13,14,22,23
Information on CB units
| Criteria . | Comments . |
|---|---|
| TNC dose/kg | Exact threshold for acceptable dose unknown but clearly varies according to HLA match (the greater the mismatch, the higher the required TNC)17 * |
| Do not use units < 2.5 × 107/kg with 1 or 2 mismatches as single-unit grafts17 * | |
| HLA match: HLA-A, -B antigens, -DRB1 alleles | A maximum of 2 mismatches is acceptable because of the very high TRM associated with greater mismatch17 * |
| TNC dose and HLA match for double unit grafts | As either unit could engraft, use same unit principles for selection of each unit of the graft10,12,18 * |
| Our policy is to give preference to HLA match above a TNC threshold of ∼ 2.0 × 107/kg (we do not consider HLA match of units to each other)† | |
| Bank of origin | Quality may vary from unit to unit and bank to bank18-20 * |
| FACT and/or AABB accredited banks are preferred | |
| Be aware that turnaround time, speed of responses, reliability of unit information, and fees for unit testing can vary† | |
| Confirmatory HLA typing from an attached segment | Only way to confirm unit identity, unless rapid HLA typing is performed after thaw21 * |
| IDMs | Ensure completeness of testing to expedite unit acquisition, and avoid shipment before results are available† |
| Hemoglobinopathy screen | Ensure screening is completed before unit shipment† |
| Criteria . | Comments . |
|---|---|
| TNC dose/kg | Exact threshold for acceptable dose unknown but clearly varies according to HLA match (the greater the mismatch, the higher the required TNC)17 * |
| Do not use units < 2.5 × 107/kg with 1 or 2 mismatches as single-unit grafts17 * | |
| HLA match: HLA-A, -B antigens, -DRB1 alleles | A maximum of 2 mismatches is acceptable because of the very high TRM associated with greater mismatch17 * |
| TNC dose and HLA match for double unit grafts | As either unit could engraft, use same unit principles for selection of each unit of the graft10,12,18 * |
| Our policy is to give preference to HLA match above a TNC threshold of ∼ 2.0 × 107/kg (we do not consider HLA match of units to each other)† | |
| Bank of origin | Quality may vary from unit to unit and bank to bank18-20 * |
| FACT and/or AABB accredited banks are preferred | |
| Be aware that turnaround time, speed of responses, reliability of unit information, and fees for unit testing can vary† | |
| Confirmatory HLA typing from an attached segment | Only way to confirm unit identity, unless rapid HLA typing is performed after thaw21 * |
| IDMs | Ensure completeness of testing to expedite unit acquisition, and avoid shipment before results are available† |
| Hemoglobinopathy screen | Ensure screening is completed before unit shipment† |
FACT indicates Foundation for the Accreditation of Cellular Therapy; and AABB, American Association of Blood Banks.
Recommendation based on peer-reviewed literature as referenced.
Recommendation is MSKCC institutional policy based on MSKCC experience, and MSKCC/New York Blood Center unpublished data.
Our institutional policy is therefore to routinely use double-unit grafts, and we have achieved a cumulative incidence of sustained donor engraftment of 94% in patients with hematologic malignancies,12 a result comparable with the engraftment reported in a large series of URD peripheral blood stem cell transplantation.24 Whether double-unit CB grafts improve transplantation outcomes in children is being addressed in the current U.S. Clinical Trials Network single- versus double-unit randomized study (BMT CTN-0501). However, this study does not address the potential benefit of double-unit grafts in adult patients. To track the unit selection process, we summarize the TNC, HLA match, bank of origin, and other relevant information of units of interest on the patient's CB Search Summary (example in Figure 1). This is updated as information becomes available to facilitate easy review of the search progress and final unit selection.
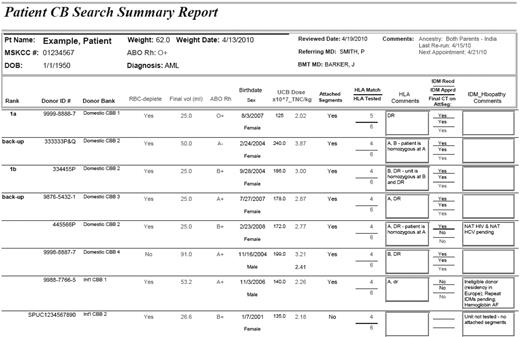
Example of CB Search Summary Report. In 1 page, the patient demographics and the information on units of interest (including the bank of origin, TNC dose, and HLA match) are summarized. The patient's weight is indicated with the date this was last updated. The date the search was most recently rerun is recorded at the top right under “Comments,” whereas the date unit information was last updated is at the bottom left hand corner. The units are listed with bank of origin on the left. The type of processing (whether the units are RBC depleted or not) is indicted, and the unit volume is shown as additional information. ABO, Rh, and donor sex are also listed for future reference. Date of birth indicates the unit collection date. The HLA match obtained from CT, whether it was confirmed to be from an attached segment, and the loci of mismatch are indicated (lowercase indicates an HLA-allele mismatch; and uppercase, an HLA-antigen mismatch). Problems with unit availability (eg, reserved on another patient's search), IDMs, or other issues making the unit ineligible (and therefore requiring a Declaration of Urgent Medical Need for use) are listed in the comments field. Finally, when all information is available, the unit rank is assigned (first column on the far left) that indicates the unit(s) that will compose the graft (unit 1 if a single-unit graft, or 1a and 1b if a double-unit graft) as well as backup units (ideally from a domestic bank). Unit selection in this example reflects our institutional practice.
Example of CB Search Summary Report. In 1 page, the patient demographics and the information on units of interest (including the bank of origin, TNC dose, and HLA match) are summarized. The patient's weight is indicated with the date this was last updated. The date the search was most recently rerun is recorded at the top right under “Comments,” whereas the date unit information was last updated is at the bottom left hand corner. The units are listed with bank of origin on the left. The type of processing (whether the units are RBC depleted or not) is indicted, and the unit volume is shown as additional information. ABO, Rh, and donor sex are also listed for future reference. Date of birth indicates the unit collection date. The HLA match obtained from CT, whether it was confirmed to be from an attached segment, and the loci of mismatch are indicated (lowercase indicates an HLA-allele mismatch; and uppercase, an HLA-antigen mismatch). Problems with unit availability (eg, reserved on another patient's search), IDMs, or other issues making the unit ineligible (and therefore requiring a Declaration of Urgent Medical Need for use) are listed in the comments field. Finally, when all information is available, the unit rank is assigned (first column on the far left) that indicates the unit(s) that will compose the graft (unit 1 if a single-unit graft, or 1a and 1b if a double-unit graft) as well as backup units (ideally from a domestic bank). Unit selection in this example reflects our institutional practice.
TNC/kg and selection of single units
There are no data to guide the dosing of TNC by actual versus ideal or adjusted body weight. Thus, TNC dose should be based on the patient's actual weight. Coordinators must ensure that the patient's weight is recent, particularly during treatment that can impact weight (eg, salvage chemotherapy that includes corticosteroids). For individual unit selection, the principles resulting from a recent New York Blood Center analysis of 1061 recipients of single-unit myeloablative CBT for the treatment of hematologic malignancies guide how to trade off TNC dose against HLA mismatch.17 This study demonstrated that the best transplantation outcomes were in recipients of 6 of 6 units regardless of precryopreservation TNC dose (median, 4.0 × 107/kg), indicating that HLA match at HLA-A and -B antigens and -DRB1 alleles, rather than high TNC dose, was the more favorable graft characteristic. Further, recipients of 4 of 6 units required a precryopreservation TNC ≥ 5.0 × 107/kg to achieve comparable TRM and disease-free survival to that of recipients of 5 of 6 units with a TNC ≥ 2.5 × 107/kg. This finding introduces a sliding scale concept in unit selection in that the greater the HLA-mismatch, the higher the required TNC dose to ensure transplantation survival; and conversely, the better the HLA match, the less important the TNC dose.
Many questions in relation to TNC remain unanswered, however. Because TNC dose is a continuous variable, any specific TNC dose cut-off is arbitrary. Based on the recent New York Blood Center analysis of single-unit CBT,17 although a lower TNC dose was required for 6 of 6 HLA-matched units, there are very little data available on transplantation outcomes with 6 of 6 HLA-matched units < 1.5 × 107/kg, for example, and we would avoid such units. Further, the impact of TNC dose and HLA match could be influenced by the use of newer fludarabine-based preparative regimens or double-unit grafts. We would suggest that TCs use a minimum cryopreserved TNC dose of 2.5 × 107/kg for a single-unit 5 of 6 graft, and 5.0 × 107/kg for a single-unit 4 of 6 graft in patients with malignancies.17 Individual units with such high cell doses are very unlikely to ever be found in a single-unit for an adult patient, and even higher TNC thresholds have been suggested for patients with nonmalignant conditions.25
We also consider the method of unit processing comparing the TNC dose of different units. We check the processing technique on the unit report against the cryopreserved volume given that red blood cell (RBC) replete units typically have a volume ≥ 75 mL. Commonly used processing methods for CB units include partial RBC depletion according to the Rubinstein et al method,26 mononuclear cell separation by automated devices such as AXP (Thermogenesis, CA, USA) or Sepax (Biosafe, Switzerland) that achieve more extensive RBC depletion, or plasma depletion only (RBC replete units). RBC replete units have a higher TNC because of the higher nucleated RBC and granulocyte content given that no cells are removed. Therefore, to permit comparison of such units with RBC depleted units, many TCs correct the TNC dose of RBC replete units (J.N.B., personal communication with multiple US investigators, 2004-2009). Unfortunately, this strategy is controversial, and the most appropriate correction factor is unknown. As these units have a TNC that is ∼ 25% higher than that of RBC depleted units, our current institutional policy is to use a correction factor of 0.75 (ie, TNC × 0.75 = corrected TNC for RBC replete units). Similarly, it could be argued that the TNC of units that have undergone mononuclear cell selection should have their TNC corrected upward to account for the loss of granulocytes during processing to permit comparison with partially RBC depleted units. We do not practice such a correction, but this problem highlights that an alternative cell dose measurement should be adopted in the future, such as the mononuclear cell content,27 or CD34+ dose (if this could be standardized), to permit unit selection according to the most relevant cellular content.
TNC/kg and selection of double-unit grafts
Our current institutional policy is to use a double-unit graft in an effort to augment engraftment and reduce both TRM and relapse. Given that either unit may engraft after a double-unit CBT, each unit of a double-unit graft is equally important. Hence, the same unit selection principles should apply to both units. We select what we consider the best unit as unit 1a, and either an equivalent or second best unit as 1b. How to trade off TNC dose versus HLA match in the setting of double-unit grafts is currently unknown. Based on the importance of HLA match demonstrated in large analyses of single-unit CBT,17 as well as a recent analysis of 84 double-unit CBT recipients at MSKCC,28 we give a strong priority to HLA match above a precryopreservation TNC threshold of ∼ 2.0 × 107/kg for each unit of a double-unit graft. This approach gives strong priority to HLA match but augments the chance of engraftment by infusing 2 units with an at least adequate dose in each unit. This recommendation may need to be revised in the future when much larger numbers of double-unit CBT recipients are available for analysis.
HLA match and single-unit selection
Multiple studies have shown that HLA mismatch at HLA-A, -B antigens and -DRB1 alleles leads to delayed engraftment, increased severity of acute graft-versus-host disease, and all large analyses of CBT outcomes have shown that HLA mismatch increases TRM and decreases survival.17,25,29 At this time, there is no information as to how to trade-off HLA-A and -B allele match against TNC dose, and as yet there are no data to suggest that matching HLA-C is important. Therefore, currently, it is standard clinical practice to assess the donor-recipient match of CB units at HLA-A and -B antigens at intermediate level resolution (taking into account the serologic equivalent of splits), and at the allele level for HLA-DRB1. We accept units that are ≥ 4 of 6 HLA-A, -B antigen and -DRB1 allele matched with the patient (ie, either an antigen or an allele mismatch is a mismatch at HLA-DRB1). As HLA-mismatch increases TRM and does not offer any benefit in disease-free survival,17 we do not recommend more mismatched CB units if better matched units with suitable cell doses are available.
As not all banks type HLA-DRB1 at high resolution, we determine how many units without high-resolution HLA-DRB1 to type according to the frequency of the patient's HLA-DRB1 type, the number of potentially suitable units available, the search urgency, and the availability of patient funds. There are minimal data concerning transplantation outcomes according to the exact loci of HLA-mismatch (eg, 2 class I mismatches vs 1 class I and 1 class II). However, our institutional policy is to avoid units with double HLA-DRB1 mismatches. Although we only strictly consider HLA-A and -B antigen match in unit selection, we still obtain HLA-A, -B, -C allele typing as the high-resolution match can be considered if there are multiple units of similar TNC dose and HLA-A, -B antigen, -DRB1 allele match available. If a single HLA mismatch is present, our institutional policy is to consider the vector (direction) of the mismatch as New York Blood Center data suggest that a unidirectional mismatch in the graft-versus-host disease direction is associated with a survival advantage compared with a single bidirectional or rejection vector mismatch.27
Finally, confirmatory HLA typing of an attached or contiguous segment is the only definitive test to confirm unit identity.21 Therefore, we give high priority to units that have an attached segment (Figure 2) for confirmatory typing, as opposed to an associated (ie, not attached) sample. A recent report by McCullough et al has highlighted the potentially disastrous problem of unit mislabeling that can only be avoided by attached segment typing.21 Although identity of units with no attached segments can be confirmed with ABO group and rapid HLA typing at thaw, this approach considerably delays unit infusion until results are obtained. Therefore, TCs must either restrict themselves to units with confirmatory typing on attached segments, or perform a rapid confirmatory type after thaw while ensuring that unit quality is not compromised because of the delayed infusion. A further problem is that some banks do not clearly indicate whether the sample used for confirmatory typing came from an attached segment or not. In addition, some banks will test a pilot sample and cross-reference the results with the maternal haplotype. This approach does not confirm the identity of the CB unit and should not substitute for attached segment testing. Another acceptable approach for confirmation of unit identity is analysis of short tandem repeat regions of an attached segment in parallel with the CB sample used for CT typing.
CB unit with attached segments for confirmatory HLA typing and other tests of unit quality. If the specimen used for confirmatory typing is obtained from an associated sample (eg, a test vial), there is a potential risk that this sample came from another unit and, therefore, the wrong unit will be shipped. Therefore, performing CT from an attached or contiguous segment (green circle) is the critical test of unit identity.
CB unit with attached segments for confirmatory HLA typing and other tests of unit quality. If the specimen used for confirmatory typing is obtained from an associated sample (eg, a test vial), there is a potential risk that this sample came from another unit and, therefore, the wrong unit will be shipped. Therefore, performing CT from an attached or contiguous segment (green circle) is the critical test of unit identity.
In the future, the HLA type of the donor's mother may have an influence on unit selection. A recent analysis of 1121 CBT recipients demonstrated lower TRM and improved survival for the 79 recipients of units in which the donor-recipient mismatched locus was matched to the donor's noninherited maternal HLA antigens.30 This suggests that fetal exposure to noninherited maternal HLA antigens could translate to the potential for permissive mismatches between donor and patient. If confirmed, such permissive mismatches could increase the number of optimal units in the global inventory and would be very attractive to incorporate into unit selection algorithms in the future. This, however, will require that banks perform maternal HLA typing and develop search algorithms that take this information into account.
HLA match and double-unit graft selection
As stated in previous sections, based on the importance of HLA match demonstrated in large analyses of single-unit CBT,17 and a recent MSKCC analysis of 84 double-unit CBT recipients,28 our institutional policy is to give a strong priority to HLA match above a TNC threshold of ∼ 2.0 × 107/kg for each unit of a double-unit graft. Further, the Avery analysis demonstrated no relationship between unit-unit HLA match and the likelihood of sustained donor engraftment. In 84 recipients of double-unit CBT transplanted for the treatment of hematologic malignancies, there was no difference in the distributions of the unit-unit HLA match in the 79 patients with sustained engraftment and the 5 patients with graft failure when analyzed at HLA-A, -B antigen, -DRB1 allele or 10 HLA-allele match. There was also no association between unit-unit HLA match and the speed of neutrophil engraftment. Therefore, we do not consider the unit-unit HLA match in the selection of a double-unit graft. This is an important issue as obeying an arbitrary unit-unit HLA match rule could result in the selection of a second unit with an inferior TNC.
Bank of origin
We consider multiple factors comparing units from different CB banks. The quality of units can vary not only from unit to unit, but from bank to bank.18 McCullough et al have reported unit quality problems in 56% of 268 units evaluated, and in 10% of cases these were considered to place the patient at risk.19 More recently, we have found that the percentage of viable CD34+ cells after thaw can vary significantly according to the bank of origin, and poor viability units were unlikely to engraft in our analysis.18 Querol et al have similarly reported variable quality between units.20 This raises the possibility that part of the benefit of double-unit CBT is that, by transplanting 2 units, we increase the chance that at least 1 unit of good quality, and thus engraftment potential, is infused. Further, given that unit quality is one of the most important considerations in CBT today, the field must determine how unit quality can be reliably measured and ensured, and how poor quality units are to be investigated and/or eliminated. Rodriguez et al and others have suggested that unit potency can be determined from analysis of cells in an attached segment.31,32 Such an approach could ensure the engraftment potential of a unit before shipment, and further validation of segment content in larger studies should be a priority.
In addition to monitoring the CD34+ viability and colony-forming unit content of the units provided to date, we also assess banks on the speed of their responsiveness, the completeness and reliability of unit information, and payment schedules. We rarely use banks that charge a fee to obtain unit information reports to determine whether a unit is of interest, or those that charge the cost of the entire unit once the unit is ordered regardless of whether it is shipped. At this time, the difference in cost among units from various banks is not considered in unit selection, but this may change in the future.
It is desirable to obtain CB units from banks that are accredited by the Foundation for the Accreditation of Cellular Therapy/Netcord and/or American Association of Blood Banks. U.S. patients with few or no satisfactory domestic units may require typing of multiple international units. Some of these units may not have attached segments to confirm unit identity and may have incomplete testing for infectious disease markers (IDMs). Therefore, information about such units must be obtained before CT is requested. This is especially important for patients with limited funds, to avoid typing units that may not be optimal for use. Unfortunately, the time taken to receive this information can vary considerably from bank to bank from a few days to more than 8 weeks.
Other factors in unit selection: IDM, sterility, and hemoglobinopathy screening
We request the screening tests as outlined by the FDA requirements.33 Complete maternal IDM testing in a Clinical Laboratory Improvement Amendments-certified laboratory is our standard. Units from mothers who are antihepatitis B core antibody positive are accepted if mothers test negative for hepatitis B surface antigen and hepatitis B virus by polymerase chain reaction (PCR). If donor mothers were screened using older techniques (eg, HIV p24 antigen) rather than HIV nucleic acid testing, the nucleic acid testing is performed if possible. Positive syphilis screening that is confirmed negative is accepted. There is controversy as to the significance of maternal cytomegalovirus (CMV) total antibody positivity. Maternal CMV serology has no relationship with CMV infection in CBT recipients,34 and testing of the unit by PCR is not approved by the FDA. Our institutional policy, however, is to avoid a unit with positive CMV PCR or positive CMV saliva culture on the baby. If maternal CMV IgM is positive, we will consider testing the unit for CMV PCR if the patient is sero-negative, although no data exist to support this approach. Recently collected units are tested for West Nile virus by nucleic acid testing and for Chagas disease by antibody screening. Foundation for the Accreditation of Cellular Therapy requirements mandate that CB units are sterile (ie, bacterial and fungal cultures negative).
Donor eligibility of the CB unit is based on the history and risk factors of the mother according to FDA guidelines. We sometimes accept units from ineligible CB donors based on urgent medical need after evaluating the potential risk associated with the reason for ineligibility versus the potential benefit of that unit's TNC and HLA match relative to other options. It is critical that the TC be notified of the specific details of ineligible units early in the search so an informed decision can be made.
Unit or newborn screening must use methodology that distinguishes hemoglobin A, A2, S, C, F, and H. If there is a family history of hemoglobinopathy, or, if any hemoglobin types except HbA and HbF are detected, further testing is required. Units reported as normal or AF are acceptable. The presence of S hemoglobin in addition to HbA and HBF indicates sickle cell trait. Units homozygous for either sickle cell disease or thalassemia, or heterozygous for both sickle cell and thalassemia, are not used. Units heterozygous for either sickle cell trait or thalassemia can be accepted if other options are limited. IDM and hemoglobinopathy screening must be completed before unit shipment.
Other graft characteristics in unit selection
As CD34+ cell measurement is not standardized between laboratories,35 we do not use CD34+ cell dose/kilogram to select between units from different banks. However, CD34+ cell dose is considered when choosing between multiple otherwise similar units from the same bank. Colony-forming unit assays are also not standardized; and similarly, we do not use them to compare units from different banks. However, we would not accept a unit with no colony growth because the engraftment potential of this unit may be severely compromised. There is no apparent decrease in hematopoietic potential in units that have been cryopreserved for more than a decade.36 However, our institutional policy is to consider unit age (ie, time in storage) given that bank practices, such as criteria for unit processing as well as cryopreservation technology and equipment, have changed over time, and these could impact unit quality.
The RBC content of a unit influences our choice. RBC replete units contain a significant load of red cell debris and free hemoglobin. These units can be associated with significant infusion reactions if not washed, but washing is more challenging than that of RBC depleted units because of the difficulty of separating the supernatant from the cells after centrifugation. Therefore, we weigh the merits of a RBC replete unit in terms of HLA match and TNC dose against these challenges.
Because there is conflicting evidence as to the value of killer cell immunoglobulin-like receptor mismatch in unrelated CBT,37,38 we do not incorporate natural killer alloreactivity into unit selection at this time. Similarly, as limited data exist for the role of recipient anti-HLA antibodies against antigens expressed in the unit, this test is also not currently included in our selection criteria for double-unit grafts. However, both of these recommendations may be modified in the future.
Summary of how we select units
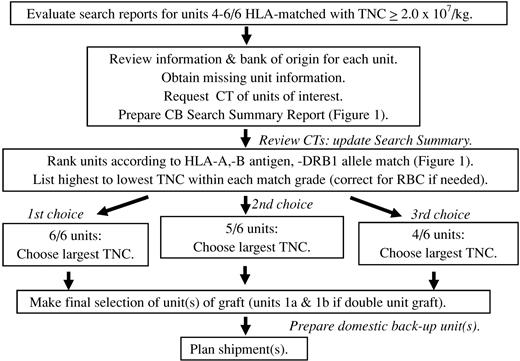
An algorithm summarizing the MSKCC approach to CB graft selection is shown in Figure 3.
Schema of how we select CB units. In addition to the HLA match, TNC dose, and bank of origin, the ability to perform confirmatory typing from an attached segment, the processing method, IDMs, hemoglobinopathy screening, and unit age are also taken into account. At least 4 units are confirmatory typed, and a higher number are requested in patients with difficult searches.
Schema of how we select CB units. In addition to the HLA match, TNC dose, and bank of origin, the ability to perform confirmatory typing from an attached segment, the processing method, IDMs, hemoglobinopathy screening, and unit age are also taken into account. At least 4 units are confirmatory typed, and a higher number are requested in patients with difficult searches.
The role of backup grafts
Our policy is to have at least 1 backup unit identified before transplantation if there are problems with unit shipping, thaw, infusion, or graft failure. It is optimal to have backup units in domestic banks, given the potential difficulties associated with international shipping at short notice. We ensure that backup units have had confirmatory HLA typing on an attached segment and are ready for shipment on transplantation day in case there are unexpected problems with the graft at thaw. These units remain reserved until the patient has engrafted.
Rerunning the search
If unit selection occurred more than 3 to 4 weeks before patient workup for transplantation, the CB search is rerun using updated patient weight to identify any additional CB units that might have become available.
Scheduling transplantation day and CB graft shipment
Multiple logistical challenges are associated with CB shipment. We submit shipment requests at least 14 calendar days before the desired graft arrival date, if possible, taking into account the speed of the bank(s) in question. Given that CB is a cryopreserved product, it is desirable to confirm receipt of a satisfactory graft before the initiation of conditioning. Routinely, we arrange units to arrive 1 to 2 days before the start of cytoreduction. However, shipment of the graft before the patient's admission raises the possibility that unit shipment (and the consequent billing) could occur on a patient whose transplantation admission is cancelled. Thus, before shipment our coordinators confirm that the patient is cleared for transplantation admission with the patient's physician. In the United States, it is also critical to ensure that insurance approval is underway, so that potential denial will not delay transplantation admission. To avoid shipment of units for patients who fail transplantation workup and have their transplantation delayed or canceled, the transplantation physician completing the patient's pretransplantation testing must be made aware of the deadline for cancellation of unit shipment. This is especially important for the shipment of international units with major time zone differences. Although the arrival time is requested to be during the working hours of the cytotherapy laboratory, a contingency plan must be in place stating how to handle a unit that arrives unexpectedly out of hours. Given that couriers may demand to take the dry shipper with them on delivery of the unit, after-hours staff must have operating procedures for this situation to eliminate the potential for unit mishandling.
On arrival, our cytotherapy laboratory staff confirms that the unit(s) arrived in satisfactory condition and that the accompanying documentation is adequate. The integrity of the dry shipper, the data logger, unit labeling, and documentation are reviewed. If the shipper is in alarm, an immediate measurement of the shipping temperature must be performed. Bank documentation must match the information already provided. For example, if the bank reported that the unit is cryopreserved in 2 bags, it must be confirmed that 2 bags arrived. Any discrepancies or quality concerns are resolved before the start of pretransplantation conditioning. Short-term storage of units is in liquid nitrogen freezers, and storage conditions must be documented.
CB unit thaw and transplantation day testing
We perform CB unit thaw and preparation for infusion according to the validated practices of the stem cell laboratory and routinely evaluate unit quality to ensure an adequate graft has been administered. Our postthaw testing includes TNC count, flow cytometric evaluation of CD34+ cell count and viability using 7-amino-actinomycin D,18 Gram stain and culture, and colony-forming unit assays. Although viability of the cells by trypan blue exclusion is still performed, we have found no correlation with this assay and either the CD34+ cell viability or unit engraftment.18 We define adequate CD34+ cell viability of a unit as a viability ≥ 75% by flow cytometry using a gating strategy that excludes only debris and incorporates all dead cells.18 Thus, we calculate viability of the total number of CD34+ cells originally present in the unit and on the day of transplantation to ensure the infusion of at least 1 unit of adequate viability. If any problems arise during unit thaw that would dictate shipment of backup graft, our coordinators initiate shipment of backup units on the same day. We also provide samples to the diagnostic molecular pathology laboratory for short tandem repeat profile of the donor, to be available for posttransplantation chimerism testing.
Graft failure
In the event of graft failure, the inpatient transplantation physician advises whether to ship the backup graft and determines the reinfusion date. As these patients can be critically ill, the appropriateness of unit shipment must be confirmed immediately before shipment.
Conclusions
In conclusion, whereas this review provides a framework for CB unit search and acquisition, it also highlights the many areas that require improvement in this process. Priorities for the field include further clarification as to who should have a CB search, including how adult CBT compares with that of URD transplantation. In addition, the complex CB search process should be streamlined so that the global CB inventory can be easily accessed with all the information needed by TCs. We need a more accurate measurement of stem cell content that would negate the need to consider the method of processing, as well as additional information as to how to balance TNC dose versus HLA match in unit selection, and need clarification of who should receive a double-unit graft. The role of high-resolution HLA typing and match at class 1 alleles is unknown, and how to incorporate the vector of mismatch or noninherited maternal HLA antigens match into unit selection needs to be further clarified. Even more important is the issue of unit quality and how engraftment potential can be determined before thaw, from testing of an attached segment, for example, so that unit quality can be part of unit release criteria. Furthermore, it must be decided how poor-quality units should be reported, investigated, and reimbursed. The need for attached segments for determination of unit identity, as well as universally recognized terminology to reflect what constitutes an attached or contiguous segment should be mandated. Finally, payment schedules should be standardized and at the very least further scrutinized.
Acknowledgments
The authors thank the search coordinators Sinda Lee, Kathleen Doshi, and Deborah Wells and Dr Nancy A. Kernan for their assistance in the development of these guidelines.
This work was supported in part by the Gabrielle's Angel Foundation for Cancer Research, the Memorial Sloan-Kettering Cancer Center Society, the Translational and Integrative Medicine Research Grant, and the National Institutes of Health (P01 CA23766; J.N.B.).
National Institutes of Health
Authorship
Contribution: J.N.B., C.B., and A.S. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Juliet N. Barker, Box 259, 1275 York Ave, New York, NY 10065; e-mail: barkerj@mskcc.org.