Abstract
Histones are released from dying cells and contribute to antimicrobial defense during infection. However, extracellular histones are a double-edged sword because they also damage host tissue and may cause death. We studied the interactions of histones with platelets. Histones bound to platelets, induced calcium influx, and recruited plasma adhesion proteins such as fibrinogen to induce platelet aggregation. Hereby fibrinogen cross-linked histone-bearing platelets and triggered microaggregation. Fibrinogen interactions with αIIbβ3 integrins were not required for this process but were necessary for the formation of large platelet aggregates. Infused histones associated with platelets in vivo and caused a profound thrombocytopenia within minutes after administration. Mice lacking platelets or αIIbβ3 integrins were protected from histone-induced death but not from histone-induced tissue damage. Heparin, at high concentrations, prevented histone interactions with platelets and protected mice from histone-induced thrombocytopenia, tissue damage, and death. Heparin and histones are evolutionary maintained. Histones may combine microbicidal with prothrombotic properties to fight invading microbes and maintain hemostasis after injury. Heparin may provide an innate counter mechanism to neutralize histones and diminish collateral tissue damage.
Introduction
In innate immune responses, neutrophils can release DNA fibers with histones after cell death to form extracellular traps (NETs).1 Microbes bind to NETs and are killed by the antimicrobial actions of histones.1,2 However, histones are a double-edged sword because they are also cytotoxic to tissue.3 When intravenously infused into mice, histones cause death within minutes.3 In septic mice, extracellular histones are found in the circulation and contribute to death.3 In septic patients, levels of plasma nucleosomes correlate with disease activity and mortality.4 Inflammation may induce thrombosis also by NETs. NETs provide a scaffold for platelet adhesion and aggregation5 and stimulate fibrin deposition.5,6 Extracellular histones and DNA are important for the prothrombotic functions because they stimulate platelet aggregation5 and coagulation,6,7 respectively.
Inside cells, histones are essential for DNA organization.8 Histones H2A, H2B, H3, and H4 build a histone core that is wrapped by DNA to form nucleosomes. Binding of histone H1 to this complex allows the formation of chromatin strands and chromosomes. Functions of extracellular histones are poorly understood.
Here, we show how platelets respond to histones in vitro and in vivo and describe the mechanism of histone-induced platelet aggregation (HiPA) and thrombocytopenia. We show that heparin, a common anticoagulant, can neutralize extracellular histones in vitro and, on infusion, prevent histone-induced thrombocytopenia and mortality in mice.
Methods
Materials
Reagents were purchased from Sigma-Aldrich or from indicated suppliers.
Mice
We used male C57/BL6 mice and Balb/c mice purchased from The Jackson Laboratory. CalDAG-GEF1−/− mice9 are on a C57BL/6J background; β3−/− mice are on a Balb/c background and were kindly provided by Dr Richard O. Hynes (Massachusetts Institute of Technology).10 For in vivo studies, mice were used at 8-12 weeks of age. Animals were bred at the Immune Disease Institute, and experimental procedures were approved by its Animal Care and Use Committee.
Isolation of murine blood, platelets, and plasma
Blood was collected from the retro-orbital plexus and anticoagulated with 2.5mM EDTA. Blood cell counts were determined by the Hemavet HV950S Hematology System (Drew Scientific). EDTA-blood was centrifuged for 10 minutes at 80g, and platelet rich plasma (PRP) was collected. For preparation of washed platelets, 1 volume of PRP and buffy coat was collected, mixed with 1 volume of Tyrode buffer containing 5 μg/mL prostaglandin E2 (PGE2) and 2 U/mL Apyrase, and centrifuged for 5 minutes at 80g. Next, PRP was collected and centrifuged for 5 minutes at 400g. The pellet was resuspended in Tyrode buffer containing 1 μg/mL PGE2 and centrifuged for 5 minutes at 400g. Platelets or PRP was mixed with Tyrode buffer to 3 × 108 platelets/mL. Control experiments showed that histones also induced platelet aggregation if 3.8% sodium citrate was used as an anticoagulant.
Isolation of human platelets
The investigation conforms to the principles outlined in the Declaration of Helsinki and received approval from the Immune Disease Institute Institutional Review Board. After explaining the nature and possible consequences of the study, we obtained informed consent from all donors. Blood donors were healthy and had not taken any medication for ≥ 10 days. Isolation of human platelets was performed as described5 ; a washing step with Tyrode buffer containing 10 μg/mL PGE1 was included to remove traces of plasma proteins.
Histones
We used either a mixture of all histones isolated from calf thymus (Worthington Biochem) or individual human recombinant histones (New England Biolabs).
Platelet aggregometry
Platelet aggregation was determined by an optical aggregation system (Chrono-Log). One volume of washed platelets was mixed with 1 volume of Tyrode buffer supplemented with 4mM CaCl2. In some experiments, we supplemented the reaction with 2.5% of autologous human plasma and 50μM of the thrombin inhibitor PPACK (Calbiochem). We added human fibrinogen, murine IgG, human recombinant VWF (Baxter) and unfractionated heparin or heparan sulfates at indicated concentrations. The peptidomimetic αIIbβ3 inhibitor tirofiban (Merck) was at 100 ng/mL. Platelet aggregation was induced with calf thymus histones or human recombinant histones at concentrations indicated in the figure legends. ADP at 50μM or collagen at 25 μg/mL (Nycomed) served as positive controls. To analyze platelet aggregates microscopically, platelets were labeled with either 5 μg/mL Calcein-red/orange-AM or in green with CellTrace CFSE (both Invitrogen) for 30 minutes at room temperature. Fluorescent images were acquired by a Zeiss Axiovert 200 inverted fluorescence microscope (objective lens, Plan Apochromat 10×/0.45) in conjunction with a monochrome camera (AxioCam MRm). Colors for fluorescence channels were assigned with Axiovision software (Axio Vs40 Version 4.6.3.0).
Fibrinogen binding to platelets
Washed platelets were mixed in a 1:1 ratio (vol/vol) with 200 μg/mL Alexa Fluor 488 conjugated to fibrinogen (Invitrogen) and 4mM CaCl2. The samples were stimulated with 50μM ADP or calf thymus histones for 5 minutes at 37°C. Fibrinogen binding to platelets was measured by flow cytometry.
Histone binding to platelets and RBCs
Calf thymus histones or BSA (Sigma-Aldrich) were fluorescently labeled with Alexa Fluor 488 dye (Invitrogen) according to the manufacturer's instructions. We mixed washed platelets with different concentrations of labeled histones or BSA. In some experiments, increasing concentrations of heparin were mixed with washed platelets before the addition of fluorescent histones. To determine histone binding to blood cells, we diluted EDTA-blood 10-fold with Tyrode buffer and added either fluorescent histones or BSA at indicated concentrations. After 5 minutes of incubation at 37°C, samples were analyzed by flow cytometry.
Platelet aggregation in whole blood
To analyze HiPA in whole blood, we mixed 2 volumes of blood with 1 volume of buffer containing histones, 6mM CaCl2, and 300μM PPACK. Samples were incubated for 5 minutes at 37°C, and cell counts were determined by the Hemavet HV950S Hematology System. To analyze platelet aggregation in blood by fluorescence microscopy, we labeled platelets in EDTA-blood with 10 μg/mL Rhodamine 6G for 10 minutes at 37°C. The platelet aggregation was induced as described but with fluorescently labeled histones. After 5 minutes at 37°C, we analyzed the samples by fluorescence microscopy.
Measurement of calcium influx
Washed platelets were loaded with 5 μg/mL of the calcium-sensitive dye Fluo-4 (Invitrogen) for 15 minutes at room temperature. Platelets were stimulated with histones for 10 minutes at 37°C. Platelets were then mixed in buffer with and without 5mM CaCl2 and were analyzed immediately by flow cytometry.
Flow chamber experiment
Glass slides were coated with 1 μg of calf thymus histones for 1 hour at 37°C and blocked with 2% BSA for an additional hour at 37°C. In the meantime, blood was collected from mice, mixed with PPACK (50μM), and recalcified with 2mM CaCl2. Rhodamine 6G (10 μg/mL) was added to label platelets. Thereafter, glass slides were perfused with the modified blood at a shear rate of 400/s, and fluorescent images were recorded.
Histone-induced thrombocytopenia or mortality
Calf thymus histones were injected retro-orbitally at 10-50 mg/kg to induce thrombocytopenia and 60 mg/kg to induce mortality. In some experiments, mice were treated with unfractionated heparin from porcine intestinal mucosa. Platelets were depleted by intravenous application of an anti-GP Ib antibody (3 mg/kg; Emfret Analytics) 4 hours before the experiment. Control mice received isotype IgG.
Bleeding time measurement
Mice were infused with 50 mg/kg histones or vehicle. After 20 minutes the tail bleeding time was determined by removing 3 mm of the distal mouse tail and immersing the tail in 37°C PBS. A complete cessation of bleeding for > 120 seconds was defined as the bleeding time. Bleeding time measurements > 900 seconds were stopped by cauterization of the tail.
Histologic analysis
Frozen sections (20 μm) of lungs were fixed with 4% paraformaldehyde for 30 minutes at 37°C. Parallel samples were subjected to immunohistochemistry and incubated with 2 μg/mL rat anti–mouse-CD41 (BD Bioscience), rabbit anti-H3 (Abcam), or isotype control antibodies (BD Bioscience). After washing, the following Alexa-conjugated secondary Abs (Invitrogen) were used: goat-anti-rat-IgG (10 μg/mL) and goat anti–rabbit-IgG (10 μg/mL). Cell nuclei were counterstained with 1 μg/mL Hoechst 33342 (Invitrogen). Sections from formalin-fixed and paraffin-embedded lungs were stained with H&E. Images of H&E staining were acquired by a Zeiss Axioplan microscope (objective lens, Plan Neofluar 10×/0.30) in conjunction with a color camera (AxioCam HRc).
Histone-induced cytotoxicity
Cells from the murine endothelial cell line bEnd3 (American Type Culture Collection; CRL-2299) were cultured to confluence and incubated with 100 μg/mL heparin or 20 μg/mL recombinant histone H4 for 30 minutes at 37°C. Cells were stained with 1μM SytoxGreen to detect nuclei of dead cells.
Statistical evaluation
Statistical analysis included mean ± SEM, ANOVA, and Student t test. Results were considered significant at P < .05.
Results
Histone-induced platelet aggregation uses αIIbβ3 integrins
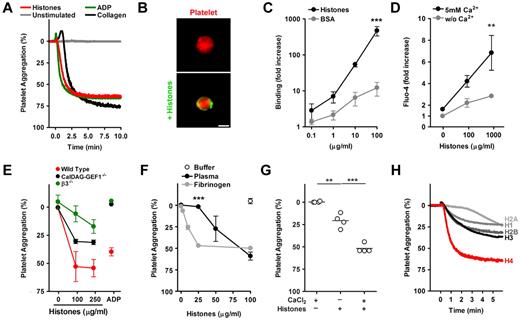
Platelet aggregation generally results from cross-linking of platelet integrin αIIbβ3 by plasma fibrinogen.11 Stimulation of murine platelets in plasma with histones, ADP, or collagen causes aggregation with similar efficiency and kinetics (Figure 1A). To decipher the mechanism of HiPA, we first tested whether histones can bind to platelets. Fluorescently labeled histones localized to the surface of washed platelets, indicating that histones and platelets interact directly (Figure 1B). Quantification showed strong binding of fluorescent histones, but not BSA, to washed platelets (Figure 1C). The binding did not require calcium (not shown) and did not appear to be saturable. Histones can induce calcium influx into different cell types,3,12,13 and we questioned whether this function applies to histone-platelet interactions as well. We loaded platelets with a calcium-sensitive fluorescent dye and incubated them with histones. Elevated fluorescence after the addition of calcium indicated histone-induced calcium influx into platelets (Figure 1D). A rise in intracellular calcium concentrations initiates a signaling cascade in platelets, which leads to the activation of αIIbβ3 integrins. CalDAG-GEF1 is a sensor of intracellular calcium implicated in this signaling process.9 We compared HiPA of platelets isolated from control mice, CalDAG-GEF1−/− mice, and β3−/− mice, which lack functional αIIbβ3 integrins (Figure 1E). Deficiency in either CalDAG-GEF1 or β3 integrins impaired platelet aggregation in response to histones. However, CalDAG-GEF1–deficient platelets showed a mild defect compared with platelets lacking β3 integrins (P < .01 at 100 μg/mL). CalDAG-GEF1– and β3-deficient platelets both did not aggregate in response to ADP. This indicates that ADP and histone-induced aggregations are not mechanistically identical.
Histone-induced platelet aggregation requires β3-integrins. (A) Histones induce platelet aggregation. PRP was mixed at t = 0 minutes with histones (200 μg/mL), ADP (50μM), or collagen (25 μg/mL) or left unstimulated. Histones induced aggregation as efficiently as ADP or collagen. (B) Histones bind to platelets. Fluorescence microscopy of platelets labeled in red incubated with BSA or histones labeled fluorescently in green. Histones localize to the platelet surface. Scale bar = 2 μm. (C) Quantification of histone or BSA binding to washed platelets by flow cytometry (***compared with BSA; n = 3). (D) Histones induce calcium influx into platelets. Platelets loaded with the calcium-sensitive dye Fluo-4 and stimulated with indicated concentrations of histones for 5 minutes. Platelets were resuspended in medium with or without CaCl2, and fluorescence was analyzed by flow cytometry (**compared with or without Ca2+; n = 6). (E) Comparison of histone-induced aggregation of control mice (C57, red), CalDAG-GEF1 (black), or β3-integrin (green) deficient platelets, 5 minutes after stimulation with indicated concentrations of histones or ADP (50μM). CalDAG-GEF1 deficiency leads to an impaired platelet aggregation in response to histones, but it is less severe than β3-integrin deficiency. (F) Histone-induced platelet aggregation requires plasma proteins. Dose-dependent response of platelets to histones in the presence of plasma (black circles), 200 μg/mL fibrinogen (gray circles), or the response of washed platelets with buffer (white circle); n = 3; ***plasma compared with fibrinogen. (G) Exogenous CaCl2 (2mM) enhanced HiPA. Data show extent of platelet aggregation 3 minutes after stimulation (n = 4). (H) Aggregation of washed platelets stimulated with 1μM recombinant histone H1, H2A, H2B, H3, or H4 in the presence of 200μg/mL fibrinogen. Histone H4 induced platelet aggregation potently. Data presented are representative of ≥ 3 independent experiments. *P < .05, **P < .01, and ***P < .001.
Histone-induced platelet aggregation requires β3-integrins. (A) Histones induce platelet aggregation. PRP was mixed at t = 0 minutes with histones (200 μg/mL), ADP (50μM), or collagen (25 μg/mL) or left unstimulated. Histones induced aggregation as efficiently as ADP or collagen. (B) Histones bind to platelets. Fluorescence microscopy of platelets labeled in red incubated with BSA or histones labeled fluorescently in green. Histones localize to the platelet surface. Scale bar = 2 μm. (C) Quantification of histone or BSA binding to washed platelets by flow cytometry (***compared with BSA; n = 3). (D) Histones induce calcium influx into platelets. Platelets loaded with the calcium-sensitive dye Fluo-4 and stimulated with indicated concentrations of histones for 5 minutes. Platelets were resuspended in medium with or without CaCl2, and fluorescence was analyzed by flow cytometry (**compared with or without Ca2+; n = 6). (E) Comparison of histone-induced aggregation of control mice (C57, red), CalDAG-GEF1 (black), or β3-integrin (green) deficient platelets, 5 minutes after stimulation with indicated concentrations of histones or ADP (50μM). CalDAG-GEF1 deficiency leads to an impaired platelet aggregation in response to histones, but it is less severe than β3-integrin deficiency. (F) Histone-induced platelet aggregation requires plasma proteins. Dose-dependent response of platelets to histones in the presence of plasma (black circles), 200 μg/mL fibrinogen (gray circles), or the response of washed platelets with buffer (white circle); n = 3; ***plasma compared with fibrinogen. (G) Exogenous CaCl2 (2mM) enhanced HiPA. Data show extent of platelet aggregation 3 minutes after stimulation (n = 4). (H) Aggregation of washed platelets stimulated with 1μM recombinant histone H1, H2A, H2B, H3, or H4 in the presence of 200μg/mL fibrinogen. Histone H4 induced platelet aggregation potently. Data presented are representative of ≥ 3 independent experiments. *P < .05, **P < .01, and ***P < .001.
We analyzed the contribution of plasma and calcium to HiPA. A dose response showed that 100 μg/mL histones stimulated platelet aggregation in the presence of plasma, whereas washed platelets did not aggregate (Figure 1F). Platelet aggregation could be restored after the addition of fibrinogen to washed platelets (Figure 1F). The addition of IgG did not have such an effect (data not shown). A lower concentration of histones was required to induce aggregation in the presence of fibrinogen compared with plasma. This could be because histones interact with plasma fibrinogen14 or that plasma neutralizes histones.14 Exogenous calcium was necessary for optimal HiPA (Figure 1G). To test the activity of individual histones, we mixed washed murine platelets with fibrinogen and tested whether recombinant histones were able to induce platelet aggregation. At equimolar concentrations, recombinant histone H4 displayed potent activity (Figure 1H), whereas recombinant histones H1, H2A, H2B, and H3, were less effective.
Taken together, these data indicate that HiPA results from histone activation of platelets followed by fibrinogen-mediated cross-linking of platelet αIIbβ3. These findings were corroborated by pharmacology because inhibition of αIIbβ3 by tirofiban blocked HiPA in human platelets (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Histones recruit fibrinogen and cause platelet microaggregation by αIIbβ3-dependent and -independent mechanisms
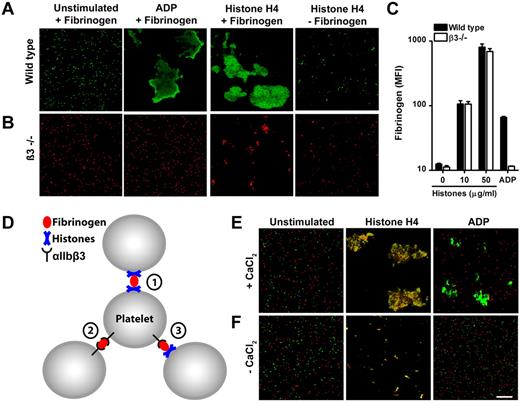
We analyzed the aggregation of fluorescently labeled wild-type (Figure 2A) and β3-deficient platelets (Figure 2B) in the presence of fibrinogen microscopically. Stimulation of wild-type cells with recombinant histone H4 or ADP caused the transformation of individual cells to large aggregates (Figure 2A). Platelets deficient in β3-integrins did not form large aggregates in response to histones or ADP (Figure 2B). Importantly, histones but not ADP induced the formation of small aggregates in the absence of β3-integrins (Figure 2B). This process was not observed when platelets were stimulated in the absence of fibrinogen (Figure 2B).
Histones recruit fibrinogen and cause platelet aggregation through mechanisms dependent and independent of αIIbβ3 integrins. Fluorescence imaging of wild-type (A) or β3-integrin–deficient (B) platelets stimulated in the presence or absence of fibrinogen with ADP (50μM) or recombinant histone H4 (10 μg/mL). Histone H4 or ADP induce large platelet aggregates of wild-type but not β3-integrin–deficient platelets. Recombinant histone H4 but not ADP induces microaggregation of β3-integrin–deficient platelets. This microaggregation depended on the presence of fibrinogen. (C) Histones induce β3-integrin–independent fibrinogen binding to platelets. Washed platelets stimulated with the indicated concentrations of histones or ADP (50μM) were incubated with fluorescent fibrinogen and analyzed by flow cytometry. (D) Hypothesized interactions of fibrinogen with αIIbβ3 and histones in HiPA. Fibrinogen can bind to αIIbβ3 and histones on platelets. Fluorescence imaging of wild-type (green) or β3-integrin–deficient (red) platelets mixed with fibrinogen in response to ADP or histones in the presence (E) or absence (F) of CaCl2. Recombinant histone H4 but not ADP induced the formation of large platelet aggregates consisting of wild-type and β3-deficient platelets. This process depended on exogenous CaCl2. Scale bar = 100 μm. Data presented are representative of ≥ 3 independent experiments.
Histones recruit fibrinogen and cause platelet aggregation through mechanisms dependent and independent of αIIbβ3 integrins. Fluorescence imaging of wild-type (A) or β3-integrin–deficient (B) platelets stimulated in the presence or absence of fibrinogen with ADP (50μM) or recombinant histone H4 (10 μg/mL). Histone H4 or ADP induce large platelet aggregates of wild-type but not β3-integrin–deficient platelets. Recombinant histone H4 but not ADP induces microaggregation of β3-integrin–deficient platelets. This microaggregation depended on the presence of fibrinogen. (C) Histones induce β3-integrin–independent fibrinogen binding to platelets. Washed platelets stimulated with the indicated concentrations of histones or ADP (50μM) were incubated with fluorescent fibrinogen and analyzed by flow cytometry. (D) Hypothesized interactions of fibrinogen with αIIbβ3 and histones in HiPA. Fibrinogen can bind to αIIbβ3 and histones on platelets. Fluorescence imaging of wild-type (green) or β3-integrin–deficient (red) platelets mixed with fibrinogen in response to ADP or histones in the presence (E) or absence (F) of CaCl2. Recombinant histone H4 but not ADP induced the formation of large platelet aggregates consisting of wild-type and β3-deficient platelets. This process depended on exogenous CaCl2. Scale bar = 100 μm. Data presented are representative of ≥ 3 independent experiments.
Fibrinogen binds to an active conformation of αIIbβ3 on activated platelets. We show that fluorescently labeled fibrinogen binds to platelets stimulated with histones independent of β3 integrins (Figure 2C). Platelets deficient in β3 integrins and stimulated with ADP served as a negative control. These results suggest that histone-induced microaggregation and macroaggregation of platelets are achieved by different mechanisms which are independent or dependent of αIIbβ3 integrins, respectively.
We deduced 3 hypothetical interplays of histones, fibrinogen and αIIbβ3 (Figure 2D). (1) Fibrinogen cross-links histones bound to platelets. This process leads to microaggregation and is independent of calcium and αIIbβ3 integrins. (2) Histones bind to platelets and induce activation of αIIbβ3 integrins, which in turn are cross-linked by fibrinogen. (3) Fibrinogen cross-links platelets with activated αIIbβ3 and histone-bearing platelets. Mechanisms 2 and 3 require calcium and αIIbβ3 integrins. To test for mechanism 3 in HiPA, we stimulated a mixture of wild-type (Figure 2E green) and β3-deficient (Figure 2E red) platelets with histones (Figure 2E). ADP served as a control in these experiments and induced aggregation only of wild-type platelets. In response to recombinant histone H4, we observed orange aggregates, which indicated the presence of wild-type and β3-deficient cells as suggested by mechanism 3. This effect was not observed in the absence of calcium (Figure 2F). We conclude that mechanisms 1, 2, and 3 probably cooperate, and fibrinogen may cross-link platelets with activated αIIbβ3 and/or histones in HiPA. Interestingly, human recombinant VWF enhanced HiPA similarly to fibrinogen (supplemental Figure 2). Histones interact with VWF15 and fibrinogen14,16 ; therefore, ligands, which bind both histones and αIIbβ3, may enable HiPA.
Extracellular histones induce thrombocytopenia in mice
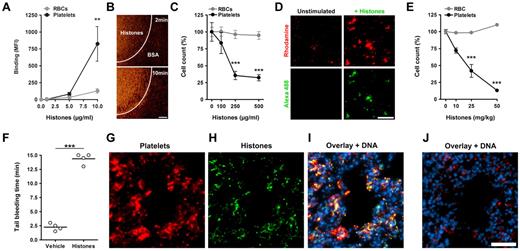
We tested the effects of histones on platelets in whole blood in vitro. Incubation of blood with fluorescent histones showed that histones bound to platelets under static (Figure 3A) or flow (Figure 3B) conditions. Incubation of blood with histones resulted in a drop in platelet but not RBC counts (Figure 3C). This in vitro thrombocytopenia reflects the loss of individual platelets because of platelet aggregation in blood17 induced by histones (Figure 3D). These results indicated that in blood histones bind preferentially to platelets over RBCs in vitro. We infused histones at sublethal concentrations intravenously into mice and sampled blood after 10 minutes. Histones decreased the platelet but not RBC counts in a dose-dependent manner (Figure 3E). At 50 mg/kg histones, which was the highest nonlethal concentration, histones depleted ∼ 90% of platelets from circulation. As would be expected, the thrombocytopenia was associated with a prolonged bleeding time (Figure 3F). Immunohistochemistry showed that, after histone infusion, platelet aggregates (Figure 3G) colocalized with histones in lungs (Figure 3H-I). Control lungs did not show platelet aggregates or histones (Figure 3J). Histones in nuclei were not stained in these experiments. In summary, histones induced platelet aggregation in vivo, thus producing a rapid and profound thrombocytopenia.
Histones induce thrombocytopenia. Histones bind to platelets in blood. (A) Fluorescent histones were mixed at the indicated concentrations with blood. Flow cytometric analysis showed that histones bind preferentially to platelets over RBCs in blood (**compared with RBCs; n = 5). (B) Perfusion of Rhodamine 6G–labeled platelets in blood over a histone-coated surface shows platelet binding to histones. Areas covered with BSA only did not bind platelets. Scale bar = 100 μm. (C) Histones induce thrombocytopenia in blood in vitro. Single platelet and RBC counts of blood mixed with indicated concentrations of histones. At concentrations of 250 μg/mL histones induced thrombocytopenia in vitro (***compared with RBCs; n = 3). (D) Microscopic analysis of Rhodamine 6G–labeled platelets in blood before (Unstimulated) and after stimulation with Alexa 488–labeled histones (+Histones). Histones induce and localize to platelet aggregates in blood. Scale bar = 50 μm. Histones induce thrombocytopenia in vivo. (E) Platelet and RBC counts of mice 10 minutes after infusion with indicated sublethal concentrations of histones (***compared with RBCs; n = 3). Histone infusion caused dose-dependent depletion of platelets from circulation. (F) Determination of tail bleeding time. Mice infused with histones (50 mg/kg) but not vehicle showed prolonged bleeding time. Histones associate with platelets in vivo. Immunostaining of lungs dissected 10 minutes after infusion with histones (G-I; 50 mg/kg) or vehicle (J). Lungs were stained for CD41 (platelets), histone H3 (histones), and DNA. Platelets (G) and histones (H) colocalized in mice infused with histones (I) but not in mice infused with vehicle (J). Under these conditions, histone staining of nuclei was below the detection limit. Scale bar = 50 μm. Data presented are representative of ≥ 3 independent experiments. **P < .01, ***P < .001.
Histones induce thrombocytopenia. Histones bind to platelets in blood. (A) Fluorescent histones were mixed at the indicated concentrations with blood. Flow cytometric analysis showed that histones bind preferentially to platelets over RBCs in blood (**compared with RBCs; n = 5). (B) Perfusion of Rhodamine 6G–labeled platelets in blood over a histone-coated surface shows platelet binding to histones. Areas covered with BSA only did not bind platelets. Scale bar = 100 μm. (C) Histones induce thrombocytopenia in blood in vitro. Single platelet and RBC counts of blood mixed with indicated concentrations of histones. At concentrations of 250 μg/mL histones induced thrombocytopenia in vitro (***compared with RBCs; n = 3). (D) Microscopic analysis of Rhodamine 6G–labeled platelets in blood before (Unstimulated) and after stimulation with Alexa 488–labeled histones (+Histones). Histones induce and localize to platelet aggregates in blood. Scale bar = 50 μm. Histones induce thrombocytopenia in vivo. (E) Platelet and RBC counts of mice 10 minutes after infusion with indicated sublethal concentrations of histones (***compared with RBCs; n = 3). Histone infusion caused dose-dependent depletion of platelets from circulation. (F) Determination of tail bleeding time. Mice infused with histones (50 mg/kg) but not vehicle showed prolonged bleeding time. Histones associate with platelets in vivo. Immunostaining of lungs dissected 10 minutes after infusion with histones (G-I; 50 mg/kg) or vehicle (J). Lungs were stained for CD41 (platelets), histone H3 (histones), and DNA. Platelets (G) and histones (H) colocalized in mice infused with histones (I) but not in mice infused with vehicle (J). Under these conditions, histone staining of nuclei was below the detection limit. Scale bar = 50 μm. Data presented are representative of ≥ 3 independent experiments. **P < .01, ***P < .001.
Heparin prevents histone-induced thrombocytopenia, tissue damage, and lethality
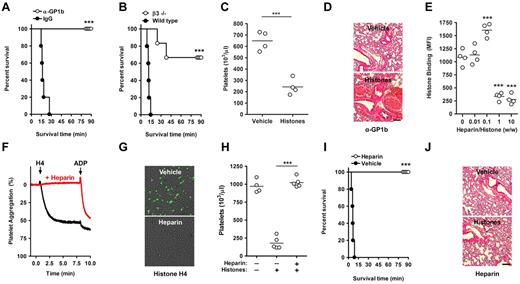
We wanted to address the role of platelets in histone-induced lethality. Death after histone infusion occurs within minutes.3 Mice immunodepleted of platelets by infusion of anti-GP1b antibodies, but not mice treated with control IgG, survived a lethal dose of histones (Figure 4A). Mice deficient in β3 integrins were protected from histone-induced death as well (Figure 4B) but not from thrombocytopenia (Figure 4C). The drop in platelet count may result from β3-independent microaggregation. Deficiency in β3-integrins might prevent the formation of large platelet aggregates and thus protect from death in this model. Neither platelet depletion (Figure 4D) nor β3-deficiency (not shown) protected mice from the previously reported histone-induced tissue damage and hemorrhage,3 which is presumably caused by cytotoxic actions of histones on endothelium. We observed that histones induced lethality in the absence of platelets or β3-integrins at higher concentrations (≥ 75 mg/kg), indicating that histone interactions with other cell types can induce death as well.
Heparin prevents histone-induced thrombocytopenia, tissue damage, and mortality. Platelets contribute to histone-induced mortality. (A) Platelet depletion protects from histone-induced death. Infusion of platelet-depleting antibodies (anti-GP1b) but not control IgG protected mice from a lethal dose of histones (60 mg/kg). (B) β3-Integrin deficiency partially protects from histone-induced death. Survival time of wild-type and β3-integrin–deficient mice infused with histones (60 mg/kg). (C) β3-Integrin deficiency does not protect from histone-induced thrombocytopenia (25 mg/kg). Platelet counts of β3−/− mice 10 minutes after infusion with vehicle or histones. (D) Platelet depletion does not protect from histone-induced tissue damage. H&E stainings of lungs from platelet-depleted mice (anti-Gp1b) 90 minutes after the infusion of vehicle or histones. Hemorrhage indicates histone-induced damage of the vascular endothelium. Scale bars = 250 μm. (E) At high concentrations, heparin prevents the interaction of histones with platelets. Flow cytometric analysis of histone binding to platelets in the presence of indicated concentrations of heparin (***compared with 0). (F) Heparin prevents HiPA. Aggregometry of platelets mixed with plasma in the presence (red) or absence (black) of heparin and in response to recombinant histone H4 or ADP. (G) Heparin neutralizes histone-induced cytotoxicity. Overlays of SytoxGreen fluorescence and phase-contrast images. A murine endothelial cell line was incubated in the presence or absence of heparin (100 μg/mL) with recombinant histone H4 (20 μg/mL). Cell death was detected by staining with the cell-impermeable DNA dye SytoxGreen. The presence of heparin prevented cytotoxicity. (H) Heparin prevents histone-induced thrombocytopenia. Platelet counts of untreated mice, mice infused with histones (50 mg/kg), and mice treated with heparin (50 mg/kg) before histone infusion. (I) Heparin prevents histone-induced lethality. Survival of mice treated with 50 mg/kg heparin or vehicle before infusion of 75 mg/kg histones. (J) Heparin prevents histone-induced hemorrhage in lungs. H&E stainings of lungs from mice treated with heparin after the infusion of vehicle or histones. Data are representative of ≥ 3 independent experiments. *P < .05, **P < .01, and ***P < .001.
Heparin prevents histone-induced thrombocytopenia, tissue damage, and mortality. Platelets contribute to histone-induced mortality. (A) Platelet depletion protects from histone-induced death. Infusion of platelet-depleting antibodies (anti-GP1b) but not control IgG protected mice from a lethal dose of histones (60 mg/kg). (B) β3-Integrin deficiency partially protects from histone-induced death. Survival time of wild-type and β3-integrin–deficient mice infused with histones (60 mg/kg). (C) β3-Integrin deficiency does not protect from histone-induced thrombocytopenia (25 mg/kg). Platelet counts of β3−/− mice 10 minutes after infusion with vehicle or histones. (D) Platelet depletion does not protect from histone-induced tissue damage. H&E stainings of lungs from platelet-depleted mice (anti-Gp1b) 90 minutes after the infusion of vehicle or histones. Hemorrhage indicates histone-induced damage of the vascular endothelium. Scale bars = 250 μm. (E) At high concentrations, heparin prevents the interaction of histones with platelets. Flow cytometric analysis of histone binding to platelets in the presence of indicated concentrations of heparin (***compared with 0). (F) Heparin prevents HiPA. Aggregometry of platelets mixed with plasma in the presence (red) or absence (black) of heparin and in response to recombinant histone H4 or ADP. (G) Heparin neutralizes histone-induced cytotoxicity. Overlays of SytoxGreen fluorescence and phase-contrast images. A murine endothelial cell line was incubated in the presence or absence of heparin (100 μg/mL) with recombinant histone H4 (20 μg/mL). Cell death was detected by staining with the cell-impermeable DNA dye SytoxGreen. The presence of heparin prevented cytotoxicity. (H) Heparin prevents histone-induced thrombocytopenia. Platelet counts of untreated mice, mice infused with histones (50 mg/kg), and mice treated with heparin (50 mg/kg) before histone infusion. (I) Heparin prevents histone-induced lethality. Survival of mice treated with 50 mg/kg heparin or vehicle before infusion of 75 mg/kg histones. (J) Heparin prevents histone-induced hemorrhage in lungs. H&E stainings of lungs from mice treated with heparin after the infusion of vehicle or histones. Data are representative of ≥ 3 independent experiments. *P < .05, **P < .01, and ***P < .001.
Histones are basic proteins and have high affinity toward heparin.18 Heparin is a highly negatively charged glycosaminoglycan (GAG) found in mast cells.19 Heparan sulfate is found on cell surfaces and is closely related to heparin. Histone binding to platelets was inhibited in the presence of heparin (Figure 4E) or heparan sulfate (supplemental Figure 3). We observed dose-dependent effects of heparin on the interaction of histones with platelets. At a ratio of 1:10 (wt/wt) of heparin to histones, the binding of histones to platelets was somewhat enhanced, whereas histone-platelet interactions were inhibited at 10-fold higher doses of heparin (Figure 4E). Heparin binds to platelets20 ; therefore, we believe that at low concentrations it may help recruit some histones to the platelet, whereas at high concentrations free heparin absorbs histones and prevents their binding to platelets.
In further experiments, we used heparin at least at a ratio of 1:1 (wt/wt) to histones. Then heparin prevented HiPA (Figure 4F) and also histone-induced cytotoxicity to endothelium (Figure 4G). In vivo, heparin prevented histone-induced thrombocytopenia (Figure 4H) and lethality (Figure 4I). Lungs of heparin-treated mice surviving the infusion of histones did not show hemorrhage and appeared similar to lungs of mice not infused with histones (Figure 4J).
Discussion
Antibodies targeting histone/DNA complexes suppress coagulation at the site of injury,6 and the supporting role of nucleic acids in blood coagulation has been established.6,7 Here, we also show that the histone-platelet interaction could potentially contribute to thrombosis. Our data show that histone can bind to platelets, induce calcium influx, and recruit plasma adhesion molecules to induce platelet aggregation. This is in agreement with the observation by Xu et al3 that infusion of histones leads to microthrombi formation in the lung.
Although histones have been reported to interact with a variety of cell types,3,12,13 we show that in blood histones bind preferentially to platelets over RBCs in vitro and in vivo. How histones bind to platelets is not clear, and a receptor for histones has not yet been identified. Histones may bind directly to the plasma membrane of cells by interactions with lipids.13,21 GAGs, including heparan sulfates on cell surfaces, may also be implicated in the interaction with histones.19 GAGs are negatively charged and thus could support binding of positively charged histones. It is conceivable that charge contributes to the binding of histones, because polycations bind to negatively charged proteoglycans on platelets and elicit platelet aggregation.22 However, despite a nearly identical charge,23 not all histones induce platelet aggregation.5 It is probable that, in addition to charge, molecular characteristics of histones, such as specific binding of platelet receptors or fibrinogen14,16 and VWF,15 are responsible for their actions.
We observed that the binding of histones to platelets was not saturable. This may indicate either that histones interact with the plasma membrane directly or that the number of binding sites increases with platelet activation. This is probable because histone-activated platelets release VWF and fibrinogen, a portion of which re-associates with platelets, thus providing new binding sites for histones.
We observed potent platelet aggregation in response to calf thymus histones and recombinant histone H4. Histones from calf thymus contain posttranslational modifications such as acetylation and methylation of positively charged amino acids. In a mixture of histones, intermolecular interactions such as dimerization of H2A-H2B or H3-H4 are also enabled. Both mechanisms are absent when platelets are stimulated with individual, recombinant histones. Posttranslational modifications or interactions between histones could regulate functions of extracellular histones. Future studies are needed to address this possibility.
The interaction of histones with different cell types results in an influx of calcium without mobilization of internal calcium stores.12 The influx is not selective for calcium ions and is accompanied by membrane depolarization.12,13 However, the mechanism is controversial and requires further investigation. On the one hand, histones bound to liposomes were reported to form pores,13 and, on the other hand, histone binding to cells may open existing channels.12 In platelets, a rise in intracellular calcium concentrations triggers activation of αIIbβ3,9 and it is probable that histones use this mechanism to activate platelets.
Because histones are also able to bind the platelet adhesion molecules fibrinogen and VWF, they are well equipped to stimulate platelet aggregation. Indeed, interactions between histones and fibrinogen contribute to HiPA in our in vitro studies, but histone-mediated αIIbβ3 activation makes this process more efficient. Future studies may show whether agents that specifically neutralize histones protect against thrombosis.
Infusion of histones induces sepsis-like symptoms.3 Three hours after administering histones, lungs show tissue damage, hemorrhage, intravascular platelet-rich thrombi, and neutrophil infiltration. Our studies show that platelets disappear from circulation within the first minutes after histone infusion, and platelets together with histones deposit in lungs. Thrombocytopenia and platelet deposition with histones occur in lungs of septic mice.24,25 Moreover, in sepsis platelets stimulate neutrophils to release histones in the form of NETs,25 indicating a positive feedback mechanism. Further studies should address whether histones also induce thrombocytopenia in sepsis.
Heparin is commonly known as an anticoagulant, but it has also been successfully used for the treatment of inflammatory conditions.19 Heparin is highly sulfated and thus is rich in negative charges. It is probable that electrostatic interactions of low specificity but high affinity are responsible for the histone-neutralizing effects of heparin. Heparin and its close relative heparan sulfate, the natural anticoagulant of endothelium, have been maintained throughout evolution and, interestingly, are present in species without a coagulation system.26 Similar to heparin or heparan sulfate, histones are highly evolutionarily conserved. Functionally, histones combine microbicidal2 and cytotoxic functions3 with prothrombotic properties.5 The exposure of histones after injury may provide a basic mechanism to prevent infections and promote local hemostasis. Heparin and heparan sulfate may be evolutionarily maintained to prevent cytotoxicity and collateral damage from histones released from injured tissue and during innate immune responses.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Lesley Cowan for help preparing the manuscript.
This work was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health (grant P01 HL056949, D.D.W.) and a fellowship from the Deutsche Forschungsgemeinschaft, Germany (FU 742/1-1, T.A. F.).
National Institutes of Health
Authorship
Contribution: T.A.F. designed and performed the experiments and wrote the manuscript; A.A.B. performed experiments; and D.D.W. wrote the manuscript and co-designed the study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Tobias A. Fuchs, Immune Disease Institute, 3 Blackfan Cir, 3rd Fl, Boston, MA 02115; e-mail: fuchs@idi.harvard.edu; and Denisa D. Wagner, Immune Disease Institute, 3 Blackfan Cir, 3rd Fl, Boston, MA 02115; e-mail: wagner@idi.harvard.edu

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal