Abstract
Follicular lymphoma in situ (FLIS) was first described nearly a decade ago, but its clinical significance remains uncertain. We reevaluated our original series and more recently diagnosed cases to develop criteria for the distinction of FLIS from partial involvement by follicular lymphoma (PFL). A total of 34 cases of FLIS were identified, most often as an incidental finding in a reactive lymph node. Six of 34 patients had prior or concurrent FL, and 5 of 34 had FLIS composite with another lymphoma. Of patients with negative staging at diagnosis and available follow-up (21 patients), only one (5%) developed FL (follow-up: median, 41 months; range, 10-118 months). Follow-up was not available in 2 cases. Fluorescence in situ hybridization for BCL2 gene rearrangement was positive in all 17 cases tested. PFL patients were more likely to develop FL, diagnosed in 9 of 17 (53%) who were untreated. Six patients with PFL were treated with local radiation therapy (4) or rituximab (2) and remained with no evidence of disease. FLIS can be reliably distinguished from PFL and has a very low rate of progression to clinically significant FL. FLIS may represent the tissue counterpart of circulating t(14;18)-positive B cells.
Introduction
Follicular lymphoma (FL) is the second most common non-Hodgkin lymphoma in Western countries, with an incidence of 2.6 per 100 000 and median age in the 6th decade,1,2 and comprises approximately 20% of all lymphomas.3 It is slightly more common in females, and the majority of patients present with advanced-stage disease.2 Generally considered to be an incurable disease, a “watchful waiting” approach of conservative management has been advocated in most cases, deferring treatment until symptoms develop.4,5 With localized disease, however, the management of FL is more controversial. Whereas some recommend radiation therapy, with or without chemotherapy, for potential cure,6,7 other studies reported no benefit from early intervention in stage I and II FL.8 Refinement of optimal treatment strategies will depend, in part, on our understanding of the pathogenesis and natural history of FL.
One of the earliest events in follicular lymphomagenesis is thought to occur in B-cell precursors developing in the bone marrow, giving rise to the t(14;18)(q32;q21) translocation because of erroneous VDJ recombination.9 This translocation juxtaposes the BCL2 gene on chromosome 18 to the immunoglobulin heavy chain gene locus, resulting in overproduction of the antiapoptotic BCL2 protein. Observed in ∼ 85% of FLs, this cytogenetic abnormality has been demonstrated through highly sensitive PCR assays to also be present at extremely low frequencies (∼ 1 in 105) in circulating B cells from healthy persons, up to 67% depending on the study, as well as in benign lymphoid tissue.10-17 The frequency of cells carrying the translocation appears to increase with age, smoking,18 and pesticide exposure,19 and such cells may be less common in ethnic groups with a lower incidence of FL.20 Furthermore, sequence analysis has demonstrated persistence and evolution of a single dominant clone or multiple t(14;18)-carrying B-cell clones over several years within a given person.21-23 An increased incidence of FL in such persons has yet to be demonstrated through prospective analysis.
Although the t(14;18)(q32;q21) chromosomal translocation is thought to occur in B-cell precursors, FL is invariably a mature B-cell neoplasm. Because normal germinal centers in lymphoid tissue lack BCL2, immunohistochemistry for this marker is valuable in diagnosing FL. In 2002, we described a series of 25 cases demonstrating abnormal BCL2 expression in follicle centers, associated with fairly preserved tissue architecture and residual reactive germinal centers.24 The term follicular lymphoma in situ (FLIS) has been proposed for this pattern of in situ localization because the cells exhibited a propensity to be confined to the germinal centers, without evidence of disseminated disease in most patients. Since then, a few case reports and a series of 13 cases have been described.25-30 The differential diagnosis of FLIS includes partial involvement by FL (PFL), and one study concluded that PFL was associated with low-stage disease.31 However, standardized criteria for the distinction of FLIS and PFL have not been applied routinely in the literature.
Since our original report, we have expanded our experience with FLIS and developed histologic criteria that can be applied in diagnostic biopsies for the distinction of FLIS and PFL.24,32 Moreover, for many cases, we have extended follow-up, allowing an assessment of the clinical implications of the FLIS lesion and the risk for subsequent FL.
Methods
Case selection
Cases were selected from biopsy specimens submitted to the Hematopathology Section at the National Cancer Institute for consultation, from 1992 through 2008. The pathology database was screened for cases that had been interpreted (by E.S.J. or S.P.) as FLIS, or focal or partial involvement by FL, grade 1 or 2 (PFL). Twenty-one of the 25 cases from the original series spanning 1992 to 2000 had slides available for reevaluation and data regarding clinical outcome.24 An additional 72 cases with a consideration of FLIS or PFL were identified from 2000 through 2008, resulting in a total of 93 cases for review. The final cohort of cases included 34 FLIS and 23 PFL; cases with PFL without follow-up were excluded.
Only cases of FL, grade 1 or 2, were eligible for the study. All cases were preserved as formalin-fixed paraffin-embedded tissue specimens. Clinical information, including follow-up, was obtained from the referring physicians when possible. The study was approved by the National Cancer Institute Institutional Review Board.
Histology and immunohistochemistry
All cases were rereviewed by A.G.J. and E.S.J. without knowledge of the clinical follow-up, and classified as FLIS or PFL according to the criteria outlined in “Histopahology and immunochemistry of FLIS and PFL.” In addition to review of hematoxylin and eosin-stained sections, immunohistochemistry was performed on deparaffinized, formalin-fixed tissue sections using a panel of antibodies: CD20 (L26), CD3 (F7.2.38), BCL2 (124), BCL6 (PG-B6p), IgD, MIB-1/Ki-67 (Dako); CD10 (58C6), Vision Biosystems; and BCL2 (E17), Epitomics (selected cases). Staining was conducted on an automated immunostainer (Ventana Medical Systems), with antigen retrieval and antibody dilutions per manufacturer's recommendations. Scoring of BCL2+ follicles was performed as described previously,24 according to absolute numbers of follicles involved (1 indicates < 5; 2, 6-10; and 3, > 10) as well as the relative proportion of follicles involved (A indicates less than half of all follicles; and B, half or more than half of all follicles). Photomicrographic images were acquired with a Nikon Eclipse 50i microscope equipped with an Olympus (Olympus America Inc) DP71 camera and software. Objectives used were: 2×/0.1 numeric aperture (NA), 4×/0.2 NA, 10×/0.45 NA, 20×/0.75 NA, and 40×/0.95 NA. Final image preparation was performed with Adobe Photoshop CS4 extended Version 11.0.2.
PCR
PCR amplification for detecting monoclonal immunoglobulin heavy (IGH@) chain gene rearrangement was performed in a subset of cases using consensus primers directed against the joining region and framework 3 (FRIII) variable region, and against the joining region and framework 2 (FRII) region, according to the method of Ramasamy et al.33 The products were analyzed on 16% polyacrylamide gels and stained with ethidium bromide. PCR for the t(14;18) translocation was performed using primers for BCL2 and JH, as described previously.24
FISH
Fluorescence in situ hybridization (FISH) analysis was performed using the BCL2 FISH DNA Probe, Split Signal (Dako North America) on paraffin-embedded sections using methods previously described.34 Each case was interpreted as positive for a BCL2 translocation if a break-apart signal was observed in > 10% of nuclei in 3 different high-power fields. All other signal constellations were regarded as negative for BCL2 gene rearrangement. A parallel section stained for BCL2 protein was examined in tandem, with scoring performed on both BCL2-positive and BCL2-negative follicles, to ascertain the distribution of cells with the translocation.
Digital acquisition of FISH images was done using a Zeiss Imager.Z2 fluorescence microscope (Carl Zeiss), ASI CCD camera CCD-1300QDS (Applied Spectral Imaging) and a Dell Precision T3500 computer. Image acquisition was done using the Case Data Manager Software (Applied Spectral Imaging); 40×/0.75 objective, 10× oculars with the use of filters specific for 4,6-diamidino-2-phenylindole, fluorescein, and rhodamine (TR1, TR2, and TR3; Chroma Technologies). Final image preparation was performed with Adobe Photoshop CS4 extended Version 11.0.2.
Results
Histopathology and immunohistochemistry of FLIS and PFL cases
FLIS and PFL may have similar features, with reactive background germinal centers present in the lesional tissue. Table 1 summarizes the criteria that were used to distinguish FLIS from PFL. In FLIS, the lymphoid architecture was intact, with patent sinuses, preserved paracortex/interfollicular regions, and scattered follicles with well-defined mantle zones. Follicles involved by FLIS were mainly identified through the use of immunohistochemical staining for BCL2 and CD10. Involved follicles were widely scattered in the lymph node (LN) and of normal size. The BCL2/ CD10+ cells, uniformly centrocytes, were confined to the germinal centers, and did not always replace the entire follicle center (Figure 1). Both BCL2 and CD10 were strongly expressed, and there was no evidence of interfollicular infiltration. The involved follicles had a low proliferation fraction, as determined by Ki-67 immunohistochemistry. The degree of involvement by FLIS within the biopsy tissue varied and was scored accordingly (Tables 2–3).24
Diagnostic features of FLIS and PFL
| FLIS . | PFL . |
|---|---|
| Architecture intact | Altered architecture |
| Follicle size normal | Follicle size often expanded |
| Involved follicles widely scattered | Involved follicles grouped together in LN |
| Intact cuff with sharp edge to GC | Blurred edge to GC and attenuated cuff |
| Very strong expression of BCL2 and CD10 | BCL2 and CD10 more variable in intensity |
| Almost pure centrocytes | Centrocytes with few centroblasts |
| Atypical cells confined to GC | Atypical cells (CD10+/BCL2+ B cells) may be found outside the GC |
| FLIS . | PFL . |
|---|---|
| Architecture intact | Altered architecture |
| Follicle size normal | Follicle size often expanded |
| Involved follicles widely scattered | Involved follicles grouped together in LN |
| Intact cuff with sharp edge to GC | Blurred edge to GC and attenuated cuff |
| Very strong expression of BCL2 and CD10 | BCL2 and CD10 more variable in intensity |
| Almost pure centrocytes | Centrocytes with few centroblasts |
| Atypical cells confined to GC | Atypical cells (CD10+/BCL2+ B cells) may be found outside the GC |
GC indicates germinal center.
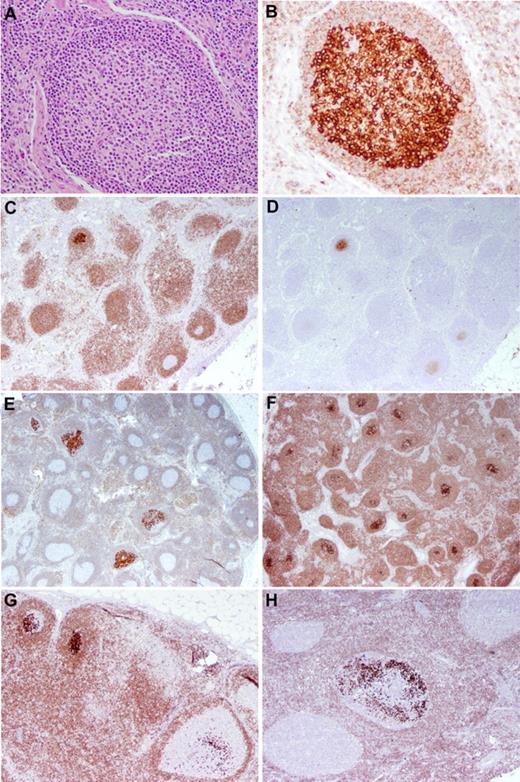
FLIS. (A-B) H&E-stained (A) and BCL2-stained (B) sections of LN showing replacement of a germinal center by centrocytes with uniformly intense positivity for BCL2. The surrounding mantle cuff is intact and shows relatively weak BCL2 positivity. Original magnification ×200. (C-D) FLIS in an LN stained for BCL2 (C) and CD10 (D). The single involved follicle is strongly positive for BCL2 and CD10. Mantle zone B cells and T cells show less intense BCL2 positivity. Normal germinal centers are BCL2− and only dimly CD10+. Original magnification ×40. (E-G) Several examples of FLIS patterns. (E-F) LNs with overall intact architecture but with the minority (E) or majority (F) of follicle centers involved by FLIS. Original magnification ×20. The individual follicles exhibit various degrees of involvement by FLIS cells (G), and a similar pattern is seen in a case from a patient who had a prior history of follicular lymphoma (H; case 23 in Table 3). Original magnification ×40.
FLIS. (A-B) H&E-stained (A) and BCL2-stained (B) sections of LN showing replacement of a germinal center by centrocytes with uniformly intense positivity for BCL2. The surrounding mantle cuff is intact and shows relatively weak BCL2 positivity. Original magnification ×200. (C-D) FLIS in an LN stained for BCL2 (C) and CD10 (D). The single involved follicle is strongly positive for BCL2 and CD10. Mantle zone B cells and T cells show less intense BCL2 positivity. Normal germinal centers are BCL2− and only dimly CD10+. Original magnification ×40. (E-G) Several examples of FLIS patterns. (E-F) LNs with overall intact architecture but with the minority (E) or majority (F) of follicle centers involved by FLIS. Original magnification ×20. The individual follicles exhibit various degrees of involvement by FLIS cells (G), and a similar pattern is seen in a case from a patient who had a prior history of follicular lymphoma (H; case 23 in Table 3). Original magnification ×40.
Scoring system of involved follicles in FLIS and PFL
| Absolute number of involved follicles |
| 1: < 5 |
| 2: 6-10 |
| 3: > 10 |
| Relative proportion of involved follicles |
| A: < 50% |
| B: ≥ 50% |
| Absolute number of involved follicles |
| 1: < 5 |
| 2: 6-10 |
| 3: > 10 |
| Relative proportion of involved follicles |
| A: < 50% |
| B: ≥ 50% |
Histologic scoring, clinical features, and follow-up of FLIS cases
| Case no. . | Age, y/sex . | Site/FLIS . | Degree of involvement by FLIS* . | Site of recurrence . | Time to FL, months† . |
|---|---|---|---|---|---|
| 20 cases with no other evidence of lymphoma | |||||
| 18 | 63/F | Thyroid, thyroiditis | 1A | — | NEL (102) |
| 2‡ | 37/M | LN, L inguinal | 1A | — | NEL (111) |
| 3‡ | 41/F | LN, L axillary | 1A | — | NEL (73) |
| 4 | 61/M | LN, R submandibular | 1A | — | NEL (79) |
| 5 | 73/F | LN, L inguinal | 1A | — | NEL (33) |
| 6 | 50/F | LN, R inguinal | 1A | — | NEL (16) |
| 7‡ | 53/F | LN, R axillary | 2A | — | NEL (74) |
| 8‡ | 23/F | LN, L inguinal | 2B | — | NEL (96) |
| 9‡ | 52/F | LN, L axillary | 2B | — | NEL (19) |
| 10 | 48/F | LN, L parotid | 2B | — | NEL (41) |
| 11 | 59/M | LN, pretracheal | 2B | — | NEL (3)§ |
| 12 | 53/F | LN, R cervical | 3A | — | NEL (48) |
| 13 | 51/M | LN, R chest | 3B | — | NEL (118) |
| 14‡ | 52/F | LN, R cervical | 3B | — | NEL (84) |
| 15 | 48/F | LN, supraclavicular | 3B | — | NEL (30) |
| 16 | 69/M | LN, mesenteric | 3B | — | NEL (12) |
| 17 | 74/F | LN, mesenteric | 3B | — | NEL (15) |
| 18 | 23/F | LN, mesenteric | 3B | — | NEL (12) |
| 19 | 42/F | LN, R axillary | 3B | — | NEL (10) |
| 20 | 31/M | Jejunum LN, mesenteric | 1B (jejunum) 3B (LN) | — | NEL (57) |
| 1 case with subsequent development of FL | |||||
| 21‡ | 76/M | LN, R inguinal | 1A | FL, low-grade/LN, R inguinal | 29 |
| 3 cases with prior or coexisting FL (BCL2+) | |||||
| 22‡ | 42/M | LN, R cervical | 1A | FL, low-grade/LN, R cervical | 0 |
| 23 | 45/M | LN, NOS | 1A | Prior FL, site unknown | 0 |
| 24 | 49/F | LN, retroperitoneal | 3A | Prior FL, low-grade/LN, L axillary | 0 |
| 2 cases with no available follow-up | |||||
| 25 | 60/M | LN, submental | 1A | — | NA |
| 26 | 42/M | LN, R thigh | 2A | — | NA |
| 3 cases with BCL2-negative FL | |||||
| 27 | 74/F | LN, L parotid | 1A | — | 0 |
| 28 | 51/M | LN, L parotid | 1A | — | 0 |
| 29 | 57/F | LN, L cervical | 1A | — | 0‖ |
| 5 cases with miscellaneous composite lymphoma | |||||
| 30 | 70/F | LN, L scalene | 1A | CLL/SLL κ¶ | DLBCL (48)¶ |
| 31‡ | 53/M | LN, bilateral pelvic | 2A | CLL/SLL | NEFL (108)# |
| 32 | 41/M | LN, L axillary | 3B | Interfollicular cHL | NEFL (21)** |
| 33 | 48/F | LN, L cervical, R axillary | 3B | Nodal marginal zone lymphoma | NEL (18)†† |
| 34‡ | 63/M | LN, scalene | 2B | LPL | NEFL (40) |
| Case no. . | Age, y/sex . | Site/FLIS . | Degree of involvement by FLIS* . | Site of recurrence . | Time to FL, months† . |
|---|---|---|---|---|---|
| 20 cases with no other evidence of lymphoma | |||||
| 18 | 63/F | Thyroid, thyroiditis | 1A | — | NEL (102) |
| 2‡ | 37/M | LN, L inguinal | 1A | — | NEL (111) |
| 3‡ | 41/F | LN, L axillary | 1A | — | NEL (73) |
| 4 | 61/M | LN, R submandibular | 1A | — | NEL (79) |
| 5 | 73/F | LN, L inguinal | 1A | — | NEL (33) |
| 6 | 50/F | LN, R inguinal | 1A | — | NEL (16) |
| 7‡ | 53/F | LN, R axillary | 2A | — | NEL (74) |
| 8‡ | 23/F | LN, L inguinal | 2B | — | NEL (96) |
| 9‡ | 52/F | LN, L axillary | 2B | — | NEL (19) |
| 10 | 48/F | LN, L parotid | 2B | — | NEL (41) |
| 11 | 59/M | LN, pretracheal | 2B | — | NEL (3)§ |
| 12 | 53/F | LN, R cervical | 3A | — | NEL (48) |
| 13 | 51/M | LN, R chest | 3B | — | NEL (118) |
| 14‡ | 52/F | LN, R cervical | 3B | — | NEL (84) |
| 15 | 48/F | LN, supraclavicular | 3B | — | NEL (30) |
| 16 | 69/M | LN, mesenteric | 3B | — | NEL (12) |
| 17 | 74/F | LN, mesenteric | 3B | — | NEL (15) |
| 18 | 23/F | LN, mesenteric | 3B | — | NEL (12) |
| 19 | 42/F | LN, R axillary | 3B | — | NEL (10) |
| 20 | 31/M | Jejunum LN, mesenteric | 1B (jejunum) 3B (LN) | — | NEL (57) |
| 1 case with subsequent development of FL | |||||
| 21‡ | 76/M | LN, R inguinal | 1A | FL, low-grade/LN, R inguinal | 29 |
| 3 cases with prior or coexisting FL (BCL2+) | |||||
| 22‡ | 42/M | LN, R cervical | 1A | FL, low-grade/LN, R cervical | 0 |
| 23 | 45/M | LN, NOS | 1A | Prior FL, site unknown | 0 |
| 24 | 49/F | LN, retroperitoneal | 3A | Prior FL, low-grade/LN, L axillary | 0 |
| 2 cases with no available follow-up | |||||
| 25 | 60/M | LN, submental | 1A | — | NA |
| 26 | 42/M | LN, R thigh | 2A | — | NA |
| 3 cases with BCL2-negative FL | |||||
| 27 | 74/F | LN, L parotid | 1A | — | 0 |
| 28 | 51/M | LN, L parotid | 1A | — | 0 |
| 29 | 57/F | LN, L cervical | 1A | — | 0‖ |
| 5 cases with miscellaneous composite lymphoma | |||||
| 30 | 70/F | LN, L scalene | 1A | CLL/SLL κ¶ | DLBCL (48)¶ |
| 31‡ | 53/M | LN, bilateral pelvic | 2A | CLL/SLL | NEFL (108)# |
| 32 | 41/M | LN, L axillary | 3B | Interfollicular cHL | NEFL (21)** |
| 33 | 48/F | LN, L cervical, R axillary | 3B | Nodal marginal zone lymphoma | NEL (18)†† |
| 34‡ | 63/M | LN, scalene | 2B | LPL | NEFL (40) |
— indicates not applicable; NOS, not otherwise specified; DLBCL, diffuse large B-cell lymphoma; NEFL, no evidence of follicular lymphoma; cHL, classic Hodgkin lymphoma; and NEL, no evidence of lymphoma.
Described in “Histology and immunochemistry” and Table 2.
Unless otherwise indicated, all patients were treated with excision and observation only.
Case included in original series.24
Patient died of germ cell tumor.
Patient treated with radiation, with no evidence of disease 88 months later.
The CLL/SLL was κ-positive; the patient developed DLBCL 48 months after diagnosis, which was λ-positive.
Patient treated with rituximab with Cytoxan, hydroxyrubicin, Oncovin, and prednisone (R-CHOP) chemotherapy.
Patient treated with adriamycin, bleomycin, vinblastine, and doxorubicin (ABVD) and radiation therapy.
Patient treated with rituximab.
For the purposes of this study, all cases that were categorized as PFL had < 25% of the LN involved by lymphoma. In PFL, the involved follicles were typically larger than uninvolved follicles within the same LN (Figure 2). The mantle zones were sometimes attenuated or disrupted, and the margin between the follicle center and the cuff was often blurred. Involved follicles tended to be grouped in one portion of the LN, with other regions uninvolved. Follicles sometimes extended beyond the LN capsule. By immunohistochemistry, staining for BCL2 and CD10 was generally variable and less intense than that seen in FLIS. In addition, single cells positive for CD10 and strongly positive for BCL2 were sometimes observed in the interfollicular regions. Whereas all cases of PFL were grade 1 or 2, centroblasts were sometimes observed in affected follicles.
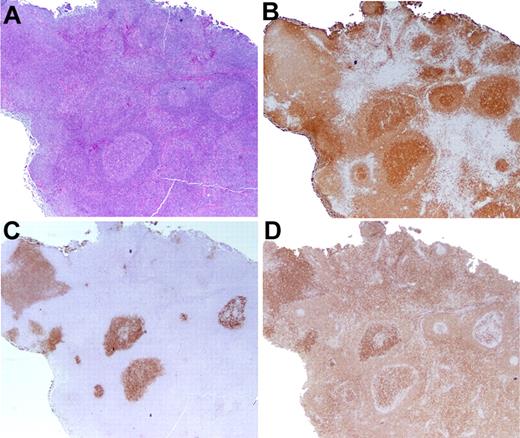
Partial LN involvement by FL. (A-B) In distinction from FLIS, the follicles are expanded in size (A), and clustered in 1 portion of the LN, as highlighted by CD20 immunohistochemistry (B). (C) CD10 staining shows that the margins of the atypical follicles are slightly blurred and not sharply defined. (D) The BCL2 stain is more variable in intensity than is typical for FLIS. Original magnification ×20.
Partial LN involvement by FL. (A-B) In distinction from FLIS, the follicles are expanded in size (A), and clustered in 1 portion of the LN, as highlighted by CD20 immunohistochemistry (B). (C) CD10 staining shows that the margins of the atypical follicles are slightly blurred and not sharply defined. (D) The BCL2 stain is more variable in intensity than is typical for FLIS. Original magnification ×20.
Based on these criteria, we reevaluated cases from our original series24 and reassigned them to these more specific categories. Eleven cases, including 2 composite with either chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) or lymphoplasmacytic lymphoma, were designated as FLIS (Table 3); 9 cases were designated as PFL (Table 4). One case was reclassified as pediatric type FL, grade 3, with partial involvement, and was not further evaluated.
Histologic scoring and clinical features of PFL cases with available follow-up
| Case no. . | Age, y/sex . | Site/PFL . | Degree of involvement by PFL* . | Treatment . | Time to FL or NEL, mo . |
|---|---|---|---|---|---|
| Cases with follow-up; not treated | |||||
| 1† | 45/F | LN, R inguinal | 1A | — | FL; LN, R inguinal (3) |
| 2 | 67/M | LN, L inguinal | 2A | — | NEL (60) |
| 3 | 76/M | LN, cervical | 2A | — | NEL (28) |
| 4† | 56/F | LN, L axillary | 3A | — | FL; LN, R axillary (13) |
| 5† | 44/M | LN, inguinal | 3A | — | B-cell lymphoma (60) |
| 6† | 41/M | LN, R axillary and L cervical | 3B | — | FL; ST, L cheek (72) |
| 7† | 75/F | LN, R axillary | 3B | — | NEL (6)‡ |
| 8 | 30/F | LN, occipital | 3B | — | FL; LN, occipital (6) |
| 9 | 56/F | LN, L cervical | 3B | — | NEL (53) |
| 10 | 48/F | LN, mesenteric | 3B | — | NEL (32) |
| 11 | 74/M | LN, R cervical | 3B | — | NEL (31) |
| 12 | 41/F | LN, R inguinal | 3B | — | NEL (10) |
| 13 | 62/F | LN, R axillary | 3B | — | NEL (13) |
| 14† | 56/M | LN, R inguinal | 3B | — | FL, LN, inguinal (3) |
| 15† | 72/M | LN, cervical | 3B | — | FL, LN parotid (0) |
| 16† | 65/F | LN, hilar | 3B | — | FL, LN, pulmonary (0) |
| 17† | 58/F | LN, R inguinal | 3B | — | FL, LN, supraclavicular (0) |
| Cases with follow-up; treated | |||||
| 18 | 57/F | LN, L cervical | 1A | Rituximab | NEL (66) |
| 19 | 75/M | ST, L occipital | 3A | XRT | NEL (86) |
| 20 | 39/F | LN, cervical | 3A | XRT | NEL (85) |
| 21 | 74/F | LN, mesenteric | 3B | Rituximab | NEL (37) |
| 22 | 40/M | LN, R inguinal | 3B | XRT | NEL (36) |
| 23 | 47/M | Tongue base | 3B | XRT | NEL (6) |
| Case no. . | Age, y/sex . | Site/PFL . | Degree of involvement by PFL* . | Treatment . | Time to FL or NEL, mo . |
|---|---|---|---|---|---|
| Cases with follow-up; not treated | |||||
| 1† | 45/F | LN, R inguinal | 1A | — | FL; LN, R inguinal (3) |
| 2 | 67/M | LN, L inguinal | 2A | — | NEL (60) |
| 3 | 76/M | LN, cervical | 2A | — | NEL (28) |
| 4† | 56/F | LN, L axillary | 3A | — | FL; LN, R axillary (13) |
| 5† | 44/M | LN, inguinal | 3A | — | B-cell lymphoma (60) |
| 6† | 41/M | LN, R axillary and L cervical | 3B | — | FL; ST, L cheek (72) |
| 7† | 75/F | LN, R axillary | 3B | — | NEL (6)‡ |
| 8 | 30/F | LN, occipital | 3B | — | FL; LN, occipital (6) |
| 9 | 56/F | LN, L cervical | 3B | — | NEL (53) |
| 10 | 48/F | LN, mesenteric | 3B | — | NEL (32) |
| 11 | 74/M | LN, R cervical | 3B | — | NEL (31) |
| 12 | 41/F | LN, R inguinal | 3B | — | NEL (10) |
| 13 | 62/F | LN, R axillary | 3B | — | NEL (13) |
| 14† | 56/M | LN, R inguinal | 3B | — | FL, LN, inguinal (3) |
| 15† | 72/M | LN, cervical | 3B | — | FL, LN parotid (0) |
| 16† | 65/F | LN, hilar | 3B | — | FL, LN, pulmonary (0) |
| 17† | 58/F | LN, R inguinal | 3B | — | FL, LN, supraclavicular (0) |
| Cases with follow-up; treated | |||||
| 18 | 57/F | LN, L cervical | 1A | Rituximab | NEL (66) |
| 19 | 75/M | ST, L occipital | 3A | XRT | NEL (86) |
| 20 | 39/F | LN, cervical | 3A | XRT | NEL (85) |
| 21 | 74/F | LN, mesenteric | 3B | Rituximab | NEL (37) |
| 22 | 40/M | LN, R inguinal | 3B | XRT | NEL (36) |
| 23 | 47/M | Tongue base | 3B | XRT | NEL (6) |
We also identified 23 more recently diagnosed cases of FLIS. Three FLIS cases were seen in association with BCL2− FL that more extensively involved the LN (Figure 3). In one case in which additional sections were available for further studies (case 28), the BCL2− FL was positive with an alternative antibody to BCL2, the E17 clone.35 Three of the newly recognized FLIS cases were composite with another non-FL lymphoma subtype.
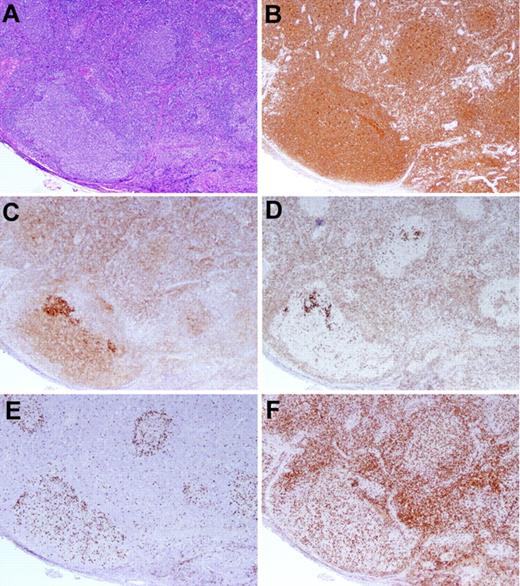
BCL2− FL with an in situ FLIS component. (A-B) The LN exhibits a proliferation of large follicles lacking polarization, highlighted by the CD20 stain (B). (C-D) A CD10 stain (C) demonstrates more intense staining of the FLIS component than the FL component, and the BCL2 stain (D) highlights the intensely staining FLIS component, whereas the FL is BCL2−. (E-F) A MIB-1 stain (E) identifies a low proliferation rate within the abnormal follicle, and a CD3 stain (F) reveals admixed T cells. Original magnification ×40. Case 29 in Table 3.
BCL2− FL with an in situ FLIS component. (A-B) The LN exhibits a proliferation of large follicles lacking polarization, highlighted by the CD20 stain (B). (C-D) A CD10 stain (C) demonstrates more intense staining of the FLIS component than the FL component, and the BCL2 stain (D) highlights the intensely staining FLIS component, whereas the FL is BCL2−. (E-F) A MIB-1 stain (E) identifies a low proliferation rate within the abnormal follicle, and a CD3 stain (F) reveals admixed T cells. Original magnification ×40. Case 29 in Table 3.
In addition to the 9 cases reclassified as PFL, we identified 14 newer cases of PFL, in which clinical follow-up was available. Of the 23 total cases of PFL, one presented in Waldeyer ring, and the remainder involved LNs. Two cases involved mesenteric LNs, 1 involved mediastinal LNs, but all others presented with peripheral adenopathy. All cases of PFL were grade 1 or 2 in the World Health Organization classification.32
FLIS composite with a disparate lymphoma subtype
In addition to the 2 composite lymphomas included in the original series (1 CLL/SLL; 1 lymphoplasmacytic lymphoma), we identified 3 newer composite cases in association with FLIS: 1 CLL/SLL (case 30), 1 interfollicular classic Hodgkin lymphoma (case 32), and 1 nodal marginal zone lymphoma (case 33). The classic Hodgkin lymphoma case was positive for Epstein-Barr virus by Epstein-Barr virus–encoded RNA in situ hybridization. In each of the composite lymphomas, the FLIS lesion was a minor component, involving only scattered germinal centers.
Clinical features of FLIS cases
Table 3 summarizes the clinical data on 34 FLIS cases, including presentation and follow-up. There were 19 females (56%). The majority presented in the fifth or sixth decade of life, and only 4 (12%) were younger than 40 years. The typical FLIS was an incidental finding in the setting of reactive follicular hyperplasia or another lymphoma (ie, composite cases). One patient (case 11) presented with a mediastinal mass, diagnosed as a germ cell tumor, but with FLIS incidentally discovered in a pretracheal LN. Almost all biopsies were of LN, with the exception of one case presenting in thyroid involved by marked lymphocytic thyroiditis. The BCL2+ cells, positive for the translocation by PCR, were confined to reactive follicles in the gland. One patient (case 20) had simultaneous involvement of mesenteric LN and jejunum, but no other evidence of lymphoma on clinical evaluation, and remains well at 57 months without therapy. Three of 4 patients with mesenteric LN involvement (cases 16-18, 20) presented with small bowel obstruction as the cause for surgical exploration and LN biopsy. For unclear reasons, all 4 mesenteric LNs were associated with a high score for FLIS involvement (3B).
In 26 cases, there was no other evidence of lymphoma in the LN involved by FLIS; and of these, 2 patients (8%) had a prior diagnosis of FL (cases 23 and 24). One additional patient (case 22) was found to have FL elsewhere at the time of presentation, in an adjacent cervical LN. Three cases (cases 27-29) had FLIS in association with BCL2 protein-negative FL more extensively involving the LN. The 2 components were not intermixed.
Among 21 patients without overt lymphoma before or at the time of diagnosis and with clinical follow-up available (median, 41 months; mean, 50.5 months; range, 10-118 months), only 1 eventually developed overt FL, 29 months later at the same anatomic site (case 21). Interestingly, this patient had a low score of FLIS involvement in the original biopsy (1A).
Clinical features of PFL cases
A total of 23 cases of PFL with available clinical follow-up were identified for comparison with FLIS. None of the patients was known to have a diagnosis of lymphoma at the time the biopsy was obtained (Table 4). On staging, 3 patients were found to have other LNs involved by FL at the time of diagnosis. Three additional patients had a positive LN biopsy within 6 months of the PFL diagnosis. Three more patients developed lymphoma at 13-72 months. In total, 9 of the 17 (53%) PFL patients who remained untreated developed evidence of FL. In 6 patients, the more fully involved LN was in the same LN region as the PFL biopsy. All patients with subsequent or concurrent FL at another site had FL, grade 1 or 2. Eight patients were managed expectantly and had no evidence of FL during the period of follow-up (median follow-up, 29.5 months; range, 6-72 months). Interestingly, none of the 6 patients who were treated, either with rituximab or radiation therapy, progressed to further disease (median follow-up, 51.5 months; range 6-86 months).
FISH studies for BCL2 in FLIS and FLIS composite cases
FISH for t(14;18) was performed for all cases of FLIS in which appropriate material for analysis was available (Table 5). Cases in which FISH could not be performed were as follows: (1) no additional material was available for FISH studies (10 cases), (2) tissue was B5-fixed (3 cases), or (3) BCL2+ follicles were lost in deeper sections (3 cases). Thirteen cases of FLIS in an otherwise reactive LN, 1 case of FLIS with BCL2− FL, and 4 cases of FLIS composite with another lymphoma were available for FISH analysis of the BCL2 gene. Technically successful analyses could be performed in 17 of 18 cases (94%). Positive results for t(14;18) involving the major breakpoint region were observed in 16 of 17 cases of FLIS (94%) with a satisfactory signal for evaluation (Figure 4). One case (case 16) did not show a translocation involving the major breakpoint region but was suspicious for t(14;18) involving the minor cluster region. Specifically, there were 2 wild-type fusion signals and an extra single red signal of a smaller size, suspicious for t(14;18) involving minor cluster region. Of note, evidence of a split in the BCL2 region by FISH was not detected in immunohistochemically BCL2− reactive follicles.
Evaluation of BCL2/IGH@ translocation in FLIS cases
| Case no. . | IGH@ rearrangement (PCR) . | BCL2/JH rearrangement (PCR) . | FISH (BCL2 break) . |
|---|---|---|---|
| 1* | ND | + | ND |
| 2* | ND | ND | + |
| 3* | − | + | + |
| 7* | − (LCM) | − (LCM) | + |
| 9* | − | − | + |
| 11 | ND | ND | Unsat |
| 13 | − | − | + |
| 16 | − | − | s/f mcr |
| 17 | − | + | + |
| 19 | ND | ND | + |
| 20 | ND | + | + |
| 21* | + (LCM) | − (LCM) | + |
| 24 | ND | − | + |
| 26* | ND | ND | + |
| 29 | ND | ND | + |
| 30 | ND | − | + |
| 32 | − | − | + |
| 33 | ND | ND | + |
| 34* | ND | + | + |
| Case no. . | IGH@ rearrangement (PCR) . | BCL2/JH rearrangement (PCR) . | FISH (BCL2 break) . |
|---|---|---|---|
| 1* | ND | + | ND |
| 2* | ND | ND | + |
| 3* | − | + | + |
| 7* | − (LCM) | − (LCM) | + |
| 9* | − | − | + |
| 11 | ND | ND | Unsat |
| 13 | − | − | + |
| 16 | − | − | s/f mcr |
| 17 | − | + | + |
| 19 | ND | ND | + |
| 20 | ND | + | + |
| 21* | + (LCM) | − (LCM) | + |
| 24 | ND | − | + |
| 26* | ND | ND | + |
| 29 | ND | ND | + |
| 30 | ND | − | + |
| 32 | − | − | + |
| 33 | ND | ND | + |
| 34* | ND | + | + |
ND indicates not done; +, positive; −, negative; LCM, area selected by laser capture microdissection; Unsat, technically unsatisfactory; and s/f mcr, suspicious for minor breakpoint cluster region.
Case included in original series.24
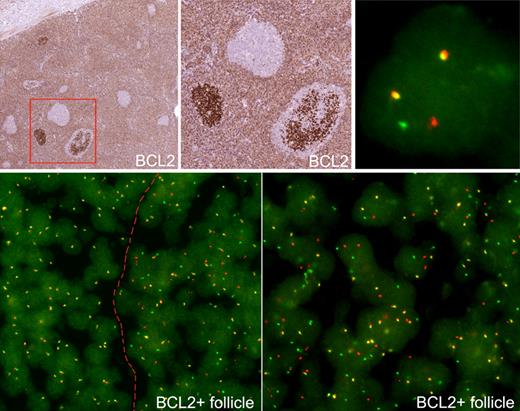
FISH of FLIS. (Top panels) Two FLIS follicles stained for BCL2. FISH was examined in follicular and interfollicular areas. Within the FLIS follicle, a cell shows a split red and green signal, indicating a BCL2 break (top right). (Bottom panels) Cells with split signals are seen only in involved follicle, to right of dotted line, and not in uninvolved areas, to left of dotted line. The involved follicle is shown at higher power (bottom right).
FISH of FLIS. (Top panels) Two FLIS follicles stained for BCL2. FISH was examined in follicular and interfollicular areas. Within the FLIS follicle, a cell shows a split red and green signal, indicating a BCL2 break (top right). (Bottom panels) Cells with split signals are seen only in involved follicle, to right of dotted line, and not in uninvolved areas, to left of dotted line. The involved follicle is shown at higher power (bottom right).
In one case of FLIS associated with BCL2− FL (case 29), a BCL2 break was observed in both the FLIS component and the BCL2− FL. Subsequent immunohistochemistry using an alternative antibody to BCL-2 (E17 clone) demonstrated BCL2 positivity in the FL component for this case.35 In all 4 cases of FLIS composite with a nonfollicular lymphoma, FISH analysis identified evidence of a BCL2 break restricted to the FLIS component.
Correlation of FISH results with PCR results for BCL-2/JH rearrangement and IGH rearrangement
In 12 cases of FLIS or FLIS composite lymphoma for which FISH had yielded positive results, PCR for BCL2/JH fusion was also performed. Of these, 4 (33%) were positive. PCR for IGH@ rearrangements showed a monoclonal pattern of IGH@ gene rearrangements in only 1 FLIS case (case 21), which had been microdissected to enrich for BCL2+ follicles. This corresponded to the one FLIS patient who subsequently developed FL (Table 3).
Discussion
The multistep pathway of tumorigenesis has parallels in most organ systems, best documented in the evolution of colonic adenocarcinoma.36 Histologic progression is a well-recognized feature of many lymphoid neoplasms, but the earliest events in lymphoid neoplasia are difficult to recognize. Indeed, the lymphoid system has no recognized “benign neoplasms, ” a fact that may be related to the propensity of lymphoid cells to circulate or home, and not remain confined to a single anatomic site.37 The 2008 World Health Organization classification recognized the increasing awareness of clonal expansions of B cells, or less often T cells, that appear to have limited potential for histologic or clinical progression.3 Monoclonal gammopathy of undetermined significance and monoclonal B-lymphocytosis are both clonal proliferations of B cells that appear to have a very limited capacity to progress to clinically significant disease.32 FLIS was first recognized in 2002,24 but we have had limited understanding of the risk for subsequent FL in these patients.
In addition, the borderlands and overlap between FLIS and LNs partially involved by FL have been ambiguous. We have reviewed cases from our files with a differential diagnosis of FLIS or PFL and applied newly developed criteria for their distinction. The criteria, when rigorously applied, appear to allow for ready distinction between these conditions. We also provide new insights regarding the clinical outcome of FLIS, identifying a very low risk for progression to FL, even lower than anticipated from our original series. This reduced risk is in part the result of a reclassification of some cases originally suspected as FLIS but reclassified when the new criteria were used by observers blinded to the clinical outcome. Of 24 cases in which FLIS was the only lesion identified in the LN biopsy, 3 patients (12%) were identified on further clinical evaluation to have FL at another site, either before or at the time of biopsy. One patient (4%) developed FL at 29 months, and 20 of 24 (83%) of patients did not develop evidence of FL during the period of follow-up (median, 41 months).
In 5 patients, FLIS was diagnosed in an LN containing a histologically unrelated type of lymphoma (composite). In all composite cases, however, the associated lymphoma was of B-cell lineage, including one case with interfollicular classic Hodgkin lymphoma. Because of the very focal nature of the FLIS lesions, we were unable to examine the clonal relationship between the FLIS component and the second B-cell lymphoma, although the histologic subtypes, CLL/SLL, lymphoplasmacytic lymphoma, and nodal marginal zone lymphoma, make it unlikely that they are clonally related. A lack of BCL2 gene translocation by FISH in the non-FL component of all tested cases is also in keeping with this notion. A relatively high incidence of FLIS was reported composite with other B-cell lymphomas in the series by Montes-Moreno et al.30 Five of their 13 cases were associated with another B-cell neoplasm. In addition, there are isolated case reports of 2 distinct in situ lymphomas in a single LN, both FLIS and in situ mantle cell lymphoma.29,38,39 These observations suggest that patients with FLIS may be at increased risk for B-cell neoplasms, possibly related to underlying genetic instability involving the IGH@ rearrangement process and B-cell development.
Interestingly, in our current report, 3 additional FLIS cases coexisted with FL negative for BCL2 protein. In a small proportion of FL negative for BCL2, BCL2 expression is not detected by immunohistochemistry because of mutations in the BCL2 gene.40 Other BCL2− FLs may lack the translocation. In one BCL2− FL composite with FLIS, we were able to confirm the presence of the t(14;18) by FISH in both components, but PCR amplification of the IG genes to examine a clonal relationship was unsuccessful. The BCL2− component was positive with the E17 monoclonal antibody, which detects an alternate epitope preserved in cases with acquired mutations in the BCL2 gene.35 Whether these are true composite lymphomas with 2 independent clones, or cases in which there is a secondary evolution in the original clone, remains to be determined. Molecular analysis of microdissected components of some composite low-grade B-cell lymphoma cases has shown unrelated clonal rearrangements.41
The diagnosis of FLIS is typically an incidental finding. The LNs are enlarged or biopsied secondary to reactive follicular hyperplasia or sometimes other pathology. One case had a LN biopsy after diagnosis of a germ cell tumor, and in 3 patients small bowel obstruction led to surgical exploration and LN biopsy. The high incidence of FLIS composite with other lymphomas also provides an explanation for LN biopsy in these patients and perhaps more likely detection of the FLIS lesion. Whether FLIS incidence is inherently increased in patients with other B-cell neoplasms requires further study.
Patients with LNs involved by PFL are at greater risk for a subsequent FL diagnosis than patients with only FLIS at diagnosis. At clinical staging or within 6 months of diagnosis, 6 of 17 (35%) patients with PFL developed FL, most often in the same LN region. Three additional PFL patients were diagnosed with FL between 13 and 72 months after the PFL biopsy. Interestingly, 6 patients with PFL treated with either local radiation therapy or rituximab remained free of clinically evident disease (median follow-up, 51.5 months; range, 6-86 months). In contrast, only 1 of 21 (5%) patients with FLIS and a negative workup for lymphoma at diagnosis developed FL. Thus, initial staging is most important in evaluating risk of subsequent FL in a patient with an LN showing FLIS.
Montes-Moreno et al, in the only other published series since our original report, identified subsequent or concurrent FL in 4 of 13 (31%) patients with FLIS/intrafollicular neoplasia.30 However, it is not clear what treatment, if any, the remaining 9 patients received after a diagnosis of FLIS because 5 were diagnosed with another subtype of B-cell lymphoma (3 patients) or classic Hodgkin lymphoma (2 patients). We conclude that conservative management of FLIS is advisable in cases where initial staging is negative for other sites of disease, as suggested by other authors.42 However, initial staging is probably indicated for all patients with FLIS because an identical histologic pattern may be seen in some patients with synchronous or prior FL (Figure 1H). In patients with overt FL at other sites, this phenomenon probably represents colonization of preexisting follicles by neoplastic FL cells, although even in this setting the presence of more than one clone carrying the BCL2/IGH@ translocation cannot be excluded.
FLIS most likely represents the tissue counterpart of FL-like B cells that have been described in the peripheral blood of healthy persons.22 A recent case report described the presence of identical BCL2/IGH@ fusion gene sequences in a mesenteric LN with a FLIS pattern and in peripheral blood B cells, with no evidence of disease progression after 2 years of observation.27 In FLIS, circulating t(14;18)-positive B cells may seed individual germinal centers and proliferate in an antigen-dependent manner. This hypothesis might explain why FLIS LNs typically show follicular hyperplasia in most of the specimens. The FLIS lesion itself is insufficient to produce LN enlargement. It may be that, in the setting of an immune response, increased trafficking of FL-like B cells leads to their accumulation in selected reactive germinal centers. Lending support to this theory is the fact that FL-like B cells increase in numbers in the peripheral blood in patients with active hepatitis C infection and decrease during periods of disease inactivity.22
The relatively low risk of progression to FL suggests that the BCL2+ B cells lack additional molecular alterations required for malignant transformation. Deregulated BCL2 expression alone does not appear to be sufficient for malignant transformation, as supported by experiments with BCL2/IGH@ transgenic mice, which develop lymphoma only after a long latency period and additional oncogenic events.43 FLIS shares some similarities with duodenal FL, a process associated with the t(14;18) that produces localized polypoid lesions in the duodenal mucosa with a very low risk of evolution to disseminated disease. In contrast to FLIS, the atypical cells in duodenal follicular lymphomas extend beyond the follicles into the adjacent lamina propria. The low risk of dissemination may be a consequence of dependence on antigen in the mucosal environment. A recent study showed that the t(14;18) was the sole karyotypic abnormality in 4 patients with duodenal FL in whom complete karyotypes could be performed.44
Like normal centrocytes, circulating t(14;18)-carrying B cells may accumulate somatic mutations in the variable genes and undergo antigen-driven clonal expansion in germinal centers.45,46 With their inherent survival advantage, they may amass additional genetic alterations, conferring malignant transformation. FL cells can also undergo extensive migration among follicles and trafficking between organs, leading to dissemination of malignant cells,47,48 before becoming clinically apparent. It has been proposed that the follicular growth pattern of FL may be a consequence of seeding of normal germinal centers by small numbers of malignant cells, which then undergo proliferation in a microenvironment-dependent manner.49 This may account for the PFL pattern, which has been associated with limited clinical stage (I/II) disease at presentation.31
In conclusion, we have proposed histologic and immunophenotypic criteria to distinguish PFL from FLIS. These criteria were applied by a panel of experts during a recent workshop on “Early Lesions in Lymphoid Neoplasia,” organized by the European Association of Hematopathology and the Society of Hematopathology, in Uppsala, Sweden, and were successfully applied to a series of cases submitted with a differential diagnosis of FLIS and PFL (Fend et al, manuscript in preparation). In the current study, PFL carried a higher risk of histologic progression in untreated patients; although somewhat surprisingly, some patients did not develop progressive disease within the period of follow-up. As strictly defined, FLIS appears to be associated with a very low rate of clinical progression, even if more than one site is involved. At the aforementioned workshop, the panel suggested that the term “FL-like B cells of undetermined significance” would be preferable for clinical usage and that the FLIS lesion alone does not meet currently accepted criteria for the diagnosis of lymphoma.50 This terminology has parallels with monoclonal gammopathy of undetermined significance and monoclonal B-cell lymphocytosis.
Presented in part at the 99th annual meeting of the United States and Canadian Academy of Pathology, Washington, DC, March 22, 2010.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute.
National Institutes of Health
Authorship
Contribution: A.G.J. and F.C.E. provided clinical, immunophenotypic, and genetic data, analyzed the results, and wrote the paper; S.D.P., M.M., M.R., and S.P. provided and analyzed data; and E.S.J. designed the research, provided and analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for A.G.J. is Department of Clinical Pathology, Cleveland Clinic, Cleveland, OH. The current affiliation for F.C.E. is Department of Dermatology, Eberhard Karls University of Tuebingen, Tuebingen, Germany.
Correspondence: Elaine S. Jaffe, National Cancer Institute, 10 Center Drive, MSC-1500, Bldg 10, Room 2B42, Bethesda, MD 20892-1500; e-mail: elainejaffe@nih.gov.
References
Author notes
A.G.J. and F.C.E. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal