Tissue hypoxia affects immunity and inflammation; however, the way in which changes in oxygen levels may modulate dendritic cell (DC) function is still not clear. Bosco et al report the gene expression profiling of hypoxic mature DCs and identify TREM-1 as a novel hypoxia-inducible gene that may amplify inflammatory responses.1
Normal oxygen delivery is essential for survival of metazoan organisms and evolutionarily conserved mechanisms have been developed to maintain oxygen homeostasis. Changes in tissue oxygen levels in pathologic conditions are caused by an imbalance between cellular oxygen demand and tissue oxygen delivery. Thus, tissue hypoxia is a hallmark of a number of pathologic conditions ranging from cancer to inflammatory diseases. Oxygen homeostasis is by and large maintained through a family of hypoxia-inducible transcription factors (HIFs), composed of a constitutively expressed HIF-1β subunit and a HIF-α subunit (HIF-1α and HIF-2α) that is tightly regulated by tissue oxygen levels.2 Accumulation of the HIF-α subunit is rapidly triggered by a decrease in oxygen levels, with consequent transcriptional activation of a number of genes whose products are involved in compensatory pathways aimed at restoring oxygen delivery and maintaining energy requirements, including erythropoiesis, angiogenesis, and glycolytic metabolism.2
Abnormal activation or deregulation of hypoxia-induced transcriptional pathways may contribute to the pathogenesis of conditions as diverse as inflammation, vascular disease, and cancer.3 A cross-talk between hypoxic and nonhypoxic signaling pathways, including pro-inflammatory cytokines, such as tumor necrosis factor-α and interleukin-1β, and bacterial products, that is, lipopolysaccharide, may further amplify inflammatory responses by activating HIF as well as other oxygen-sensitive transcription factors, for example nuclear factor-κB (NF-κB). Indeed, accumulating evidence indicates that NF-κB and HIF-1α are linked in a regulatory loop that substantiates the involvement of hypoxia in innate immunity and inflammation.4 Likewise, hypoxia-triggered inflammation may play a role in providing a nurturing environment for cancer initiation and progression.
To what extent does tissue hypoxia affect immune response and inflammatory processes? Over the past several years, the role of hypoxia in differentially regulating the function of cells involved in innate and adaptive immunity has been increasingly appreciated. HIF-1 provides an adequate energy supply and promotes bactericidal activities of myeloid cells,5 at least in part by transcriptional activation of the inducible nitric oxide synthase gene.6 HIF-1 promotes neutrophil survival in an NF-κB–dependent fashion7 and increases neutrophil blood vessel extravasation. Conversely, hypoxia may down-regulate adaptive immunity, for instance, by signaling through the extracellular adenosine receptors and by inhibiting interferon-γ production and T-cell activation.8
In contrast, the effects of the hypoxic microenvironment on DC function are still poorly understood. DCs are a heterogeneous group of professional antigen-presenting cells that are essential to link innate to adaptive immunity, by recognizing danger signals and triggering T-cell responses. Evidence has been provided that hypoxia may either impair9 or promote10 DC function. In this issue, Bosco et al1 provide a detailed analysis of the transcriptional profile of monocyte-derived mature DCs cultured under hypoxic conditions (H-mDCs). They shed some light on genes that are induced under hypoxic conditions and that may contribute to the activation of adaptive pathways essential for survival and function of H-mDCs, such as genes involved in glycolysis, cell metabolism, and angiogenesis. However, the majority of genes up-regulated in H-mDCs encoded for proteins that are involved in immune regulation, inflammatory responses, and cell migration and adhesion, clearly indicating a functional distinction between normoxic and H-mDCs.
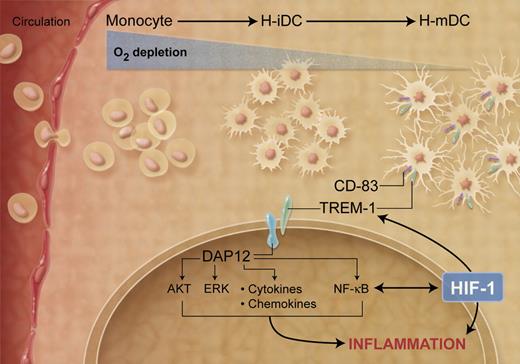
Among the genes induced in H-mDCs, Bosco et al1 identified a cluster of genes coding for immune-related cell-surface receptors and further characterized the expression and regulation of TREM-1, which was highly and selectively expressed in H-mDCs (see figure). TREM-1 is a surface molecule expressed on neutrophils and a subset of monocytes, which is down-regulated during monocyte differentiation into DCs.11 Thus, expression of TREM-1 on CD-83+ DCs may represent a novel marker that characterizes the H-mDC population. TREM-1 lacks an intracellular signal transduction domain and associates with the adaptor protein DAP12, which triggers downstream signaling. TREM-1 expression is up-regulated by lipopolysaccharide and other microbial products and it functions as an amplifier of the inflammatory response in the context of microbial infections.11 Bosco et al1 show that HIF-1 is, at least in part, implicated in the hypoxic-mediated transcriptional activation of TREM-1, consistent with the presence of a putative hypoxia response element in the promoter region. Notably, cross-linking of TREM-1 was associated with induction of DAP12-mediated signaling pathways leading to activation of AKT, ERK-1, and IκBα and increased production of pro-inflammatory cytokines, indicating that TREM-1 may be involved in amplifying hypoxic-dependent inflammatory responses. TREM-1 ligand is still unknown, so it is unclear how hypoxic conditions trigger TREM-1/DAP12 signaling cascade. Notably, TREM-1 signaling was primarily activated under chronic, rather than acute, hypoxic conditions, emphasizing the requirement for sustained stimulation to activate TREM-1 expression and further indicating a functional distinction between normoxic and H-mDCs.
Effects of decreased oxygen concentrations on DC maturation and function. (Professional illustration by A. Y. Chen.)
Effects of decreased oxygen concentrations on DC maturation and function. (Professional illustration by A. Y. Chen.)
The findings reported by Bosco et al1 in this issue raise a number of questions. What are the functional consequences of TREM-1 expression in H-mDCs? Does TREM-1 expression affect the response of mDCs to hypoxia? In which other cell types is TREM-1 induced by hypoxia? More importantly, what is the role of TREM-1 in human pathologic conditions known to be associated with hypoxia? Bosco et al1 provide evidence that this pathway may be activated in synovial fluid from children with Juvenile Idiopathic Arthritis, an inflammatory condition associated with hypoxia. Previously, TREM-1 expression had been implicated in the pathogenesis of sterile inflammatory conditions, including rheumatoid arthritis. Further studies are required to fully appreciate the role of TREM-1 expression in human inflammatory diseases associated with hypoxia either as a marker of H-mDCs or as a potential therapeutic target.
Overall, this study highlights several important points. The first is the essential contribution of tissue hypoxia to the initiation and amplification of inflammatory processes, emphasizing its potential involvement in a broad range of human diseases. The second is the heterogeneous response of distinct cellular components to the hypoxic inflammatory microenvironment, which may differentially modulate the inflammatory response. Finally, the recurrent implication of hypoxia-triggered inflammation in distinct, and apparently unrelated, human diseases provides an opportunity to identify novel targets and develop more effective therapies potentially active in a broad range of pathologic conditions.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■
Acknowledgments
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
National Institutes of Health

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal