Abstract
Actin filament remodeling regulates several endothelial cell (EC) processes such as contraction, migration, adhesion, and shape determination. Mitogen-activated protein kinase (MAPK)–activated protein kinase 2 (MK2)–mediated phosphorylation of heat-shock protein 27 kDa (HSP27) promotes actin filament remodeling, but little is known about the regulation of this event in ECs. We found that tumor necrosis factor-α (TNF-α) SUMOylated MK2 at lysine (K)-339 affected EC actin filament organization and migration. Loss of the MK2 SUMOylation site (MK2-K339R) increased MK2 kinase activity and prolonged HSP27 phosphorylation, enhancing its effects on actin filament-dependent events. Both TNF-α–mediated EC elongation and steady laminar shear stress–mediated EC alignment were increased by MK2-K339R. Moreover, kinase-dead dominant-negative MK2 (DN-MK2) inhibited these effects. Cell migration is a dynamic process regulated by actin filament remodeling. Both wild-type MK2 (WT-MK2) and DN-MK2 significantly enhanced TNF-mediated inhibition of EC migration, and MK2-K339R further augmented this effect. Interestingly, the p160-Rho–associated coiled-coil kinase (ROCK) inhibitor Y-27632 reversed this effect by MK2-K339R, which strongly suggests that both excessive and insufficient levels of actin filament remodeling can block EC migration. Our study shows that MK2 SUMOylation is a new mechanism for regulating actin filament dynamics in ECs.
Introduction
Endothelial cell (EC) migration is an essential process of angiogenesis and vascular repair and requires a complex series of coordinated processes. Of particular importance is the dynamic regulation of actin filament assembly and disassembly and the interaction between actin filaments and myosin thick filaments. EC migration is partly regulated through the mitogen-activated protein kinase (MAPK) p38-dependent pathway that induces actin filament reorganization and stress fiber formation.1 Actin filaments are involved in making firm cell-substrate adhesion and providing contractile force for tail retraction, both contributing to forward movement during migration.2 Activation of p38 leads to the activation of MAPK-activated protein kinase-2 (MK2), which then phosphorylates heat-shock protein-27 kDa (HSP27).3,4 HSP27 is a phosphorylation-regulated actin filament capping protein that normally inhibits actin polymerization in its nonphosphorylated form. MK2-mediated phosphorylation of HSP27 releases this inhibition and promotes actin filament remodeling and EC migration.5 Phosphorylation of HSP27 has been linked to processes such as the migration, proliferation, and differentiation of cells.6 Disruption of balance of the HSP27 phosphorylation and dephosphorylation states can lead to either insufficient actin filaments, which prevents forward movement, or excessive actin filaments, which also inhibits cell movement by impeding the release of focal adhesions.2 Therefore, the coordinated regulation of actin filament assembly and disassembly through MK2-mediated HSP27 phosphorylation is a critical component of actin filament dynamics that regulates endothelial migration and vascular repair.
Many studies on EC migration have primarily focused on the role of vascular endothelial growth factor-A (VEGF) in activating this MK2-HSP27 pathway to promote actin filament dynamics.1,7 VEGF is a potent angiogenic agent that can induce EC migration and proliferation. Interestingly, tumor necrosis factor-α (TNF-α) has also been shown to activate this pathway and to induce actin filament remodeling,8,9 but it has the opposite effect on migration.10 However, the mechanisms and signal transduction pathways leading to TNF-mediated reduction of EC migration are poorly understood.
Although the basic mechanism of the MK2-HSP27 pathway in cell migration has been elucidated, the dynamic regulation of MK2 kinase activity to function as an “on-off” switch for actin filament remodeling and EC migration remains poorly understood. In the past decade, changes in protein functions by posttranslational modifications such as phosphorylation, ubiquitination, SUMOylation, and acetylation have come into focus as important regulators of signal transduction in different pathologies. Of particular interest is SUMOylation, a unique posttranslational modification akin to ubiquitination that conjugates small, ubiquitin-like proteins called SUMO (Small Ubiquitin-like MOdifier) to target proteins. It is a reversible modification that affects targeted proteins' subcellular localization, degradation, protein partnering, DNA binding, and transcription factor regulation.11 Interestingly, a previous study has shown that TNF-α can induce promyelocytic leukemia protein SUMOylation in endothelial cells.12 However, the role of SUMOylation in kinase regulation remains relatively unknown. In this study, we identified a SUMOylation site on MK2 at the lysine (K)-339 residue and found that MK2 SUMOylation at this site regulated the role of MK2 as an “on-off” switch of HSP27 phosphorylation, which then regulated actin filament remodeling and EC migration.
Methods
Materials and reagents
Polyclonal anti–phospho-MK2 (Thr334) (#3041), anti–MK2 (#3042), and anti–HSP27 (#2402) were obtained from Cell Signaling Technology. Anti–phospho-HSP27 (Ser78) (#05-645) was obtained from Millipore. MK2 short interfering RNA (siRNA; SI02223697) and siRNA negative control siRNA (1027281) were purchased from QIAGEN. The target sequence for the MK2 siRNA was 5′-CTACGAGCAGATCAAGATAAA-3′. The antibodies used for immunoprecipitation were anti–Flag M2 (Sigma-Aldrich) and normal mouse immunoglobulin G1 (IgG1) control (sc-3877; Santa Cruz Biotechnology). Human recombinant TNF-α was purchased from Calbiochem (#654205). p38 inhibitor 203580 (S8307) and Rho-associated, coiled-coil containing protein kinase (ROCK) inhibitor Y-27632 (Y0503) were both purchased from Sigma-Aldrich. Constitutively active MKK3 (CA-MKK3)13 (Addgene plasmid 14670) was subcloned from Flag-tagged pRc/RSV into Xpress-tagged pcDNA3.1 vector.
Cell culture
Human umbilical vein endothelial cells (HUVECs) were obtained from collagenase-digested umbilical veins14 and collected in M200 medium supplemented with LSGS (Gibco) and 2% fetal calf serum (Gibco). HUVECs were cultured on 0.2% gelatin-precoated dishes.
Generation of MK2 mutants
Potential SUMOylation sites of MK2 were determined and scored using the SUMOplot software (2009 version) from Abgent. Using site-directed mutagenesis (Stratagene), we generated the MK2-K339R SUMOylation–defective mutant by mutating the lysine-339 site to an arginine (AAG to AGG). The dominant-negative (DN) mutant for kinase activity was also generated using site-directed mutagenesis by mutating the lysine-79 residue to an arginine (AAG to AGG) as described previously.15
Immunoprecipitation and immunoblotting
Cells were collected in phosphate-buffered saline (PBS) containing 10mM N-ethylmaleimide, and cell extracts were prepared in modified radioimmunoprecipitation assay 1 buffer (50mM Tris-HCl, pH 7.4, 150mM NaCl, 1mM EDTA, 1% Nonidet P-40, 0.1% sodium dodecyl sulfate [SDS], 1mM dithiothreitol, 1:200-diluted protease inhibitor cocktail [Sigma-Aldrich], 1mM phenylmethanesulfonyl fluoride, 10mM N-ethylmaleimide, and 0.1mM iodoacetamide). Immunoprecipitation with a mouse monoclonal anti–Flag M2 (Sigma-Aldrich) and normal mouse IgG1 control (sc-3877; Santa Cruz Biotechnology) was performed as described previously.16 Proteins were pulled down with a mixture of protein A (15918-014; Invitrogen) and G (15920-010; Invitrogen) agarose beads, released in 2× SDS sample buffer, resolved by SDS-polyacrylamide gel electrophoresis (SDS-PAGE), transferred onto a Hybond enhanced chemiluminescence nitrocellulose membrane, and visualized by using the enhanced chemiluminescence detection reagents (Amersham Pharmacia Biotech) according to the manufacturer's instructions. For transient expression experiments, cells were transfected with appropriate plasmids or siRNA using Lipofectamine (Invitrogen), immunoprecipitated, and then immunoblotted with the antibody of interest.
Evaluation of MK2 SUMOylation and kinase activity
For the SUMOylation assay, Flag-tagged wild-type MK2 (WT-MK2) or MK2-K339R was transfected using Lipofectamine (Invitrogen) along with the SUMO E2 ligase Ubc9 and hemagglutinin (HA)-tagged SUMO3 protein to induce SUMOylation in Chinese hamster ovary (CHO) or HeLa cells. After 24 hours of transfection, cell lysates were immunoprecipitated with the anti–Flag M2 antibody for MK2 and then immunoblotted with the respective antibodies. Kinase activity of SUMOylated-MK2 was evaluated using in vitro kinase assays as described previously.17 In brief, MK2 immunoprecipitants from the SUMOylation assay were combined with kinase reaction buffer (25mM HEPES buffer, pH 7.5, 10mM MgCl2, 10mM MnCl2, 15μM cold ATP, 0.5 mCi/mL of [γ-32P] ATP) and recombinant HSP27 (SPP-715; Stressgen Biotechnologies), allowed to react for 20 minutes at 30°C with agitation, and stopped with the addition of 1× SDS sample buffer. Samples were then separated by 15% SDS-PAGE, transferred overnight at 4°C to nitrocellulose membrane, and then exposed to X-ray film between 2 enhancers for 3-6 hours.
Adenovirus-mediated gene transfer
Adenovirus vectors for LacZ (Ad-LacZ), wild-type MK2 (Ad-WT-MK2), DN-MK2 (Ad-DN-MK2), and the MK2-K339R SUMOylation–defective mutant (Ad-MK2-K339R) were constructed as described previously.18 Fully confluent HUVECs were transducted with adenovirus in fresh medium for 24 hours before being treated with TNF-α or exposed to steady laminar shear stress.
[3H]Thymidine incorporation assay
Measurement of [3H] thymidine incorporation into DNA was performed as described previously.19
Flow apparatus
To expose a large number of cells to flow, we used a cone and plate flow apparatus identical to the one used previously.20 Confluent HUVECs cultured in 100-mm dishes were exposed to laminar shear stress in a flow apparatus placed in a cell culture incubator with 5% CO2 and at 37°C for long-term experiments (shear stress = 24 dynes/cm2).
Fluorescence microscopy and data quantification
After adenovirus transduction, cells were fixed in 3.7% formaldehyde in PBS, permeabilized with 0.5% Triton X-100, and then stained with Alexa Fluor 488–labeled phalloidin (A12379; Invitrogen) for 10 minutes to visualize EC actin filaments. Nonstimulated confluent HUVECs that have reached contact inhibition are polygonal. After TNF-α stimulation, the morphology of these ECs becomes elongated with stress fibers aligned along the cell's longitudinal axis.9 Cells with axial ratios (long axis/short axis) larger than 3 were counted in randomly selected fields and expressed as percentages of the total cells counted.21 For the flow-induced EC alignment experiments, 24 dynes/cm2 laminar shear stress was applied to ECs using a cone and plate flow apparatus20 for 6 hours. For the quantification of EC alignment, ECs with their long axes oriented within ± 15 degrees relative to the direction of flow were defined as aligned and percentages of such cells within randomly selected fields were calculated.22
Wound-healing assay
After adenovirus transduction, monolayer cultures were scratched with a P200 pipette tip, washed twice with PBS, then treated with TNF-α (10 ng/mL), and tracked for 12 hours using time-lapse microscopy with an Olympus IX81 microscope equipped with an environmental chamber (Precision Plastics) and phase-contrast optics. Some cultures were treated with vehicle or ROCK inhibitor Y-27632 (10μM) for 1 hour before TNF-α treatment.
Statistical analysis
Data are reported as means ± standard deviation or means ± standard error of the mean (SEM), as indicated. Statistical analysis was performed with the StatView 4.0 package (Abacus Concepts). Differences were analyzed with a 1-way or a 2-way repeated-measure analysis of variance as appropriate, followed by a Scheffé correction for multiple comparisons.
Results
TNF-α induces MK2-mediated HSP27 phosphorylation and MK2 SUMOylation
Both TNF-α treatment and p38 activation, which activates MK2 and phosphorylates HSP27, have been reported to induce actin filament remodeling in ECs.8,9 We first verified the importance of p38-mediated MK2 activation in HSP27 phosphorylation in HUVECs under TNF-α. Confluent HUVECs were treated with TNF-α (10 ng/mL) and MK2 activation was investigated. TNF-α induced rapid activation of endogenous MK2 and subsequent phosphorylation of HSP27 (Figure 1A). This HSP27 phosphorylation was abolished by the p38-specific inhibitor SB203580 (Figure 1A) and MK2-specific siRNA (siMK2; Figure 1B). Interestingly, immunoblotting for MK2 revealed a 12-kDa band shift after TNF-α stimulation (Figure 1A long exposure). SB203580 (Figure 1A) and siMK2 (Figure 1B) both blocked the formation of this higher-molecular-weight MK2 band under TNF-α stimulation. We hypothesized that this band shift may have been due to SUMOylation because SUMO proteins are ∼ 12 kDa in size11 and TNF-α has previously been demonstrated to induce promyelocytic leukemia protein SUMOylation in ECs.12 Sentrin-specific protease 2 (SENP2) is a deSUMOylation enzyme that is important both for processing new SUMO proteins for conjugation and for deconjugating SUMO from SUMOylated proteins.23-25
TNF-α induces MK2 SUMOylation and MK2-mediated HSP27 phosphorylation. (A) HUVECs were pretreated with the p38-specific inhibitor SB203580 or vehicle for 30 minutes before being stimulated by TNF-α (10 ng/mL) for 0, 10, and 60 minutes. Cell lysates were probed by immunoblotting with various antibodies (IB). Transient HSP27 phosphorylation was observed in vehicle-treated control cells only. Long exposure of the anti–MK2 immunoblot shows 12-kDa band shifts for MK2 (46 kDa) after TNF-α stimulation in vehicle-treated but not SB203580-treated cells. (B) HUVECs were transfected with MK2 siRNA or scrambled siRNA for 48 hours, stimulated with TNF-α for 0, 10, and 60 minutes, and cell lysates probed with various antibodies (IB). The MK2 12-kDa band shift is not detected in cells treated with MK2 siRNA (siMK2). (C) HUVECs were transduced with Ad-SENP2 or Ad-LacZ (50 MOI) and treated with TNF-α and analyzed as in (A). Note reduced expression of the high-molecular-weight MK2 bands. (D) Quantification of SUMOylated MK2. The total density of the boxed areas for MK2 and the band-shift zone was determined using ImageJ Version 1.43u software, and the density ratio between the band-shift area versus the MK2 area for each sample lane was determined. For standardization, each ratio was divided by the ratio of the Ad-LacZ control at time 0. For (A-D), representative images from 1 of 3 independent experiments and quantitative data are shown (n = 3). Values are means ± SEM. *P < .05; **P < .01.
TNF-α induces MK2 SUMOylation and MK2-mediated HSP27 phosphorylation. (A) HUVECs were pretreated with the p38-specific inhibitor SB203580 or vehicle for 30 minutes before being stimulated by TNF-α (10 ng/mL) for 0, 10, and 60 minutes. Cell lysates were probed by immunoblotting with various antibodies (IB). Transient HSP27 phosphorylation was observed in vehicle-treated control cells only. Long exposure of the anti–MK2 immunoblot shows 12-kDa band shifts for MK2 (46 kDa) after TNF-α stimulation in vehicle-treated but not SB203580-treated cells. (B) HUVECs were transfected with MK2 siRNA or scrambled siRNA for 48 hours, stimulated with TNF-α for 0, 10, and 60 minutes, and cell lysates probed with various antibodies (IB). The MK2 12-kDa band shift is not detected in cells treated with MK2 siRNA (siMK2). (C) HUVECs were transduced with Ad-SENP2 or Ad-LacZ (50 MOI) and treated with TNF-α and analyzed as in (A). Note reduced expression of the high-molecular-weight MK2 bands. (D) Quantification of SUMOylated MK2. The total density of the boxed areas for MK2 and the band-shift zone was determined using ImageJ Version 1.43u software, and the density ratio between the band-shift area versus the MK2 area for each sample lane was determined. For standardization, each ratio was divided by the ratio of the Ad-LacZ control at time 0. For (A-D), representative images from 1 of 3 independent experiments and quantitative data are shown (n = 3). Values are means ± SEM. *P < .05; **P < .01.
To verify the identity of this suspected endogenous MK2 SUMOylation band, we transduced ECs with Ad-LacZ or Ad-SENP2, stimulated them with TNF-α, and evaluated changes in this band shift (Figure 1C-D). As suspected, transduction of Ad-SENP2 was able to reduce the expression of the higher-molecular-weight MK2 band, which is quantified in Figure 1D. These results support the idea that the MK2 band shift was due to SUMOylation. The inhibitory effect of SENP2 expression appeared to be partial. We suggest that this was due to limited specificity of this enzyme to MK2 deSUMOylation and that other SENP isoforms are involved in the process, as is the case with other SUMOylated substrates.24,25 There are 6 isoforms of human SENPs (1-3,5-7), and there are numerous examples of cooperative behavior among SENPs in deSUMOylating substrates.23-25 We tested this possibility using Ad-SENP1 and observed a similar reduction in the expression of the MK2 SUMOylation band, although to a lesser extent compared with SENP2 (supplemental Figure 1A-B, available on the Blood Web site; see the Supplemental Materials link at the top of the online article), suggesting the cooperative behavior of SENPs in deSUMOylating substrates including MK2. Furthermore, we observed MK2 SUMOylation in 2 other human endothelial cells: human aortic endothelial cells and human pulmonary artery endothelial cells, suggesting that this TNF-α–induced MK2 SUMOylation is not unique to HUVECs (supplemental Figure 1C). These data demonstrate the importance of MK2 in HSP27 phosphorylation under TNF-α stimulation in ECs, as well as the possible role of SUMOylation in regulating MK2 activity.
Identification of MK2 SUMOylation sites
The results of our studies have shown for the first time that MK2, which phosphorylates HSP27 and regulates actin dynamics, is a target of SUMOylation. Therefore, we sought to identify the site of SUMOylation. The SUMOylation motif has been identified as ΨKxE,11 in which Ψ represents a bulky aliphatic amino acid (isoleucine, leucine, or valine), K is the lysine residue to which the SUMO protein attaches, x represents any amino acid, and E is glutamic acid. Using Abgent's SUMOplot predictive software, we identified K339 to be a putative MK2 SUMOylation site (Figure 2A). We then used site-directed mutagenesis to replace this lysine with arginine and generated an MK2 SUMOylation–defective mutant (MK2-K339R). SUMOylation occurs in an E1-E2-E3 mechanism akin to ubiquitination, although studies have shown that the SUMO E2–conjugating enzyme Ubc9 itself is sufficient to SUMOylate the substrate.11 To evaluate SUMOylation of MK2-K339R, we cotransfected CHO cells with either Flag-tagged WT-MK2 or MK2-K339R together with Ubc9 and SUMO3. Immunoprecipitation (IP: Flag-MK2) of WT-MK2 and subsequent immunoblotting (IB: HA-SUMO3) for HA-tagged SUMO3 revealed a prominent 12-kDa SUMOylation band shift (Figure 2B lane 3). We also detected MK2 SUMOylation in HeLa cells but to a lesser extent compared with CHO cells (supplemental Figure 2 lanes 6-7). On the other hand, the band shift was greatly reduced for the MK2-K339R mutant protein (Figure 2B lane 4), suggesting that K339 is a major SUMOylation site of MK2. However, there appear to be other minor SUMOylation sites, because there are weakly labeled super-shifted bands. In addition, because there are 3 isoforms of SUMO proteins (SUMO1-3), we tested the possibility that MK2 SUMOylation may be specific to a particular isoform. As shown in supplemental Figure 2, we found that all 3 SUMO protein isoforms could conjugate to the MK2 protein for SUMOylation, a phenotype we previously reported with ERK5.26 Because there was no difference in the SUMOylation of MK2 between the 3 SUMO isoforms, we concluded that all 3 could be involved in endogenous MK2 SUMOylation in ECs.
Identification of MK2 SUMOylation sites. (A) Diagram of MK2 protein structure showing a possible SUMOylation site identified by Abgent's SUMOplot software. The lysine-339 (K339) residue was identified with a score of 0.93. (B) MK2 SUMOylation at K339 was demonstrated in CHO cells expressing Flag-tagged WT-MK2, HA-tagged SUMO3, and the SUMO E2 ligase Ubc9. MK2 SUMOylation was detected by immunoprecipitating Flag-tagged MK2, followed by immunoblotting for HA-tagged SUMO3. Note the greatly reduced SUMOylation for the K339R MK2 mutant protein (lane 4). Also note that no SUMOylation occurred without Ubc9 expression. (B) Representative images from 1 of 3 independent experiments are shown (n = 3).
Identification of MK2 SUMOylation sites. (A) Diagram of MK2 protein structure showing a possible SUMOylation site identified by Abgent's SUMOplot software. The lysine-339 (K339) residue was identified with a score of 0.93. (B) MK2 SUMOylation at K339 was demonstrated in CHO cells expressing Flag-tagged WT-MK2, HA-tagged SUMO3, and the SUMO E2 ligase Ubc9. MK2 SUMOylation was detected by immunoprecipitating Flag-tagged MK2, followed by immunoblotting for HA-tagged SUMO3. Note the greatly reduced SUMOylation for the K339R MK2 mutant protein (lane 4). Also note that no SUMOylation occurred without Ubc9 expression. (B) Representative images from 1 of 3 independent experiments are shown (n = 3).
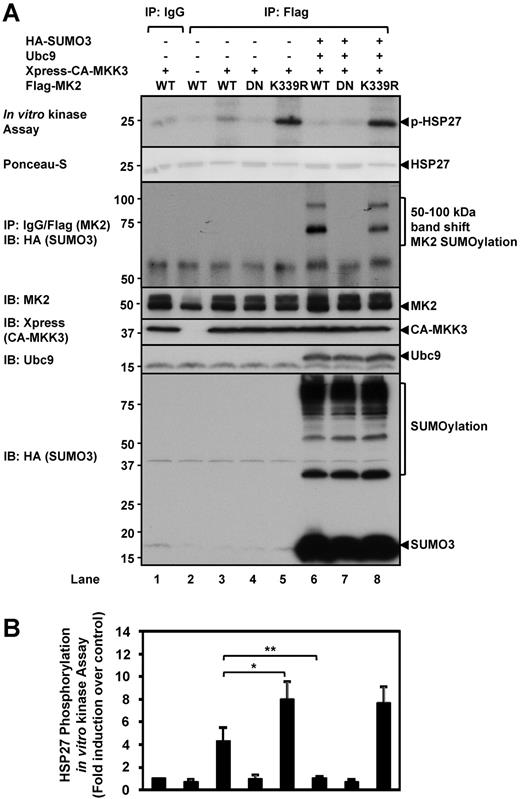
Inhibitory role of SUMOylation on MK2 kinase activity
It is not known if SUMOylation affects MK2 kinase activity. To test this possibility, we first SUMOylated MK2 in CHO cells and then immunoprecipitated the MK2 for an in vitro kinase assay using recombinant HSP27 as a substrate (Figure 3). CA-MKK3 was overexpressed in these cells to strongly activate MK2 through p3813 for the evaluation of MK2 kinase activity. CA-MKK3 was necessary to activate MK2 for in vitro HSP27 phosphorylation (Figure 3 lane 3 IB: MK2). Interestingly, the MK2-K339R mutant showed an increased MK2 kinase activity compared with WT-MK2 (Figure 3A lane 5 vs lane 3 top panel). Furthermore, SUMOylation of WT-MK2 reduced its kinase activity (Figure 3A lane 6 top panel), while the K339R mutant was unaffected (Figure 3A lane 8 top panel). These data demonstrate the inhibitory effect of SUMOylation on MK2 kinase activity and the higher kinase activity of the SUMOylation-defective mutant.
MK2 SUMOylation inhibits kinase activity. (A) The effect of SUMOylation on MK2 kinase activity was evaluated by in vitro assays using recombinant HSP27 as a substrate. A representative autoradiogram is shown, along with a SUMOylation assay to show MK2 SUMOylation expression levels. For details, please see “Methods.” Note the increase of HSP27 phosphorylation by MK2-K339R (lane 5) versus WT-MK2 (lane 3). Also note the inhibition of HSP27 phosphorylation by SUMOylated WT-MK2 (lane 6) and that this effect was not seen in the SUMOylation-deficient K339R mutant (lane 8). (B) Densitometry quantification of HSP27 phosphorylation. (A-B) Representative images from 1 of 3 independent experiments and quantitative data are shown (n = 3). Values are means ± SEM. *P < .05; **P < .01.
MK2 SUMOylation inhibits kinase activity. (A) The effect of SUMOylation on MK2 kinase activity was evaluated by in vitro assays using recombinant HSP27 as a substrate. A representative autoradiogram is shown, along with a SUMOylation assay to show MK2 SUMOylation expression levels. For details, please see “Methods.” Note the increase of HSP27 phosphorylation by MK2-K339R (lane 5) versus WT-MK2 (lane 3). Also note the inhibition of HSP27 phosphorylation by SUMOylated WT-MK2 (lane 6) and that this effect was not seen in the SUMOylation-deficient K339R mutant (lane 8). (B) Densitometry quantification of HSP27 phosphorylation. (A-B) Representative images from 1 of 3 independent experiments and quantitative data are shown (n = 3). Values are means ± SEM. *P < .05; **P < .01.
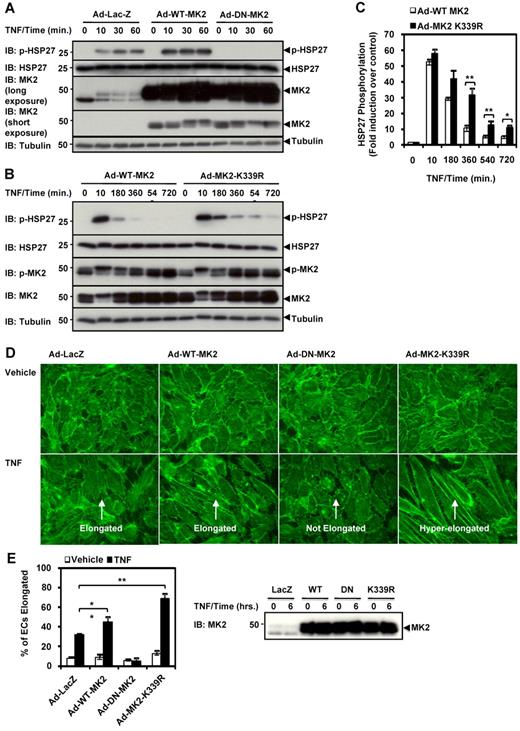
MK2 SUMOylation inhibits TNF-α–mediated HSP27 phosphorylation and EC elongation
Because MK2-mediated HSP27 phosphorylation was previously demonstrated to induce actin filament remodeling in ECs,27,28 and because SUMOylation inhibited MK2 kinase activity (Figure 3), we postulated that the MK2-K339R SUMOylation–defective mutant would enhance MK2 kinase activity and increase HSP27 phosphorylation, which would promote actin filament remodeling in ECs. Adenoviral transduction of the kinase-dead dominant-negative MK2 (K79R)15 abolished HSP27 phosphorylation (Figure 4A), confirming our results regarding the importance of MK2 in phosphorylating HSP27 (Figure 1). Next we compared the effect of transduction of Ad-WT-MK2 with that of the MK2-K339R SUMOylation–defective mutant (Ad-MK2-K339R) for HSP27 phosphorylation under TNF-α stimulation (Figure 4B). Compared with WT-MK2, the MK2-K339R SUMOylation–defective mutant exhibited a sustained phosphorylation of HSP27 at various time points over the 6-hour time course, although the maximum phosphorylation level at the 10-minute time point did not show significant differences (Figure 4C).
MK2 SUMOylation inhibits TNF-α–induced HSP27 phosphorylation and actin filament remodeling in ECs. (A-B) HUVECs were transduced with the appropriate adenoviral constructs (Ad-LacZ, Ad-WT-MK2, Ad-DN-MK2, or Ad-MK2-K339R at 50 MOI) for 24 hours, stimulated with TNF-α (10 ng/mL) for 0-60 minutes, and then harvested for immunoblotting. Note the sustained phosphorylation of HSP27 by Ad-MK2-K339R compared with Ad-WT-MK2, and that this effect was inhibited by Ad-DN-MK2. (C) Densitometry quantification of HSP27 phosphorylation data from experiments similar to the one shown in panel B. (D) HUVECs were transduced with adenoviruses under the same conditions, stimulated with TNF-α (10 ng/mL) for 6 hours, then fixed and stained with Alexa Fluor 488–phalloidin for actin filament visualization. Note the hyperelongation of ECs transduced with Ad-MK2-K339R vs Ad-WT-MK2 when stimulated with TNF-α. Also note the lack of elongation in ECs transduced with Ad-DN-MK2 despite TNF-α stimulation. (E) Quantification of cell elongation. Cells with axial ratios (long axis/short axis) larger than 3 were counted in randomly selected fields and expressed as percentages of the total cells counted. MK2 expression for panel D is shown on the right. (A-E) Representative images from 1 of 3 independent experiments and quantitative data are shown (n = 3). Values are means ± SEM. *P < .05; **P < .01.
MK2 SUMOylation inhibits TNF-α–induced HSP27 phosphorylation and actin filament remodeling in ECs. (A-B) HUVECs were transduced with the appropriate adenoviral constructs (Ad-LacZ, Ad-WT-MK2, Ad-DN-MK2, or Ad-MK2-K339R at 50 MOI) for 24 hours, stimulated with TNF-α (10 ng/mL) for 0-60 minutes, and then harvested for immunoblotting. Note the sustained phosphorylation of HSP27 by Ad-MK2-K339R compared with Ad-WT-MK2, and that this effect was inhibited by Ad-DN-MK2. (C) Densitometry quantification of HSP27 phosphorylation data from experiments similar to the one shown in panel B. (D) HUVECs were transduced with adenoviruses under the same conditions, stimulated with TNF-α (10 ng/mL) for 6 hours, then fixed and stained with Alexa Fluor 488–phalloidin for actin filament visualization. Note the hyperelongation of ECs transduced with Ad-MK2-K339R vs Ad-WT-MK2 when stimulated with TNF-α. Also note the lack of elongation in ECs transduced with Ad-DN-MK2 despite TNF-α stimulation. (E) Quantification of cell elongation. Cells with axial ratios (long axis/short axis) larger than 3 were counted in randomly selected fields and expressed as percentages of the total cells counted. MK2 expression for panel D is shown on the right. (A-E) Representative images from 1 of 3 independent experiments and quantitative data are shown (n = 3). Values are means ± SEM. *P < .05; **P < .01.
It has been reported that confluent HUVECs that have reached contact inhibition normally exhibit “cobblestone” morphology, but that TNF-α–induced actin filament remodeling causes ECs to elongate.9 Thus, we evaluated the effect of sustained HSP27 phosphorylation on EC elongation by expressing the MK2-K339R mutant. ECs were transduced with adenovirus (Ad-LacZ, Ad-WT-MK2, Ad-DN-MK2, or Ad-MK2-K339R), stimulated with TNF-α for 6 hours, stained with fluorescent phalloidin, and then quantified for EC elongation (Figure 4D-E). After TNF-α stimulation, only 32.4% ± 0.4% of the cells became elongated (Figure 4D Ad-LacZ + TNF), whereas nonstimulated cells were polygonal and their actin filaments were organized in short, unaligned stress fibers, as described previously9 (Figure 4D Ad-LacZ + Vehicle). We found that transduction of Ad-DN-MK2 completely inhibited TNF-α–mediated actin EC elongation (Figure 4D Ad-DN-MK2 + TNF). Interestingly, while the transduction of Ad-WT-MK2 enhanced TNF-α–mediated cell elongation with increased cortical actin staining, these features were significantly more prominent in cells expressing the SUMOylation-deficient Ad-MK2-K339R mutant (Figure 4D-E). These results suggest that MK2 SUMOylation has a negative impact on TNF-mediated actin filament remodeling. The expression levels of Ad-WT-MK2, Ad-DN-MK2, and Ad-MK2-K339R at a multiplicity of infection (MOI) of 50 were similar (Figure 4E).
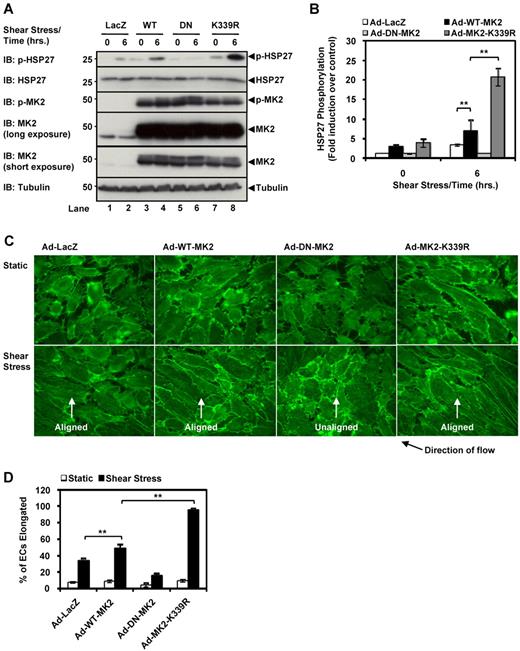
MK2 SUMOylation inhibits EC alignment under shear stress
Steady laminar shear stress has been well established as a potent activator of MAPK signaling and actin filament remodeling in ECs.29 Long-term exposure of ECs to laminar shear stress causes them to shift from their “cobblestone” shape to an elongated shape aligned in the direction of flow through the rearrangement of actin filaments,30 similar to the effect of TNF-α stimulation on EC morphology (Figure 4D). Therefore, to generalize the role of MK2 SUMOylation on actin filament remodeling, we examined another actin-dependent process and investigated if shear stress-induced EC alignment was affected by MK2 SUMOylation. Because p38 has been implicated in actin filament remodeling in ECs through the p38-MK2-HSP27 pathway,31 we hypothesized that expressing the SUMOylation-deficient MK2 K339R mutant would enhance EC alignment in response to laminar flow due to the increase in HSP27 phosphorylation and the subsequent increase in actin filament remodeling. HUVECs were exposed to laminar flow for 6 hours using a cone and plate flow apparatus20 to induce weak EC alignment so that differences in alignment between the MK2 mutants were more pronounced. As seen in Figure 5A-B, immunoblotting of EC lysates shows an increase in HSP27 phosphorylation for the Ad-MK2-K339R mutant compared with the Ad-MK2-WT and Ad-LacZ samples, whereas Ad-DN-MK2 inhibited laminar flow–induced MK2 activation. In addition, phalloidin staining for actin filaments in these cells revealed a complete inhibition of laminar flow–mediated EC alignment by Ad-DN-MK2, but highly increased alignment by Ad-MK2-K339R compared with Ad-WT-MK2 or by Ad-LacZ (Figure 5C-D). These data establish the importance of MK2 SUMOylation in actin filament remodeling in a physiologically relevant model and under a different stimulus, and agree with our data showing that MK2 SUMOylation inhibits actin filament remodeling.
MK2 SUMOylation inhibits endothelial cell alignment by shear stress. (A) HUVECs were transduced with the appropriate adenoviral constructs (Ad-LacZ, Ad-WT-MK2, Ad-DN-MK2, or Ad-K339R) for 24 hours, stimulated under steady laminar shear stress for 6 hours (24 dynes/cm2), and then harvested for immunoblotting for HSP27 phosphorylation. Note the increased HSP27 phosphorylation in cells transduced with Ad-MK2-K339R (lane 8) compared with Ad-WT-MK2 (lane 4). Once again, this effect was inhibited by Ad-DN-MK2 (lane 6). (B) Quantification of HSP27 phosphorylation data from experiments similar to the one shown in panel A. (C) HUVECs transduced under the same conditions as in panel A were stained with Alexa Fluor 488–phalloidin to visualize actin filaments and cell alignment. Note the alignment in the direction of flow of cells transduced with Ad-MK2-K339R under shear stress versus Ad-WT-MK2. Also note the lack of alignment of ECs transduced with Ad-DN-MK2 despite shear stress stimulation. MK2 expression for panel C is shown in panel A (IB: MK2). (D) Quantification of EC alignment under steady laminar shear stress. For each experiment, a total of 200 cells from randomly chosen fields were examined. We defined aligned cells as those with their long axes oriented within ± 15 degrees relative to the direction of flow, and percentages of such cells are shown. (A-D) Representative images from 1 of 3 independent experiments and quantitative data are shown (n = 3). Values are means ± SEM. *P < .05; **P < .01.
MK2 SUMOylation inhibits endothelial cell alignment by shear stress. (A) HUVECs were transduced with the appropriate adenoviral constructs (Ad-LacZ, Ad-WT-MK2, Ad-DN-MK2, or Ad-K339R) for 24 hours, stimulated under steady laminar shear stress for 6 hours (24 dynes/cm2), and then harvested for immunoblotting for HSP27 phosphorylation. Note the increased HSP27 phosphorylation in cells transduced with Ad-MK2-K339R (lane 8) compared with Ad-WT-MK2 (lane 4). Once again, this effect was inhibited by Ad-DN-MK2 (lane 6). (B) Quantification of HSP27 phosphorylation data from experiments similar to the one shown in panel A. (C) HUVECs transduced under the same conditions as in panel A were stained with Alexa Fluor 488–phalloidin to visualize actin filaments and cell alignment. Note the alignment in the direction of flow of cells transduced with Ad-MK2-K339R under shear stress versus Ad-WT-MK2. Also note the lack of alignment of ECs transduced with Ad-DN-MK2 despite shear stress stimulation. MK2 expression for panel C is shown in panel A (IB: MK2). (D) Quantification of EC alignment under steady laminar shear stress. For each experiment, a total of 200 cells from randomly chosen fields were examined. We defined aligned cells as those with their long axes oriented within ± 15 degrees relative to the direction of flow, and percentages of such cells are shown. (A-D) Representative images from 1 of 3 independent experiments and quantitative data are shown (n = 3). Values are means ± SEM. *P < .05; **P < .01.
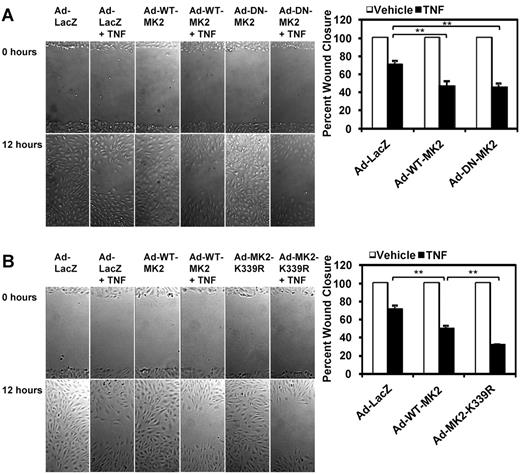
TNF-α–mediated inhibition of EC migration is enhanced by the loss of MK2 SUMOylation
Cell migration is a dynamic process regulated by the assembly and disassembly of actin filaments throughout the cell. Whereas active filament assembly occurs at the leading edge of the cell, pushing the plasma membrane forward, contraction and disassembly of stress fibers occur at the rear of the cell.2 In the context of angiogenesis and vascular repair,1,7 tight regulation of these processes by the MK2-HSP27 pathway under VEGF must enable ECs to appropriately migrate. However, TNF-α activates this same MK2-HSP27 pathway to promote actin filament remodeling but has the opposite effect by inhibiting migration.10 Because we have shown that MK2 SUMOylation has an inhibitory effect on TNF-mediated actin filament remodeling and subsequent EC elongation (Figure 4), we hypothesized that sustained actin filament polymerization by the MK2 SUMOylation–deficient K339R mutant would inhibit EC migration under TNF-α by preventing actin filament turnover and creating a stiff cell cortex.
Wound-healing assays were used to determine the effect of MK2 SUMOylation on EC migration. First we compared the effects of WT-MK2 versus DN-MK2 under TNF-α (10 ng/mL) on wound closure. Basal EC migration was unaffected by the transduction of Ad-WT-MK2 and Ad-DN-MK2 compared with the Ad-LacZ control (Figure 6A). The addition of TNF-α inhibited the migration of ECs expressing Ad-LacZ by approximately 30% compared with vehicle (% wound closure: Ad-LacZ 100% vs 71.1% ± 4.2%). Interestingly, TNF-α–induced inhibition of EC migration was further enhanced in ECs expressing WT-MK2 or DN-MK2 by the same extent (Ad-WT-MK2 47.1% ± 5.1% vs Ad-DN-MK2 45.4% ± 4.3%). These data suggest that under TNF-α, decreased actin filament dynamics by sustained inhibition of MK2 kinase activity by DN-MK2 and/or increased actin polymerization by sustained activation of MK2 kinase by WT-MK2 can inhibit cell movement by deregulating the coordinated “on-off” role of MK2 on actin dynamics.
TNF-α–mediated inhibition of EC migration is enhanced by the loss of MK2 SUMOylation. (A-B) HUVECs were transduced with the appropriate adenoviral constructs (Ad-LacZ, Ad-WT-MK2, Ad-DN-MK2, or Ad-K339R) for 24 hours. Confluent monolayers were “wounded” with a P200 pipette tip and wound closure in the presence or absence of TNF-α (10 ng/mL) was recorded using time-lapse microscopy at 5 different locations per sample for 12 hours with a 30-minute interval. Quantification for the percenthe percentage wound closure can be seen on the right. Note the inhibition by TNF-α on wound closure. Also note the similar effects of Ad-WT-MK2 and Ad-DN-MK2 on enhancing TNF-α–mediated inhibition of wound closure (A). This effect was further enhanced by Ad-MK2-K339R under TNF-α stimulation compared with Ad-WT-MK2 (B). (A-B) Representative images from 1 of 3 independent experiments and quantitative data are shown (n = 3). Values are means ± standard deviation. *P < .05; **P < .01.
TNF-α–mediated inhibition of EC migration is enhanced by the loss of MK2 SUMOylation. (A-B) HUVECs were transduced with the appropriate adenoviral constructs (Ad-LacZ, Ad-WT-MK2, Ad-DN-MK2, or Ad-K339R) for 24 hours. Confluent monolayers were “wounded” with a P200 pipette tip and wound closure in the presence or absence of TNF-α (10 ng/mL) was recorded using time-lapse microscopy at 5 different locations per sample for 12 hours with a 30-minute interval. Quantification for the percenthe percentage wound closure can be seen on the right. Note the inhibition by TNF-α on wound closure. Also note the similar effects of Ad-WT-MK2 and Ad-DN-MK2 on enhancing TNF-α–mediated inhibition of wound closure (A). This effect was further enhanced by Ad-MK2-K339R under TNF-α stimulation compared with Ad-WT-MK2 (B). (A-B) Representative images from 1 of 3 independent experiments and quantitative data are shown (n = 3). Values are means ± standard deviation. *P < .05; **P < .01.
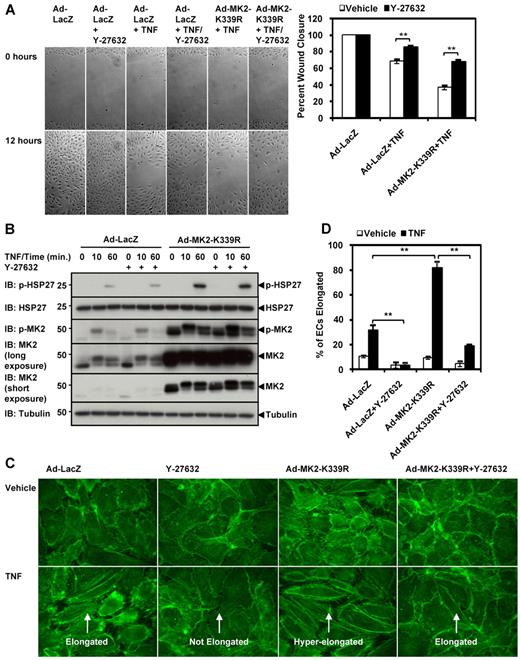
Next we elucidated the role of MK2 SUMOylation in EC migration (Figure 6B). The extent of inhibition of wound closure by TNF-α was further enhanced by overexpressing WT-MK2 or the SUMOylation-deficient K339R mutant in ECs, but the mutant had a more severe inhibitory effect than wild-type MK2 (Ad-WT-MK2 50.1% ± 3.2% vs Ad-MK2-K339R 32.0% ± 0.4%). To clarify whether excessive actin filament bundle formation by the K339R mutant was the reason for its inhibition of EC migration, we down-regulated the RhoA-ROCK pathway.32,33 It has been shown that the ROCK inhibitor Y-27632 can increase cell migration in certain circumstances.34-36 Specifically, Y-27632 at a concentration of 10μM has been shown to have no effect on basal EC migration, but abolishes VEGF-enhanced migration completely.37 Similarly, we found that Y-27632 did not affect basal EC migration (Figure 7A Ad-LacZ + Y-27632), but reversed TNF-α–mediated inhibition of EC migration (Ad-LacZ + TNF 68.0% ± 2.6% vs Ad-LacZ + TNF + Y-27632 85.3% ± 2.1%). Correspondingly, MK2-K339R-mediated inhibition of EC migration under TNF-α stimulation was also relieved by Y-27632 (Ad-MK2-K339R + TNF 36.5% ± 2.9% vs Ad-MK2-K339R + TNF + Y-27632 67.6% ± 2.4%). Y-27632 did not affect TNF-α–mediated MK2 activation or HSP27 phosphorylation (Figure 7B), but did affect actin filament organization (Figure 7C). In ECs pretreated with Y-27632 and stimulated by TNF-α, longitudinal actin filament bundles in the central part of the cell were diminished. Furthermore, to verify that our EC migration results were not dependent on proliferation, we examined the effects of the MK2-K339R SUMOylation–defective mutant on HUVEC proliferation using a [3H] thymidine incorporation assay and found that it had no significant effect on HUVEC proliferation under TNF-α stimulation (supplemental Figure 1D). These data suggest the importance of SUMOylation in regulating EC migration in part by controlling the extent of actin filament dynamics.
ROCK inhibitor Y-27632 reverses effects of MK2-K339R. (A) HUVECs were transduced with the appropriate adenoviral constructs (Ad-LacZ, Ad-WT-MK2, Ad-DN-MK2, or Ad-K339R) for 24 hours. Confluent monolayers were wounded as in Figure 6, pretreated with the ROCK inhibitor Y-27632 (10μM) for 1 hour, and wound closure with or without TNF-α (10 ng/mL) recorded as in Figure 6. Quantification for the percentage wound closure can be seen on the right. Note the lack of effect of Y-27632 on vehicle wound closure, but that the ROCK inhibitor reversed TNF-α–mediated inhibition of EC migration. Also note the reversal effect of Y-27632 on the Ad-MK2-K339R–mediated inhibition of EC migration under TNF-α stimulation. (B) HUVECs were transduced with Ad-LacZ or Ad-MK2-K339R, stimulated by TNF-α (10 ng/mL) for 0-60 minutes with or without Y-27632 pretreatment, and then harvested for immunoblotting. Note that Y-27632 had no effect on MK2 expression, MK2 activation, or HSP27 phosphorylation. (C) HUVECs were prepared under the same conditions as in (B), stimulated with or without TNF-α (10 ng/mL) for 6 hours, and stained with Alexa-Fluor 488 phalloidin. Note the loss of elongated ECs under TNF-α stimulation when Y-27632 was added. Moreover, Y-27632 caused a loss of hyperelongated ECs in the Ad-MK2-K339R–transduced culture. (D) Quantification of cell elongation performed as in Figure 4E. Panels A-D show representative images from 1 of 3 independent experiments and quantitative data (n = 3). Values are means ± SEM. *P < .05; **P < .01.
ROCK inhibitor Y-27632 reverses effects of MK2-K339R. (A) HUVECs were transduced with the appropriate adenoviral constructs (Ad-LacZ, Ad-WT-MK2, Ad-DN-MK2, or Ad-K339R) for 24 hours. Confluent monolayers were wounded as in Figure 6, pretreated with the ROCK inhibitor Y-27632 (10μM) for 1 hour, and wound closure with or without TNF-α (10 ng/mL) recorded as in Figure 6. Quantification for the percentage wound closure can be seen on the right. Note the lack of effect of Y-27632 on vehicle wound closure, but that the ROCK inhibitor reversed TNF-α–mediated inhibition of EC migration. Also note the reversal effect of Y-27632 on the Ad-MK2-K339R–mediated inhibition of EC migration under TNF-α stimulation. (B) HUVECs were transduced with Ad-LacZ or Ad-MK2-K339R, stimulated by TNF-α (10 ng/mL) for 0-60 minutes with or without Y-27632 pretreatment, and then harvested for immunoblotting. Note that Y-27632 had no effect on MK2 expression, MK2 activation, or HSP27 phosphorylation. (C) HUVECs were prepared under the same conditions as in (B), stimulated with or without TNF-α (10 ng/mL) for 6 hours, and stained with Alexa-Fluor 488 phalloidin. Note the loss of elongated ECs under TNF-α stimulation when Y-27632 was added. Moreover, Y-27632 caused a loss of hyperelongated ECs in the Ad-MK2-K339R–transduced culture. (D) Quantification of cell elongation performed as in Figure 4E. Panels A-D show representative images from 1 of 3 independent experiments and quantitative data (n = 3). Values are means ± SEM. *P < .05; **P < .01.
VEGF induces MK2-mediated HSP27 phosphorylation and MK2 SUMOylation but not EC elongation
In addition to TNF-α, MK2 can also be regulated by several stimulants including lipopolysaccharide, VEGF, and anisomycin, all of which activate the canonical p38-MK2 pathway.38 Among them, however, only VEGF and TNF-α have been reported to activate the MK2-HSP27 pathway in ECs and to regulate actin filament remodeling.3,4,6,8,9,38 We have tested the effects of VEGF on MK2 SUMOylation and HSP27 phosphorylation in HUVECs and indeed found that HSP27 phosphorylation was strongly induced by MK2 along with MK2 SUMOylation (supplemental Figure 3A). However, unlike TNF-α, VEGF was only able to weakly induce MK2 SUMOylation and could not sustain HSP27 phosphorylation for more than an hour (supplemental Figure 3A). Other studies on VEGF-induced HSP27 phosphorylation have not examined this long-term phosphorylation event.1,39,40 We also tested the effect of VEGF on the regulation of actin filament remodeling by examining EC elongation, as we did in the experiment shown in Figure 4D. Consistent with the Western blot results on HSP27 phosphorylation and MK2 SUMOylation, a short-term stimulation with VEGF for 1 hour showed a mild increase in stress fibers, as has also been shown by others,1,37,39 but without any significant EC elongation at any time points up to 6 hours (supplemental Figure 3B-C). These data suggest that although VEGF is involved in the activation of the MK2-HSP27 pathway, it only weakly induces MK2 SUMOylation and does not cause the excessive actin filament remodeling that is seen with TNF-α. Because the limited extent of stress fiber development and cell elongation exhibited by cells treated with VEGF is a pro-motility phenotype, VEGF appears to promote cell motility (unlike TNF-α).
Discussion
Posttranslational modifications have emerged as important regulators of signaling due to the vast array of changes that they can induce to physiologic and pathologic pathways in a transient fashion. SUMOylation is one of the most dynamic posttranslational modifications in nature, with a diverse repertoire of protein effects ranging from protein localization to transcriptional regulation to DNA binding.11,41 However, the role of this modification on modulating kinase activity remains relatively unknown, especially in the context of actin filament remodeling. After identifying the putative K339 site on MK2, we found that the modification by SUMOylation regulates MK2 kinase activity, which then regulates actin filament remodeling and EC migration under shear stress and TNF-α stimulation. TNF-α is a major inflammatory cytokine involved in the progression and development of endothelial dysfunction in atherosclerosis. It plays a role in compromising endothelial functions by inhibiting physiologic processes such as migration, barrier function, and vasoregulation while activating pathologic processes such as inflammation and apoptosis.42,43 Although many studies have focused on the role of TNF-α–induced vascular inflammation and apoptosis, the mechanisms and signal transduction pathways of TNF-α–induced EC migration are poorly understood. Previous studies on EC migration, however, have primarily focused on the role of VEGF in activating the MK2-HSP27 pathway that promotes actin filament remodeling in ECs.1,7 Interestingly, TNF-α has also been shown to activate the MK2-HSP27 pathway and to remodel actin filament organization,8,9 yet it has the opposite effect on EC migration.10 Studies have shown that TNF-α opposes VEGF's angiogenic effects by down-regulating the activity and expression of VEGF-receptor 2 (VEGFR2),44,45 but the effect of TNF-α–induced actin filament remodeling under MK2-HSP27 signaling on blocking EC migration has not been investigated. In this study, we introduce a novel concept that SUMOylation plays a role in actin filament dynamics by regulating MK2 kinase activity. Supporting this idea are the results that: (1) SUMOylation inhibits MK2 kinase activity, (2) TNF-α induces MK2 SUMOylation, (3) loss of the MK2 K339 SUMOylation site enhances MK2-mediated phosphorylation of HSP27, and (4) the MK2-K339R mutant enhances actin filament remodeling and (5) augments TNF-α–mediated inhibition of EC migration.
Actin filament remodeling is an important process in maintaining the integrity of the endothelium in response to vascular injuries. A coordinated remodeling of actin filaments is required for directed and efficient cell motility in vascular wound healing and angiogenesis. One major regulatory pathway of migration is the p38-MK2-HSP27 pathway, in which MK2-mediated phosphorylation of HSP27 frees actin filaments for polymerization and actin filament remodeling.5 Interruption of this signaling cascade by the p38 inhibitor SB203580,1 MK2 siRNA,39 or the nonphosphorylatable HSP27 mutant1,46 all inhibited EC migration, underlying the importance of this pathway. In this study, we found that MK2 played a critical role in actin filament remodeling in ECs by TNF-α and shear stress, because the expression of kinase-dead dominant-negative MK2 and MK2 siRNA both inhibited HSP27 phosphorylation and actin filament remodeling. On the other hand, expression of the MK2-K339R SUMOylation–defective mutant enhanced and sustained HSP27 phosphorylation. In addition, actin filament remodeling was inhibited by DN-MK2 but increased by WT-MK2 and further enhanced by the K339R mutant.
It has been reported that shear stress is a potent activator of the p38-MK2-HSP27 pathway and induces rearrangement of the actin cytoskeleton and reorientation of ECs in the direction of flow.29,31 Activation of MK2 by shear stress leads to the phosphorylation of HSP27 and relieves its actin filament capping activity, increasing the number of dynamic actin filament ends that may be necessary for rearranging actin filament organization in response to flow.47 We found that DN-MK2 blocked EC alignment by laminar flow. However, WT-MK2 was strongly activated by laminar flow, which then induced HSP27 phosphorylation and EC alignment. Moreover, compared with WT-MK2, the MK2-K339R SUMOylation–deficient mutant further enhanced HSP27 phosphorylation and EC alignment, highlighting the importance of SUMOylation in regulating MK2 kinase activity and actin filament remodeling.
Retraction of the tail is a critical process during cell migration,48,49 and the actin cytoskeleton regulators RhoA and ROCK are the key players in this process.32,33 The cycle of assembly and disassembly of focal adhesions and actin bundles is required for cell migration, but the mechanistic insight of this complex process remains unclear.48,49 It has been reported that overactivation of RhoA inhibits cell migration,50 and that inhibition of actin filament remodeling with the ROCK inhibitor Y-27632 actually increases cell migration speed,34-36 which we show in Figure 7. When we examined the role of MK2 in TNF-α–mediated EC migration, we found that TNF-α (10 ng/mL) inhibited migration and that WT-MK2 enhanced this effect. Interestingly, both WT-MK2 and DN-MK2 inhibited EC migration under TNF-α stimulation. These results may suggest that whereas TNF-α overactivates WT-MK2 to tip actin dynamics toward polymerization and hinders EC migration, DN-MK2 blocks the actin filament remodeling necessary for cell locomotion. Loss of MK2 SUMOylation with the K339R mutant may cause sustained and excessive actin filament formation, which further inhibits EC migration compared with WT-MK2. We found that ROCK inhibition restored the K339R mutation–mediated inhibition of EC migration, strongly supporting this concept.
In summary, we demonstrate the critical role of SUMOylation in actin filament remodeling and EC migration through the modulation of MK2 kinase activity. In elucidating the importance of MK2 SUMOylation in regulating HSP27 phosphorylation and EC migration under TNF-α, we have uncovered a new mechanism of kinase regulation in ECs, SUMOylation, which then regulates actin filament dynamics.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the National Institutes of Health (NIH) to J.A. (HL-088637, HL-064839, HL-077789, and HL-102746) and K.F. (HL-077789, HL-064839, HL-102746). J.A. is a recipient of Established Investigator Awards of the American Heart Association (AHA; 0740013N). C.-H.W. is supported by an AHA postdoctoral fellowship (0625957T) and an AHA Scientist Development Grant (0930560N). N.-T.L. is supported by an AHA postdoctoral fellowship (10POST4360007).
National Institutes of Health
Authorship
Contribution: E.C. performed experiments; E.C., K.-S.H., C.-H.W., H.L., N.-T.L., and T.N.T. analyzed data and critically edited the paper; and E.C., K.F., and J.A. designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Current address of C.-H.W. is Department of Pharmacology, College of Medicine, Yeungnam University, Daegu, Korea.
Correspondence: Jun-ichi Abe, MD, PhD, Aab Cardiovascular Research Institute and Department of Medicine, University of Rochester School of Medicine and Dentistry, 601 Elmwood Ave, Box CVRI, Rochester, NY 14642; e-mail: Jun-ichi_Abe@urmc.rochester.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal