Abstract
After administration of granulocyte colony-stimulating factor (G-CSF), there is a marked, albeit transient, drop in circulating neutrophils. To determine the role of leukocyte integrins in this disappearance, a dog having canine leukocyte adhesion deficiency (CLAD) or CLAD dogs who had undergone gene correction either by matched littermate allogeneic transplant or autologous gene therapy were evaluated. Shortly after G-CSF administration, a dramatic, yet transient, neutropenia was observed in the control littermates. This neutropenia was not as marked in the CLAD dogs. In all instances, it was CD18+ neutrophils that preferentially egressed from the circulation. The association of CD18 with this rapid loss suggested leukocyte integrin activation after G-CSF administration. To determine the activation status of the integrin, a monoclonal antibody recognizing the activated α-subunit cation binding domain (mAb24) was used to evaluate human leukocytes after G-CSF administration. Mirroring the dramatic decrease in circulating neutrophil numbers, there was a dramatic and specific increase in the activation of the α-subunit after G-CSF expression on polymorphonuclear leukocytes. This activation, like the drop in neutrophil count, was transient. These results demonstrate that the leukocyte integrin on circulating neutrophils is transiently activated after G-CSF administration and mediates the transient neutropenia observed after G-CSF administration.
Introduction
For more than 20 years, granulocyte colony-stimulating factor (G-CSF) has been administered therapeutically to treat neutropenia1-3 and to mobilize hematopoietic stem cells into the circulation.4 In rats,5 humans,6,7 and nonhuman primates,8,9 a dramatic, yet transient, loss of circulating neutrophils has been reported shortly after G-CSF administration. As many as 80% to 90% of circulating neutrophils disappear from the circulation within the first 30 minutes of its administration, with the majority of the neutrophils appearing in the lung and spleen.9 Circulating neutrophil counts return to baseline levels by 60 minutes. The mechanism for this transient sequestration remains unclear.
Leukocyte cell adhesion molecules are heterodimeric receptors that form a subfamily within the integrin supergene family.10 They share a common β-subunit (CD18) and are designated according to their differing α-subunits as lymphocyte-function associated antigen 1 (α-chain CD11a), complement receptor 3 (α-chain CD11b), or complement component 3 receptor 4 (α-chain CD11c), or α-chain CD11d. Ligand specificity is dictated by the distinctive α-subunits. Monoclonal antibody 24 (mAb24) defines a unique epitope present on the α-subunits and recognizes the occupied divalent cation binding site of the activated integrin.11,12 Binding of divalent cations, such as Mn2+ and Mg2+, alters the conformation of lymphocyte-function associated antigen 1 on T cells to favor binding to its ligand, intercellular adhesion molecule-1. The divalent cation Ca2+, however, acts as a negative regulator, maintaining leukocyte integrin function in an inactive state.12
The CD18 leukocyte integrin β-chain on neutrophils has been shown to mediate neutrophil trafficking to inflammatory sites.13 Genetic leukocyte adhesion deficiencies (LADs) resulting from mutations in CD18 have been described in children, cattle, and dogs.14 Lack of CD18 results in the inability of neutrophils to migrate to sites of infection, leading to severe life-threatening bacterial infections. Canine LAD-1 (CLAD) in Irish setter dogs has been corrected by both allogeneic bone marrow transplantation15,17 and by gene transfer.18 Low numbers of CD18+ neutrophils have been sufficient to correct the phenotype.16
To determine whether the leukocyte integrin on neutrophils mediated the egress, or disappearance, of neutrophils from the circulation in response to G-CSF, we used a unique resource, CLAD animals with both CD18+ and CD18− neutrophils after matched littermate allogeneic transplant or gene therapy.15-17 Healthy littermate CLAD carriers, which express CD18 on the leukocyte surface, served as controls. In addition, human leukocytes were evaluated for integrin activation using mAb24 before and after G-CSF administration. Total white blood cell counts and numbers and percentages of canine CD18+ and human mAb24+ activated neutrophils were measured before and after G-CSF administration.
Methods
Dogs
Dogs were housed in National Institutes of Health facilities in Bethesda, MD, in accordance with National Institutes of Health guidelines and approved by the American Association for Accreditation of Laboratory Animal Care. Animal study protocols were approved by the Institutional Animal Care and Use Committees of the National Cancer Institute, National Institutes of Health, Bethesda, MD and performed in accordance with the principles outlined in the Guide for Laboratory Animals Facilities and Care of the National Academy of Sciences, National Research Council. One CLAD dog and 3 CLAD dogs, which had been transplanted to achieve gene-corrected chimerism, were used for these studies. Two of the 3 CLAD dogs (T1-Pilot and T2-Frodo) had received matched littermate allogeneic hematopoietic stem cell transplants (References 15 and 17, respectively). The remaining transplanted CLAD dog (T3-Ash) received autologous, gene-corrected CD34+ hematopoietic stem cells.18 Three healthy CLAD littermate carrier dogs (C1, C2, and C3) served as controls.
Canine cytokine treatment and blood sample collection
Human recombinant G-CSF (Neupogen, Amgen) was administered at 10 μg/kg subcutaneously to all 7 animals. Peripheral blood samples (EDTA) were taken immediately before G-CSF administration and at 15, 30, 60, 120, and 240 minutes, and at 24 hours after G-CSF administration. Total WBC counts and differentials were assessed using a Sysmex XT-2000i analyzer equipped with the veterinary application package. To determine absolute neutrophil counts, the WBC count was multiplied by the percentage of cells falling within a subpopulation based on flow analysis. For example, the value of 69.1% and 64.7% for the anti-neut+CD18− subpopulation of T1 (see Figure 2A) was multiplied by the total WBC count for each time point (ie, 8710 and 5990/μL, respectively). The 6020- and 3875-μL anti-neut+CD18− represented a 35% drop. Statistical analysis was performed using Microsoft Excel. To determine statistical significance, a 2-tailed paired t test was performed.
Human cytokine treatment and blood sample collection
An angiocatheter was placed and 3 mL EDTA-anticoagulated and 10- to 12-mL heparinized blood samples were collected before subcutaneous administration of 480 μg of human recombinant G-CSF (Neupogen) in the upper arm of healthy human subjects, in accordance with an National Institutes of Health Clinical Center-approved protocol (99-CC-0168) permitting G-CSF administration to research volunteers. EDTA and heparinized blood samples were collected again at 15, 30, 60, 90, 120, and 240 minutes and at 24 hours. The catheter was flushed with saline and heparin after each sample collection. Total WBC counts and differentials were assessed using a Cell-Dyn Sapphire (Abbott Laboratories) cell analyzer.
Immunophenotyping
Canine EDTA-anticoagulated blood samples and human heparinized samples were collected at selected time points. Red blood cells were removed by lysis, and the canine leukocytes stained with an unconjugated mouse anticanine CD18 monoclonal antibody (CA1.4E9; Serotec) labeled using a Zenon AlexaFluor-647 mouse IgG1 labeling kit (Invitrogen) The human leukocytes were brought up in PBS plus 1% BSA plus 1mM MgCl2 (no Ca2+) and stained for 20 minutes at 37°C with mAb24 (CD11 antibody catalog no. NB100-2623, Novus Biologicals), labeled using a Zenon AlexaFluor-647 mouse IgG1 labeling kit (Invitrogen). Phorbol 12-myristate 13-acetate (PMA; 10 ng/mL) was added to the human leukocytes for 15 minutes at 37°C before antibody staining to serve as a positive control for leukocyte integrin activation. Leukocyte subsets for canine cells were determined using subset-specific antibodies. Monocytes were detected by a FITC-labeled mouse anti–human anti-CD14 monoclonal antibody (TÜK4; Dako North America), which cross-reacts with canine CD14. Neutrophils were identified by staining with an antineutrophil antibody (CAD048A; VMRD) labeled with PE. Leukocyte subsets for human cells were determined using forward scatter versus side scatter. Isotype controls were included for each labeled antibody isotype when appropriate. For dead cell discrimination, cells were incubated in PBS buffer containing 1% weight/volume BSA plus 1 μg/mL 7-amino actinomycin D (Sigma-Aldrich). Cells were analyzed by flow cytometry on a BD Biosciences FACSCalibur using Cellquest Version 3.3 software. Additional analyses of the data were performed using FlowJo Version 8.8.6 software (TreeStar Inc). Data are given as mean ± SD, unless otherwise stated.
Results
Transient canine neutropenia
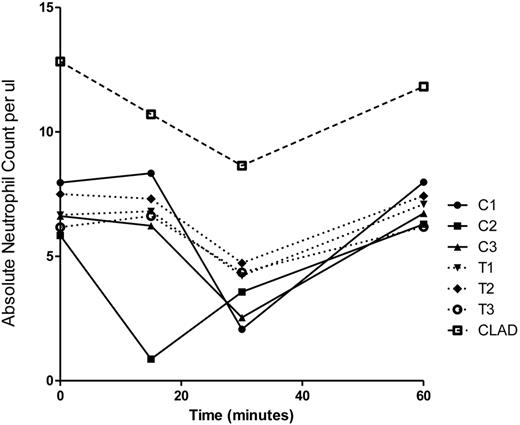
In the 3 carrier (C) dogs, there was a 60% decrease in circulating neutrophils 30 minutes after G-CSF administration; the absolute neutrophil count decreased from a baseline of 6806 ± 1072/μL to 2727 ± 767/μL (Figure 1). The 3 transplanted (T) CLAD dogs had only a 35% decline in neutrophil numbers at 30 minutes, from a baseline of 6777 ± 672/μL to 4433 ± 265/μL (Figure 1). The one CLAD puppy had a decline from 12 830 to 8640 neutrophils/μL at 30 minutes, representing a 33% decline in neutrophil count. The neutrophil nadir occurred 30 minutes after G-CSF administration in 6 of the 7 dogs, and 15 minutes after G-CSF in 1 dog (C2). In all 7 dogs, the neutrophil count returned to baseline by 60 minutes after G-CSF administration. By 24 hours, the neutrophil levels of all 7 animals had increased approximately 3-fold from baseline (data not shown).
Absolute neutrophil count per microliter of blood in individual control dogs (C), transplanted chimeric dogs (T), and a single CLAD dog before and for the first 60 minutes after G-CSF administration.
Absolute neutrophil count per microliter of blood in individual control dogs (C), transplanted chimeric dogs (T), and a single CLAD dog before and for the first 60 minutes after G-CSF administration.
Canine flow cytometric evaluation
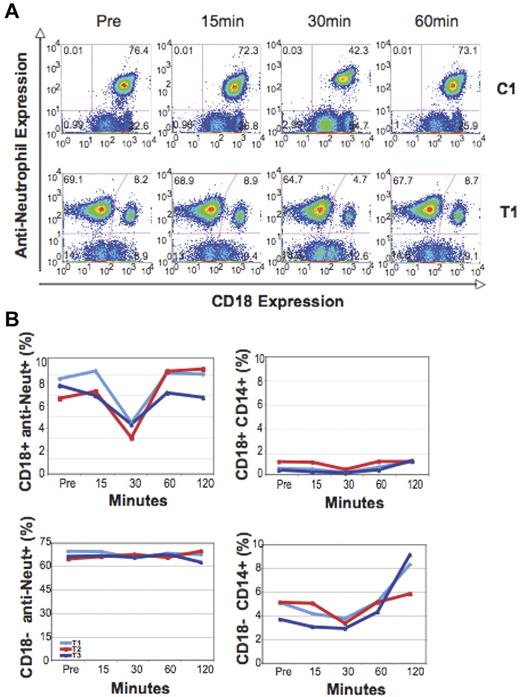
Immunophenotyping using an anti-CD18 and a canine-specific antineutrophil PE-conjugated antibody indicated that predominantly CD18+ neutrophils disappeared from the circulation after G-CSF administration. Before G-CSF administration, the transplanted CLAD dogs had 7.5% ± 0.8% CD18+ peripheral blood neutrophils, such that there were 664 ± 61 CD18+, anti-neut+ cells/μL. At the nadir point after G-CSF, the percentage of CD18+ anti-neut+ neutrophils declined significantly to 4.2% ± 0.7%, or 261 ± 23 cells/μL (P = .008). This represented approximately a 60% decrease in circulating CD18+ neutrophil number. There was no change in mean fluorescent intensity (MFI) of CD18 expression after G-CSF expression for the littermate controls or gene-corrected transplanted dogs (data not shown). A slight decline in CD14+CD18+ monocytes, from 6.2% ± 1.5% to 4.0% ± 1.2%, was also observed. There was a slight drop in CD18− anti-neut+ numbers from a pre-G-CSF value of 5875 ± 701/μL to 4093 ± 407/μL (P = .02) at the nadir of 30 minutes. This represented approximately a 30% decline in CD18- anti-neut+ cell number. In the control healthy littermates, a significant decrease in CD18+, anti-neut+ cells, from a pre-G-CSF value of 6670 ± 1488/μL to a post-G-CSF nadir of 1688 ± 1125/μL, was seen (P = .002), representing approximately a 75% drop in neutrophil number. The time course of the change in leukocyte CD18+ expression in a control (C1) and a transplanted CLAD dog (T1) after G-CSF administration is shown in Figure 2A. There was a preferential decline in CD18+ neutrophils in all dogs. In the partially corrected CLAD dogs, a slight 30% decline in the number of CD18− neutrophils was seen at 30 minutes. The CLAD puppy also demonstrated a slight 26% decline in CD18− neutrophils/μL, from 12 448 to 9237 cells/μL, at 30 minutes after G-CSF administration. In all animals, the percentage of CD18+ neutrophils returned to baseline by 60 minutes. Figure 2B demonstrates the selective decline of CD18+/anti-neut+ neutrophils and CD18+/CD14+ monocytes for all 3 transplanted CLAD dogs after G-CSF administration.
Analysis of percentage change in CD18+ and CD18− antineutrophil+ and CD14+ subsets in the circulation over time. (A) Flow cytometric analysis of the proportion of anti-neutrophil–expressing CD18+ and CD18− cells after G-CSF administration in a control dog (C1) and a transplanted dog (T1). (B) Graphic analysis of all 3 transplanted dogs (T1-T3) after G-CSF administration, demonstrating changes in the percentage of CD18+ and CD18− anti-neutrophil+ and CD14+ leukocyte subsets over time.
Analysis of percentage change in CD18+ and CD18− antineutrophil+ and CD14+ subsets in the circulation over time. (A) Flow cytometric analysis of the proportion of anti-neutrophil–expressing CD18+ and CD18− cells after G-CSF administration in a control dog (C1) and a transplanted dog (T1). (B) Graphic analysis of all 3 transplanted dogs (T1-T3) after G-CSF administration, demonstrating changes in the percentage of CD18+ and CD18− anti-neutrophil+ and CD14+ leukocyte subsets over time.
Transient human neutropenia
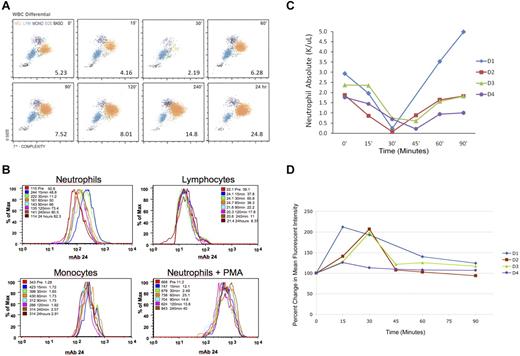
After subcutaneous administration of 480 μg G-CSF, there was a rapid loss in circulating neutrophils (Figure 3A) in donor 1 (D1), with a drop in neutrophil count from 2930/μL to 234/μL by 30 minutes. This represented a > 90% decrease in circulating neutrophil count. Counts returned to baseline by 60 minutes. Eosinophil, basophil, and lymphocyte numbers remained unchanged, although there was a drop in monocyte numbers from a baseline of 477/μL to 225/μL at 30 minutes, with a return to baseline by 60 minutes. At 24 hours, the WBC count was 24 800/μL with a neutrophilia of 20 200/μL. Similar results were observed in an additional 3 research donors. The mean (SD) absolute neutrophil baseline value for the 4 research donors was 2240(530)/μL with a significant (P = .005) nadir of 310(290)/μL. Figure 3C shows the drop in neutrophil counts for the 4 donors over time.
Analysis of neutrophil disappearance and integrin activation in human subjects following G-CSF administration. (A) WBC differential of donor 1 (D1) human leukocyte subsets at designated times after G-CSF administration, showing the disappearance of neutrophils 30 minutes after G-CSF administration. WBC is given as number of leukocytes × 103/μL. Orange represents neutrophils; blue, lymphocytes; purple, monocytes; green, eosinophils; and black, basophils. (B) Flow cytometric staining of human leukocytes using mAb24 (a monoclonal Ab that specifically recognizes the activated leukocyte integrin bound to Mg2+) for D1 over time. Cells were identified as neutrophils, monocytes, or lymphocytes based on size and granularity by flow cytometry. Colors designate that time samples were collected. Leukocytes exposed to PMA were used as a positive control. (C) Absolute neutrophil count of 4 human donors for 90 minutes after G-CSF administration. Neutrophil count is given as number of neutrophils × 103/μL. (D) Percentage change of MFI from baseline of neutrophils stained with mAb24 after G-CSF administration for the same 4 donors.
Analysis of neutrophil disappearance and integrin activation in human subjects following G-CSF administration. (A) WBC differential of donor 1 (D1) human leukocyte subsets at designated times after G-CSF administration, showing the disappearance of neutrophils 30 minutes after G-CSF administration. WBC is given as number of leukocytes × 103/μL. Orange represents neutrophils; blue, lymphocytes; purple, monocytes; green, eosinophils; and black, basophils. (B) Flow cytometric staining of human leukocytes using mAb24 (a monoclonal Ab that specifically recognizes the activated leukocyte integrin bound to Mg2+) for D1 over time. Cells were identified as neutrophils, monocytes, or lymphocytes based on size and granularity by flow cytometry. Colors designate that time samples were collected. Leukocytes exposed to PMA were used as a positive control. (C) Absolute neutrophil count of 4 human donors for 90 minutes after G-CSF administration. Neutrophil count is given as number of neutrophils × 103/μL. (D) Percentage change of MFI from baseline of neutrophils stained with mAb24 after G-CSF administration for the same 4 donors.
Human flow cytometric evaluation
Human leukocytes were stained before and after G-CSF administration using mAb24, and expression levels were determined. Figure 3B represents the results for D1. Before G-CSF administration, the MFI of neutrophils, lymphocytes, and monocytes were 115, 22.1, and 343, respectively. Fifteen minutes after exposure to G-CSF, the MFI for the neutrophils peaked at 244 (a 112% increase), with lymphocyte and monocyte MFI changing slightly (to 24.1 and 423, respectively). Within 60 minutes, the neutrophil MFI had returned close to baseline (161) and was at baseline by 24 hours (114). As a positive control, neutrophils were exposed to PMA at each time point to achieve maximum stimulation of integrin activation. At all time points, the neutrophils before and after G-CSF administration could be further stimulated with PMA beyond their initial values to a MFI ranging from 668 to 843. Figure 3D shows the percentage change from baseline for the neutrophil MFI over time. The peak elevation in MFI corresponds with the disappearance of the neutrophils from the circulation of the 4 research donors, with a return to baseline values at 60 minutes. The MFI for both the monocyte and lymphocyte populations remained level after G-CSF administration (data not shown).
Discussion
The transient loss of neutrophils from the circulation after G-CSF administration has been well documented in multiple species.5-9 We demonstrated that neutrophils accumulate predominantly in the spleen and lung after G-CSF administration.9 This transient sequestration is observed whether G-CSF is administered subcutaneously or intravenously19 before the increase in neutrophil count hours later. This increase in neutrophil number is the result of an increase proliferation in all stages of granulopoiesis, especially a greater input to the myeloblast compartment, with no alteration in neutrophil half-life and an early egression of neutrophils from the spleen.20 The mechanism behind this transient loss of neutrophils, however, remains unclear. Various adhesion proteins were observed to be up-regulated on neutrophils after G-CSF exposure,7,8 but none could be identified as exclusively involved in the transient neutropenia.
Mutations in the leukocyte integrin CD18 in humans result in type 1 LAD (LAD-1), which leads to the development of severe bacterial infections because of failure of neutrophils to effectively egress from the circulation. LAD-1 typically results in death from infection. Canine LAD in Irish setters14 has been successfully treated using reduced intensity allogeneic bone marrow transplantation from normal matched littermates15-17 and ex vivo hematopoietic stem cell gene therapy with CD18 cDNA.18 CLAD animals treated with both therapies typically have a mixed population of CD18+ and CD18− leukocytes, providing the opportunity to investigate the role of CD18 in neutrophil loss after G-CSF administration.
Similar to results in other species, G-CSF was found to induce transient neutropenia in healthy CLAD carrier dogs 30 minutes after its administration. Unlike the healthy CLAD carrier dogs, however, CLAD dogs that were chimeric for CD18 expression had only a slight decline in neutrophil count after G-CSF administration (Figure 1). This decline was preferentially associated with the loss of neutrophils expressing CD18 (Figure 2). There was a slight decline in CD18+ monocytes and no decline in CD18+ lymphocytes. The slight decline in monocytes is of interest in that it has been shown in mice that a subset of monocytes bind G-CSF at low levels21 and that a population of human monocytes express the G-CSF receptor.22 It is notable that, in both the CLAD dog and the CD18− neutrophil population of the transplanted dogs, there was a 30% decline in neutrophil number 30 minutes after G-CSF administration. Although the effect was not as marked as in the CD18+ neutrophil population, it appears as if G-CSF may also have an alternative pathway to elicit neutrophil margination. This is not surprising as it has been observed that CLAD dogs are able to produce pus. Others have shown the possibility of CD18-independent pathways for neutrophil migration.23,24 The failure of CD18− neutrophils to exit the circulation efficiently after G-CSF administration, however, indicates that CD18 expression plays a critical role in the transient neutropenia.
Leukocyte integrins are heterodimers of distinctive α-subunits (CD11a, CD11b, CD11c, and CD11d) and a conserved β-subunit (CD18).10 There is a conformational change involved in integrin activation through the binding of divalent cations, such as Mg2+. This conformational activation is recognized by a novel antibody mAb24.11,12 Use of this antibody to evaluate G-CSF-stimulated human neutrophils revealed an increase in antibody binding to circulating neutrophils within 15 minutes of G-CSF administration, based on shifts in mean fluorescence intensity. At 60 minutes, the mean fluorescence intensity had started to return to baseline, reaching baseline 24 hours later. This increase in integrin activation mirrors the decline in absolute neutrophil count, such that > 90% of neutrophils were no longer circulating 30 minutes after G-CSF administration. The rapid decline in neutrophil count shortly after G-CSF administration coincides with the conformational change in the leukocyte integrin, resulting in the binding of neutrophils to the endothelium of the lung and spleen.
The molecular mechanism by which G-CSF switches leukocyte integrin from a resting low-affinity state to an active high-affinity conformation is unclear. Recently, Kindlin-3 has been implicated as an important protein downstream from the G protein-coupled receptor signal transduction pathway for integrin conformational activation in LAD type 3. In mice, Kindlin-3 now also appears to be important in leukocyte adhesion to endothelium,25 and mutations of the human gene encoding Kindlin-3 are associated with a disorder involving severe bleeding, infection, and osteopetrosis,26 which can be corrected by bone marrow transplantation.27 It may be possible that G-CSF signals through Kindlin-3 and that this pathway is responsible for the neutrophil trafficking pattern seen within the first 30 minutes of G-CSF administration. In addition, G-CSF is known to induce in vivo activation of neutrophils by mobilization of secretory vesicles 30 minutes after injection of G-CSF.28 This mobilization may, in turn, be associated with the rapid activation of the neutrophil integrin.
For greater than 20 years, G-CSF has been used to treat neutropenia associated with myeloablative therapies and congenital disorders, as well as to mobilize granulocytes for transfusion or hematopoietic stem and progenitor cells for transplantation. The effects of G-CSF in healthy volunteers have been shown to be well tolerated, transient, and self-limited. These effects, however, may prove to be more complex than previously appreciated. The most commonly reported side effects associated with the use of G-CSF in healthy volunteers include bone pain, headache, fatigue, nausea, insomnia, anorexia, and mylagias.29,30 It has also been reported that the spleen size of healthy donors increases31 and that a transient dose-dependent decrease in arterial oxygenation occurs32 after G-CSF administration. The sequestration of neutrophils in both the spleen and lungs may be associated with these adverse effects.9 The findings presented here demonstrate that G-CSF activates the leukocyte integrin present on neutrophils and possibly monocytes, resulting in their loss from the circulation. This activation is transient, with neutrophils returning to baseline circulating values within one hour of G-CSF administration. The reason why neutrophils return to an inactive configuration despite continued in vivo exposure to G-CSF remains a puzzle. One explanation is that the divalent cation Ca2+ may interfere with continued activation of the neutrophils, resulting in their return to an inactive state.12 Additional studies of changes in the conformational state of the leukocyte integrin after initial and continuing exposure to G-CSF are needed.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Earl West and the staff of the Department of Transfusion Medicine Cell Processing Laboratory for performing the complete blood counts, Cathy DeJesus and Meredith Adams for help in data analysis and collecting dog blood samples, and Dr Tanya Burkholder and her staff for performing the timed collections on the dogs.
This work was supported by the Hematology Branch, National Heart, Lung, and Blood Institute, Experimental Transplantation and Immunology Center for Cancer Research, National Cancer Institute, and the Department of Transfusion Medicine, Clinical Center, National Institutes of Health.
National Institutes of Health
Authorship
Contribution: R.E.D. designed and performed research, analyzed data, and wrote the paper; L.T. performed research, analyzed data, and wrote the paper; Y.Y.Y. performed research and analyzed data; T.R.B. designed research and wrote the paper; and S.F.L. and D.D.H. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Robert E. Donahue, VMD Hematology Branch, National Heart, Lung, and Blood Institute, 5 Research Ct, Rockville, MD 20850; e-mail: donahuer@nhlbi.nih.gov.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal