Abstract
Platelet activation and thrombus formation are under the control of signaling systems that integrate cellular homeostasis with cytoskeletal dynamics. Here, we identify a role for the ribosome protein S6 kinase (S6K1) and its upstream regulator mTOR in the control of platelet activation and aggregate formation under shear flow. Platelet engagement of fibrinogen initiated a signaling cascade that triggered the activation of S6K1 and Rac1. Fibrinogen-induced S6K1 activation was abolished by inhibitors of Src kinases, but not Rac1 inhibitors, demonstrating that S6K1 acts upstream of Rac1. S6K1 and Rac1 interacted in a protein complex with the Rac1 GEF TIAM1 and colocalized with actin at the platelet lamellipodial edge, suggesting that S6K1 and Rac1 work together to drive platelet spreading. Pharmacologic inhibitors of mTOR and S6K1 blocked Rac1 activation and prevented platelet spreading on fibrinogen, but had no effect on Src or FAK kinase activation. mTOR inhibitors dramatically reduced collagen-induced platelet aggregation and promoted the destabilization of platelet aggregates formed under shear flow conditions. Together, these results reveal novel roles for S6K1 and mTOR in the regulation of Rac1 activity and provide insights into the relationship between the pharmacology of the mTOR system and the molecular mechanisms of platelet activation.
Introduction
Platelets represent a specialized set of peripheral blood cells that are optimally configured for adhesion, secretion and aggregation at sites of vascular injury.1,2 The exposure of platelets to extracellular matrix proteins such as collagen or laminin, or endogenous agonists such as ADP or thromboxanes, mediates hemostasis by activating signaling pathways that ultimately result in platelet adhesion and aggregation.3 On the engagement of the adhesive proteins fibrinogen and fibronectin, platelet tyrosine kinases such as Src, Syk and FAK are recruited to the platelet cytosolic cell surface to initiate signaling pathways to drive platelet cytoskeletal reorganization through the Rho family small GTPase Rac1.3-5 Rac1 regulates actin polymerization at the cell membrane to drive the growth and extension of platelet lamellipodiae that form the basis for platelet spreading.4 The molecular mechanisms by which tyrosine kinases ultimately activate Rac1 remain ill-defined.
The 70 kDa ribosome S6 protein kinase (S6K1) regulates the ribosome S6 protein to integrate processes of protein translation with cell growth and cell proliferation.6 In cultured cells as well as in vivo, mitogenic signals triggered by nutrients and growth factors initiate a complex sequence of signaling events to activate the mammalian target of rapamycin (mTOR), a serine/threonine kinase which regulates S6K1 phosphorylation and activation.7 Treatment of cells with rapamycin (Sirolimus) or other inhibitors of mTOR blocks S6K1 Thr389 phosphorylation and inhibits S6K1 activation.8 The ability of mTOR inhibitors to arrest the growth of transformed tumor cells with dysregulated mTOR signaling has led to their development as antineoplastic agents that are currently used to treat several malignancies.9 Imbalances in the mTOR pathway are also involved in obesity, diabetes, inflammatory diseases and cardiac hypertrophy, and pharmacologic intervention of mTOR has been proposed as a potential treatment for these conditions.6
In addition to controlling protein translation and cell growth, S6K1 and mTOR have roles in chemotaxis, cell migration, and tumor cell invasion.10-12 Inhibition of mTOR and S6K1 with rapamycin blocks the growth factor and nutrient mediated migration of intestinal cells,13 smooth muscle cells,14 and carcinoma cells on surface substrates such as fibronectin.15-17 As these cells migrate, integrin-mediated signals trigger an activation of mTOR and S6K1, which in turn regulate the remodeling of the actin cytoskeleton to control cell motility. The manner in which mTOR pathways direct actin remodeling and cell movement are not understood but may involve a colocalization of S6K1 with actin stress fibers18 as well as actin remodeling proteins such as Rac1. For instance, S6K1 interacts with Rac1 in transfected HEK 293 cells,19 and rapamycin can prevent cell migration through inhibition of the small GTPases RhoA, Cdc42, and Rac1.20 Furthermore, S6K1 and mTOR work with Rac1 to reorganize the actin cytoskeleton and direct the migration of ovarian cancer21 and colorectal cancer cells.22 Rac1 activity is also regulated by the tuberous sclerosis protein TSC2, a downstream target of Akt that suppresses mTOR and S6K1 activity to control tumor cell polarity and migration.23
Despite known functions of S6K1 and mTOR in cell migration and chemotaxis, the roles of these signaling kinases in motility-related platelet processes in hemostasis and thrombosis remain unexplored. This is of clinical relevance as mTOR inhibitors are used as chemotherapy drugs for a growing number of malignancies.24 mTOR inhibitors such as rapamycin are also potent immunosuppressive and antiproliferative agents which prevent the rejection of transplanted organs as well the restenosis of intravascular stents.25-27 The exact mechanisms by which mTOR inhibitors act as immunosuppressants are not completely understood but may involve inhibition of peripheral blood cells with immunologic roles such as macrophages28 and neutrophils29 as well as platelets.30 In this study, we investigate the role of mTOR/S6K1-mediated Rac1 activation in platelet spreading and platelet aggregation. We find that platelets use an mTOR/S6K1 pathway to activate Rac1 and regulate platelet spreading on fibrinogen. Moreover, mTOR has a role in glycoprotein GPVI-mediated platelet aggregation as well as maintaining the integrity of platelet aggregates formed under physiologic shear conditions. Our findings provide novel insights into the role of the mTOR signaling system in platelet biology and contribute to an understanding of how mTOR and S6K1 function in regulating Rac1 activation.
Methods
Reagents
EHT 1864, PP2, BAY 61-3606, apyrase, and fatty-acid–free BSA and all other reagents were from Sigma-Aldrich except for as noted. WYE-354 and Ku-0063794 were from Chemdea. Human fibrinogen was from Enzyme Research. Collagen was from Chrono-Log. Rapamycin and RAD001 were from LC Labs. MCF10A cells were from ATCC and provided by the National Cancer Institute Physics-Oncology Program. Cells were cultured in DMEM supplemented with 5% horse serum (Invitrogen) and hydrocortisone, EGF, insulin, and cholera toxin. D-phenylalanyl-L-prolyl-L-arginine chloromethyl ketone (PPACK) anticoagulant was from Calbiochem. Plasmids encoding GST and PAK-CRIB-GST were provided by L. Machesky (Beatson Institute for Cancer Research, Glasgow, United Kingdom). pGEX-Rac1 was from Addgene. Anti–Syk-pTyr323 (2715), Syk (2712), LAT-pTyr171 (3581), LAT (9166), FAK-pTyr576/577 (3281), FAK (3285), mTOR (2983), Raptor (2280), Rictor (2114), GβL (3274), GSK3β-pSer9 (9336), and S6K1-pThr389 (9234) were from Cell Signaling. Anti-Rac1 (23A8) and Raptor (1H6.2) were from Millipore. Anti-S6K1 (sc-230), GSK3β (sc-9166), Rictor (sc-271081), TIAM1 (sc-872), mouse (sc-2025), and rabbit (sc-2345) IgGs, and secondary goat anti–rabbit and anti–mouse HRP secondary antibodies were from Santa Cruz Biotechnology.
Preparation of human washed platelets
Human venous blood was drawn in accordance with an Oregon Health & Science University IRB–approved protocol from healthy donors into sodium citrate and acid/citrate/dextrose as previously described.4 Platelet-rich plasma (PRP) was prepared by centrifugation of anticoagulated blood at 200g for 10 minutes. Platelets were further purified from PRP by centrifugation at 1000g in the presence of prostacyclin (0.1 μg/mL). Purified platelets were resuspended in modified HEPES/Tyrode buffer (129mM NaCl, 0.34mM Na2HPO4, 2.9mM KCl, 12mM NaHCO3, 20mM HEPES, 5mM glucose, 1mM MgCl2; pH 7.3) containing 0.1 μg/mL prostacyclin. Platelets were washed once by centrifugation and resuspended in modified HEPES/Tyrode buffer at indicated concentrations.
Static adhesion assays
Glass coverslips were coated with human fibrinogen (100 μg/mL) followed by surface blocking with BSA (5 mg/mL). Inhibitors or vehicle were added to platelets in solution (2 × 107/mL) with 2 U/mL apyrase for 10 minutes before exposure to immobilized fibrinogen at 37°C. After 45 minutes, nonadherent platelets were discarded and surface-bound platelets were washed 3 times with PBS. Coverslips were fixed in 4% paraformaldehyde and washed with PBS. Platelets were imaged using Kohler illuminated Nomarski differential interference contrast (DIC) optics with a Zeiss 63× oil immersion 1.40 NA plan-apochromat lens on a Zeiss Axiovert 200M microscope as previously described.4
Western blotting and immunoprecipitations
For platelet lysate experiments, 24-well tissue culture treated plates (Corning) were coated with human fibrinogen (50 μg/mL) followed by surface blocking with BSA (5 mg/mL). Platelets (300 μL, 5 × 108/mL in modified HEPES/Tyrode buffer with 2 U/mL apyrase) were incubated in fibrinogen-coated dishes for 45 minutes at 22°C. Nonadherent platelets were discarded and surface-bound platelets were washed 3 times in 1 mL PBS before collection into 100 μL lysis buffer (1% Nonidet P-40, 1% N-octyl glucoside, 150mM NaCl, 10mM Tris/HCl, 1mM EGTA, 10mM MgCl2, 1mM phenylmethylsulfonyl fluoride, 10 μg/mL leupeptin, 2mM orthovanadate, and Sigma Phosphatase Inhibitor Cocktail, pH 7.4). Lysates were denatured in an equal volume of Laemmli sample buffer (Bio-Rad) with 0.5M dithiothreitol (100°C, 5 minutes), separated by SDS-PAGE, transferred to polyvinylidene difluoride membranes, and immunoblotted with indicated antibodies and HRP-conjugated secondary antibodies as previously described.31 Protein was detected using ECL (Amersham Biosciences). Blot films were digitally scanned and analyzed for band intensity with ImageJ Volume 1.44 software. Band densitometry statistical analysis was performed using a paired t test between vehicle and each test condition for the antigen specified (supplemental Figure 2, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). For coimmunoprecipitation studies, platelets treated as above were lysed in 1 mL of M-PER Mammalian Protein Extraction Reagent (Pierce) supplemented with 1mM DTT, 1mM phenylmethylsulfonyl fluoride, 10 μg/mL leupeptin, and Phosphatase Inhibitor Cocktail. Immunoprecipitation lysates were precleared with 25 μL Protein A/G PLUS-Agarose (Santa Cruz Biotechnology) before incubation with 10 μg of indicated antisera or control IgGs overnight at 4°C, followed by the addition of 25 μL Protein A/G agarose. Captured protein complexes were washed 3 times in 1 mL of M-PER and eluted into 50 μL of Laemmli sample buffer. To capture S6K1 from platelet lysates, M-PER lysates as above were incubated with 1 μg of Rac1-GST or GST alone conjugated to glutathione sepharose for 1 hour, then washed and eluted into Laemmli sample buffer with 0.5M DTT.
Rac1 activity assays
Rac1 activation was measured through capture of Rac1-GTP using the CRIB domain of PAK1 as previously described.4 Platelets (5 × 107/mL in modified HEPES/Tyrode buffer) were incubated at 37°C for 45 minutes in 35-mm dishes coated with human fibrinogen (100 μg/mL) or BSA in the presence of apyrase (2 U/mL) and inhibitors as indicated. Nonadherent platelets were discarded and surface-bound platelets were washed 3 times in 1 mL of PBS and collected into 1 mL of lysis buffer (as in “Western blotting and immunoprecipitations”). Lysates were incubated with 50 μL of a 50% slurry of glutathione-sepharose beads (GE Healthcare Life Sciences) conjugated to GST-PAK CRIB (60 minutes, 4°C). Beads were washed in lysis buffer and bound protein was eluted by the addition of 50 μL of Laemmli sample buffer with 0.5M DTT.
Confocal microscopy
Purified human platelets (1 × 107/mL) were spread on coverglass coated with 25 μg/mL fibrinogen for 45 minutes at 37°C. Adherent platelets were washed 3 times with PBS before fixation with 4% paraformaldehyde, then washed in PBS and permeabilized with blocking buffer (PBS, 0.1% SDS + 1% BSA) for 1 hour. Platelets were stained with anti-S6K1 (1:50) or anti-TIAM1 (1:50) and anti-Rac1 (1:100) in blocking buffer overnight at 4°C. Invitrogen Alexa Fluor secondary antibodies (1:200) and Alexa Fluor 647 phalloidin (1:100) were added in blocking buffer for 1 hour. Coverslips were mounted with Fluoromount G (Southern Biotech) on glass slides and visualized with a 60× oil immersion objective on an Olympus Fluo-View FV300 laser scanning confocal microscope.
Platelet aggregation and shape change
For platelet aggregation studies, 300 μL of purified human platelets (2 × 108/mL) were treated with inhibitors as indicated in the presence of 2 U/mL apyrase. Platelet aggregation was triggered by 10 μg/mL collagenand monitored under continuous stirring at 1200 rpm at 37°C by measuring changes in light transmission using a PAP-4 aggregometer as previously described.4
Platelet aggregate stability under flow
Glass capillary tubes (Vitrocom) were coated with collagen (100 μg/mL) and surface blocked with denatured BSA. PPACK-anticoagulated blood was perfused at 37°C to form platelet aggregates, which were then chased with modified HEPES/Tyrodes buffer containing 3 mg/mL fibrinogen containing indicated treatments. Aggregate dissociation was imaged in real time using Kohler illuminated Nomarski DIC optics with a Zeiss 40×0.75 NA EC Plan Neofluar lens on a Zeiss Axiovert 200M microscope. Time-lapse images were recorded with a Zeiss Axiocam MRm camera using Slidebook 5.0 (Intelligent Imaging Innovations Inc). To compute dissociation, images were manually outlined and quantified by determining the number of pixels within each outline using a Java plug-in for ImageJ as described previously.4
Results
S6K1 is activated upstream of Rac1 and platelet lamellipodia formation
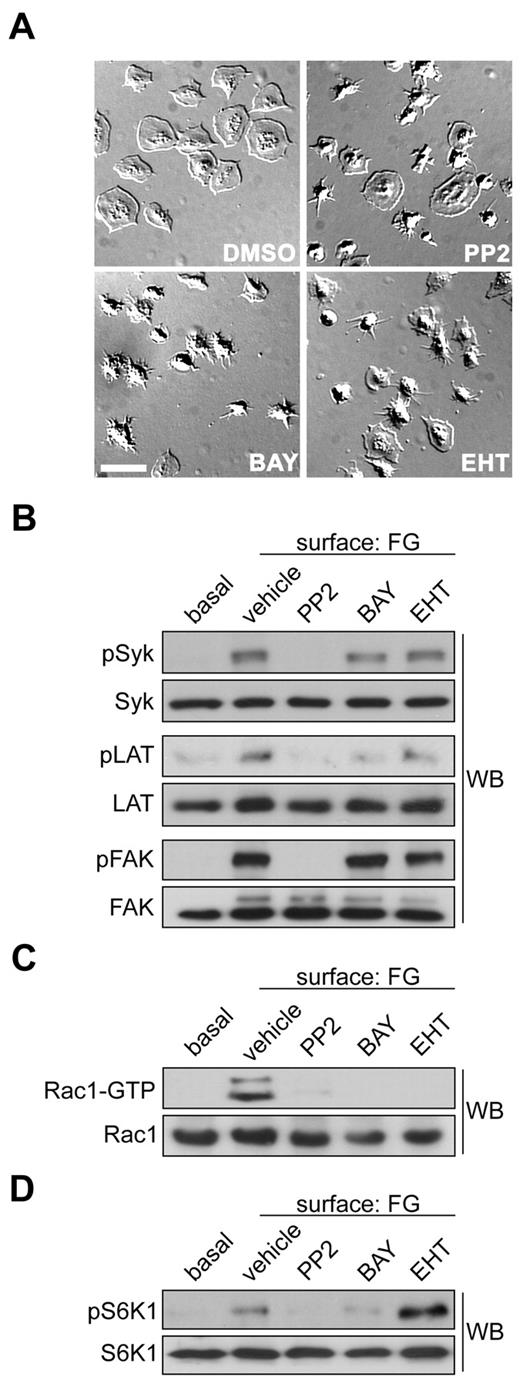
Previously, we determined that the small GTPase Rac1 is required for actin remodeling events that drive platelet lamellipodia formation and thrombus stability under shear.4 As platelets adhere to an immobilized surface of fibrinogen, the platelet integrin αIIbβ3 recruits and activates tyrosine kinases such as Src and Syk at its intracellular domains to ultimately lead to the activation of Rac1.4,32 To better understand how these tyrosine kinase pathways activate Rac1 to mediate platelet spreading, we first examined the effects of Src and Syk kinase inhibition on platelet lamellipodia formation. Human platelets were purified from freshly drawn blood and allowed to bind to an immobilized surface of fibrinogen. Surface-bound platelets were washed, fixed, and then analyzed by Kohler-illuminated Normarski DIC microscopy. As seen in Figure 1A, pretreatment of platelets with PP2 or BAY 61-3606, which inhibit Src or Syk kinases, respectively, blocked the formation of platelet lamellipodia and prevented the spreading of platelets on fibrinogen. Platelet lamellipodia formation and spreading was similarly blocked by treatment with the Rac inhibitors EHT 1864 (Figure 1A) and NSC23766 (supplemental Figure 1). Western blot analyses of platelet lysates confirmed that PP2 blocked Src kinases as evidenced by loss of the Src-dependent phosphorylation of Syk Tyr323 (Figure 1B). Moreover, inhibition of Src kinases with PP2 abrogated the phosphorylation of the downstream effector FAK at Tyr576/577 (Figure 1B). The Syk inhibitor BAY61-3606 led to the inhibition Syk activation as seen by blockade of LAT Tyr171 phosphorylation. Rac1 appeared to have no role in these tyrosine kinase pathways as the Rac1 inhibitor EHT 1864 did not affect Syk, FAK or LAT phosphorylation.
S6K1 is activated upstream of Rac1 in platelets. (A) Representative DIC images of purified human platelets (2 × 107/mL) treated with vehicle (DMSO), the Src inhibitor PP2 (20μM), the Syk inhibitor BAY 61-3606 (1μM) or the Rac1 inhibitor EHT 1864 (50μM), on a surface of fibrinogen (FG). Scale bar = 10 μm. (B) Lysates from quiescent platelets in solution (basal) or FG-surface-attached platelets were analyzed for Src, Syk and FAK activation by Western blotting (WB) for Syk-pTyr323, LAT-pTyr171 and FAK-pTyr576/577. (C) Lysates were incubated with glutathione-sepharose conjugated to GST-PAK-CRIB to capture activated GTP-bound Rac1. Captured GTP-Rac1 and total Rac1 inputs were analyzed by Western blotting. (D) Platelets were treated with inhibitors as above and analyzed for S6K1 activation by Western blotting for S6K1-pThr389. PP2 and BAY 61-3606 decreased S6K1 phosphorylation by 86.9% and 66.9%, respectively (n = 3, P < .05). EHT 1864 increased pS6K1 levels by 121% relative to vehicle (n = 3, P < .05).
S6K1 is activated upstream of Rac1 in platelets. (A) Representative DIC images of purified human platelets (2 × 107/mL) treated with vehicle (DMSO), the Src inhibitor PP2 (20μM), the Syk inhibitor BAY 61-3606 (1μM) or the Rac1 inhibitor EHT 1864 (50μM), on a surface of fibrinogen (FG). Scale bar = 10 μm. (B) Lysates from quiescent platelets in solution (basal) or FG-surface-attached platelets were analyzed for Src, Syk and FAK activation by Western blotting (WB) for Syk-pTyr323, LAT-pTyr171 and FAK-pTyr576/577. (C) Lysates were incubated with glutathione-sepharose conjugated to GST-PAK-CRIB to capture activated GTP-bound Rac1. Captured GTP-Rac1 and total Rac1 inputs were analyzed by Western blotting. (D) Platelets were treated with inhibitors as above and analyzed for S6K1 activation by Western blotting for S6K1-pThr389. PP2 and BAY 61-3606 decreased S6K1 phosphorylation by 86.9% and 66.9%, respectively (n = 3, P < .05). EHT 1864 increased pS6K1 levels by 121% relative to vehicle (n = 3, P < .05).
To study the pathways by which platelet tyrosine kinases trigger Rac1 activation, we measured the activation state of this small GTPase through capture of GTP-Rac1 by the CRIB domain of the p21-activated kinase PAK. Platelet spreading on fibrinogen was marked by an increase in Rac1 activation as evidenced by the precipitation of GTP-Rac1 from lysates of activated platelets versus resting platelets (Figure 1C). Pretreatment of platelets with PP2 or BAY61-3606 blocked Rac1 activation, indicating that Src or Syk kinases are needed for Rac1 activation in response tofibrinogen. Rac1 activation was also inhibited by EHT 1864. To test the hypothesis that S6K1 plays a role in Rac1-based platelet lamellipodia formation we next looked at S6K1 phosphorylation in fibrinogen-activated platelets. Quiescent platelets in solution had no detectable S6K1 activation as demonstrated by Western blotting for S6K1 phosphorylation at Thr389 (Figure 1D). Spreading on a surface of fibrinogen increased the phosphorylation of S6K1 Thr389. Src and Syk inhibitors, which prevented platelet spreading (Figure 1A), blocked S6K1 activation. However, EHT 1864, which also inhibits platelet spreading, did not block S6K1 activation, suggesting that S6K1 is activated upstream of Rac1 in spreading platelets (Figure 1D).
S6K1 and Rac1 colocalize at the leading edge of activated platelets
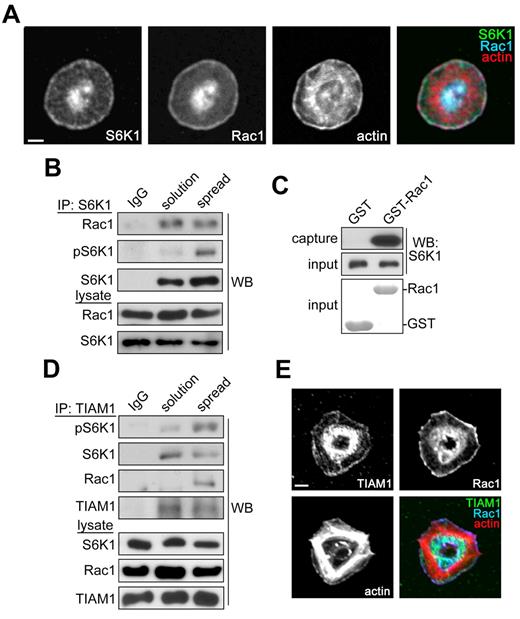
To better understand how S6K1 coordinates the activation of Rac1 in platelets, we examined the localization of these proteins in fibrinogen-activated platelets. Purified human platelets were spread on fibrinogen-coated coverglass and stained for S6K1, Rac1 and actin. Laser scanning confocal immunofluorescence microscopy showed that S6K1 localized to the actin-rich leading edge of activated platelets (Figure 2A). S6K1 colocalized with Rac1 and lamellipodial actin at the leading edge of platelets, suggesting that S6K1 may interact with Rac1 in a lamellipodial signaling complex. To test this hypothesis, we immunoprecipitated S6K1 from human platelets and detected coimmunoprecipitating Rac1 by Western blot. Anti-S6K1 precipitated S6K1 from both quiescent and activated platelets (Figure 2B). S6K1 immunoprecipitates from spread platelets showed more S6K1 phosphorylation than those from quiescent platelets. Immunoprecipitation of S6K1 supported the cocapture of Rac1 from platelets in solution as well as fibrinogen-activated platelets. To determine whether Rac1 itself can support an interaction with S6K1, we incubated platelet lysates with glutathione sepharose conjugated to Rac1-GST. As seen in Figure 2C, Rac1-GST captured S6K1 from platelet lysates while GST alone failed to interact with S6K1. Previous work has established that Rac1 activity in tumor cells is regulated in part by TIAM1,33 a Rac guanine nucleotide exchange factor (GEF) that is known to interact with S6K1.34 To determine whether S6K1 and Rac1 are associated with TIAM1 in platelets, we next immunoprecipitated TIAM1 from platelet lysates with sc-872, an antibody that specifically captures TIAM1 from platelet lysates (supplemental Figure 3). TIAM1 was precipitated at similar levels from quiescent platelets in solution as well as platelets spread on fibrinogen (Figure 2D). As seen in Figure 2D, platelet activation by a surface of fibrinogen increased the association of Rac1 with TIAM1. Moreover, platelet activation was associated with an increase in phosphorylation of S6K1 associated with TIAM1. Like S6K1, TIAM1 also colocalized with Rac1 at the platelet lamellipodial edge (Figure 2E). Together, these data suggest that TIAM1 may associate with an S6K1-containing Rac1 signaling complex which localizes to the lamellipodial edge of platelets to regulate the spreading of platelets on fibrinogen.
S6K1 and Rac1 localize to the lamellipodial edge of spreading platelets. (A) Purified human platelets were spread on coverglass coated with 25 μg/mL fibrinogen. After 45 minutes, platelets were fixed, stained for S6K1, Rac1, and actin, and visualized by confocal microscopy. Scale bar = 2 μm. (B) S6K1 was immunoprecipitated (IP) from lysates of quiescent platelets in solution or platelets spread on fibrinogen and analyzed for coprecipitating Rac1 by Western blot. Nonspecific rabbit immunoglobulins (IgG) were used as negative control for immunoprecipitations. Total S6K1 and Rac1 levels in whole-platelet lysates serve as input controls. (C) Platelet lysates were incubated with GST or Rac1-GST glutathione sepharose and captured S6K1 was analyzed by Western blot. Coomassie-stained GST and Rac1-GST inputs are shown. (D) TIAM1 was immunoprecipitated from platelet lysates as above and examined for coprecipitating S6K1 and Rac1 by Western blot. (E) Localization of TIAM1, Rac1 and actin in fibrinogen-activated human platelets visualized by confocal microscopy. Scale bar = 2 μm. Western blot, IP, protein capture, and imaging results are representative of 3 independent experiments.
S6K1 and Rac1 localize to the lamellipodial edge of spreading platelets. (A) Purified human platelets were spread on coverglass coated with 25 μg/mL fibrinogen. After 45 minutes, platelets were fixed, stained for S6K1, Rac1, and actin, and visualized by confocal microscopy. Scale bar = 2 μm. (B) S6K1 was immunoprecipitated (IP) from lysates of quiescent platelets in solution or platelets spread on fibrinogen and analyzed for coprecipitating Rac1 by Western blot. Nonspecific rabbit immunoglobulins (IgG) were used as negative control for immunoprecipitations. Total S6K1 and Rac1 levels in whole-platelet lysates serve as input controls. (C) Platelet lysates were incubated with GST or Rac1-GST glutathione sepharose and captured S6K1 was analyzed by Western blot. Coomassie-stained GST and Rac1-GST inputs are shown. (D) TIAM1 was immunoprecipitated from platelet lysates as above and examined for coprecipitating S6K1 and Rac1 by Western blot. (E) Localization of TIAM1, Rac1 and actin in fibrinogen-activated human platelets visualized by confocal microscopy. Scale bar = 2 μm. Western blot, IP, protein capture, and imaging results are representative of 3 independent experiments.
mTOR regulates S6K1 phosphorylation, Rac1 activation, and platelet spreading
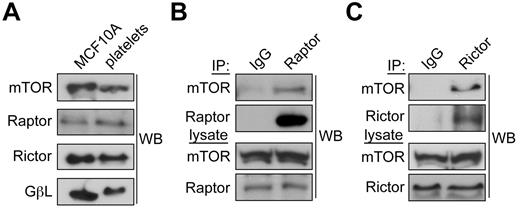
mTOR is a serine/threonine protein kinase that mediates cellular responses to growth factors, cell cycle transitions, cell growth and cell migration through 2 separate protein complexes, the mTOR complex 1 (mTORC1) and the mTOR complex 2 (mTORC2).7 These complexes are distinguished by their uniquely associated regulatory proteins, Raptor and Rictor, respectively.7 To first determine whether mTORC1 and mTORC2 protein complexes are present in platelets, we prepared lysates from human platelets as well as MCF10A cells. Lysates were normalized for total protein content and separated by gel electrophoresis and blotted for the presence of the mTOR complex components mTOR, Raptor, Rictor and GβL. As seen in Figure 3A, mTOR, Raptor and Rictor were found in comparable levels in cytosolic lysates from both platelets and MCF10A cells. GβL, a G-protein β-subunit-like protein component of both mTORC1 and mTORC2 was also present in both cell types, though in slightly lower amounts in platelets. To next determine whether mTORC1 and mTORC2 complexes are intact in human platelets, we performed coimmunoprecipitation experiments. Platelet lysates were incubated with antibodies against Raptor, Rictor, or control IgGs and analyzed for coprecipitating mTOR by Western blot. As seen in Figures 3B and C, Raptor and Rictor both coprecipitated mTOR, indicating that mTORC1 and mTORC2 complexes are present in platelets.
mTORC1 and mTORC2 complexes are present in human platelets. (A) Centrifugally cleared lysates-(50 μg) from human platelets or MCF10A breast epithelial cells were analyzed by Western blot for levels of mTOR, Raptor, Rictor, or GβL. (B) mTORC1 complexes were identified by immunoprecipitating Raptor from platelet lysates and Western blotting for associated mTOR. (C) mTORC2 complexes were identified by immunoprecipitation of Rictor followed by Western blotting for coprecipitating mTOR. Nonspecific immunoglobulins (IgG) served as a negative control for immunoprecipitations. Immunoprecipitation results are representative of 3 independent experiments.
mTORC1 and mTORC2 complexes are present in human platelets. (A) Centrifugally cleared lysates-(50 μg) from human platelets or MCF10A breast epithelial cells were analyzed by Western blot for levels of mTOR, Raptor, Rictor, or GβL. (B) mTORC1 complexes were identified by immunoprecipitating Raptor from platelet lysates and Western blotting for associated mTOR. (C) mTORC2 complexes were identified by immunoprecipitation of Rictor followed by Western blotting for coprecipitating mTOR. Nonspecific immunoglobulins (IgG) served as a negative control for immunoprecipitations. Immunoprecipitation results are representative of 3 independent experiments.
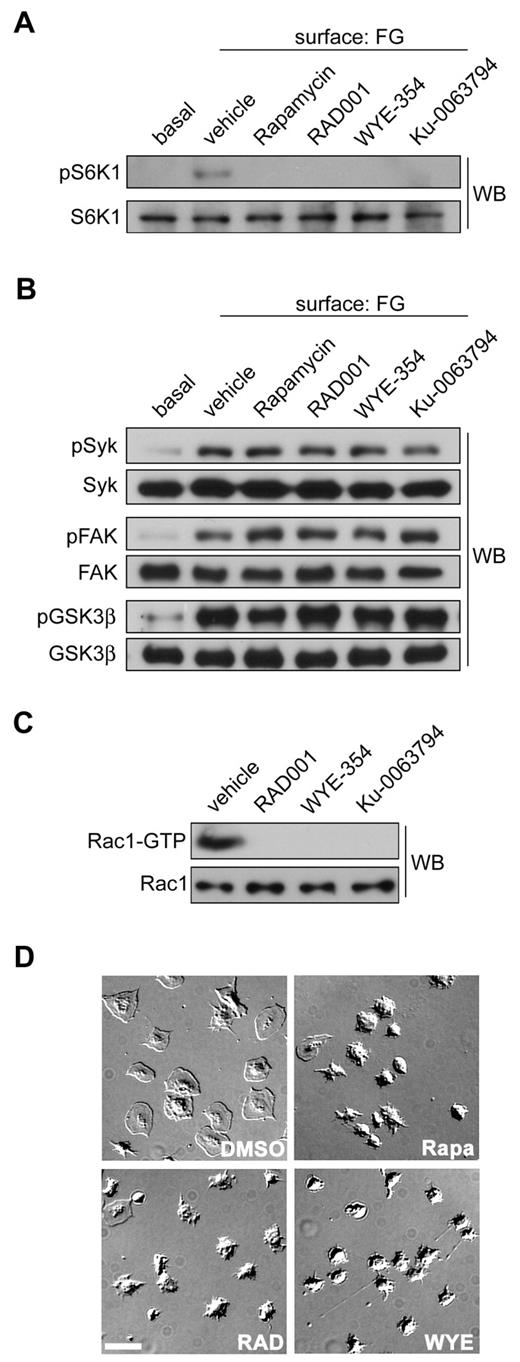
To determine whether S6K1 and mTOR have roles in platelet function, we next studied the effects of mTOR and S6K1 inhibition on platelet activation. Several agents block S6K1 activity through inhibition of mTORC1, most notably rapamycin and the rapalogue compounds such as RAD001 (Everolimus). These agents bind to FK-binding protein 12 (FKBP12) to form a complex that allosterically inhibits mTORC1 and subsequently S6K1 activation.6 mTORC1 and S6K1 are also inhibited by mTOR substrate inhibitors that engage the active site of mTOR directly.8 These next generation mTOR inhibitors include compounds such as WYE-35435 and Ku-0063794.8,36 As seen in Figure 4A, pretreatment of platelets with any of these agents inhibited platelet S6K1 phosphorylation in response to spreading on immobilized fibrinogen. mTOR inhibitors abrogated S6K1 activation but had no effect on early signaling pathways of platelet activation; rapamycin, RAD001, WYE-354 and Ku-0063794 had no significant effect on Src or FAK activation or the PI3K/Akt-mediated phosphorylation GSK3β (Figure 4B).37 However, mTOR/S6K1 inhibitors blocked Rac1 activation (Figure 4C). To confirm that this Rac1 inhibition had a consequence to platelet function, we used DIC microscopy to analyze platelet spreading on fibrinogen after pretreatment with mTOR inhibitors. As seen in Figure 4D, mTOR/S6K1 inhibitors blocked lamellipodia formation and platelet spreading on a surface of fibrinogen.
mTOR and S6K1 inhibition blocks Rac1 activation and platelet spreading. (A) Purified human platelets were treated with apyrase (2 units/mL) and vehicle (DMSO), Rapamycin (1μM), RAD001 (1 μM), WYE-354 (10μM), or Ku-0063794 (10μM) before spreading on a surface of fibrinogen. Lysates from surface-bound platelets were analyzed for S6K1 activation by Western blotting for S6K1-pThr389. (B) Western blot analysis of Syk-pTyr323, FAK-pTyr576/577, and GSK3β-pSer9 phosphorylation of fibrinogen-activated platelets pretreated with Rapamycin, RAD001, WYE-354, or Ku-0063794. (C) Lysates from surface-bound platelets were incubated with PAK-CRIB-GST to capture activated Rac1. Captured GTP-Rac1 and total Rac1 levels were determined by Western blot. (D) Representative DIC images of human platelets spread on coverglass coated with 100 μg/mL fibrinogen after treatment with apyrase and vehicle (DMSO), Rapamycin, RAD001, or WYE-354. Scale bar = 10 μm. Results are representative of 3 independent experiments.
mTOR and S6K1 inhibition blocks Rac1 activation and platelet spreading. (A) Purified human platelets were treated with apyrase (2 units/mL) and vehicle (DMSO), Rapamycin (1μM), RAD001 (1 μM), WYE-354 (10μM), or Ku-0063794 (10μM) before spreading on a surface of fibrinogen. Lysates from surface-bound platelets were analyzed for S6K1 activation by Western blotting for S6K1-pThr389. (B) Western blot analysis of Syk-pTyr323, FAK-pTyr576/577, and GSK3β-pSer9 phosphorylation of fibrinogen-activated platelets pretreated with Rapamycin, RAD001, WYE-354, or Ku-0063794. (C) Lysates from surface-bound platelets were incubated with PAK-CRIB-GST to capture activated Rac1. Captured GTP-Rac1 and total Rac1 levels were determined by Western blot. (D) Representative DIC images of human platelets spread on coverglass coated with 100 μg/mL fibrinogen after treatment with apyrase and vehicle (DMSO), Rapamycin, RAD001, or WYE-354. Scale bar = 10 μm. Results are representative of 3 independent experiments.
mTOR controls platelet aggregation and aggregate stability under flow
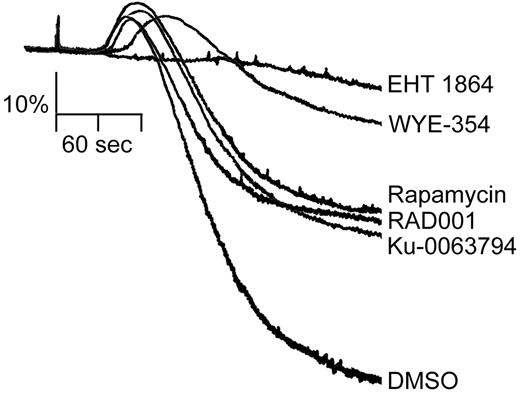
We next examined the role of S6K1 in the process of platelet shape change and aggregation using a Born aggregometer. This apparatus measures light transmission through a platelet suspension, with an increase in optical density correlating to platelet sphericity and shape change and a decrease indicative of platelet aggregation. The Rac1 inhibitor EHT 1864 abrogated the shape change and aggregation of platelets stimulated with collagen in the presence of the ADP scavenger, apyrase (Figure 5). mTOR/S6K1 inhibitors also significantly decreased the magnitude and time course of both the shape change and aggregation in response to 10 μg/mL collagen (Figure 5).
Inhibitors of mTOR and S6K1 block platelet aggregation. Washed human platelets (2 × 108/mL) were stimulated with 10 μg/mL collagen in the presence of 2 U/mL apyrase and the change in optical density indicative of platelet aggregation was recorded after 10 minutes of preincubation with EHT 1864 (50μM), WYE-354 (10μM), Ku-0063794 (10μM), Rapamycin (1μM), RAD001 (1μM), or vehicle (DMSO). One experiment representative of 3 separate experiments is shown.
Inhibitors of mTOR and S6K1 block platelet aggregation. Washed human platelets (2 × 108/mL) were stimulated with 10 μg/mL collagen in the presence of 2 U/mL apyrase and the change in optical density indicative of platelet aggregation was recorded after 10 minutes of preincubation with EHT 1864 (50μM), WYE-354 (10μM), Ku-0063794 (10μM), Rapamycin (1μM), RAD001 (1μM), or vehicle (DMSO). One experiment representative of 3 separate experiments is shown.
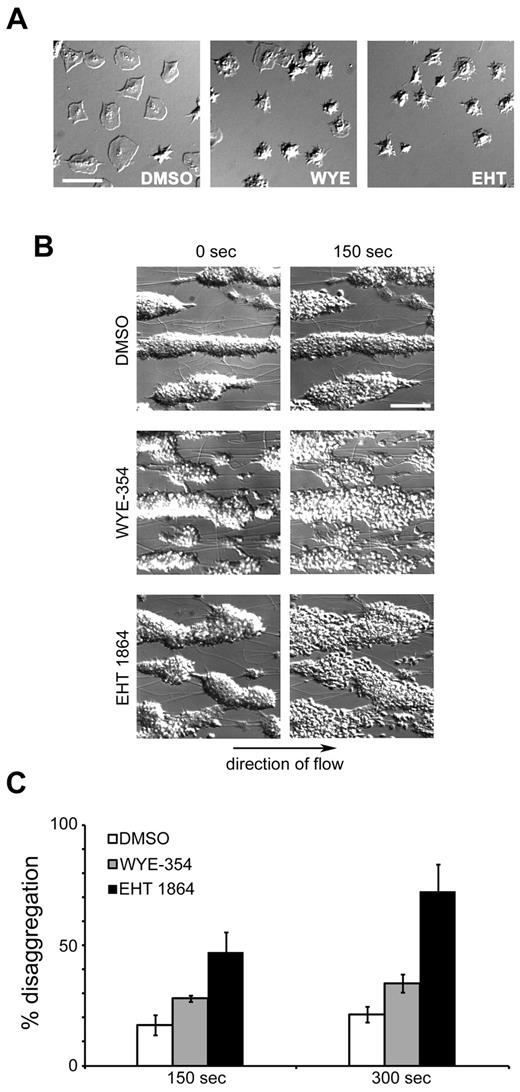
The role of S6K1 and mTOR in platelet activation and aggregation prompted us to investigate the function of these signaling proteins in maintaining platelet spreading. Purified human platelets were first spread on an immobilized surface of fibrinogen. After 30 minutes, nonadherent platelets were washed away and spread platelets were exposed to vehicle (DMSO), the mTOR/S6K1 inhibitor WYE-354 or the Rac1 inhibitor EHT 1864. As seen in Figure 6A, DMSO treatment did not reverse the spreading of individual platelets on fibrinogen. Treatment with WYE-354 or EHT 1864, however, caused a lamellipodial withdrawal of individual platelets previously spread on fibrinogen. We next aimed to determine whether mTOR/S6K1 plays a role in platelet aggregate stability under shear flow. Platelet aggregates were formed after perfusion of PPACK-anticoagulated blood over a surface of collagen. After a secondary perfusion with buffer containing fibrinogen and vehicle alone, platelet aggregates remained stable. Aggregate stability was assessed during secondary perfusion of buffer containing physiologic concentrations of fibrinogen. Our data show that while platelet aggregates remained stable in the presence of vehicle, the mTOR/S6K1 inhibitor WYE-354 led to aggregate instability at 150 seconds, with further instability at 300 seconds (Figure 6C). Quantification of the surface area changes of these aggregates revealed an increase in coverage of 28.8% after 150 seconds (P < .005 vs vehicle) extending to 37.0% at 300 seconds (P < .05 vs vehicle). EHT 1864 similarly destabilized platelet aggregates within 150 seconds under shear flow conditions (Figure 6C).
mTOR is required for platelet aggregate stability under flow. (A) Purified human platelets (2 × 107/mL) were spread on glass coverslips coated with 100 μg/mL fibrinogen. After 30 minutes, unbound platelets were removed and activated platelets were treated with vehicle (DMSO), WYE-354 (10μM), or EHT 1864 (50μM) for an additional 30 minutes. Platelet spreading and lamellipodial withdrawal were evaluated by DIC microscopy. Scale bar = 10 μm. (B) PPACK-anticoagulated blood was perfused over collagen for 4 minutes to produce platelet aggregates that were subsequently perfused with buffer containing fibrinogen for an additional 4 minutes before perfusion with buffer, fibrinogen, and vehicle (DMSO), WYE-354 (10μM), or EHT 1864 (50μM). Representative DIC images of platelet aggregates are shown. Arrow indicates direction of flow. Scale bar = 50 μm. (C) Quantification of platelet disaggregation promoted by WYE-354 or EHT 1864 under flow (n = 3). Data are represented as mean ± SEM.
mTOR is required for platelet aggregate stability under flow. (A) Purified human platelets (2 × 107/mL) were spread on glass coverslips coated with 100 μg/mL fibrinogen. After 30 minutes, unbound platelets were removed and activated platelets were treated with vehicle (DMSO), WYE-354 (10μM), or EHT 1864 (50μM) for an additional 30 minutes. Platelet spreading and lamellipodial withdrawal were evaluated by DIC microscopy. Scale bar = 10 μm. (B) PPACK-anticoagulated blood was perfused over collagen for 4 minutes to produce platelet aggregates that were subsequently perfused with buffer containing fibrinogen for an additional 4 minutes before perfusion with buffer, fibrinogen, and vehicle (DMSO), WYE-354 (10μM), or EHT 1864 (50μM). Representative DIC images of platelet aggregates are shown. Arrow indicates direction of flow. Scale bar = 50 μm. (C) Quantification of platelet disaggregation promoted by WYE-354 or EHT 1864 under flow (n = 3). Data are represented as mean ± SEM.
Discussion
Here we identify mTOR and its effector S6K1 as mediators of Rac1 signaling in platelet activation, platelet spreading and the maintenance of platelet aggregate stability under flow. As platelets engaged a surface of immobilized fibrinogen, a signaling cascade triggered through tyrosine kinases activated S6K1 to switch on Rac1 to drive platelet spreading. Inhibition of mTOR, S6K1 or Rac1 had no effect on early, upstream tyrosine kinase activation of Src, Syk and FAK, demonstrating that the activation of S6K1 and Rac1 occur after the initiation of these tyrosine kinase pathways in platelets. mTOR/S6K1 inhibitors, however, blocked Rac1 activation in response to the binding of platelets to immobilized fibrinogen. In accordance with the hypothesis that mTOR and S6K1 activate Rac1, the Rac1 inhibitor EHT 1864 did not block S6K1 activation by fibrinogen. Rather, Rac1 inhibition robustly increased S6K1 Thr389 phosphorylation (Figure 1E), raising the possibility that Rac1 may also regulate a negative feedback loop to dephosphorylate S6K1 during platelet activation. Our model of platelet cytoskeletal reorganization in which S6K1 drives Rac1 activation is further evidenced by imaging analyses showing that S6K1 colocalized with Rac1 and actin at the leading lamellipodial edge of activated platelets. Moreover, Rac1 coprecipitated with S6K1 in activated platelets, suggesting that S6K1 acts proximal to Rac1 to control platelet activation. S6K1 may work together with the Rac1 GEF TIAM1,34 as TIAM1 coprecipitated both Rac1 and S6K1 from platelet lysates. Current models of TIAM function hypothesize that TIAM1 recruits and activates Rac1 at the cytosolic surface of the plasma membrane.33 As platelet activation increases the association of TIAM1 with Rac1 and phosphorylated S6K1 (Figure 2D), TIAM1 may recruit a Rac1-S6K1 complex to the plasma membrane to help drive lamellipodia formation. In support of this model, confocal microscopy revealed that TIAM1 colocalized with Rac1 at the lamellipodial edge of activated platelets (Figure 2E). Importantly, the effects observed in this study occurred with platelets in presence of the ADP scavenger apyrase, an agent known to reduce the fibrinogen-mediated activation of platelet tyrosine kinase pathways.38 The extent to which mTOR influences tyrosine kinase, S6K1 and Rac activation and platelet function in the absence of ADP inhibitors remains to be determined. Interestingly, earlier studies of the S6K1-Rac1 axis revealed that Cdc42 and Rac1 are required for S6K1 activation in transfected NIH 3T3 cells.19 In platelets, however, Rac1 is not required for S6K1 activation, but rather the contrary. These disparities may be explained in part by the roles of Rac1 and S6K1 in cell cycle transitions in nucleated, dividing cells. In this study, the anucleate and nontransformed nature of platelets facilitates the examination of the roles of S6K1/mTOR in fundamental cell biologic processes such as cytoskeletal reorganization free from nuclear events.
The formation of platelet plugs at sites of vascular injury consists of 3 sequential phases of thrombus initiation, propagation and perpetuation.1,2,39 Intriguingly, these processes are interdependent, as many of the signaling systems at the core of thrombus initiation must remain active to maintain thrombus integrity and to remodel thrombi in processes of wound healing. For instance, continuous PI3K signaling is required for thrombus stabilization.39 During thrombus perpetuation, fibrin formation and other postaggregation phenomena maintain the activation of signaling programs such as the PI3K system through integrins to keep thrombi stable. Previously, we showed that Rac1 plays a critical role in thrombus formation in vivo.4 Here, we show that like PI3K, continuous Rac1 activation is also required to maintain the stability of platelet aggregates under physiologic conditions of shear, as EHT 1864 disaggregates platelet clusters within seconds of administration (Figure 6B). Our hypothesis that mTOR and S6K1 control platelet Rac1 activity to mediate platelet aggregate stability is similarly supported through these assays using the mTOR inhibitor WYE-354. Like Rac1, continuous mTOR activation was also required to maintain the integrity of platelet aggregates formed on a collagen matrix under shear. mTOR activity was also required for collagen-stimulated platelet aggregation in solution (Figure 5). It is noteworthy that our aggregation experiments were performed in the presence of inhibitors of ADP, as previous studies have linked the inhibition of mTOR with ADP secretion and ADP-triggered platelet aggregation.40
mTOR and S6K1 prototypically control cell size and cell proliferation by regulating mRNA translation through substrates such as the ribosome S6 protein and the 4E binding protein 4E-BP1.6 Intriguingly, platelets synthesize proteins in an mTOR-dependent manner on binding to fibrinogen.41,42 Platelet engagement of fibrinogen promotes the synthesis of several proteins including Bcl-3,41 a B cell lymphoma oncoprotein that can interact with the tyrosine kinase Fyn.42 Here, however, we find that translation of Bcl-3 mRNA is not likely to have any instantaneous effects on platelet Rac1 activity as puromycin, which also inhibits Bcl-3 translation in platelets,41,42 had no effect on platelet spreading or aggregation (supplemental Figures 4-5). Previous work has also established that rapamycin can inhibit the retraction of platelet fibrin clots by blocking the translation of Bcl-3 mRNA in platelets over a time course of minutes to several hours in the process of thrombus consolidation and wound healing.43 Here, our data suggests that mTOR and S6K1 also have more immediate effects in thrombus formation and in the maintenance of platelet aggregate stability. Like Rac1 inhibitors, mTOR inhibitors also disrupt platelet lamellipodia formation, platelet aggregate formation and platelet aggregate stability within seconds to minutes through a mechanism that may not be dependent on mRNA translation.
mTORC1 inhibitors such as rapamycin are used as chemotherapeutic agents for the treatment of renal cell44 and other carcinomas.24 Rapamycins selectively block cancer cell growth and induce apoptosis of transformed tumor cells with dysregulated mTOR signaling with minimal effects on healthy cells and only mild physiologic complications.45 The majority of studies investigating the antineoplastic effects of mTOR inhibition have focused on the antiproliferative actions of the rapamycins. However, rapamycins also inhibit cell migration and have been proposed to block tumor cell growth by halting tumor cell chemotactic responses to nutrients and growth factors such as IGF-I.15,17,46 In tumor cells, the effects of rapamycin on chemotaxis are in part mediated by an inhibition of FAK through mTORC1, as rapamycin inhibits IGF-I–induced F-actin reorganization and phosphorylation of focal adhesion proteins in an mTORC1-dependent manner.16 Here, we find that mTOR inhibitors have no effect on platelet FAK activation, suggesting that an mTORC1 activation of FAK may be specific to tumor cells and other transformed cells with irregular mTOR signaling. mTOR also has roles in the migration of intestinal cells13 and smooth muscle cells14 as well as tumor cells. Such migratory processes are driven by an interaction of cell surface integrins with immobilized substrate proteins such as fibronectin and fibrinogen. In cultured cells, the engagement of integrins α5β1 and αvβ3 by fibronectin activates an mTOR/S6K1 signal that is required to drive cell migration.14,47 The mechanisms by which mTOR and S6K1 are involved in directing the motility of these cells, however, have not been described. Here, we demonstrate that mTOR and S6K1 are required for Rac1 signaling which prompts cytoskeletal remodeling events that form the basis for cell motility of platelets triggered by fibrinogen binding to integrin αIIbβ3. As an integrin-mediated activation of S6K1 drives Rac1 processes in platelets, S6K1/mTOR may similarly have Rac1-related roles in migratory processes of other cell types such as tumor cells and other peripheral blood cells with immune functions such as macrophages and neutrophils.
In addition to their use as chemotherapeutic agents, rapamycins are also potent immunomodulators used to prevent organ rejection in transplant recipients.48 The mechanisms by which rapamycins suppress the immune system are not fully understood, but may involve immunoregulators such as B cells, neutrophils,29 and perhaps platelets.30 While our work ascribes a role for mTOR/S6K1 in mediating Rac1-based cytoskeletal reorganization in platelets, similar mechanisms may be present in other peripheral blood cells. Our results may in part explain how rapamycins block immune responses by preventing a Rac1-mediated activation of chemotaxis in peripheral blood cells involved in immunity. The antiproliferative actions of rapamycins are further exploited in drug-eluting intravascular stents to prevent restenosis.25,27 As thrombosis is a common long-term complication of stent implantation, antiplatelet regimens are often prescribed to patients after stent insertion.26 Intriguingly, rapamycin-eluting stents show reduced platelet binding and activation.49 Our data suggests that rapamycins and other mTORC1 inhibitors may play a role in inhibiting platelet function in regions proximal to drug-eluting stents and may provide insights into future treatments for stent thrombosis.
In conclusion, our results uncover a novel mechanism by which tyrosine kinase systems work through S6K1/mTOR to control Rac1 activity and platelet action. While we have determined that S6K1 and Rac1 activation occur downstream of platelet tyrosine kinases, fundamental questions remain about how integrin-triggered tyrosine kinase pathways can activate S6K1 in platelets. Future work will aim to identify the precise signaling network that leads to and from tyrosine kinases to mTOR/S6K1 and then to Rac1 to drive platelet activation. While Rac1 associates with S6K1 in platelet lysates, the nature of how S6K1, Rac1 and TIAM1 may all come together remains an open question. Furthermore, exactly how S6K1 may help to promote the exchange of GDP for GTP by Rac1 is also mysterious. It is possible that undetermined partners or substrates of mTOR or S6K1 may be present in a complex with Rac1 to control platelet action. The hypothesis that platelet S6K1/mTOR can work directly with Rac1 or a Rac-associated guanine nucleotide exchange factor such as TIAM1 is currently under investigation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank M. Larson (Augustana College), M. Berny-Lang (Harvard), and D. Greenberg, M. Hinds, and A. Gruber (OHSU) for helpful discussions.
This work was supported by National Institutes of Health grants T32HL007781 (J.E.A.), U54CA143906 (O.J.T.M.), and R01HL101972 (O.J.T.M.). C.P.L. is an Oregon State University Johnson Scholar.
The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Cancer Institute or the National Institutes of Health.
National Institutes of Health
Authorship
Contribution: J.E.A. designed the project, performed research, analyzed data, and wrote the manuscript; G.W.T. designed and performed the aggregation experiments under flow, analyzed data, and assisted in writing the manuscript; C.P.L. conducted DIC microscopy experiments; J.P. carried out Western blotting and platelet aggregation experiments; and O.J.T.M. supervised the research and assisted in writing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Joseph E. Aslan, 3303 SW Bond Ave, Center for Health and Healing CH13B, Oregon Health & Science University, Portland, OR 97239; e-mail: aslanj@ohsu.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal