Abstract
Endothelial cells (ECs) in blood vessels under formation are stabilized by the recruitment of pericytes, both in normal tissues and during angiogenesis in pathologic situations, including neoplasia. In the tumor vasculature, besides supporting the functionality of blood flow, pericytes protect ECs from antiangiogenic therapies, and have thus been implicated in clinical resistance to vascular targeting drugs. However, the molecular nature of the crosstalk between pericytes and ECs is largely unchartered. Herein, we identified pericyte-induced survival signals in ECs by isolation of vascular fragments derived from tumors that had been genetically or pharmacologically engineered to be either pericyte-rich or pericyte-poor. Pericytes induced the antiapoptotic protein Bcl-w in tumor ECs both in vivo and in vitro, thereby conveying protection from cytotoxic damage. The pericyte-dependent survival signaling in ECs was consequential to enforcement of an autocrine loop involving VEGF-A expression in ECs. Through molecular and functional studies, we delineated a signal transduction pathway in ECs downstream of integrin αv involving activation of NF-κB as the initiating event of the protective crosstalk from pericytes. Our elucidation of pericyte-derived pro-survival signaling in tumor ECs has potentially important implications for clinical development of antiangiogenic drugs, and suggests new therapeutic targets for rational multitargeting of cancer.
Introduction
Cancer results from the concerted action of malignant cells interacting with the many cellular and protein components of the tumor stroma.1 Different strategies to therapeutically target the tumor stroma are currently being pursued. Most notably, drugs targeting endothelial cells (ECs) of the angiogenic tumor vasculature have been introduced into clinical practice after successful preclinical and clinical trials.2 Despite abundant evidence that angiogenesis is instrumental for the growth of solid tumors, the clinical benefit from drugs incorporating antiangiogenic activity have thus far proved limited, typically improving progression-free survival by 2-6 months with only minimal benefit in terms of overall survival.3 In addition, recent preclinical studies suggest that tumors can acquire resistance to blockade of angiogenic growth factors, and as a consequence attain an increased invasive and metastatic potential.4,5 Evidently, we must learn more about the biology underlying the positive effects of targeted antivascular therapies to more accurately be able to predict in which context to use a particular drug, as well as which patients will benefit the most from treatment.
Endothelial cells in small blood vessels and capillaries interact with pericytes, cells of mesenchymal origin that provide important support for blood vessel formation and function.6 Numerous studies describe the significance of members of the platelet-derived growth factor (PDGF) family for recruitment to and productive association of pericytes with the blood vessel endothelium during embryonal development.7 Strikingly, mice lacking components of the PDGF-B/PDGFR-β signaling axis are essentially devoid of pericytes, and as a consequence die from microhemorrhaging because of malformed blood vessels. The importance of PDGF signaling for pericytes in tumor blood vessels is further illustrated by recent work. Studies of genetically manipulated mice demonstrate the significance of endothelium-derived PDGF-BB for a normal integration of pericytes into tumor blood vessels.8 In addition, ectopic expression of PDGF-BB by tumor cells results in increased recruitment of mural cells to blood vessels on establishment of subcutaneous tumors, whereas inhibition of PDGFR signaling decreases the extent of pericyte coverage in tumors.9-12 The notion of targeting pericyte function, for example, via their PDGF receptors, to gain enhanced efficacy of antiangiogenic treatment regimens is supported by reports of beneficial effects of combining PDGFR inhibitors with antiangiogenic drugs or regimens.11-15 However, despite the tight physical and functional association between ECs and pericytes, there is an evident paucity of information about the signals exchanged between the 2 cell types.
Herein, we have delineated a molecular mechanism involved in conferring pericyte-dependent protection from cytotoxic damage in tumor ECs. The expression of genes involved in survival signaling was assessed in tumor ECs from situations of poor pericyte coverage, after pharmacologic disruption of EC-pericyte association, or of rich pericyte coverage, after ectopic expression of PDGF-BB by tumor cells. Association with pericytes was found to enhance the transcription of autocrine VEGF-A in tumor ECs, leading to increased expression of the antiapoptotic protein Bcl-w, ultimately resulting in protection from drug-induced apoptosis. The stimulation of VEGF-A and Bcl-w expression was consequential to EC signaling by integrin αv via NF-κB, as blockade of these pathways resulted in abolished pericyte support to ECs. Taken together, our findings open new avenues for interference with blood vessel growth for the treatment of cancer, and may instruct the use of antiangiogenic agents currently available in the clinic.
Methods
Mice, tumors and treatments
All animal studies described were approved by the Karolinska Instituet committee for animal care, application N146/08. RIP1-Tag2 mice were treated with imatinib (Glivec/Gleevec, Novartis; 150 mg/kg a day divided in a morning dose of 50 mg/kg and an afternoon dose of 100 mg/kg) dissolved in PBS, or with CP-673,451 (Pfizer, 50 mg/kg a day) dissolved in PEG-400 for 5 consecutive days by gavage at 12 weeks of age. C57Bl/6 mice were subcutaneously injected with 106 cells of the following tumor cell lines: B16 melanoma (mock-transfected) or B16-PDGFB (B16 cells transfected with the full-length human Pdgfb gene).10 Tumors were grown for 16-20 days, at which time they reached a size of 200-500 mm3.
EC isolation
Mice were heart perfused with PBS, and after excision and mincing of tumors, tissue was subjected to digestion with collagenase type II (Worthington Biochemical), collagenase IV (Worthington Biochemical), and DNase I (Sigma-Aldrich) dissolved in DMEM with 5% Cell Dissociation buffer (PBS-based; Gibco/Invitrogen) for 18 minutes at 37°C with constant stirring. After lysis of red blood cells by 10 minutes incubation in Pharm Lyse buffer (BD Biosciences) at room temperature, ECs from RIP1-Tag2 mice (10 mice/group) were purified using FACS for cells positively labeled by PE-conjugated antibodies against CD31 (clone MEC 13.3; BD Biosciences). ECs were isolated from B16 and B16-PDGFB tumors (4 mice/group) using magnetic beads (Dynabeads) coated with biotinylated CD31 antibodies (clone MEC 13.3; BD Biosciences). The purity of the fractions was analyzed by RT-PCR using the following markers and primers:
Flk1 (ECs) forward GGAGGAGTACAACACCACGG
reverse TTGAGGAGCTTTCACCGAAC
CD31 (ECs) forward AGAGACGGTCTTGTCGCAGT
reverse TACTGGGCTTCGAGAGCATT
Insulin (PNET cells from RIP1-Tag2 mice)
forward GCAAGCAGGTCATTGTTTCA
reverse GGGACCACAAAGATGCTGTT
Zeocin resistance gene (included in the vector transfected into B16 tumor cells)
forward GTTCTCCCGGGACTTCGT
reverse GACACGACCTCCGACCAC
PDGFRβ (pericytes) forward CACCTTCTCCAGTGTGCTGA
reverse GGAGTCCATAGGGAGGAAGC
CD68 (macrophages) forward CCAATTCAGGGTGGAAGAAA
reverse ATGGGTACCGTCACAACCTC
L19 (reference) forward AGCCACGCTTTCATACTGCT
reverse TTCAGCTTGTGGATGTGCTC
GAPDH (reference) forward ACCCAGAAGACTGTGGATGG
reverse CACATTGGGGGTAGGAACAC
Gene expression analysis
RNA was isolated using the RNeasy mini kit with DNase treatment (QIAGEN). cDNA was prepared from RNA using iScript cDNA Synthesis kit (Bio-Rad). Gene expression analysis was performed by quantitative RT-PCR using Quantitect primer assays (QIAGEN) with L19 as universal reference genes. Transcript levels were expressed as percent of L19 using the formula: % of expression = 2–(Ct(GOI)−Ct(L19)) × 100.
Immunostaining
After heart perfusion with PBS and 4% paraformaldehyde, tumors were excised and embedded in Optimal Cutting Temperature freezing medium. Tissue sections were air-dried, postfixed with ice-cold acetone for 10 minutes, and blocked using serum-free protein block (DAKO) for 60 minutes at room temperature. Next, immunostaining was performed using the following primary antibodies: CD31 (1 μg/mL, #553370; BD Pharmingen), PDGFRβ (1 μg/mL, #3169; Cell Signaling), NG-2 (1 μg/mL, AB5320; Millipore), Bcl-w (1 μg/mL, AB1723, Chemicon Intl), and vitronectin (10 μg/mL, MAB38751; R&D Systems). Detection was performed using secondary antibodies conjugated to Alexa Fluor 594 or Alexa Fluor 488 (1:1000; Invitrogen). Mounting medium containing DAPI (Vector Laboratories) was used.
In situ hybridization
Double in situ hybridization was used to detect mRNA for VEGF-A and for Flk1 (used as an EC marker) on RIP1-Tag2 frozen tumor samples. The protocol was adapted from Widenfalk et al.16,17 Oligonucleotide probes complementary to VEGF-A and Flk1 were used (1 μg/mL). The VEGF-A probe was biotinylated and further detected using streptavidin conjugated to Alexa 594 (Invitrogen). The Flk1 probe was FITC-labeled. The probes in forward orientation were used as negative controls and gave no specific signal (data not shown).
Microscope image acquisition
Imaging was performed using a Nikon Eclipse E800 microscope equipped with Nikon Plan Fluor objectives (10×/0.30 NA, 20×/0.50 NA, and 40×/0.75 NA), at room temperature in air using Alexa 594- and Alexa 488-coupled secondary antibodies. Images were acquired using a SPOT RTKE camera using the SPOT 1.0 advanced software.
Cell lines
Mouse pancreatic islet endothelial cells MS1 (Mile Sven 1, CRL-2279; ATCC) were routinely maintained in culture on gelatin-coated flasks in MV2 EC growth medium (PromoCell). MS1 cells stably expressing a green monomeric GFP-like protein (copGFP) under the CMV promoter were generated through lentiviral infection according to the manufacturer′s instructions (copGFP Control Lentiviral Particles; Santa Cruz Biotechnology). In brief, cells were infected for 24 hours in complete EC growth medium containing 5 μg/mL polybrene (Santa Cruz Biotechnology). Cells stably expressing copGFP were selected and maintained by adding 5 μg/mL of puromycin (Invitrogen) to the culture media (puromycin was removed during experiments). HBVP cells (human brain vascular pericytes, SC1200; ScienCell) were routinely maintained in pericyte medium (PM, SC1201; ScienCell).
Transfection of cells and assessment of growth characteristics
Knock-down of Bcl-w was achieved by transfection of Bcl-w Accell SMART pool according to the manufacturer's instructions (Dharmacon). A nontargeting pool of siRNA was used as a negative control. Successful reduction of Bcl-w by > 70% was verified by qPCR and Western blot (data not shown). MS1 cells were transfected with the siRNA and allowed 72 hours before experiments. The growth rate of control siRNA or Bcl-w siRNA transfected MS1 cells was assessed by counting in a cell counter (Z2 Coulter Counter; Beckman Coulter) after 48 hours of culturing in serum-deprived medium with or without the addition of 50 ng/mL vinblastine (Sigma-Aldrich). Similarly, MS1 cells cultured for 48 hours with or without vinblastine were subjected to analysis of the apoptotic rate by the in situ cell death detection kit, fluorescein, performed according to the manufacturer's instructions (Roche Applied Science).
An expression vector containing the IκB-α cDNA containing S32G and S36A mutations (IKB-α super-repressor) or VEGF-A was transfected into MS1 cells using Lipofectamine LTX (Invitrogen). Forty-eight hours posttransfection, cells were seeded into mono- or cocultures with HBVP, or seeded for experiments, as described.
Coculture system of endothelial cells and pericytes
MS1 ECs stably expressing GFP were seeded alone or in the presence of HBVP pericytes in a 1:1 ratio in 6-well plates or chamber slides precoated with gelatin (for investigations on the role of vitronectin in conferring survival signals to ECs, cells were coated either on plastic or in wells precoated with 5 μg/mL vitronectin (R&D Systems) and put in EC growth medium, allowing them to attach overnight. Alternatively, pericytes were seeded in a transwell culture insert separated from the ECs seeded in the well below by a membrane with 0.4 μm pore size. The following day, the medium was replaced by starvation medium (basal EC medium containing 0.1% BSA) and kept for 3 more days. At the end of the assay, ECs and pericytes were separated by FACS sorting based on GFP expression in the ECs.
In vitro treatments
Vinblastine (50 ng/mL, Sigma-Aldrich) was used as a cytotoxic agent. The following concentrations were used for treatments of in vitro cultures of MS1 and/or HBVP cells: vinblastine (50 ng/mL; Sigma-Aldrich), N-a-tosyl-L-phenylalanine chloromethyl ketone (TPCK, 3μM; Sigma-Aldrich), the small molecule inhibitor of VEGFR2 AG-028262 (3nM; Pfizer), soluble Flt1/Fc chimera (1 μg/mL, 321-FL; R&D Systems), and integrin αv-neutralizing antibodies (10 μg/mL, CBL1346Z; Millipore). Vinblastine was added on day 3 of cocultures and kept for 2 days. The VEGF/VEGFR inhibitors or the antibodies to integrin αv were added on the second day of coculture, 5 hours after starting the starvation, and were kept for 2-3 days.
Detection of apoptosis
Apoptosis was detected by the in situ cell death detection kit, TMR red, performed according to the manufacturer's instructions (Roche Applied Science). The number of viable cells was taken as the total number of cells subtracted by the number of apoptotic cells.
Western blotting
MS1 cells sorted from mono- or cocultures with HBVP were lysed in 20mM Tris-HCl (pH 7.5), 150mM NaCl, 5mM EDTA, 0.5% deoxycholic acid, and 0.5% Triton X-100 containing complete protease inhibitor (Roche Applied Science). Protein (30 μg) was loaded onto SDS-PAGE gels (Invitrogen), and subsequently transferred onto a nitrocellulose membrane using the iBlot system (Invitrogen). Western blotting was performed using antibodies against Bcl-w (AB1723, 5μg/mL; Chemicon) and VEGF-A (MAB493, 1 μg/mL; R&D Systems) with β-actin (GTX26276, 1:5000; GeneTex) serving as loading control.
Statistical analysis
Data are shown as mean ± SEM. Statistical analysis was performed using an un-paired, 2-tailed Student t test with α = 0.05.
Results
Pharmacologic disruption of endothelial cell-pericyte attachment diminishes survival signaling in tumor endothelial cells
To investigate the consequence of pericyte attachment on EC survival pathways, we made use of the widely studied RIP1-Tag2 mouse model of pancreatic neuroendocrine tumorigenesis (PNET).18 Starting at 12 weeks of age, RIP1-Tag2 mice were treated for 1 week with the small molecule tyrosine kinase inhibitors imatinib or CP-673,451.19,20 The 2 drugs have a nonoverlapping spectrum of kinase inhibition with potent blocking of PDGFRβ as the only common feature in cell-based assays. Importantly, neither imatinib, nor CP-673,451 has been found to inhibit the VEGFR kinases at relevant doses.20,21 Examination of tumors from mice treated with either imatinib or CP-673,451 revealed a partial detachment of pericytes from ECs, in agreement with previous studies (Figure 1).11,12 Treatment with CP-673,451 for 1 week resulted in a consistent reduction of cells expressing several different pericyte markers, including PDGFRβ, desmin, α-SMA and NG2 by an average of 55% (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). After disaggregation of the tumor tissue into a single cell suspension, tumor ECs were purified by FACS or magnetic bead enrichment of CD31+ cells. The purity of the EC preparations was validated by RT-PCR. Flk1 mRNA was exclusively found in the EC fraction, and not in the nonendothelial fraction (other cell [OC]), whereas the mRNA of markers for the transformed islet β-cells (Ins1), pericytes (Pdgfrb) and macrophages (Cd68) were only found in the OC fraction, confirming the specificity of the purification procedure (supplemental Figure 1B). Next, we performed gene expression analyses of the EC fractions obtained from PNET from RIP1-Tag2 mice treated or not with imatinib (ECcontrol and ECimatinib, respectively). We focused our study on genes with a documented role in cell survival and specifically assessed the transcriptional abundance of > 30 members of the Bcl-2 family. The analysis revealed an appreciable and consistent decrease in the expression of the antiapoptotic gene Bcl-w (Bcl2l2) in ECimatinib compared with ECcontrol (Figure 2A). No change was observed in the expression of Bcl-w in cells of the OC fraction on treatment with imatinib, although the mRNA of Bcl-w was much more abundant in OCs compared with ECs (Figure 2B). Similar results were obtained after treatment with CP-673,451 (data not shown). The finding that Bcl-w expression in ECs was dependent on pericyte association led us to explore the localization of Bcl-w protein in PNET of RIP1-Tag2 mice. Double-label immunostaining for the EC marker CD31 and Bcl-w revealed that Bcl-w protein was abundant in CD31+ cells localized in vascular structures within tumors and angiogenic islets (Figure 2C). Expression of Bcl-w in endothelial cells persisted in a minor subset of vessels in tumors derived from mice treated with imatinib or CP-673,451 (Figure 2C). Notably, Bcl-w was expressed to a lower degree in the cancer cells, indicating posttranscriptional regulation of Bcl-w protein in a cell type-specific manner, given the lack of concordance between mRNA abundance and protein levels (Figure 2A-C).
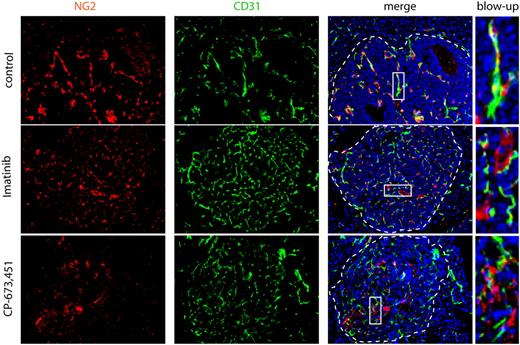
Pericytes are dissociated from ECs on treatment with PDGFR inhibitors. Treatment of RIP1-Tag2 mice with imatinib or CP-673,451 induces pericyte detachment in PNET (arrows). RIP1-Tag2 mice were treated with imatinib (150 mg/kg a day; n = 10) or CP-673,451 (50 mg/kg a day; n = 5) for 5 days by oral gavage. Pictures show immunostaining for CD31 (green) and NG2 (red) on cryosections. Arrows indicate tightly associated (control) or dissociated (imatinib and CP-673,451) pericytes in the tumor vasculature. Dotted line marks tumor:exocrine pancreas boundary. Cell nuclei (DAPI), blue. Boxes indicate areas shown in higher magnification in the right panel. Original magnification, 200×. The panels are representative of at least 5 fields in 5 tissue sections taken from 5 mice.
Pericytes are dissociated from ECs on treatment with PDGFR inhibitors. Treatment of RIP1-Tag2 mice with imatinib or CP-673,451 induces pericyte detachment in PNET (arrows). RIP1-Tag2 mice were treated with imatinib (150 mg/kg a day; n = 10) or CP-673,451 (50 mg/kg a day; n = 5) for 5 days by oral gavage. Pictures show immunostaining for CD31 (green) and NG2 (red) on cryosections. Arrows indicate tightly associated (control) or dissociated (imatinib and CP-673,451) pericytes in the tumor vasculature. Dotted line marks tumor:exocrine pancreas boundary. Cell nuclei (DAPI), blue. Boxes indicate areas shown in higher magnification in the right panel. Original magnification, 200×. The panels are representative of at least 5 fields in 5 tissue sections taken from 5 mice.
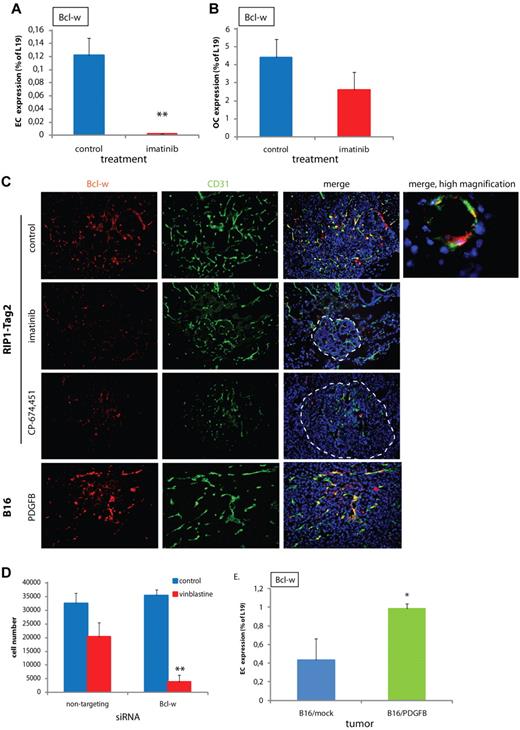
Bcl-w is a pericyte-induced antiapoptotic protein in ECs. (A-B) Expression of Bcl-w in ECs (A) or OCs (B) comprising cancer cells, pericytes, macrophages, and infrequent cancer-associated fibroblastic cells fractionated from PNET tumors excised from RIP1-Tag2 mice that had been treated with control or imatinib, as assessed by quantitative RT-PCR (n = 10 in each group, data shown are representative for 2 independent EC isolations). **P < .01 vs control, Student t test. (C) Immunostaining of sections from PNET of RIP1-Tag2 mice or B16/PDGFB tumors for Bcl-w (red) and CD31 (green). Cell nuclei (DAPI), blue. Original magnification, 200× (for high magnification image, 630×). The panels are representative of at least 5 fields in 5 tissue sections taken from 5 mice. (D) Number of MS1 pancreatic islet ECs after siRNA-mediated knockdown of Bcl-w and treatment with control or vinblastine (50 ng/mL). A nontargeting siRNA was used as a control. Data shown are representative for 3 independent experiments. **P < .01 vs nontargeting/vinblastine, Student t test. (E) Expression of Bcl-w in isolated ECs from B16/mock and B16/PDGFB tumors assessed by quantitative RT-PCR (n = 4 in each group, data shown are representative for 2 independent EC isolations). *P < .05 vs B16/mock, Student t test.
Bcl-w is a pericyte-induced antiapoptotic protein in ECs. (A-B) Expression of Bcl-w in ECs (A) or OCs (B) comprising cancer cells, pericytes, macrophages, and infrequent cancer-associated fibroblastic cells fractionated from PNET tumors excised from RIP1-Tag2 mice that had been treated with control or imatinib, as assessed by quantitative RT-PCR (n = 10 in each group, data shown are representative for 2 independent EC isolations). **P < .01 vs control, Student t test. (C) Immunostaining of sections from PNET of RIP1-Tag2 mice or B16/PDGFB tumors for Bcl-w (red) and CD31 (green). Cell nuclei (DAPI), blue. Original magnification, 200× (for high magnification image, 630×). The panels are representative of at least 5 fields in 5 tissue sections taken from 5 mice. (D) Number of MS1 pancreatic islet ECs after siRNA-mediated knockdown of Bcl-w and treatment with control or vinblastine (50 ng/mL). A nontargeting siRNA was used as a control. Data shown are representative for 3 independent experiments. **P < .01 vs nontargeting/vinblastine, Student t test. (E) Expression of Bcl-w in isolated ECs from B16/mock and B16/PDGFB tumors assessed by quantitative RT-PCR (n = 4 in each group, data shown are representative for 2 independent EC isolations). *P < .05 vs B16/mock, Student t test.
Taken together, detachment of pericytes from tumor ECs by the use of 2 different pharmacologic inhibitors lead to decreased EC expression of Bcl-w, suggesting a role for Bcl-w in promoting EC survival. To address the hypothesis that Bcl-w acts as an antiapoptotic factor in ECs, we transfected MS1 mouse pancreatic islet ECs with siRNA targeting Bcl-w and assessed EC apoptosis and viability on treatment with vinblastine, a drug known to produce cytotoxic effects on ECs both in vitro and in vivo.22-24 While knockdown of Bcl-w did not change the growth rate (Figure 2D) or apoptotic rate (supplemental Figure 1C; nontargeting siRNA, control: 2.3 ± 0.5 TUNEL+ cells/field vs Bcl-w siRNA, control: 2.1 ± 0.6 TUNEL+ cells/field) of untreated MS1 cells, diminished expression of Bcl-w sensitized MS1 cells to the damaging action of vinblastine, as assessed by inhibition of cell growth (Figure 2D) and induction of apoptosis (supplemental Figure 1C; nontargeting siRNA, vinblastine: 10.9 ± 2.3 TUNEL+ cells/field vs Bcl-w siRNA, vinblastine: 22.8 ± 3.9 TUNEL+ cells/field, P < .05, Student t test).
Expression of the antiapoptotic protein Bcl-w is increased in tumor ECs with improved pericyte coverage
To address whether improved pericyte coverage enhanced the expression of Bcl-w in tumor ECs, we made use of a tumor model in which the tumor cells ectopically express PDGF-BB, the main ligand for PDGFRβ. Tumors derived from mouse melanoma B16 cells transfected with Pdgfb (B16/PDGFB) exhibit a 45% increase in the number of pericytes lining the microvasculature, compared with mock-transfected tumors (B16/mock;10 and data not shown). Pure preparations of ECs from B16/mock and B16/PDGFB tumors (supplemental Figure 1D) were subjected to quantitative RT-PCR analysis to assess the expression of Bcl-w. ECs from B16/PDGFB tumors exhibited a 2.3-fold higher expression of Bcl-w compared with ECs from B16/mock tumors (Figure 2E). Immunostaining confirmed EC localization of the Bcl-w protein in B16/PDGFB tumors (Figure 2C).
Thus, EC expression of the antiapoptotic protein Bcl-w was increased concomitant with augmented pericyte coverage, in further support of the hypothesis that mural cell association improves EC survival through paracrine regulation of gene expression in tumor ECs.
Autocrine signaling by VEGF-A is regulated in a pericyte-dependent manner in tumor ECs
Recently, EC homeostasis and survival was found to be regulated by autocrine stimulation of VEGFRs by VEGF-A.25 In PNET from RIP1-Tag2 mice, VEGF-A mRNA was readily detected in a subset of microvessels, as demonstrated by double in situ hybridization for VEGF-A and Flk1 (Figure 3A). In addition to ECs, tumor cells also produced abundant levels of VEGF-A transcript, as expected (Figure 3A). To investigate whether pericyte attachment contributes to the EC expression of VEGF-A, we assessed the abundance of VEGF-A transcript in EC preparations from conditions of poor or rich pericyte association. As seen in Figure 3B and C, tumor ECs from imatinib-treated RIP1-Tag2 mice expressed significantly lower levels of endogenous VEGF-A, and ECs from B16/PDGFB tumors expressed appreciably higher levels of VEGF-A, than the respective control preparations of ECs. In contrast, global levels of VEGF-A in the tumors were not regulated in a pericyte-dependent manner (data not shown). In addition, the unchanged expression of VEGF-A and Bcl-w in cultured MS1 cells after treatment with imatinib or CP-673,451 ruled out direct effects of the tyrosine kinase inhibitors on EC expression of either Bcl-w or VEGF-A (Figure 3D and data not shown).
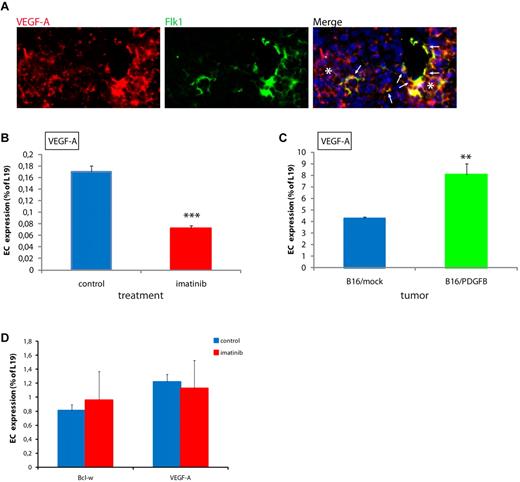
Pericyte association regulates expression of VEGF-A in ECs. (A) VEGF-A is expressed in a subset of ECs (arrows) in PNET from RIP1-Tag2 mice, as assessed by double in situ hybridization for VEGF-A (red) and Flk1 (green). Note that VEGF-A is abundantly expressed by tumor cells, as expected (stars). Cell nuclei (DAPI), blue. Original magnification, 400×. The panel is representative of at least 5 fields in 5 tissue sections taken from 5 mice. (B) Expression of VEGF-A is decreased in ECs in PNET from imatinib-treated RIP1-Tag2 mice compared with controls, as assessed by quantitative RT-PCR. ***P < .001 vs control, Student t test. (C) Expression of VEGF-A is increased in ECs in B16/PDGFB tumors compared with B16/mock, as assessed by quantitative RT-PCR. **P < .01 vs B16/mock, Student t test. (D) Treatment of MS1 pancreatic islet ECs with 3μM imatinib did not alter the expression of VEGF-A or Bcl-w, as assessed by quantitative RT-PCR.
Pericyte association regulates expression of VEGF-A in ECs. (A) VEGF-A is expressed in a subset of ECs (arrows) in PNET from RIP1-Tag2 mice, as assessed by double in situ hybridization for VEGF-A (red) and Flk1 (green). Note that VEGF-A is abundantly expressed by tumor cells, as expected (stars). Cell nuclei (DAPI), blue. Original magnification, 400×. The panel is representative of at least 5 fields in 5 tissue sections taken from 5 mice. (B) Expression of VEGF-A is decreased in ECs in PNET from imatinib-treated RIP1-Tag2 mice compared with controls, as assessed by quantitative RT-PCR. ***P < .001 vs control, Student t test. (C) Expression of VEGF-A is increased in ECs in B16/PDGFB tumors compared with B16/mock, as assessed by quantitative RT-PCR. **P < .01 vs B16/mock, Student t test. (D) Treatment of MS1 pancreatic islet ECs with 3μM imatinib did not alter the expression of VEGF-A or Bcl-w, as assessed by quantitative RT-PCR.
An in vitro coculture system confirms pericyte-induced survival signals in ECs
To investigate the molecular mechanism for the pericyte-induced survival signals in ECs, we devised an in vitro coculture system consisting of primary human brain vascular pericytes (HBVP) and GFP-labeled MS1 cells. When seeded on a gelatin substrate, ECs lined up in cord-like structures with pericytes in close apposition (supplemental Figure 2A). Immunostaining demonstrated readily detectable levels of Bcl-w protein within the cultured ECs, both in mono-culture and in coculture with pericytes, indicating that pericyte association is not needed to maintain basal levels of Bcl-w expression in ECs (supplemental Figure 2B). After 4 days of coculture, the MS1 cells were separated from HBVP by FACS and the expression of pro-survival genes compared with MS1 cells purified from mono-cultures was assessed. In close agreement with the findings in tumors in vivo, the presence of pericytes produced a significant up-regulation of Bcl-w and VEGF-A in ECs (Figure 4A-B). Importantly, VEGF-A and Bcl-w were also increased at the protein level in ECs after coculture with HBVP (Figure 4C). Using a transwell coculture system, we assessed the need for physical contact for the pericyte-dependent regulation of survival genes in ECs. Separation of HBVP and MS1 cells by a membrane permeable to proteins, but not cells, rendered pericytes unable to induce VEGF-A or Bcl-w expression in ECs, indicating that the 2 cell types need to be juxtaposed for the observed crosstalk to take place (Figure 4A-B). Additional time course experiments revealed that induction of VEGF-A was observed after 3 days in physical cocultures of HBVP and MS1 cells, whereas stimulation of Bcl-w expression required 4 days, suggesting that internal VEGF-A signaling in ECs acts upstream of Bcl-w (data not shown). To further demonstrate a causal relationship between autocrine VEGF-A signaling and induction of Bcl-w expression, we made use of pharmacologic inhibitors against the VEGF/VEGFR system. MS1 mono-cultures or cocultures with HBVP were treated with saturating concentrations of soluble VEGFR1 (sFlt1), a ligand trap that solely blocks extracellular sources of VEGF-A, or with AG-028262, a small molecule kinase inhibitor with potent and selective activity against both external and internal sources for VEGFR activation. Treatment with sFlt1 did not affect the induction of Bcl-w in MS1 cells by HBVP, indicating that VEGF-A acting in a paracrine manner is not part of the signaling events leading to up-regulation of Bcl-w (Figure 4D). In contrast, the addition of AG-028262 to MS1 and HBVP cocultures completely abolished the augmented expression of Bcl-w elicited by pericyte association, establishing Bcl-w as a downstream target gene of autocrine VEGF-A signaling (Figure 4D). To assess the functional importance of the presence of pericytes in terms of protection from apoptosis, we treated MS1 mono-cultures or MS1/HBVP cocultures with vinblastine and followed the viability of MS1 cells (defined as the total number of cells reduced by the number of cells currently undergoing apoptosis). As seen in Figure 4E, MS1 cells from pericyte and EC cocultures displayed a significantly higher viability in the face of cytotoxic challenge than mono-cultured MS1 cells (Figure 4E). The survival benefit provided by HBVPs was partly, but statistically nonsignificantly, reduced by the addition of sFlt1, consistent with previous findings that pericytes can provide paracrine stimulation of ECs in part by production of VEGF-A26 (Figure 4E). Strikingly, however, inclusion of AG-028262 completely eliminated pericyte support during vinblastine treatment, demonstrating that the autocrine VEGF-A signaling in ECs evoked by pericytes was instrumental for the observed survival benefit of MS1 cells cocultured with HBVP (Figure 4E).
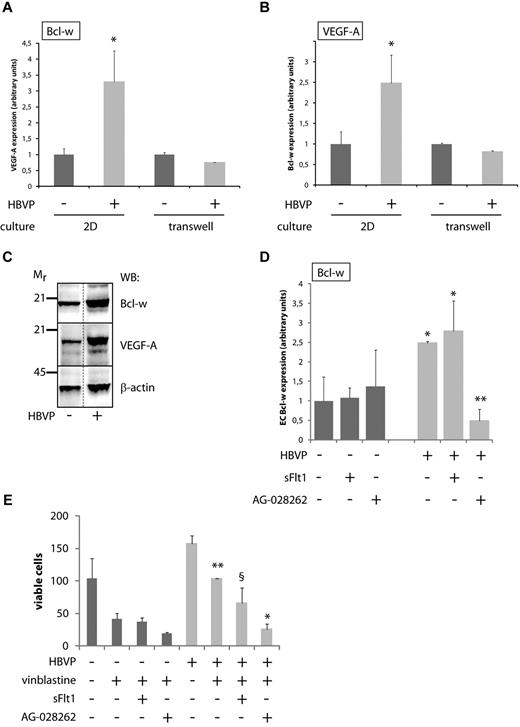
A coculture system reveals the functional significance of crosstalk between ECs and pericytes. (A,B) Quantitative RT-PCR analysis of Bcl-w (A) and VEGF-A (B) expression in MS1 endothelial cells cultured alone or together with HBVPs for 96 hours, either as 2D cultures allowing physical contact between ECs and pericytes or in transwell cultures where the 2 cell types are separated by a membrane with pore size 0.4 μm. Data shown represent the mean of 9 and 2 independent experiments (2D cultures and transwell cultures, respectively). *P < .05 vs MS1 mono-culture. (C) Western blot (WB) analysis of Bcl-w and VEGF-A in cell lysates from MS1 endothelial cells cultured alone or together with HBVPs for 96 hours. Mr indicates molecular weight (kDa). Western blot for β-actin was used as a loading control. Dotted line marks the removal of one irrelevant lane from the original photograph. (D) Quantitative RT-PCR analysis of Bcl-w expression in MS1 endothelial cells cultured alone or together with HBVPs for 96 hours with the addition of the VEGF inhibitors sFlt1 (a ligand trap acting extracellularly) or AG-028262 (an intracellular inhibitor blocking VEGF signaling via its receptor tyrosine kinases). *P < .05 vs MS1 mono-culture; **P < .01 vs MS1/HBVP coculture. (E) Quantification of the number of viable MS1 endothelial cells cultured alone or together with HBVPs for 96 hours with the addition of the cytotoxic drug vinblastine (50 ng/mL) and the VEGF inhibitors sFlt1 (blocking only extracellular sources of VEGF-A) or AG-028262 (blocking both extra- and intracellular sources of VEGF-A). *P < .05 vs vinblastine-treated coculture; **P < .01 vs vinblastine-treated mono-culture; and §, not statistically significant vs vinblastine-treated coculture. HBVP indicates human brain vascular pericytes.
A coculture system reveals the functional significance of crosstalk between ECs and pericytes. (A,B) Quantitative RT-PCR analysis of Bcl-w (A) and VEGF-A (B) expression in MS1 endothelial cells cultured alone or together with HBVPs for 96 hours, either as 2D cultures allowing physical contact between ECs and pericytes or in transwell cultures where the 2 cell types are separated by a membrane with pore size 0.4 μm. Data shown represent the mean of 9 and 2 independent experiments (2D cultures and transwell cultures, respectively). *P < .05 vs MS1 mono-culture. (C) Western blot (WB) analysis of Bcl-w and VEGF-A in cell lysates from MS1 endothelial cells cultured alone or together with HBVPs for 96 hours. Mr indicates molecular weight (kDa). Western blot for β-actin was used as a loading control. Dotted line marks the removal of one irrelevant lane from the original photograph. (D) Quantitative RT-PCR analysis of Bcl-w expression in MS1 endothelial cells cultured alone or together with HBVPs for 96 hours with the addition of the VEGF inhibitors sFlt1 (a ligand trap acting extracellularly) or AG-028262 (an intracellular inhibitor blocking VEGF signaling via its receptor tyrosine kinases). *P < .05 vs MS1 mono-culture; **P < .01 vs MS1/HBVP coculture. (E) Quantification of the number of viable MS1 endothelial cells cultured alone or together with HBVPs for 96 hours with the addition of the cytotoxic drug vinblastine (50 ng/mL) and the VEGF inhibitors sFlt1 (blocking only extracellular sources of VEGF-A) or AG-028262 (blocking both extra- and intracellular sources of VEGF-A). *P < .05 vs vinblastine-treated coculture; **P < .01 vs vinblastine-treated mono-culture; and §, not statistically significant vs vinblastine-treated coculture. HBVP indicates human brain vascular pericytes.
Taken together, our observations from studies using a newly established coculture system of ECs and pericytes confirm that mural cells confer a survival advantage to ECs through regulation of autocrine VEGF-A signaling and Bcl-w expression.
Pericyte-induced survival signaling in ECs is mediated by NF-κB
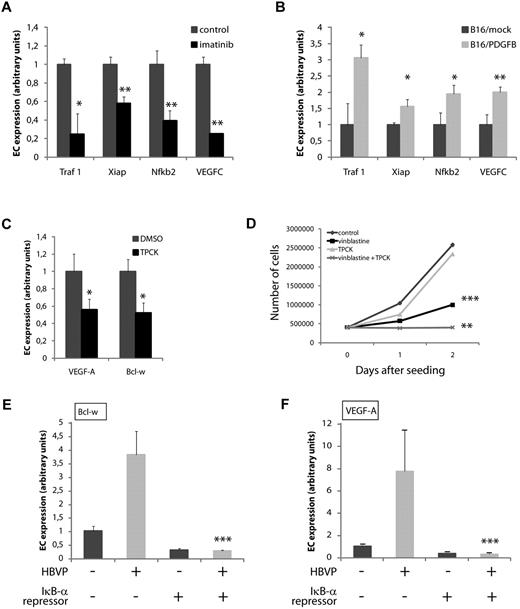
Next, to gain insight into the mediators involved in inducing VEGF-A and Bcl-w in ECs as a result of pericyte contact, we took a candidate approach. Since hypoxia is a strong inducer of VEGF-A, we analyzed the expression of other hypoxia-regulated genes, including Glut1, by quantitative RT-PCR. However, no change in the expression of genes related to hypoxia was observed in ECs isolated from pericyte-poor PNET excised from imatinib-treated RIP1-Tag2 mice, or from pericyte-rich B16/PDGFB tumors (data not shown). In contrast, the expression of target genes of NF-κB, a known regulator of VEGF-A transcription,27,28 was altered in ECs in a pericyte-dependent manner. The expression of the NF-κB target genes Traf1, Xiap, Vegfc, and Nfkb2 was reduced in tumor ECs from RIP1-Tag2 mice after treatment with imatinib (Figure 5A). Reciprocally, the expression of the panel of genes induced by NF-κB was higher than in controls in tumor ECs from B16/PDGFB tumors that harbor increased pericyte content (Figure 5B). Furthermore, treatment of MS1 cells with the NF-κB inhibitors N-a-tosyl-L-phenylalanine chloromethyl ketone (TPCK) or pyrrolinedithiocarbamate lowered the basal expression of both VEGF-A and Bcl-w, indicating that these genes are downstream target genes for NF-κB in ECs (Figure 5C and data not shown). In addition, TPCK sensitized MS1 cells to the cytotoxic action of vinblastine, but did not alter the growth rate of ECs when added alone, demonstrating an involvement of NF-κB signaling in protection from apoptosis during stressed conditions (Figure 5D). To investigate whether NF-κB activity was instrumental for the induction of VEGF-A and Bcl-w by pericytes, we transfected MS1 cells with an IκB-α super-repressor, which carries mutations in 2 crucial serine residues (S32 and S36), leading to an inability to provoke the release, activation and subsequent nuclear translocation of NF-κB.29 Strikingly, pericytes were unable to evoke expression of VEGF-A and Bcl-w in ECs transfected with the IκB-α super-repressor (Figure 5E-F). In summary, NF-κB activity was instrumental in the pericyte-induced regulation of VEGF-A and Bcl-w in ECs.
Pericyte-induced expression of survival genes in ECs requires NF-κB activity. (A,B) Expression of target genes of the transcription factor NF-κB was modulated in ECs from tumors in a pericyte-dependent manner, as assessed by quantitative RT-PCR on purified ECs from PNET from RIP1-Tag2 mice treated with control or imatinib (A), and from B16/mock or B16/PDGFB tumors (B). *P < .05 vs control; *P < .01 vs control. (C,D) The NF-κB inhibitor TPCK (3μM) reduced the basal expression of Bcl-w and VEGF-A in MS1 ECs (D) and sensitized MS1 cells to the action of vinblastine (50 ng/mL; D). *P < .05 vs DMSO; **P < .01 vs vinblastine; ***P < .001 vs control. (E,F) Pericytes are unable to modulate the expression of Bcl-w (E) or VEGF-A (F) in ECs on expression of the IκB-α super-repressor protein in MS1 cells. ***P < .001 vs coculture. HBVP indicates human brain vascular pericytes.
Pericyte-induced expression of survival genes in ECs requires NF-κB activity. (A,B) Expression of target genes of the transcription factor NF-κB was modulated in ECs from tumors in a pericyte-dependent manner, as assessed by quantitative RT-PCR on purified ECs from PNET from RIP1-Tag2 mice treated with control or imatinib (A), and from B16/mock or B16/PDGFB tumors (B). *P < .05 vs control; *P < .01 vs control. (C,D) The NF-κB inhibitor TPCK (3μM) reduced the basal expression of Bcl-w and VEGF-A in MS1 ECs (D) and sensitized MS1 cells to the action of vinblastine (50 ng/mL; D). *P < .05 vs DMSO; **P < .01 vs vinblastine; ***P < .001 vs control. (E,F) Pericytes are unable to modulate the expression of Bcl-w (E) or VEGF-A (F) in ECs on expression of the IκB-α super-repressor protein in MS1 cells. ***P < .001 vs coculture. HBVP indicates human brain vascular pericytes.
Pericyte-dependent survival signaling in ECs requires the activation of integrin αv
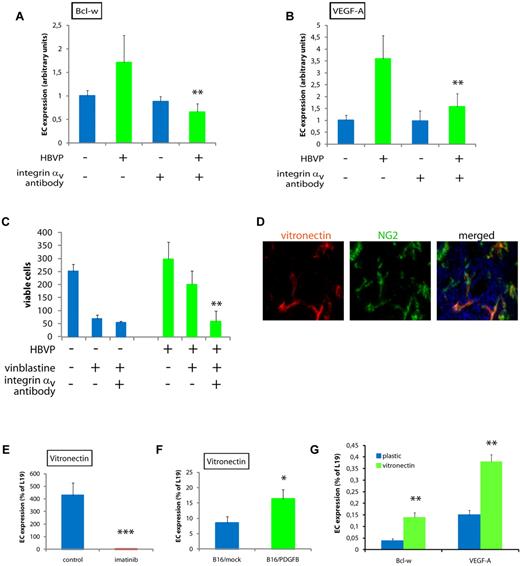
Survival signaling and activation of NF-κB is known to occur downstream of activated integrins in ECs.30 Therefore, we investigated whether the effects of pericytes on EC survival gene expression were dependent on signaling by integrin αv. Indeed, in the presence of neutralizing integrin αv antibodies, pericytes were no longer able to induce expression of Bcl-w or VEGF-A in ECs in cocultures (Figure 6A-B). In addition, the survival advantage conferred by pericytes to ECs during treatment with vinblastine was abolished by the addition of integrin αv-blocking antibodies (Figure 6C). The major ligand for integrin αv is vitronectin. Based on this, and an earlier report that vitronectin protects ECs against apoptosis,31 we assessed whether pericytes are a major source of vitronectin in the tumor models used. Strikingly, in PNET from RIP1-Tag2 mice, vitronectin was predominantly observed surrounding pericytes (Figure 6D). In addition, quantitative RT-PCR analysis of the nonEC fraction of PNET from RIP1-Tag2 mice treated with imatinib revealed that vitronectin was expressed at exceedingly low levels compared with control mice (Figure 6E). Conversely, the OC fraction of B16/PDGF-B tumors contained higher levels of vitronectin transcript (Figure 6F). In addition, plating of MS1 cells on vitronectin, as opposed to on plastic, mediated an increased transcription of both VEGF-A and Bcl-w (Figure 6G). Taken together, our data indicate that pericyte-derived survival signals to ECs are conferred by PDGFR-dependent expression and deposition of vitronectin in the vascular basement membrane, and subsequent ligation-stimulated signaling via integrin αv of ECs in the angiogenic tumor vasculature. A schematic cartoon depicting the regulatory pathway induced by pericytes in tumor ECs leading to enhanced survival, as elucidated by our work, is shown in Figure 7.
The crosstalk between pericytes and ECs is mediated by integrin αv activity. (A,B) Quantitative RT-PCR analysis of Bcl-w (A) and VEGF-A (B) expression in MS1 endothelial cells cultured alone or together with HBVPs for 96 hours in the presence or absence of neutralizing antibodies against integrin αv. Data shown are representative of 2 independent experiments. **P < .01 vs MS1/HBVP coculture. (C) Quantification of the number of viable MS1 endothelial cells cultured alone or together with HBVPs for 96 hours with the addition of the cytotoxic drug vinblastine (50 ng/mL) in the presence or absence of neutralizing antibodies against integrin αv. **P < .01 vs vinblastine coculture. (D) Immunostaining of tissue sections from PNET of RIP1-Tag2 mice for the integrin αv ligand vitronectin (green) and the pericyte marker PDGFRβ (red). Cell nuclei (DAPI), blue. Original magnification, 400×. The panels are representative of at least 5 fields in 5 tissue sections taken from 5 mice. (E,F) Quantitative RT-PCR analysis of the expression of vitronectin in nonendothelial cells of PNET from RIP1-Tag2 mice treated or not with imatinib (E), and of B16/mock or B16/PDGFB tumors (F). *P < .05 vs B16/mock; ***P < .001 vs control. (G) Quantitative PCR analysis of expression of Bcl-w and VEGF-A in MS1 cells cultured on plastic or vitronectin. **P < .01 vs plastic.
The crosstalk between pericytes and ECs is mediated by integrin αv activity. (A,B) Quantitative RT-PCR analysis of Bcl-w (A) and VEGF-A (B) expression in MS1 endothelial cells cultured alone or together with HBVPs for 96 hours in the presence or absence of neutralizing antibodies against integrin αv. Data shown are representative of 2 independent experiments. **P < .01 vs MS1/HBVP coculture. (C) Quantification of the number of viable MS1 endothelial cells cultured alone or together with HBVPs for 96 hours with the addition of the cytotoxic drug vinblastine (50 ng/mL) in the presence or absence of neutralizing antibodies against integrin αv. **P < .01 vs vinblastine coculture. (D) Immunostaining of tissue sections from PNET of RIP1-Tag2 mice for the integrin αv ligand vitronectin (green) and the pericyte marker PDGFRβ (red). Cell nuclei (DAPI), blue. Original magnification, 400×. The panels are representative of at least 5 fields in 5 tissue sections taken from 5 mice. (E,F) Quantitative RT-PCR analysis of the expression of vitronectin in nonendothelial cells of PNET from RIP1-Tag2 mice treated or not with imatinib (E), and of B16/mock or B16/PDGFB tumors (F). *P < .05 vs B16/mock; ***P < .001 vs control. (G) Quantitative PCR analysis of expression of Bcl-w and VEGF-A in MS1 cells cultured on plastic or vitronectin. **P < .01 vs plastic.
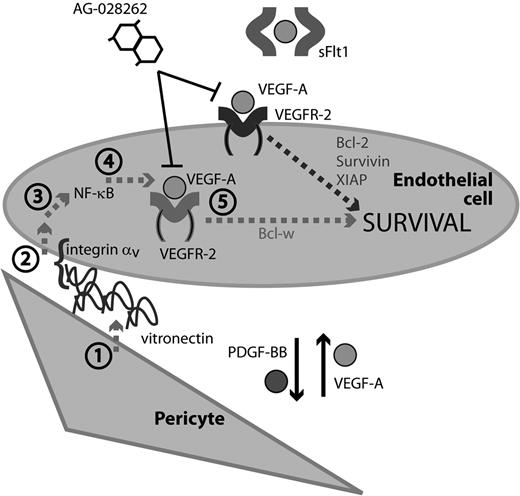
A model of pericyte-induced EC survival signaling. Endothelial cells and pericytes communicate through the reciprocal exchange of growth factors. PDGF-BB produced by ECs acts to recruit pericytes along growing vascular sprouts. In turn, pericytes confer survival advantage to ECs through PDGF-dependent secretion of vitronectin, (1) which through an integrin αv (2) and NF-κB (3) mediated signaling pathway results in up-regulation of intracrine VEGF-A signaling (4) and Bcl-w expression. (5) Previously described survival factors induced by the paracrine action of pericyte- or tumor cell-derived VEGF-A, such as survivin, Bcl-2, and XIAP, are presumably sensitive to the action of both intra- and extra-cellularly acting VEGFR inhibitors (AG-028262 and sFlt1, respectively), whereas the pericyte-induced autocrine signaling by VEGF-A is only sensitive to intra-cellularly acting inhibitors.
A model of pericyte-induced EC survival signaling. Endothelial cells and pericytes communicate through the reciprocal exchange of growth factors. PDGF-BB produced by ECs acts to recruit pericytes along growing vascular sprouts. In turn, pericytes confer survival advantage to ECs through PDGF-dependent secretion of vitronectin, (1) which through an integrin αv (2) and NF-κB (3) mediated signaling pathway results in up-regulation of intracrine VEGF-A signaling (4) and Bcl-w expression. (5) Previously described survival factors induced by the paracrine action of pericyte- or tumor cell-derived VEGF-A, such as survivin, Bcl-2, and XIAP, are presumably sensitive to the action of both intra- and extra-cellularly acting VEGFR inhibitors (AG-028262 and sFlt1, respectively), whereas the pericyte-induced autocrine signaling by VEGF-A is only sensitive to intra-cellularly acting inhibitors.
Discussion
Despite the tight association between the 2 cell types, little is known about the molecular nature of the crosstalk engaging pericytes and ECs during tissue homeostasis or neo-angiogenesis. Newly formed vascular sprouts recruit pericytes primarily through secretion of PDGF-BB by endothelial tip cells.32 Pericytes respond by migrating along the matrix-retained gradient of PDGF-BB, and by a proliferative burst as they reach the tip of the sprout.32,33 Reciprocally, pericytes up-regulate expression of cell-bound VEGF-A as they mature and are recruited into newly formed vessels.26 We found that pericytes, in addition to producing VEGF-A that acts in a paracrine fashion, stimulate autocrine expression of VEGF-A by tumor ECs, suggesting that the 2 different mechanisms for pericyte-dependent VEGF-A stimulation of ECs are functionally distinct. In agreement with this notion, mice harboring conditional ablation of Vegfa in ECs exhibit progressive endothelial degeneration despite normal levels of exogenously derived VEGF-A.25 Recently, EC expression of VEGF-A was found to be regulated in an integrin α3β1-dependent manner, implying that the composition of the vascular basement membrane modulates the phenotype of ECs.34 Similarly, we demonstrate here that blockade of integrin αv abolishes pericyte-induced control of autocrine VEGF-A and survival in tumor ECs. In the context of vascular maturation, it is interesting to note that the recruitment of pericytes to angiogenic sprouts is closely correlated to enhanced basement membrane production.35 We found that pericytes need to be in apposition to ECs to confer induction of VEGF-A and Bcl-w, possibly because of the requirement to deposit basement membrane products within close range of the EC. However, it may be that actual physical contact between pericytes and ECs is not needed in the context of tumor angiogenesis, as it has been noted that pericytes in malignant tissues are only loosely attached to the vasculature.36
More work is needed to conclusively identify the ligand(s) responsible for integrin αv ligation, but pericytes abundantly and selectively expressed vitronectin in the tumor models usedin our studies. Interestingly, adhesion of ECs to vitronectin through integrin αvβ3 protects from apoptosis in an NF-κB dependent manner,30,31 in strong concordance with our observation that pericyte association governs the transcriptional activity of direct NF-κB targets, including VEGF-A, in tumor ECs in vivo.27,28 Thus, by tying together previous independent observations of regulation of EC survival by vitronectin, integrin αvβ3, NF-κB, and autocrine VEGF-A, we identify pericytes as key orchestrators of tumor EC survival by triggering EC-matrix interactions.
Stimulation of ECs by extracellularly provided VEGF-A leads to the production of several antiangiogenic signaling proteins in parallel, including Bcl-2, survivin and XIAP37,38 (Figure 7). The primary cell-autonomous function of VEGF-A in ECs is thought to be promotion of survival, although the molecular mechanism has not been fully explored.25 We provide evidence that autocrine VEGF-A sustained by pericytes leads to induction of the antiapoptotic protein Bcl-w. Thus, survival signaling in ECs occurring in response to paracrine and autocrine stimulation with VEGF-A appears to be mediated through distinct downstream pathways, the identity of which should be the topic for further studies. Mice deficient for Bcl-w do not display any overt vascular abnormalities, and are alive and fertile.39 Whether the lack of Bcl-w in the knock-out mice sensitizes ECs to the action of antiangiogenic therapies, or whether regulation of other Bcl-family members compensates for the lack of Bcl-w in ECs, deserves renewed attention in light of our findings.
Vascular regression after exposure to hyperoxia leads to a selective ablation of immature, nonpericyte covered capillaries.40 Furthermore, several studies demonstrate detrimental effects of pericyte targeting through the use of PDGFR inhibitors on tumor EC survival in the face of antiangiogenic treatment regimens, resulting in therapeutic synergy.11-15 Indeed, combined treatment of RIP1-Tag2 mice with imatinib and metronomic, antiangiogenic chemotherapy results in a significant increase in tumor EC apoptosis.11 In addition to conferring protection from apoptosis to ECs, pericytes also control EC proliferation. Thus, ectopic expression of PDGF-BB in HT29 (colo-rectal carcinoma) or FG (pancreatic adenocarcinoma) xenografts inhibits angiogenesis and tumor growth by reducing EC proliferation, whereas mice lacking a proper pericyte coverage display vascular hyper-proliferation and/or dilation.33,41,42 These observations, together with the work presented here, are consistent with a model in which pericyte association governs the balance between a proliferative and a pro-survival phenotype of tumor ECs. In further agreement with this model, conditional repression of NF-κB activity in ECs, or suppression of autocrine VEGF-A expression in ECs consequent to deletion of integrin α3β1, results in an increased sensitivity to apoptosis and/or a hyper-proliferative vasculature in implanted tumors.34,43 Further studies to investigate whether modulation of pericyte recruitment or function can be used as a strategy to modify EC behavior during various pathologic settings, including ischemic disease and retinopathy are warranted. In this context it is important to note that the description of pericyte-dependent effects on the EC phenotype may vary depending on whether a pharmacologic or genetic approach is taken to reduce pericyte coverage of blood vessels; pharmacologic agents may have off-target effects or produce incomplete functional ablation, whereas germ line genetic defects may be subject to compensatory adaptations during embryogenesis.
To avoid the risk of inducing compensatory survival pathways in ECs, we chose to use pharmacologic tools to diminish pericyte association, with the corollary objective to gain additional insight into the clinical use of drugs incorporating inhibitory action against the PDGF and/or the VEGF system.
Antiangiogenic agents targeting VEGF/VEGFR signaling, including bevacizumab, sunitinib and sorafenib, have made their way into the clinic during recent years. Sunitinib and sorafenib are small molecule VEGFR inhibitors that include inhibitory action against PDGFR, thus encompassing dual pericyte and EC targeting capabilities in a single drug. The preclinical data presented herein, indicating that pericytes protect tumor ECs from apoptosis through induction of an autocrine VEGF-A signaling loop, lead to the prediction that inhibitors of the VEGF pathway acting intracellularly, such as sunitinib and sorafenib, would be superior to antibody therapy by bevacizumab, which acts exclusively on paracrine VEGF-A signaling. Nevertheless, the efficacies of bevacizumab, sunitinib and sorafenib are comparable in terms of prolongation of progression-free survival in renal cell carcinoma patients.44-46 It may be that off-target effects of the more broadly acting ATP-competitive small molecules mandate that they be dosed in a sub-optimal manner, thus giving rise to incomplete inhibition of VEGF signaling, whereas administration of an antibody with complete specificity for blockade of VEGF-A would result in a higher degree of signal inhibition. Thus, a treatment regimen combining inhibition of pericyte and EC function through the use of concomitant administration of a VEGF-targeting antibody and a PDGF-BB/PDGFRβ-targeting antibody may hold promise for improved efficacy by blocking both paracrine and autocrine VEGF signaling in ECs. In addition, our studies identify inhibition of NF-κB and Bcl-w as new approaches to improve antivascular therapy in malignant disease by interfering with specific aspects of EC-pericyte crosstalk. Given the recent pre-clinical reports of acquired resistance leading to increased metastatic dissemination after anti-VEGF treatment,5 our identification of pericyte-induced survival signaling pathways in tumor ECs delineate potential novel strategies for overcoming the impediments to realizing the full potential of antiangiogenic therapies against cancer.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Rune Toftgård, Karolinska Institutet, for providing the IκB-α super-repressor; Arne Östman, Karolinska Institutet, for providing the B16/PDGFB cells; and W. Gregory Roberts and Dana Hu-Lowe at Pfizer for providing CP-673,451 and AG-028262, respectively.
K.P. is the recipient of a Young Investigator Award from the Swedish Cancer Society. This work was supported by grants to K.P. from the Swedish Cancer Society, the Swedish Research Council (project # K2011-67X-21 865-01-6), the Swedish Childhood Cancer Society, KI Cancer network, Jeansson's foundation, Magn Bergvalls foundation, and Åke Wiberg's foundation. In addition, support was provided by a Linnaeus grant to the STARGET consortium from the Swedish Research Council, and by strategic funds to the BRECT network from Karolinska Institutet and Stockholm County.
Authorship
Contribution: M.F., E.C., P.R., and K.P. performed experiments; M.F. and K.P. designed the research and analyzed results; and M.F., P.R., D.H., and K.P. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Kristian Pietras, Karolinska Institutet Department of Medical Biochemistry and Biophysics, Stockholm, SE-171 77, Sweden; e-mail: kristian.pietras@ki.se.