Abstract
We conducted a multi-institutional randomized study to determine whether high-dose daunorubicin would be as effective as standard-dose idarubicin in remission-induction therapy for newly diagnosed adult patients younger than 65 years of age with acute myeloid leukemia. Of 1064 patients registered, 1057 were evaluable. They were randomly assigned to receive either daunorubicin (50 mg/m2 daily for 5 days) or idarubicin (12 mg/m2 daily for 3 days) in combination with 100 mg/m2 of cytarabine by continuous infusion daily for 7 days as induction therapy. Complete remission was achieved in 407 (77.5%) of 525 patients in the daunorubicin group and 416 (78.2%) of 532 in the idarubicin group (P = .79). Patients achieving complete remission received intensive postremission therapy that consisted of either 3 courses of high-dose cytarabine or 4 courses of standard-dose therapy. Overall survival rates at 5 years were 48% for the daunorubicin group and 48% for the idarubicin group (P = .54), and relapse-free survival rates at 5 years were 41% and 41% (P = .97), respectively. Thus, high-dose daunorubicin and standard-dose idarubicin were equally effective for the treatment of adult acute myeloid leukemia, achieving a high rate of complete remission and good long-term efficacy. This study is registered at http://www.umin.ac.jp/ctrj/ as C000000157.
Introduction
The combination of anthracycline and cytarabine (Ara-C) with or without other antileukemia drugs is a standard induction therapy for acute myeloid leukemia (AML),1-3 and a combination of daunorubicin at a dose of 45 to 50 mg/m2 given daily for 3 days and Ara-C at a dose of 100 to 200 mg/m2 given daily for 7 days generally has been used. In the late 1980s, however, idarubicin was introduced into clinics, and 3 randomized studies comparing idarubicin with daunorubicin reported significantly higher complete remission (CR) rates in favor of idarubicin.4-6 A meta-analysis also confirmed a superior effect of idarubicin at a dose of 10 to 12 mg/m2 for 3 days versus daunorubicin at a dose of 45 to 60 mg/m2 for 3 days in the achievement of CR.7 Nevertheless, the long-term follow-up of the above-mentioned 3 randomized studies comparing idarubicin with daunorubicin revealed that the idarubicin group had better overall survival (OS) than the daunorubicin group in only 1 study.8
The Japan Adult Leukemia Study Group (JALSG) used idarubicin and Ara-C as induction therapy in the AML95 and AML97 studies,9-11 after idarubicin was registered and approved for the national health insurance system in 1995. Both studies resulted in satisfactorily high CR rates (80% and 79%, respectively); however, these CR rates were not superior to those of our earlier AML87, AML89, and AML92 studies, which used daunorubicin in combination with other antileukemia drugs.12-14 In these 3 previous studies, daunorubicin and other drugs were administered in a response-oriented individualized manner; that is, additional drugs were given for a few days when the bone marrow at day 8 was not hypoplastic, containing a substantial number of blasts. Therefore, the total doses of daunorubicin administered during the first course of induction therapy were 240 to 280 mg/m2 given for more than 5 to 7 days, which was more than the conventional dose of 40 to 60 mg/m2 given for 3 days. Usui et al also reported that the optimal dose of daunorubicin in their induction therapy for newly diagnosed adult AML was approximately 280 mg/m2 (40 mg/m2 for 7 days).15
Because there had been no prospective randomized study comparing a higher dose of daunorubicin with the standard dose of idarubicin (12 mg/m2) in adult AML, in the present multi-institutional randomized study, we prospectively compared idarubicin (12 mg/m2 for 3 days) with daunorubicin (50 mg/m2 for 5 days), in combination with Ara-C (100 mg/m2 for 7 days), as induction therapy for previously untreated adult AML. High-dose daunorubicin resulted in the same CR rate and predicted 5-year OS compared with standard-dose idarubicin.
Methods
Patients
From December 2001 to December 2005, 1064 newly diagnosed adult patients 15 to 64 years of age with de novo AML were consecutively registered from 129 participating institutions. AML was first diagnosed by the French-American-British (FAB) classification at each institution. Peripheral blood and bone marrow smears from all registered patients were sent to Nagasaki University and examined by May-Giemsa, peroxidase, and esterase staining. Next, diagnosis was reevaluated by the central review committee. Patients with the FAB M3 subtype were not registered in the present study. Eligibility criteria included adequate function of liver (serum bilirubin level < 2.0 mg/dL), kidney (serum creatinine < 2.0 mg/dL), heart, and lung and an Eastern Cooperative Oncology Group performance status between 0 and 3. Patients were not eligible if they had prediagnosed myelodysplastic syndrome, but they were eligible if they had no definite diagnosis of myelodysplastic syndrome confirmed by bone marrow histologic analysis even when they had a previous history of hematologic abnormality. Cytogenetic abnormalities were grouped by standard criteria and classified according to the Medical Research Council classification.16 The study was approved by the institutional review boards at each participating institution. Written informed consent was obtained from all patients before registration in accordance with the Declaration of Helsinki. The study was registered at http://www.umin.ac.jp/ctr/ as C000000157.
Treatments
Patients were randomly assigned by use of a centralized computer system to receive either idarubicin or daunorubicin. Randomization was stratified by age (younger or older than 50 years) and type of AML (FAB classification). All patients received 100 mg/m2/d Ara-C by 24-hour continuous infusion from days 1 to 7. In the idarubicin group, patients received 12 mg/m2/d idarubicin for 3 days, and in the daunorubicin group, they received 50 mg/m2/d daunorubicin for 5 days. If patients did not achieve CR by the first course, the same induction therapy was repeated after an approximately 3- to 4-week interval. If patients did not achieve CR with 2 courses, they were judged as failure cases.
All patients who achieved CR were again randomized to receive either 4 courses of conventional consolidation therapy or 3 courses of high-dose Ara-C therapy. In the conventional consolidation-therapy group, the first course consisted of mitoxantrone (7 mg/m2 by 30-minute infusion on days 1 to 3) and Ara-C (200 mg/m2 by 24-hour continuous infusion on days 1 to 5). The second course consisted of daunorubicin (50 mg/m2 by 30-minute infusion on days 1 to 3) and Ara-C (200 mg/m2 by 24-hour continuous infusion on days 1 to 5). The third course consisted of aclarubicin (20 mg/m2 by 30-minute infusion on days 1 to 5) and Ara-C (200 mg/m2 by 24-hour continuous infusion on days 1 to 5). The fourth course consisted of Ara-C (200 mg/m2 by 24-hour continuous infusion on days 1 to 5), etoposide (100 mg/m2 by 1-hour infusion on days 1 to 5), vincristine (0.8 mg/m2 by bolus injection on day 8), and vindesine (2 mg/m2 by bolus injection on day 10). Each consolidation was administered as soon as possible after the neutrophils, white blood cells (WBCs), and platelets recovered to more than 1.5 × 109/L, 3.0 × 109/L, and 100 × 109/L, respectively. In the high-dose Ara-C group, 3 courses of 2.0 g/m2 Ara-C were given by 3-hour infusion every 12 hours on days 1 to 5. Each course was administered 1 week after the neutrophils, WBCs, and platelets recovered to the above counts.
The best supportive care, including administration of antibiotics and platelet transfusions, was given as indicated. When patients had life-threatening documented infections during neutropenia, the use of granulocyte colony-stimulating factor was permitted.
After completion of consolidation therapy, no patients received further chemotherapy. Allogeneic stem cell transplantation (SCT) was offered during the first CR to patients 50 years of age or younger and with a histocompatible donor in the intermediate or adverse cytogenetic risk groups.
Definitions and study end points
Responses were evaluated according to the recommendations of the International Working Group.17 CR was defined as the presence of all of the following: fewer than 5% blasts in bone marrow, no leukemic blasts in peripheral blood, recovery of peripheral neutrophil counts to more than 1.0 × 109/L and platelet counts to more than 100 × 109/L, and no evidence of extramedullary leukemia. Relapse after CR was defined as the presence of at least 1 of the following: reappearance of leukemic blasts in the peripheral blood, recurrence of more than 5% blasts in the bone marrow not attributable to any other cause (eg, bone marrow regeneration after consolidation therapy), and appearance of extramedullary leukemia.
This was a multi-institutional, randomized, phase 3 study with a 2 × 2 factorial design. The primary end point of the first randomization was CR rate. The result of the second randomization is reported here in part but will be presented fully in a separate paper. OS was calculated from the date of entry into the study until death due to any cause and was censored at the last follow-up. Relapse-free survival (RFS) for patients who achieved CR was measured from the date of CR until the date of AML relapse or death of any cause and was censored at the last follow-up. Patients who underwent allogeneic SCT were not censored at the date of SCT.
Statistical analysis
This study was prospectively powered to demonstrate noninferiority of daunorubicin compared with idarubicin. With a sample size of 420 patients per group (840 in total), the study had a power of 90% at a 1% level of significance to demonstrate noninferiority (assuming an 80% CR rate for both groups). Statistical testing for the noninferiority trial was performed according to the method of Blackwelder.18 The Kaplan-Meier method was used to estimate probabilities of OS and RFS.19 To test factors that predict CR, the χ2 test and Wilcoxon rank sum test were used for univariate analysis, and the multiple logistic regression model was used for multivariate analysis. For comparison of OS and RFS, the log-rank test was used for univariate analysis and the proportional hazard model of Cox for multivariate analysis.20,21 Cumulative rates of CR, neutrophil recovery, and platelet recovery were estimated according to the Kaplan-Meier method and were evaluated with the log-rank test. The JMP program (SAS Institute Inc) was used for these analyses. All analyses were performed according to the intention-to-treat principle. All statistical tests except the method of Blackwelder were 2-sided, and the significance level was set at .05.
Results
Patient characteristics
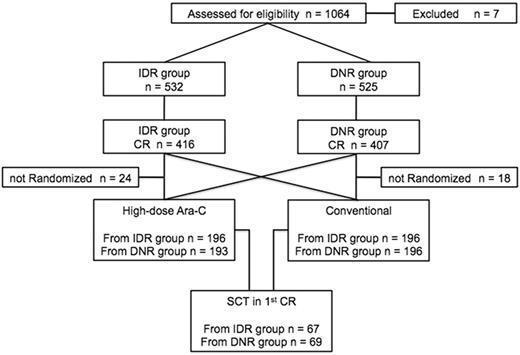
Among 1064 registered patients, 7 did not meet the inclusion criteria (misdiagnosis, 1; infectious complication, 1; without therapy, 1; and withdrawal of consent, 4). The study population thus comprised 1057 patients (Figure 1). Patient characteristics are presented in Table 1. Median age was 47 years (range, 15-64 years). Cytogenetics data were available for 1021 patients (96.6%). Among these, 247 (24.2%) were classified in the favorable-risk group, 681 (66.7%) in the intermediate-risk group, and 93 (9.1%) in the adverse group. Five hundred thirty-two patients were assigned to the idarubicin group and 525 to the daunorubicin group. The 2 groups were well balanced with regard to pretreatment characteristics such as age, initial WBC counts, FAB classification, and cytogenetic prognostic grouping.
CONSORT flow diagram. IDR indicates idarubicin; DNR, daunorubicin; CR, complete remission; Ara-C, cytarabine; and SCT, stem cell transplantation.
CONSORT flow diagram. IDR indicates idarubicin; DNR, daunorubicin; CR, complete remission; Ara-C, cytarabine; and SCT, stem cell transplantation.
Patient characteristics
| . | IDR group (n = 532) . | DNR group (n = 525) . | P . |
|---|---|---|---|
| Median age, y (range) | 47 (15-64) | 47 (15-64) | .781 |
| ≤ 50 | 310 | 306 | .996 |
| > 50 | 222 | 219 | |
| Median WBC count, ×109/L (range) | 13.7 (0.1-382) | 15.3 (0.1-334) | .769 |
| ≤ 20 × 109/L | 304 | 297 | .427 |
| 20= ≤ 50 × 109/L | 95 | 104 | |
| > 50 × 109/L | 125 | 121 | |
| Unknown | 8 | 3 | |
| FAB type | |||
| M0 | 30 | 30 | .997 |
| M1 | 95 | 94 | |
| M2 | 232 | 233 | |
| M4 | 100 | 100 | |
| M5 | 56 | 51 | |
| M6 | 17 | 16 | |
| M7 | 2 | 1 | |
| Cytogenetic group | |||
| Favorable | 128 | 119 | .561 |
| Intermediate | 335 | 346 | |
| Adverse | 49 | 44 | |
| Unknown | 20 | 16 | |
| MPO-positive blasts, % | |||
| < 50 | 169 | 187 | .330 |
| ≥ 50 | 307 | 292 | |
| Unknown | 56 | 46 | |
| Performance status | |||
| 0, 1, 2 | 512 | 509 | .524 |
| 3 | 20 | 16 | |
| . | IDR group (n = 532) . | DNR group (n = 525) . | P . |
|---|---|---|---|
| Median age, y (range) | 47 (15-64) | 47 (15-64) | .781 |
| ≤ 50 | 310 | 306 | .996 |
| > 50 | 222 | 219 | |
| Median WBC count, ×109/L (range) | 13.7 (0.1-382) | 15.3 (0.1-334) | .769 |
| ≤ 20 × 109/L | 304 | 297 | .427 |
| 20= ≤ 50 × 109/L | 95 | 104 | |
| > 50 × 109/L | 125 | 121 | |
| Unknown | 8 | 3 | |
| FAB type | |||
| M0 | 30 | 30 | .997 |
| M1 | 95 | 94 | |
| M2 | 232 | 233 | |
| M4 | 100 | 100 | |
| M5 | 56 | 51 | |
| M6 | 17 | 16 | |
| M7 | 2 | 1 | |
| Cytogenetic group | |||
| Favorable | 128 | 119 | .561 |
| Intermediate | 335 | 346 | |
| Adverse | 49 | 44 | |
| Unknown | 20 | 16 | |
| MPO-positive blasts, % | |||
| < 50 | 169 | 187 | .330 |
| ≥ 50 | 307 | 292 | |
| Unknown | 56 | 46 | |
| Performance status | |||
| 0, 1, 2 | 512 | 509 | .524 |
| 3 | 20 | 16 | |
Values are number of patients unless otherwise indicated.
IDR indicates idarubicin; DNR, daunorubicin; WBC, white blood cell count; FAB, French-American-British classification; and MPO, myeloperoxidase.
Response to induction therapy
Overall, of 1057 evaluable patients, 823 (77.9%) achieved CR. Of 532 patients in the idarubicin group, 416 (78.2%) achieved CR, and of 525 in the daunorubicin group, 407 (77.5%) obtained CR (P = .79). Noninferiority for the primary end point was assessed by determining whether the lower bound of the 95% confidence interval (CI) of the difference between the CR rates for the daunorubicin and idarubicin groups was less than −10%. The CR rate of the daunorubicin group was noninferior to that of the idarubicin group (Table 2). In the idarubicin group, 341 patients (64.1%) achieved CR after the first course, and in the daunorubicin group, 321 (61.1%) did so (P = .39). The average period to achieve CR was 33.8 days (95% CI 32.9 to 34.6 days) in the idarubicin group and 32.4 days (95% CI 31.6 to 33.2 days) in the daunorubicin group (P = .038). CR rates related to FAB classification, age, and cytogenetics are shown in Table 3. Although they were few, patients with FAB M6 responded better to idarubicin: 78% of 17 patients in the idarubicin group and 38% of 16 in the daunorubicin group achieved CR (P = .037). There were no differences in CR rate between the 2 groups in other FAB subtypes, cytogenetic risk groups, age, myeloperoxidase positivity of blasts, initial WBC count, or performance status (Table 3). Overall, logistic regression analysis revealed that induction regimen was not an independent prognostic factor but that cytogenetic group and percentage of myeloperoxidase-positive blasts were significant independent factors for achieving CR (Table 4). A cutoff value of WBCs at 20 or 50 × 109/L did not change the result.
Results of induction therapy
| . | IDR group, n (%) . | DNR group, n (%) . |
|---|---|---|
| Patients | 532 | 525 |
| CR | 416 (78.2) | 407 (77.5) |
| CR by 1 course | 341 (64.1) | 321 (61.1) |
| CR by 2 courses | 75 (14.1) | 86 (16.4) |
| 95% CI | 74.5-81.5 | 73.8-80.9 |
| . | IDR group, n (%) . | DNR group, n (%) . |
|---|---|---|
| Patients | 532 | 525 |
| CR | 416 (78.2) | 407 (77.5) |
| CR by 1 course | 341 (64.1) | 321 (61.1) |
| CR by 2 courses | 75 (14.1) | 86 (16.4) |
| 95% CI | 74.5-81.5 | 73.8-80.9 |
IDR indicates idarubicin; DNR, daunorubicin; and CR, complete remission.
CR rates by induction therapy
| . | CR rate, % . | P . | |
|---|---|---|---|
| IDR group (n = 532) . | DNR group (n = 525) . | ||
| FAB type | |||
| M0 | 43 | 63 | .195 |
| M1 | 86 | 79 | .236 |
| M2 | 80 | 82 | .718 |
| M4 | 81 | 79 | .86 |
| M5 | 77 | 75 | .96 |
| M6 | 76 | 38 | .037 |
| M7 | 50 | 100 | .999 |
| Cytogenetic group | |||
| Favorable | 91 | 96 | .134 |
| Intermediate | 79 | 76 | .359 |
| Adverse | 51 | 43 | .534 |
| Unknown | 50 | 69 | .257 |
| Age, y | |||
| ≤ 50 | 83 | 77 | .108 |
| > 50 | 73 | 78 | .225 |
| Myeloperoxidase-positive blasts, % | |||
| < 50 | 68 | 66 | .709 |
| ≥ 50 | 87 | 88 | .699 |
| WBC at diagnosis, ×109/L | |||
| ≤ 20 | 79 | 76 | .767 |
| 20= ≤ 50 | 82 | 82 | .993 |
| > 50 | 74 | 77 | .824 |
| Performance status | |||
| 0, 1, 2 | 79 | 78 | .762 |
| 3 | 80 | 75 | .999 |
| . | CR rate, % . | P . | |
|---|---|---|---|
| IDR group (n = 532) . | DNR group (n = 525) . | ||
| FAB type | |||
| M0 | 43 | 63 | .195 |
| M1 | 86 | 79 | .236 |
| M2 | 80 | 82 | .718 |
| M4 | 81 | 79 | .86 |
| M5 | 77 | 75 | .96 |
| M6 | 76 | 38 | .037 |
| M7 | 50 | 100 | .999 |
| Cytogenetic group | |||
| Favorable | 91 | 96 | .134 |
| Intermediate | 79 | 76 | .359 |
| Adverse | 51 | 43 | .534 |
| Unknown | 50 | 69 | .257 |
| Age, y | |||
| ≤ 50 | 83 | 77 | .108 |
| > 50 | 73 | 78 | .225 |
| Myeloperoxidase-positive blasts, % | |||
| < 50 | 68 | 66 | .709 |
| ≥ 50 | 87 | 88 | .699 |
| WBC at diagnosis, ×109/L | |||
| ≤ 20 | 79 | 76 | .767 |
| 20= ≤ 50 | 82 | 82 | .993 |
| > 50 | 74 | 77 | .824 |
| Performance status | |||
| 0, 1, 2 | 79 | 78 | .762 |
| 3 | 80 | 75 | .999 |
CR indicates complete remission; IDR, idarubicin; DNR, daunorubicin; FAB, French-American-British classification; and WBC, white blood cell count.
Factors that predicted CR in all evaluable patients by multivariate analysis
| Variables . | Odds ratio . | P . |
|---|---|---|
| Cytogenetic group | ||
| Favorable | 10.39 | < .0001 |
| Intermediate | 4.67 | < .0001 |
| Myeloperoxidase-positive blast ≥ 50% | 2.64 | < .0001 |
| Induction therapy: IDR arm | 0.97 | .854 |
| Variables . | Odds ratio . | P . |
|---|---|---|
| Cytogenetic group | ||
| Favorable | 10.39 | < .0001 |
| Intermediate | 4.67 | < .0001 |
| Myeloperoxidase-positive blast ≥ 50% | 2.64 | < .0001 |
| Induction therapy: IDR arm | 0.97 | .854 |
CR indicates complete remission; and IDR, idarubicin.
OS and RFS
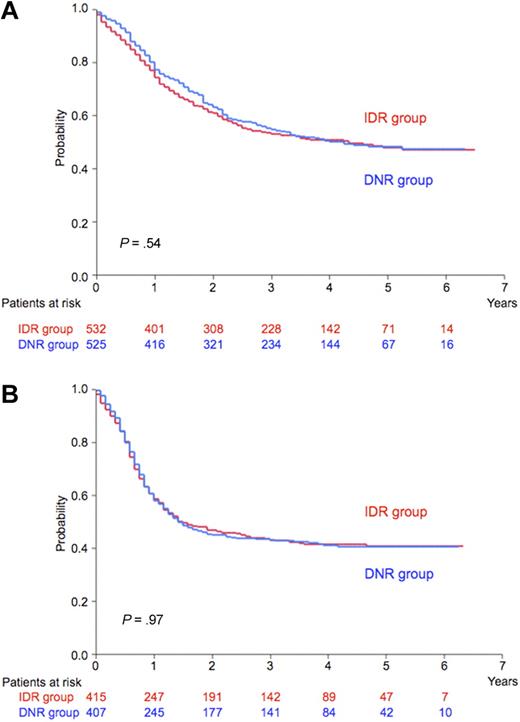
At a median follow-up of 48 months, 5-year predicted OS rates were 48% for the idarubicin group (95% CI 43% to 53%) and 48% for the daunorubicin group (95% CI 43% to 53%; P = .54; Figure 2A), and 5-year predicted RFS rates of CR patients were 41% (95% CI 36% to 46%) and 41% (95% CI 35% to 45%), respectively (P = .97; Figure 2B). Significant unfavorable prognostic features for OS by the Cox proportional hazard model were adverse cytogenetic risk group, age greater than 50 years, WBC count more than 20 × 109/L, myeloperoxidase-positive blasts less than 50%, and FAB classification of either M0, M6, or M7; for RFS, the significant unfavorable prognostic features were adverse cytogenetic risk group, WBC count more than 20 × 109/L, myeloperoxidase-positive blasts less than 50%, lactate dehydrogenase of 500 IU/L or more, and age greater than 50 years. Induction regimen was not an independent prognostic factor for either OS or RFS by this multivariate analysis.
OS and RFS. (A) Predicted 5-year overall survival (OS) was 48% for the idarubicin group (IDR; n = 532; red line) and 48% for the daunorubicin group (DNR; n = 525; blue line; P = .54). (B) Predicted 5-year relapse-free survival (RFS) was 41% for the idarubicin group (IDR; n = 416; red line) and 41% for the daunorubicin group (DNR; n = 407; blue line; P = .97).
OS and RFS. (A) Predicted 5-year overall survival (OS) was 48% for the idarubicin group (IDR; n = 532; red line) and 48% for the daunorubicin group (DNR; n = 525; blue line; P = .54). (B) Predicted 5-year relapse-free survival (RFS) was 41% for the idarubicin group (IDR; n = 416; red line) and 41% for the daunorubicin group (DNR; n = 407; blue line; P = .97).
Adverse events
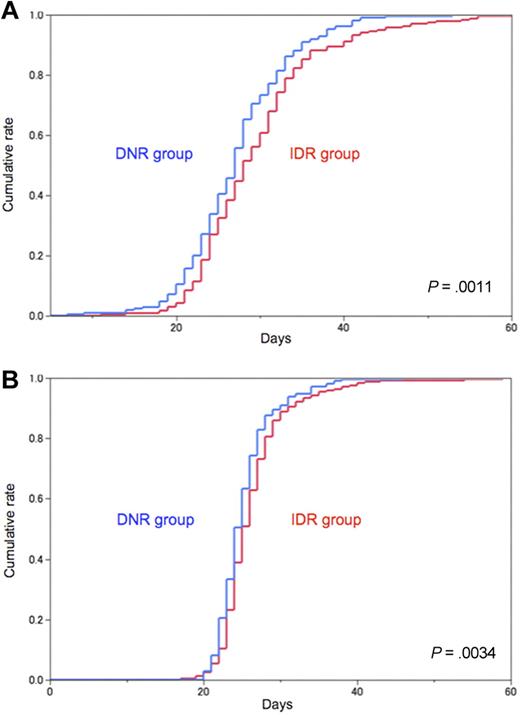
Patients receiving idarubicin required a slightly but significantly longer time to recover from neutropenia and thrombocytopenia. Median duration with a neutrophil count less than 1.0 × 109/L was 28 days for the idarubicin group and 27 days for the daunorubicin group (P = .0011; Figure 3A). Median duration with a platelet count less than 100 × 109/L was 25 days for the idarubicin group and 24 days for the daunorubicin group (P = .0034; Figure 3B). Sepsis occurred more frequently in the idarubicin group than in the daunorubicin group (8.7% and 4.9%, respectively; P = .02). Early death within 60 days occurred more frequently in the idarubicin group than in the daunorubicin group (4.7% and 2.1%, respectively; P = .03; Table 5).
Hematologic recovery. (A) Day of recovery from neutropenia after the first induction course. Neutropenia was defined as neutrophil count < 1.0 × 109/L. Median duration until recovery was 28 days for the idarubicin group (IDR; red line) and 27 days for the daunorubicin group (DNR; blue line; P = .0011). (B) Day of recovery from thrombocytopenia after the first induction course. Thrombocytopenia was defined as platelet count < 100 × 109/L. Median duration until recovery was 25 days for the idarubicin group (IDR; red line) and 24 days for the daunorubicin group (DNR; blue line; P = .0034).
Hematologic recovery. (A) Day of recovery from neutropenia after the first induction course. Neutropenia was defined as neutrophil count < 1.0 × 109/L. Median duration until recovery was 28 days for the idarubicin group (IDR; red line) and 27 days for the daunorubicin group (DNR; blue line; P = .0011). (B) Day of recovery from thrombocytopenia after the first induction course. Thrombocytopenia was defined as platelet count < 100 × 109/L. Median duration until recovery was 25 days for the idarubicin group (IDR; red line) and 24 days for the daunorubicin group (DNR; blue line; P = .0034).
Adverse events (World Health Organization grades 3 to 5) after the start of induction therapy
| . | IDR group, no. of patients (%) . | DNR group, no. of patients (%) . | P . |
|---|---|---|---|
| Sepsis | 46 (8.7) | 26 (4.9) | .021 |
| Early death* | 25 (4.7) | 11 (2.1) | .026 |
| Bleeding | 19 (3.6) | 23 (4.4) | .532 |
| Febrile neutropenia | 416 (78.2) | 406 (77.4) | .761 |
| Acute cardiac toxicity | 10 (1.9) | 4 (0.8) | .112 |
| Late-onset cardiac failure | 2 (0.38) | 2 (0.38) | .998 |
| . | IDR group, no. of patients (%) . | DNR group, no. of patients (%) . | P . |
|---|---|---|---|
| Sepsis | 46 (8.7) | 26 (4.9) | .021 |
| Early death* | 25 (4.7) | 11 (2.1) | .026 |
| Bleeding | 19 (3.6) | 23 (4.4) | .532 |
| Febrile neutropenia | 416 (78.2) | 406 (77.4) | .761 |
| Acute cardiac toxicity | 10 (1.9) | 4 (0.8) | .112 |
| Late-onset cardiac failure | 2 (0.38) | 2 (0.38) | .998 |
IDR indicates idarubicin; and DNR, daunorubicin.
Death within 60 days after the start of induction therapy.
Postremission therapy
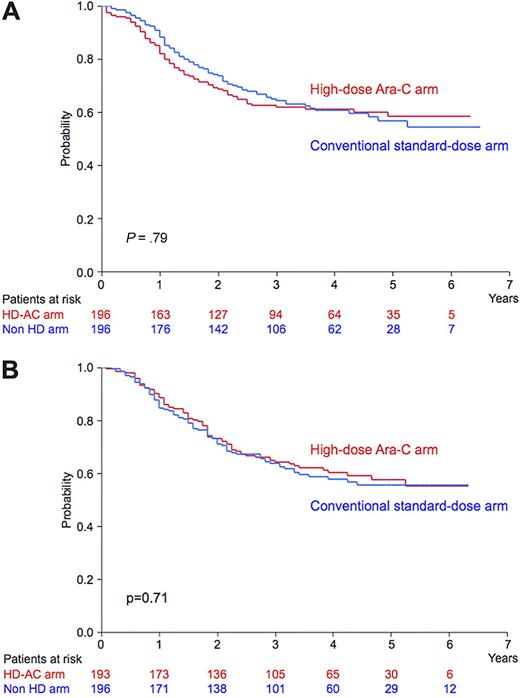
Of the 823 CR patients, 781 were randomly assigned to receive either 4 courses of conventional standard-dose consolidation therapy (392 patients) or 3 courses of high-dose Ara-C therapy (389 patients), and 136 patients (16% of CR patients) underwent allogeneic SCT in the first CR. There was no significant difference in OS or RFS by postremission therapy between the idarubicin and daunorubicin groups (Table 6). In the idarubicin group, predicted 5-year OS rates were 57% for the conventional standard-dose consolidation arm (95% CI 49% to 65%) and 58% for the high-dose Ara-C arm (95% CI 51% to 66%; P = .79; Figure 4A). In the daunorubicin group, predicted 5-year OS rates were 56% (95% CI 48% to 63%) and 58% (95% CI 50% to 65%; P = .71; Figure 4B), respectively. If 2 groups were evaluated together, predicted 5-year OS rates were 56% (95% CI 51% to 62%) and 58% (95% CI 53% to 62%; P = .95), and predicted 5-year RFS rates were 39% (95% CI 34% to 44%) and 43% (95% CI 38% to 48%), respectively (P = .72). The detailed results of this consolidation phase will be reported in a separate paper.22
Effect of induction therapy on outcome by postremission therapies
| Consolidation arm . | 5-year OS . | 5-year RFS . | ||
|---|---|---|---|---|
| IDR group . | DNR group . | IDR group . | DNR group . | |
| Conventional standard-dose, % | 57 | 56 | 41 | 37 |
| P | .759 | .332 | ||
| High-dose Ara-C, % | 58 | 58 | 42 | 44 |
| P | .725 | .658 | ||
| Allogeneic SCT in first CR, % | 59 | 59 | 58 | 64 |
| P | .469 | .394 | ||
| Consolidation arm . | 5-year OS . | 5-year RFS . | ||
|---|---|---|---|---|
| IDR group . | DNR group . | IDR group . | DNR group . | |
| Conventional standard-dose, % | 57 | 56 | 41 | 37 |
| P | .759 | .332 | ||
| High-dose Ara-C, % | 58 | 58 | 42 | 44 |
| P | .725 | .658 | ||
| Allogeneic SCT in first CR, % | 59 | 59 | 58 | 64 |
| P | .469 | .394 | ||
Number of patients in the conventional standard-dose arm was 196 in the IDR group and 196 in the DNR group; in the high-dose Ara-C arm, the numbers were 196 and 193, respectively; and in the SCT group, the numbers were 67 and 69, respectively, as shown in Figure 1.
OS indicates overall survival; RFS, relapse-free survival; IDR, idarubicin; DNR, daunorubicin; Ara-C, cytarabine; and CR, complete remission.
OS of CR patients randomized to receive consolidation therapy. (A) In the idarubicin group, predicted 5-year OS was 58% for the high-dose Ara-C arm (n = 196; red line) and 57% for the conventional standard-dose arm (n = 196; blue line; P = .79). (B) In the daunorubicin group, predicted 5-year OS was 58% for the high-dose Ara-C arm (n = 193; red line) and 56% for the conventional standard-dose arm (n = 196; blue line; P = .71). Ara-C indicates cytarabine; HD-AC arm, high-dose Ara-C arm; and Non HD arm, conventional standard-dose arm.
OS of CR patients randomized to receive consolidation therapy. (A) In the idarubicin group, predicted 5-year OS was 58% for the high-dose Ara-C arm (n = 196; red line) and 57% for the conventional standard-dose arm (n = 196; blue line; P = .79). (B) In the daunorubicin group, predicted 5-year OS was 58% for the high-dose Ara-C arm (n = 193; red line) and 56% for the conventional standard-dose arm (n = 196; blue line; P = .71). Ara-C indicates cytarabine; HD-AC arm, high-dose Ara-C arm; and Non HD arm, conventional standard-dose arm.
Discussion
The present randomized study demonstrates that if the dose intensity is increased appropriately, daunorubicin is as effective as a standard dose of idarubicin for adults less than 65 years of agewho have been newly diagnosed with AML. Remission-induction therapy with 50 mg/m2 of daunorubicin for 5 days resulted in almost the same CR rate and long-term outcome as seen with 12 mg/m2 of idarubicin for 3 days in combination with 100 mg/m2 of Ara-C for 7 days. Generally, daunorubicin is used at a dose of 45 to 50 mg/m2 for 3 days in combination with 100 to 200 mg/m2 of Ara-C for 7 days, and 50% to 70% of newly diagnosed adult patients with AML achieve CR. As stated in the “Introduction,” JALSG used a response-oriented individualized induction therapy in the AML87, AML89, and AML92 studies for AML, which permitted the additional daunorubicin and other antileukemia drugs to be administered according to bone marrow status on day 8 or later.12-14 The CR rates in these 3 studies ranged from 77% to 80%, and the median total dose of daunorubicin was 240 mg/m2.
On the basis of these experiences and also because of the regulation of our national medical insurance system, we used a dose and schedule of daunorubicin of 50 mg/m2 for 5 days, that is, a total dose of 250 mg/m2. In addition,we avoided higher daily doses, such as 80 mg/m2 for 3 days, because higher plasma concentration might cause more cardiotoxicity in older patients.23
Three randomized studies in the early 1990s4-6 and subsequent studies24,25 and meta-analyses7 reported a superior effect of idarubicin (12 to 13 mg/m2 × 3 days) over that of daunorubicin (45 to 50 mg/m2 × 3 days), in combination with Ara-C, and AML patients receiving idarubicin obtained 70% to 80% CR without a significant increase in toxic mortality, whereas those receiving daunorubicin achieved 58% to 65% CR.4-6 However, because the duration of neutropenia and thrombocytopenia was longer in the idarubicin groups, it was questioned whether the doses used in these comparisons were equivalent in terms of levels of toxicity and whether any observed advantage represented an inherent biological advantage of idarubicin rather than biological dose equivalence.1,2
In these randomized studies, Wiernik et al reported that patients with initial WBC counts > 50 × 109 cells/L obtained only 32% CR by the daunorubicin regimen compared with 68% CR by the idarubicin regimen, whereas patients with WBC counts < 50 × 109/L obtained 65% and 69% CR, respectively.5 Berman et al also reported that patients in the idarubicin group did well regardless of their initial WBC count, whereas patients in the daunorubicin group had a decreased response rate as the WBC count increased.4 In the present study, however, a total of 250 mg/m2 of daunorubicin resulted in almost the same CR rate as a total dosage of 36 mg/m2 of idarubicin regardless of initial WBC counts and other prognostic factors such as cytogenetics, age, and FAB classification except M6. Although among patients with FAB M6, 16 patients in the daunorubicin group had a significantly lower CR rate than 17 patients in the idarubicin group, we have no clear explanation for this observation, because the small number of patients made further analysis difficult. Thus, the increased total dosage of daunorubicin administered in 5 days would be responsible for almost the same satisfactory CR rate and long-term outcome as idarubicin administered in 3 days in the present study. As for adverse events, the recovery from neutropenia and thrombocytopenia was slightly but significantly delayed in the idarubicin group, and sepsis and early mortality occurred more frequently in the idarubicin group, as shown in Figure 3 and Table 5.
Before we initiated the present AML201 study, there was no evidence that a higher dose of daunorubicin was more effective than its standard dose because of the lack of a prospective randomized study. In the sequential studies reported by Southwest Oncology Group, however, the CR rate with daunorubicin at a dose of 70 mg/m2 was better than that with 45 mg/m2.26,27 Very recently, 2 groups reported that a higher dose of daunorubicin improved the CR rate and OS in prospective randomized studies.28,29 A collaborative group composed of the Dutch-Belgian Cooperative Trial Group for Hemato-Oncology, the German AML Study Group, and the Swiss Group for Clinical Cancer Research compared 3-day daunorubicin at 90 mg/m2 with 3-day daunorubicin at 45 mg/m2, in combination with 7-day Ara-C, in elderly patients 60 to 83 years of age who had AML or high-risk refractory anemia and reported a higher CR rate for the escalated-treatment group (52% vs 35%, P = .002).28 Although survival end points did not differ significantly overall, among patients 60 to 65 years of age, the CR rate (73% vs 51%) and OS rate (38% vs 23%) were significantly higher for the 90-mg/m2 group. The Eastern Cooperative Oncology Group also compared 3-day daunorubicin at 90 mg/m2 with 3-day daunorubicin at 45 mg/m2, in combination with 7-day Ara-C, in patients 17 to 60 years of age with AML and reported a higher CR rate (70.6% vs 57.3%, P < .001) and longer OS (median 23.7 vs 15.7 months, P = .003) for the high-dose group.29 Given these previous reports and the present report, the optimal total dose of daunorubicin is still to be explored but may rest somewhere between 250 and 270 mg/m2. Because we used the FAB classification in the present study, we did not include either patients with 20% to 30% of blasts in the bone marrow or those with refractory anemia with excess blasts; therefore, it is unclear whether the present result is applicable to those patients.
Idarubicin is a derivative of daunorubicin and differs from its parent compound by the deletion of a methoxy group at position 4 of the chromophore ring. In vitro and preclinical data have shown that idarubicin is more lipophilic, is faster in cellular uptake, exhibits increased cellular retention, is lower in susceptibility to P-glycoprotein–dependent resistance, and is less cardiotoxic than daunorubicin. Both idarubicin and daunorubicin undergo conversion to their respective alcohol metabolites, idarubicinol and daunorubicinol. Unlike the latter, idarubicinol has a prolonged plasma half-life and is thought to have a pharmacologic advantage.30-33
The pediatric Berlin-Frankfurt-Münster group previously compared idarubicin 12 mg/m2 for 3 days with daunorubicin 30 mg/m2 twice daily for 3 days, in combination with Ara-C and etoposide, and reported almost the same CR rates (85% vs 86%, respectively) and predicted 5-year event-free survival (55% vs 49%, respectively, P = .29) in newly diagnosed childhood AML.34 Furthermore, daunorubicin at a dose of 60 mg/m2 for 3 days and idarubicin at a dose of 12 mg/m2 for 3 days achieved similar CR rates in the studies by Eastern Cooperative Oncology Group that consisted of a large number of adult patients.35,36
Recently, the French Acute Leukemia Association reported a randomized study comparing standard doses of idarubicin (12 mg/m2 for 3 days) with high doses of daunorubicin (80 mg/m2 for 3 days) or idarubicin (12 mg/m2 for 4 days) for remission induction in newly diagnosed elderly patients 50 to 70 years of age (median 60 years old) with AML.37 CR rates were significantly higher for the standard-dose idarubicin group (83%) than for the high-dose daunorubicin group (70%, P = .007) but not for the high-dose idarubicin group (78%, P = .12). Although OS, relapse incidence, and event-free survival were not different among the 3 arms of the study, daunorubicin (80 mg/m2 for 3 days) did not improve the CR rate of elderly AML patients to the level of the standard-dose idarubicin regimen.
With regard to adverse events, recovery from myelosuppression was faster and sepsis was less frequent in the daunorubicin group. Both acute and late-onset cardiotoxicity were reported only in a small number of patients in both groups. Given that there was no increase in severe cardiac toxicities in patients receiving high-dose daunorubicin (90 mg/m2 for 3 days) compared with standard-dose daunorubicin (45 mg/m2 for 3 days) in the Eastern Cooperative Oncology Group study (7.9% and 7.2%, respectively),29 daunorubicin may not necessarily be administered for 5 days as in the present study (50 mg/m2 for 5 days), although further follow-up observation is needed for late-onset cardiotoxicity.
Since the landmark study of the Cancer and Leukemia Group B,38 it has been believed that high-dose Ara-C is superior to consolidation therapy with intermediate (400 mg/m2 for 5 days) or conventional (100 mg/m2 for 5 days) doses of Ara-C. In the present study, we prospectively compared high-dose Ara-C with consolidation therapy that included a conventional dose of Ara-C and non–cross-resistant agents. Our results clearly demonstrate that there is no difference in RFS and OS between the 2 consolidation arms, regardless of whether idarubicin or daunorubicin is used as induction chemotherapy.
In conclusion, the intensified dose of daunorubicin in the present setting, that is, 50 mg/m2 for 5 days, proved to be biologically equivalent in terms of efficacy and no more toxic in terms of myelosuppression than the standard dose and schedule of idarubicin, that is, 12 mg/m2 for 3 days, for remission-induction therapy in newly diagnosed younger patients (15 to 64 years old, median 47 years) with AML.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the clinicians and the leaders of the 129 institutions who entered their patients into the JALSG AML201 study and provided the necessary data to make this study possible. The authors are indebted to Miki Nishimura, who died recently, for her major contributions to the design, conduct, and performance of this study.
This work was supported in part by grants from the Ministry of Health, Labor, and Welfare of Japan.
Authorship
Contribution: S.O. designed and performed research, collected and interpreted data, and wrote the manuscript; S.M. designed and performed research, analyzed data, and participated in writing the manuscript; H.F., H.K., K.S., N.U., H.O., K.M., C.N., Y.M., A.F., T. Nagai, T.Y., M. Taniwaki, M. Takahashi, F.Y., Y.K., N.A., H.S., and H.H. performed research; S.H. analyzed data; K.O. and T. Naoe conducted and performed research; and R.O. conducted research, interpreted data, and participated in writing the manuscript.
For a complete list of the members of the JALSG, see the supplemental Appendix (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Shigeki Ohtake, MD, PhD, Department of Clinical Laboratory Science, Kanazawa University Graduate School of Medical Science, 5-11-80 Kodatsuno, Kanazawa, 920-0942, Japan; e-mail: sohtake@med3.m.kanazawa-u.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal