Abstract
During the course of multiple myeloma (MM), new monoclonal proteins of an isotype distinct from the original clone, referred to as secondary monoclonal gammopathy of undetermined significance (MGUS), have been described. We report on the frequency, characteristics, and outcome of secondary MGUS. Of the 1942 patients with MM, 128 (6.6%) developed a secondary MGUS, at a median of 12 months from the diagnosis of MM. The median duration of secondary MGUS was 5.9 months. Secondary MGUS was more common in patients after stem cell transplantation than in those who had not undergone such treatment (22.7% vs 1.6%, P < .001). Overall survival was significantly superior in MM patients who developed secondary MGUS compared with the rest of the cohort (73 vs 38 months, respectively; P < .001). The time of onset and the duration of secondary MGUS, as well as failure to resolve spontaneously, had an effect on overall survival and require further study.
Introduction
Monoclonal gammopathy of undetermined significance (MGUS) is a premalignant plasma cell disorder.1-3 It is present in 3%-4% of the general population over the age of 50 years.4 The prevalence of MGUS increases with age5-9 and toxin exposure.10 MGUS progresses to multiple myeloma (MM) or related malignancy at a rate of 1% per year.3,11,12 At 25 years of follow-up, the probability of progression is 11% with adjustment for competing causes of mortality.13-15 Osteoporosis, neuropathy, and thrombophlebitis have been associated with MGUS.16
Previous studies have found that during the course of MM, new monoclonal gammopathies of an isotype (heavy and/or light chain) distinct from the original MM can emerge.17,18 This entity, termed secondary MGUS,17 has been hypothesized to be caused by recapitulation of early B-cell ontogeny after stem cell transplantation (SCT).18 Previous investigations suggest that the appearance of a secondary MGUS is associated with better outcome.19,20 We studied the frequency, characteristics, and natural history of secondary MGUS in MM.
Methods
We identified 2088 cases of MM seen at the Mayo Clinic, Rochester, MN, between January 1, 1990 and December 31, 2009, diagnosed according to standard criteria.3,21 We excluded 62 patients in whom immunofixation was either negative or not obtained within 30 days of diagnosis of MM, 53 with biclonal MM, 3 with amyloidosis, 16 who refused research authorization, and 12 without adequate data. The study was approved by the Mayo Institutional Review Board.
The diagnosis of secondary MGUS required (1) current diagnosis of MM and (2) a new monoclonal (M) protein with heavy and/or light chain immunoglobulin distinct from the initially diagnosed MM. All data were collected by detailed review of medical and laboratory records and the Mayo Clinic MM clinical and hematopoietic SCT databases. The status of MM at the time of secondary MGUS occurrence was assessed according to standard criteria.22
Statistical analysis
Two-sided Fisher exact test was used to test for differences between categorical variables. Time-to-event analyses were performed by the Kaplan-Meier method, and survival curves were compared with the 2-tailed log rank test. Multivariate analysis was performed with the Cox proportional hazards model.
Results and discussion
Frequency of secondary MGUS
We studied 1942 patients diagnosed with MM, with a median follow-up of 7 years. A secondary MGUS occurred in 128 (6.6%). Secondary MGUS was more common in patients who had undergone SCT than in those who had not (104 [22.7%] of 458 patients versus 24 [1.6%] of 1484, respectively; P < .001). Among patients who were followed up at least 4 times over 2 years (n = 439), the corresponding rates were 59 (36.2%) of 163 versus 14 (5%) of 276, respectively. Among patients who were followed up at least 8 times over 2 years (n = 248), the corresponding rates were 33 (36.2%) of 91 versus 12 (7.6%) of 157, respectively.
The median time from diagnosis of MM to secondary MGUS was 12 months (95% confidence interval 2-63 months). Among SCT patients, a secondary MGUS occurred 12 months or more after transplantation in only 15 patients (14%). The median duration of secondary MGUS was 5.9 months. Of the 128 individuals identified with secondary MGUS, 34 (27%) had multiple secondary MGUS of various isotypes.
Characteristics of secondary MGUS
Patient characteristics are given in Table 1. Most secondary MGUS M proteins were small, detectable by immunofixation only in 84 patients (66%), 0.2 to 0.9 g/dL in 29 patients (23%), and ≥ 1 g/dL in 15 patients (12%). Cytogenetic data to classify the underlying MM were available in 107 patients who developed secondary MGUS; 26 patients were hyperdiploid, and 18 had an immunoglobulin heavy chain (IgH) translocation, including 9 with t(11;14) and 6 with t(4;14) translocations. In 37 patients, results were normal, and 26 had other cytogenetic abnormalities. In most patients (87%), the underlying MM was responsive to therapy at the time of secondary MGUS diagnosis.
Secondary MGUS patient characteristics
| Characteristic . | Median (range) or no. of patients (%) . |
|---|---|
| Age, y | 64 (33-83) |
| MM stage at time of secondary MGUS diagnosis | |
| Nonresponsive/progressive | 16 (13) |
| Stable plateau | 5 (4) |
| PR | 45 (35) |
| VGPR | 31 (24) |
| NCR | 8 (6) |
| CR | 23 (18) |
| Secondary MGUS Ig type | |
| IgA | 7 (6) |
| IgM | 85 (66) |
| IgG | 27 (21) |
| Light chain only | 9 (7) |
| Months from MM diagnosis till onset of secondary MGUS | 12 (1-189) |
| No. of isotypes of secondary MGUS | |
| 1 | 94 (73) |
| ≥ 2 | 34 (27) |
| Characteristic . | Median (range) or no. of patients (%) . |
|---|---|
| Age, y | 64 (33-83) |
| MM stage at time of secondary MGUS diagnosis | |
| Nonresponsive/progressive | 16 (13) |
| Stable plateau | 5 (4) |
| PR | 45 (35) |
| VGPR | 31 (24) |
| NCR | 8 (6) |
| CR | 23 (18) |
| Secondary MGUS Ig type | |
| IgA | 7 (6) |
| IgM | 85 (66) |
| IgG | 27 (21) |
| Light chain only | 9 (7) |
| Months from MM diagnosis till onset of secondary MGUS | 12 (1-189) |
| No. of isotypes of secondary MGUS | |
| 1 | 94 (73) |
| ≥ 2 | 34 (27) |
PR indicates partial remission; VGPR, very good partial remission; NCR, near complete remission; and CR, complete remission.
Survival of MM patients with a secondary MGUS
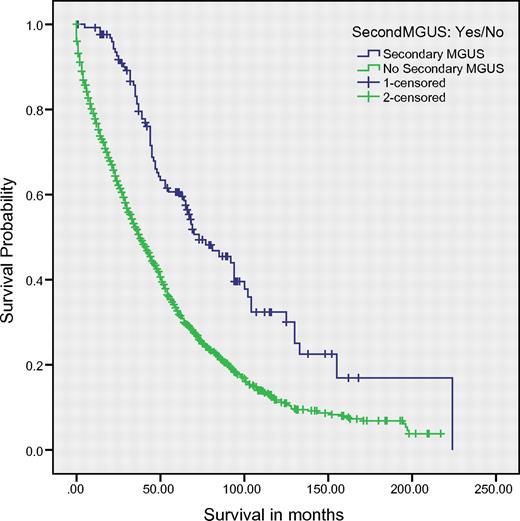
The median overall survival (OS) of the study cohort was 41 months. OS was significantly superior among patients who developed secondary MGUS compared with the rest of the cohort (73 vs 38 months, respectively; P < .001; Figure 1). On multivariate analysis in a model that contained age, International Staging System stage, SCT (yes/no), secondary MGUS, and serum creatinine, the presence of secondary MGUS retained independent prognostic value (P = .002); all other variables were significant at P < .001 with the exception of serum creatinine, which was significant at P = .043. Survival was then studied after analysis was restricted to patients diagnosed since the year 2000 (n = 1088); OS was superior in patients with second MGUS (n = 86) versus those without (n = 1002; median 77 vs 51 months, respectively; P < .001). On multivariate analysis, secondary MGUS and date of diagnosis of MM were independently predictive of OS (P < .001 for both factors).
OS among all patients. Median overall survival was 73 months in patients with secondary MGUS versus 38 months in the rest of the cohort (P < .001). Cum. survival indicates cumulative survival.
OS among all patients. Median overall survival was 73 months in patients with secondary MGUS versus 38 months in the rest of the cohort (P < .001). Cum. survival indicates cumulative survival.
On landmark analysis at 12 months, OS remained significantly superior in patients who developed secondary MGUS compared with the rest of the cohort (73 vs 53 months, respectively; P < .001). A landmark analysis performed at 16 months found similar results, with a median OS of 77 versus 55 months, respectively (P < .001).
Among patients who did not undergo SCT (n = 1484), OS was significantly better in those who developed a secondary MGUS (n = 24) than in the rest (n = 1460; 49 vs 31 months, respectively; P = .01). The results were identical when the survival analysis was repeated after the deletion of patients who had achieved complete response, or complete response plus near complete response. Among MM patients who underwent SCT (n = 458), there was no significant difference in OS in those who developed a secondary MGUS (n = 104) versus the rest (n = 354; 73 vs 68 months, respectively; P = NS).
Median OS was significantly superior (94 vs 62 months; P = .04) among patients in whom the secondary MGUS was detected > 12 months after initial diagnosis compared with those in whom the secondary MGUS occurred sooner. Similarly, the occurrence of a secondary MGUS > 6 months after transplantation was associated with better OS (median 102 vs 68 months; P = .02). In a Cox proportional hazards model, a longer duration of secondary MGUS was associated with significantly inferior survival (P = .034). Similarly, among patients with secondary MGUS, there was also a trend to better OS in those in whom the MGUS resolved (n = 111) than in those with persistent MGUS (n = 17; 81 vs 48 months, respectively; P = .07).
The present study shows that secondary MGUS occurs in ∼ 7% of patients with MM, and the occurrence appears several fold higher after SCT than in patients who do not undergo SCT. Because this was not a prospective study in which patients were tested at predefined regular intervals for the occurrence of a second M protein, the rates of secondary MGUS that we report likely underestimate the true occurrence of this process. Furthermore, we were not able to identify new clones that secrete the same M protein type as the MM, but only those that have a different heavy and/or light chain isotype.
Secondary MGUS was a favorable prognostic factor for OS, independent of year of diagnosis, age, stage, and renal function. This is consistent with findings from other studies in SCT patients.20,23,24 The failure of a secondary MGUS to spontaneously resolve and the duration of secondary MGUS may affect OS, but this requires further study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This research was supported in part by grants CA 107476, CA 62242, CA100707, and CA 83724 from the National Cancer Institute, Rockville, MD. Additional support was received from the Jabbs Foundation, Birmingham, United Kingdom, and the Henry J. Predolin Foundation, Madison, WI.
National Institutes of Health
Authorship
Contribution: R.K.W. and S.V.R. analyzed data and wrote the manuscript; D.R.L, A.D., S.K., R.A.K., and H.M.L. contributed to the analysis, provided critical review, and edited the manuscript; and all authors reviewed and approved the final manuscript.
Conflict-of-interest statement: The authors declare no competing financial interests.
Correspondence: S. Vincent Rajkumar, MD, Division of Hematology, Mayo Clinic, Rochester, MN 55905; e-mail: rajkumar.vincent@mayo.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal