Abstract
A long outstanding problem is the resolution of the full potential of hematopoietic precursors. The commonly used allotypic marker Ly5 permits the tracing of lymphoid and granulocyte-macrophage (GM) output. Here we present a novel eGFP allele that allows the quantitative analysis of red blood cell (RBC) origin at the single-cell level. The miR-144/451 locus is required for erythroid development and homeostasis. Taking advantage of the fact that miR-451 is specifically and highly expressed in the erythroid lineage, we inserted an eGFP expression cassette into the miR-144/451 locus. In miR-144/451+/eGFP animals, accumulation of eGFP is exclusively observed during terminal erythroid differentiation. Expression of miR-144/451eGFP ignites immediately before the CFU-E stage and results in strong and complete labeling of all mature RBCs in circulation. Using competitive reconstitution experiments in the Ly5 transplant model, we show that eGFP linearly correlates with Ly5 expression. Thus, the miR-144/451eGFP allele represents a novel tool for the resolution of erythroid potential.
Introduction
The study of blood cell formation has for many years spearheaded the investigation of adult stem cells. In the bone marrow an enigmatic, self-renewing, and rare hematopoietic stem cell (HSC) population gives rise to all blood cell lineages.1 Traditionally, the study of hematopoietic stem and progenitor cells has profited from the relative ease of transplanting small amounts of stem cells into lethally irradiated recipients. Usage of mouse transplantion models enables the in vivo long-term qualitative and quantitative analysis of hematopoietic lineage determination.2 The CD45/Ly5 congenic strains constitute the most widely adopted transplantion mouse model for tracking lineage output in peripheral blood.3 However, the Ly5 antigen is expressed exclusively on nucleated cells, precluding the evaluation of donor-derived contribution to the erythrocytic and thrombocytic lineages. Several other transplant models are available that take advantage of naturally occurring isoforms (eg, Hbbs/d, Gpi1a/b),4,5 as well as transgenes under control of ubiquitous promoters (eg, ActbGFP, H2BGFP, and H2Kb-GFP).6-8 However, these methods have proved to be unsuitable or relatively inefficient in assessing erythroid output because of an absence of expression in mature RBCs or laborious methods of detection.
The vertebrate-specific miR-144/451 cluster, encoding miR-144 and miR-451, regulates erythroid development, homeostasis and susceptibility to oxidative stress.9-11 The maturation and accumulation of miR-451 is mediated by a unique biogenesis pathway that is strictly dependent on Ago2's endonuclease activity.12-14 Here we report a novel erythroid reporter allele based on the miR-144/451 noncoding RNA locus.
Methods
Flow cytometry
Cells were stained and analyzed and/or sorted using FACSCanto or FACSAria cytometers (BD Biosciences) as described.15-23 Where appropriate, RBCs were lysed with an NH4Cl-based solution (ACK lysis buffer) and nonspecific binding was reduced by preincubation with Fc receptor block (FcγII/III). Dead cells and duplets were excluded by 7-aminoactinomycin D (Sigma-Aldrich) staining and FSC/SSC profile, respectively. Data were analyzed off-line using FlowJo Flow Cytometry analysis software (TreeStar Inc). Supplemental Tables 1 and 2 detail surface marker profiles and antibody information (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Bone marrow transplantations
Young (8-12 weeks) female C57Bl/6 Ly5.1/Ly5.2 recipient mice were lethally irradiated in a single dose (875 Rad). Donor bone marrow cells were isolated from age- and sex-matched (8-10 weeks) congenic Ly5.1 or miR-144/451+/eGFP: Ly5.2 mice and 2 × 106 nucleated cells were transplanted by tail vein injection. Bone marrow reconstituted mice were maintained on medicated water (Baytril; Bayer) for 4 weeks after reconstitution and peripheral blood was analyzed 8 weeks after transfer.
Results and discussion
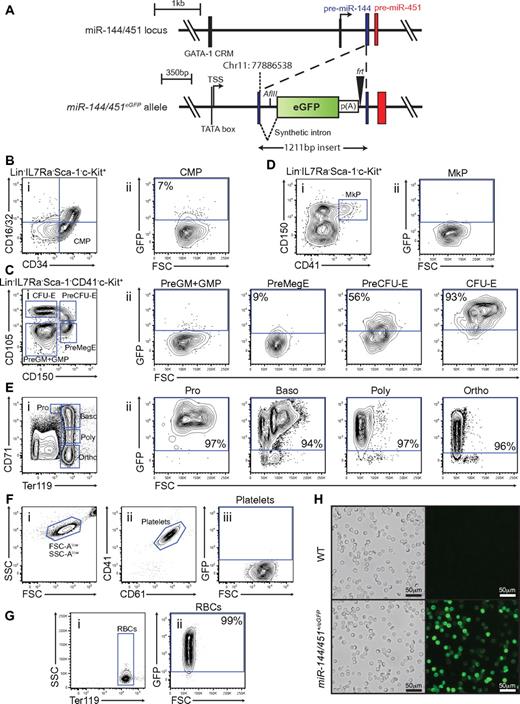
The miR-144/451 cluster represents a promising candidate locus to engineer a reporter for specifically marking terminal differentiated erythrocytes, and erythroid lineage-committed hematopoietic precursors. Firstly, expression of miR-144/451 are dependent on the erythroid transcription factor GATA-1.24 Secondly, they are restricted to the erythroid lineage.9 And finally, miR-451 accumulates to high levels in developing erythroblasts indicating extensive transcriptional activity of the locus.9,14 To investigate the expression pattern in detail, we generated mice carrying an enhanced green fluorescent protein (eGFP) reporter cassette in the stem loop of precursor-miR-144 (Figure 1A, supplemental Figure 1). The insertion of the cassette disrupted expression of both miR-144 and miR-451 from the locus (supplemental Figure 1). As expected, homozygous miR-144/451eGFP/eGFP mice displayed the same phenotype as the previously described miR-144/451−/− animals9 (supplemental Figure 2). However, despite disruption of expression from one allele of the miR-144/451 locus and concomitant decrease in miRNA dosage, heterozygous miR-144/451+/eGFP animals did not show any physiologic and erythropoietic abnormalities (supplemental Figure 2). More importantly, we found that expression of the reporter faithfully distinguished hematopoietic cells expressing miR-144 and miR-451. Strong reporter expression was observed exclusively in Ter119+ erythroblasts among terminal differentiated blood cell lineages and conversely, miR-144 and miR-451 expression fractionated with eGFP expression in sorted miR-144/451+/eGFP bone marrow cells (supplemental Figure 3).
miR-144/451eGFP expression in the hematopoietic system. (A) Schematic overview of the miR-144/451 locus before (top) and after (bottom) insertion of the eGFP expression cassette. The upstream GATA-1 cis-regulatory motif (CRM) and the transcriptional start site (TSS) are indicated. The integration site (chromosome 11: 77886538) of the eGFP cassette within the pre-miR-144 (blue) is shown. The black triangle symbolizes the remaining frt site after Flp-mediated excision of the neor cassette and the white rectangle indicates the SV40 poly(A) sequence. The synthetic intronic sequence introduced to ensure efficient nuclear processing and export of the transcript and the newly introduced AflII restriction site used for Southern analysis are shown. (B) Small population of GFPlow cells observed in the CMP population. (i) Live Lin−, IL-7R−, Sca-1−, c-kit+ gated cells stained for CD34 and CD16/32. The blue gate indicates the CMP subset.17 (ii) Cells gated as CMP as a function of FSC-A and GFP intensity. (C) MiR-144/451eGFP expression is strongly induced on erythroid lineage commitment. (i) Live Lin−, IL-7R−, Sca-1−, c-kit+, CD41− gated cells stained for CD150 and CD105. The blue gates indicate granulo-monocyte precursor (GMP) and pre-granulo-monocyte precursor (Pre-GM), pre-megakaryocyte-erythroid precursor (Pre-MegE), Pre-colony forming-unit erythroid precursor (Pre-CFU-E), colony forming unit erythroid (CFU-E), respectively.19 (ii) The respective populations identified in panel i plotted as a function of FSC-A and GFP intensity. (D) Megakaryocytic precursors (MkP) do not express the reporter. (i) Live Lin−, IL-7R<−, Sca-1−, c-kit+ cells stained for CD150 and CD41. The blue gate indicates the MkP subset.19 (ii) Cells gated as MkP as a function of FSC-A and GFP. (E) All terminal erythropoietic subpopulations are strongly GFP-positive. (i) Live unfractionated bone marrow cells stained for CD71 and Ter119. The blue gates indicate proerythroblast (Pro), basophilic erythroblast (Baso), poly chromatophilic erythroblast (Poly), and ortho-chromatophilic erythroblast (Ortho) populations, respectively.20 (ii) The respective populations identified in panel i plotted as a function of FSC-A and GFP intensity. (F) Mature platelets in the peripheral blood are GFP-negative. (i) EDTA-anticoagulated blood cells plotted as a function of FSC-A and SSC-A. Note the logarithmic axis. (ii) FSC-Alow, SSC-Alow gated cells stained with CD41 and CD61. The blue gate identifies small mature platelets. (iii) Cells gated as platelets as a function of FSC-A and GFP. (G) Mature erythrocytes in peripheral blood are GFP-positive. (i) EDTA-anticoagulated peripheral blood cells stained for Ter119. The blue gate indicates mature RBCs. (ii) Cells gated as RBCs as a function of FSC-A and GFP. (H) Blood smears of fixed peripheral blood show strong endogenous GFP signal from all RBCs. Fluorescence micrographs and corresponding bright field images are shown for wild-type and miR-144/451+/eGFP. All FACS plot shown are representative of stained miR-144/451+/eGFP bone marrow cells, except panels F and G, which are miR-144/451+/eGFP peripheral blood cell stains. The gates for GFP-positive cells in FSC-A vs GFP plots are defined by taking the respective cell population background fluorescence into account using wild-type littermate control stained cells. The percentages shown in the plots indicate the average GFP-positive fraction of the population in question, based on 3 independent experiments.
miR-144/451eGFP expression in the hematopoietic system. (A) Schematic overview of the miR-144/451 locus before (top) and after (bottom) insertion of the eGFP expression cassette. The upstream GATA-1 cis-regulatory motif (CRM) and the transcriptional start site (TSS) are indicated. The integration site (chromosome 11: 77886538) of the eGFP cassette within the pre-miR-144 (blue) is shown. The black triangle symbolizes the remaining frt site after Flp-mediated excision of the neor cassette and the white rectangle indicates the SV40 poly(A) sequence. The synthetic intronic sequence introduced to ensure efficient nuclear processing and export of the transcript and the newly introduced AflII restriction site used for Southern analysis are shown. (B) Small population of GFPlow cells observed in the CMP population. (i) Live Lin−, IL-7R−, Sca-1−, c-kit+ gated cells stained for CD34 and CD16/32. The blue gate indicates the CMP subset.17 (ii) Cells gated as CMP as a function of FSC-A and GFP intensity. (C) MiR-144/451eGFP expression is strongly induced on erythroid lineage commitment. (i) Live Lin−, IL-7R−, Sca-1−, c-kit+, CD41− gated cells stained for CD150 and CD105. The blue gates indicate granulo-monocyte precursor (GMP) and pre-granulo-monocyte precursor (Pre-GM), pre-megakaryocyte-erythroid precursor (Pre-MegE), Pre-colony forming-unit erythroid precursor (Pre-CFU-E), colony forming unit erythroid (CFU-E), respectively.19 (ii) The respective populations identified in panel i plotted as a function of FSC-A and GFP intensity. (D) Megakaryocytic precursors (MkP) do not express the reporter. (i) Live Lin−, IL-7R<−, Sca-1−, c-kit+ cells stained for CD150 and CD41. The blue gate indicates the MkP subset.19 (ii) Cells gated as MkP as a function of FSC-A and GFP. (E) All terminal erythropoietic subpopulations are strongly GFP-positive. (i) Live unfractionated bone marrow cells stained for CD71 and Ter119. The blue gates indicate proerythroblast (Pro), basophilic erythroblast (Baso), poly chromatophilic erythroblast (Poly), and ortho-chromatophilic erythroblast (Ortho) populations, respectively.20 (ii) The respective populations identified in panel i plotted as a function of FSC-A and GFP intensity. (F) Mature platelets in the peripheral blood are GFP-negative. (i) EDTA-anticoagulated blood cells plotted as a function of FSC-A and SSC-A. Note the logarithmic axis. (ii) FSC-Alow, SSC-Alow gated cells stained with CD41 and CD61. The blue gate identifies small mature platelets. (iii) Cells gated as platelets as a function of FSC-A and GFP. (G) Mature erythrocytes in peripheral blood are GFP-positive. (i) EDTA-anticoagulated peripheral blood cells stained for Ter119. The blue gate indicates mature RBCs. (ii) Cells gated as RBCs as a function of FSC-A and GFP. (H) Blood smears of fixed peripheral blood show strong endogenous GFP signal from all RBCs. Fluorescence micrographs and corresponding bright field images are shown for wild-type and miR-144/451+/eGFP. All FACS plot shown are representative of stained miR-144/451+/eGFP bone marrow cells, except panels F and G, which are miR-144/451+/eGFP peripheral blood cell stains. The gates for GFP-positive cells in FSC-A vs GFP plots are defined by taking the respective cell population background fluorescence into account using wild-type littermate control stained cells. The percentages shown in the plots indicate the average GFP-positive fraction of the population in question, based on 3 independent experiments.
We next wanted to perform an in-depth analysis of reporter expression in miR-144/451+/eGFP animals. Reporter expression was absent in the HSC population and all lymphoid- and GM-committed precursors in the bone marrow, as well as in developing T-cell progenitors in the thymus (supplemental Figure 4). Instead, we could detect minor reporter expression in the common myeloid precursor (CMP) and pre-megakaryocyte-erythrocyte precursor (PreMegE) populations (Figure 1B-C). Notably, as cells committed to the erythroid lineage by proceeding through PreMegE, PreCFU-E, and finally CFU-E stages, a dramatic increase in eGFP expression from ∼ 9% to ∼ 93% of the population was seen (Figure 1C). On the contrary, the low level of eGFP expression at the PreMegE stage was completely extinguished as cells differentiated further into megakaryocytic progenitors (MkP; Figure 1D). The strong reporter expression observed at the CFU-E stage was fully maintained as cells went through terminal erythroid differentiation in the bone marrow (Figure 1E). Finally, we bled the mice and examined the peripheral blood for traces of eGFP. In agreement with the absence of reporter expression in the megakaryocytic precursors, also fully differentiated platelets were GFP-negative (Figure 1F). Astoundingly, when examining mature erythrocytes in the peripheral blood we found that all cells (> 99%) were GFP-positive (Figure 1G-H).
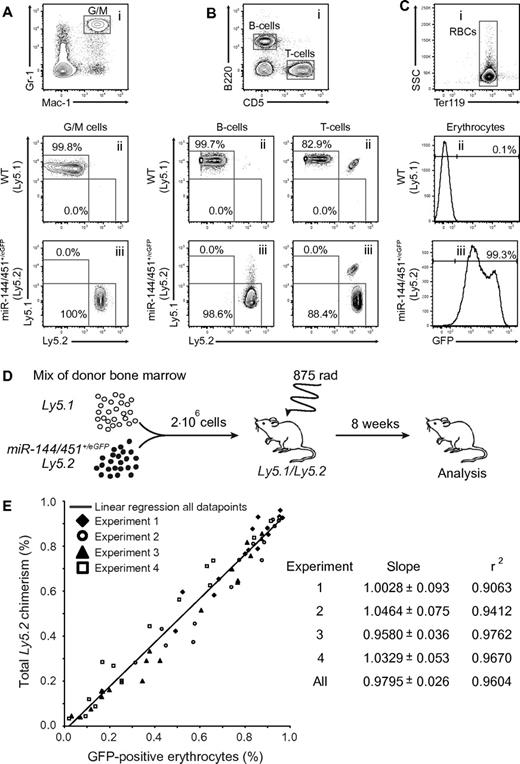
To examine whether the properties of the miR-144/451+/eGFP hematopoietic system could be faithfully recapitulated on bone marrow transplantation we took advantage of the widely used CD45/Ly5 transplantion model. Lethally irradiated recipients were injected with either control (Ly5.1) or experimental (miR-144/451+/eGFP; Ly5.2) bone marrow followed by peripheral blood analysis 8 weeks after transplantation. Similar to WT, miR-144/451+/eGFP cells could fully reconstitute the GM lineage and the B- and T-cell lineage (Figure 2A-B). Importantly, the reconstitution with miR-144/451+/eGFP cells also resulted in > 99% GFP-positive erythrocytes in the peripheral blood of the recipients (Figure 2C). Next, we wanted to examine the quantitative nature of the miR-144/451+/eGFP reporter in a competitive transplantation assay. Bone marrow transplantations were performed with diverse mixtures of control (Ly5.1) or experimental (miR-144/451+/eGFP; Ly5.2) cells (Figure 2D). Remarkably, analysis of peripheral blood in 4 independent experiments revealed a near-perfect linear correlation (r2 > 0.9) between the relative contribution of leukocytes, as measured by Ly5.2 chimerism, and GFP-positive RBCs (Figure 2E). The 2 parameters are directly proportional as the slopes approximate a value of 1 in each experiment (Figure 2E). Hence, the relative fraction of GFP-erythrocytes can be used as a novel direct measure of the HSC output in the erythroid lineage.
miR-144/451eGFP is a quantitative marker of erythroid lineage output.MiR-144/451+/eGFP cells can give rise to (A) G/M cells, (B) B and T cells, and (C) erythroid cells, and marks all erythrocytes in the peripheral blood of bone marrow–transplanted lethally irradiated Ly5.1/5.2 recipients. (i) Representative FACS plots of peripheral blood cells stained for (A) Gr-1 and Mac-1, (B) B220 and CD5, and (C) Ter119. (A-B) Representative FACS plots show the gated populations stained for Ly5.1 and Ly5.2 isolated from animals reconstituted with (ii) Ly5.1 or (iii) miR-144/451+/eGFP:Ly5.2 cells, respectively. Note that a minor population (∼ 15%) of radioresistant recipient-derived T cells can be detected in both wild-type and miR-144/451+/eGFP injected animals. (C) Bottom histograms show gated RBCs plotted for GFP expression (x-axis) from animals reconstituted with (ii) Ly5.1 or (iii) miR-144/451+/eGFP:Ly5.2 cells, respectively. (A-C) Numbers indicate mean fraction of the various gated cell populations based on 5 independent measurements. (D) Experimental setup to evaluate miR-144/451eGFP expression as a quantitative erythroid lineage marker. Lethally irradiated Ly5.1/5.2 recipients were injected with 2 million nucleated whole bone marrow cells representing diverse mixtures of Ly5.1 or miR-144/451+/eGFP:Ly5.2 cells. Eight weeks after transplantation, the fraction of GFP-positive RBCs and Ly5.2 single-positive leukocytes was measured in the peripheral blood. (E) Near-perfect linear correlation between Ly5 chimerism and GFP-positive RBCs. Plot indicates fractions of GFP-positive erythrocytes (x-axis) and Ly5.2 single-positive leukocytes (y-axis) for individual transplanted mice as in panel D. Empty and filled symbols represent individual animals in 4 independent transplantation experiments. The solid line represents simple linear regression based on data points from all experiments. The slope, standard error, and r squared (r2) value computed for each individual experiment as well as for all data points are listed.
miR-144/451eGFP is a quantitative marker of erythroid lineage output.MiR-144/451+/eGFP cells can give rise to (A) G/M cells, (B) B and T cells, and (C) erythroid cells, and marks all erythrocytes in the peripheral blood of bone marrow–transplanted lethally irradiated Ly5.1/5.2 recipients. (i) Representative FACS plots of peripheral blood cells stained for (A) Gr-1 and Mac-1, (B) B220 and CD5, and (C) Ter119. (A-B) Representative FACS plots show the gated populations stained for Ly5.1 and Ly5.2 isolated from animals reconstituted with (ii) Ly5.1 or (iii) miR-144/451+/eGFP:Ly5.2 cells, respectively. Note that a minor population (∼ 15%) of radioresistant recipient-derived T cells can be detected in both wild-type and miR-144/451+/eGFP injected animals. (C) Bottom histograms show gated RBCs plotted for GFP expression (x-axis) from animals reconstituted with (ii) Ly5.1 or (iii) miR-144/451+/eGFP:Ly5.2 cells, respectively. (A-C) Numbers indicate mean fraction of the various gated cell populations based on 5 independent measurements. (D) Experimental setup to evaluate miR-144/451eGFP expression as a quantitative erythroid lineage marker. Lethally irradiated Ly5.1/5.2 recipients were injected with 2 million nucleated whole bone marrow cells representing diverse mixtures of Ly5.1 or miR-144/451+/eGFP:Ly5.2 cells. Eight weeks after transplantation, the fraction of GFP-positive RBCs and Ly5.2 single-positive leukocytes was measured in the peripheral blood. (E) Near-perfect linear correlation between Ly5 chimerism and GFP-positive RBCs. Plot indicates fractions of GFP-positive erythrocytes (x-axis) and Ly5.2 single-positive leukocytes (y-axis) for individual transplanted mice as in panel D. Empty and filled symbols represent individual animals in 4 independent transplantation experiments. The solid line represents simple linear regression based on data points from all experiments. The slope, standard error, and r squared (r2) value computed for each individual experiment as well as for all data points are listed.
In summary, analysis of the miR-144/451eGFP allele revealed unprecedented reporter expression in the entire pool of mature erythrocytes both in terms of specificity and reporter intensity. High transcriptional activity of the locus during terminal erythroid differentiation results in a substantial accumulation of eGFP protein, which in turn concedes detection of RBCs throughout their extended lifetime in circulation. Equivalent to Ly5 mediated tracking of leukocyte output, the miR-144/451eGFP reporter enables single-cell resolution and quantification of in vivo erythroid lineage output from a subset of hematopoietic stem and progenitor cells. Moreover, the absence of reporter expression in early progenitors makes the allele compatible with the usage of already existing GFP-based reporters to isolate HSC subsets for subsequent analysis of erythroid potential. Thus, the miR-144/451eGFP allele represents a novel quantitative in vivo erythroid lineage assay that will be a valuable tool in the study of blood formation and hematopoietic stem and progenitor cell heterogeneity.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge the services of Daniel Bilbao-Cortes and Jeanette Rientjes at EMBL Flow Cytometry Core facility and Monash University's Gene Recombineering facility, respectively.
Authorship
Contribution: K.D.R. contributed to the design, execution, and analysis of the experiments, and wrote the final version of the manuscript; and D.O.C. conceived and supervised this study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dónal O'Carroll, EMBL, Mouse Biology Unit, Via Ramarini 32, Monterotondo Scalo 00015, Italy; e-mail: ocarroll@embl.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal