Somatic mutations are important factors in tumorigenesis. In this issue of Blood, Zhang et al have identified recurrent mutations in key signaling pathways in high-risk childhood B-cell precursor acute lymphoblastic leukemia (BCP-ALL) to be exploited in the development of new therapeutic approaches.1
The treatment of childhood ALL is a success story of the past 40 years, with > 85% overall survival at 5 years. Nevertheless, 20% of patients will relapse. As survival after relapse is poor, it is important that those patients at high risk of relapse are identified at the time of diagnosis. Age, white blood cell count, minimal residual disease, and cytogenetics2 are currently the gold standard to determine the risk groups used to stratify patients for treatment. Authors from this paper by Zhang et al have pioneered the use of genomic technologies to revolutionize our understanding of the genetics of childhood ALL. Their initial study showed that deletions, amplifications, point mutations, and structural rearrangements in those genes encoding the principal regulators of B-lymphocyte development and differentiation were present in ∼ 40% of childhood BCP-ALL. These abnormalities lead to direct disruption of pathways controlling B-cell development and differentiation, thus contributing to BCP-ALL pathogenesis.3 Simultaneously, deletions of other significant genes were identified by others (see figure).4,5 Further, alterations of IKZF1 (which encodes the lymphoid transcription factor IKAROS) were shown to be a marker of poor prognosis in BCP-ALL.6,7 Associations were also found with mutations of JAK2 and overexpression of the cytokine receptor CRLF2, providing potential therapeutic targets.8-10 Authors from this paper by Zhang et al also used gene expression profiling to further resolve the genetic basis of high-risk disease.11 They defined 8 cluster groups (termed ROSE clusters) within which patients shared patterns of gene expression.
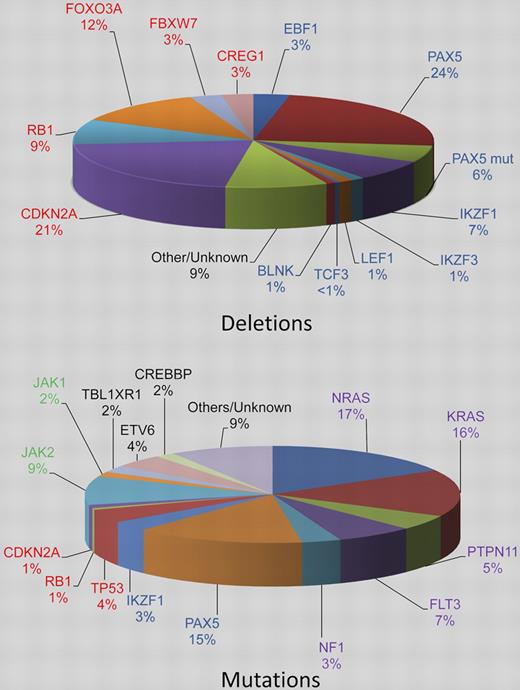
Pie charts estimating the relative incidences of deletions and somatic mutations in the key signaling pathways in childhood BCP-ALL. The genes are color coded according to the pathway to which they belong: B-cell differentiation and development, blue; TP53/RB1, red; Ras signaling, purple; JAK/STAT, green, noncanonical pathways and other/unknown genes, black. Deletions in the top chart are shown for the B-cell differentiation and development and TP53/RB1 pathways only and the percentages are estimated from data in 3 publications.3-5 The PAX5 mutation rate stated by Mullighan et al3 is given (PAX5 mut). The relative incidences of the mutations in the bottom chart are estimated from Zhang et al.1
Pie charts estimating the relative incidences of deletions and somatic mutations in the key signaling pathways in childhood BCP-ALL. The genes are color coded according to the pathway to which they belong: B-cell differentiation and development, blue; TP53/RB1, red; Ras signaling, purple; JAK/STAT, green, noncanonical pathways and other/unknown genes, black. Deletions in the top chart are shown for the B-cell differentiation and development and TP53/RB1 pathways only and the percentages are estimated from data in 3 publications.3-5 The PAX5 mutation rate stated by Mullighan et al3 is given (PAX5 mut). The relative incidences of the mutations in the bottom chart are estimated from Zhang et al.1
Somatic mutations affecting key genes involved in pathways relating to the development of BCP-ALL are known.12 However, for many of these mutations, the clinical and biologic significance, as well as their relationship to one another and other genetic changes, remain unknown. To begin to address these issues, Zhang et al have reported the first large-scale sequence analysis in ALL.1 They sequenced 120 selected candidate cancer genes in a cohort of 187 high-risk patients treated on the Children's Oncology Group P9906 protocol. The genes were selected on the basis that they were in pathways that were known to be involved in ALL or other cancers, either from genomic studies, expression profiling, or mutational analysis. Such a targeted approach precludes the finding of as yet unknown mutations in BCP-ALL or cancer in general, which might emerge from whole exome sequencing. However, selected coverage of the majority of known significant genes among a well-annotated cohort provides a rational basis on which to build further detailed studies. A total of 179 somatic mutations in 31 genes were identified, 19 of which were recurrently mutated in this patient cohort. However 11 of these 19 genes accounted for 81% of all mutations detected. The most frequently mutated genes were NRAS, KRAS, PAX5, and Janus kinases in > 10% of patients each. When these mutations were combined with copy number alterations,7 the following 4 known cancer signaling pathways—B-cell development and differentiation, Ras signaling, JAK/STAT signaling, and the TP53/RB1 tumor suppressor—were involved in 68%, 54%, 11%, and 54% of cases, respectively. In a subset of cases, multiple genes from the 4 individual signaling pathways were mutated. More surprising was the finding that the mutations of the Ras signaling pathway were not mutually exclusive; 5 patients had multiple mutations of NRAS or KRAS. These data suggest a strong selection for mutations within these signaling pathways and, as not all genes in these pathways were sequenced, these results likely provide an underestimate of the mutation rates in high-risk BCP-ALL. With the exception of the B-cell development and differentiation pathways, the frequency of alterations was higher in this high-risk patient cohort than unselected BCP-ALL patients.9,12 There was a striking difference in frequency of mutations within the 4 major pathways between the ROSE cluster groups. For example, virtually all patients in the R8 subgroup, which is associated with a high incidence of relapse, had mutations in the B-cell development and differentiation pathways. They had a higher frequency of mutations in TP53/RB1 and JAK pathways, while the rate of RAS mutations was lower than other subgroups. Notably, each ROSE cluster group was also characterized by distinct patterns of copy number alterations. IKZF1 deletions and rearrangements leading to CRLF2 overexpression were particularly prevalent and significantly associated with cluster group R8. Collectively, these findings indicate that the genetic profile, as defined by the differential gene expression patterns and genomic aberrations, contributes to patient outcome.
In addition to these mutations in key pathways, inactivating mutations were observed in other noncanonical pathways, including ETV6 and CREBBP. Using a similar targeted sequencing approach, authors from this paper by Zhang et al have recently reported the strong association between mutations in the histone acetyltransferase, CREBBP, and relapsed BCP-ALL.13 These findings endorse the value of comprehensive evaluation of sequence alterations toward yielding additional biologic insights into this disease.
This study has extended the prior knowledge of the genetics of high-risk childhood BCP-ALL. It has begun to decipher the interrelationships between different genetic abnormalities and place them into clinical context. Sequencing of the entire coding genome is likely to add to these findings, while the study of unselected patient cohorts is required to fully establish the clinical relevance. In the meantime, this demonstration that the genetic basis of high-risk BCP-ALL is truly multifactorial has highlighted potential novel therapeutic approaches, for example, targeting of the Ras signaling pathway.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal