Abstract
Tetraspanin CD151 is highly expressed in endothelial cells and regulates pathologic angiogenesis. However, the mechanism by which CD151 promotes vascular morphogenesis and whether CD151 engages other vascular functions are unclear. Here we report that CD151 is required for maintaining endothelial capillary-like structures formed in vitro and the integrity of endothelial cell-cell and cell-matrix contacts in vivo. In addition, vascular permeability is markedly enhanced in the absence of CD151. As a global regulator of endothelial cell-cell and cell-matrix adhesions, CD151 is needed for the optimal functions of various cell adhesion proteins. The loss of CD151 elevates actin cytoskeletal traction by up-regulating RhoA signaling and diminishes actin cortical meshwork by down-regulating Rac1 activity. The inhibition of RhoA or activation of cAMP signaling stabilizes CD151-silenced or -null endothelial structure in vascular morphogenesis. Together, our data demonstrate that CD151 maintains vascular stability by promoting endothelial cell adhesions, especially cell-cell adhesion, and confining cytoskeletal tension.
Introduction
Tetraspanin CD151 forms stable and stoichiometric associations with laminin-binding integrins and regulates cellular functions, such as cell-matrix adhesion strengthening, epithelial cell-cell adhesion, and cell migration.1-4 In endothelial cells (ECs), cell surface CD151 is localized at basolateral surfaces and forms tetraspanin-enriched microdomain (TEM) with other tetraspanins and integrins.5-7 The CD151-containing TEM on ECs is critical for the proper function of adhesion proteins, such as ICAM-1 and VCAM-1, and is needed for the transendothelial migration of lymphocytes.8 In addition, CD151 regulates EC migration5,6,9 and promotes vascular morphogenesis both in vitro5,9,10 and in vivo.11 Pathologic angiogenesis becomes deficient in CD151-knockout mice.9 However, the mechanism by which CD151 regulates various vascular functions remains largely unknown. Mechanistic studies from epithelial and carcinoma cells indicate that CD151-α3β1 integrin complex regulates cell-cell adhesion, probably by organizing or stabilizing cell-cell junctional complexes.12,13 CD151 overexpression leads to PKC- and Cdc42-dependent reorganization of actin cytoskeleton,2 whereas CD151 silencing results in higher RhoA activity and more stress fiber formation,13 suggesting that CD151 modulates the epithelial cytoskeletal machinery. In ECs, CD151 up-regulates eNOS, Akt, and Rac activities9,11 and promotes TEM-metalloprotease association.14
Endothelial cell-cell and cell-matrix adhesions are required for the formation and maintenance of blood vessels.15 VE-cadherin is linked to actin cytoskeleton by α-, β-, γ-, and p120-catenin to form adherens junctions (AJs).16 Integrins bridge intracellular actin fibers to extracellular matrices to anchor ECs to the basement membrane.17 Rho small GTPase-mediated cytoskeletal reorganizations are also important for blood vessels. For example, Rac1- and Cdc42-dependent protrusions are essential for vessel structure formation and integrity, whereas RhoA-mediated retraction destabilizes angiogenic vessels and induces regression.18-20 Rho GTPases and cadherin-mediated cell-cell adhesion are mutually regulated. Activation of RhoA induces the disassembly of cell-cell adhesion via myosin II-mediated cytoskeletal tension,21 whereas AJs inhibit RhoA signaling by recruiting p190RhoGAP into the junctions.22
Herein we found that the loss of CD151 expression disrupts endothelial stability in vascular morphogenesis and other vascular events. At the molecular level, lack of CD151 leads to decreased cAMP/PKA signaling and deregulated Rac1 and RhoA signaling. At the cellular level, attenuated endothelial cell-cell and cell-matrix adhesions coupled with elevated cytoskeletal tension directly destabilize endothelial structure. Our study emphasizes that CD151 balances cell adhesion and cytoskeletal tension in ECs to maintain vascular stability.
Methods
Reagents, animals, and cell culture
Information regarding reagents, animals, and cell culture is provided in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The Animal Care and Use Committee of the University of Tennessee approved the mouse protocol.
RNAi
A retrovirus-delivered shRNA system described previously4,23 was used to knock down CD151 expression in human dermal microvascular endothelial cells (HMECs) and human umbilical vein endothelial cells (HUVECs). The target sequences were as follows: AGTACCTGCTGTTTACCTACA for CD151 knockdown (CD151 KD) and GCGAGACCATGCCTCCAACAT for nonsilencing control (MOCK). Stable transductants were obtained after retrovirus transduction and puromycin selection, followed by flow cytometry to sort CD151-silenced cells, and then maintained by 0.2 μg/mL puromycin.
Endothelial network formation on 3D matrices
Matrigel was plated in 48-well plates and incubated at 37°C for 1 hour for gelation. ECs were seeded onto Matrigel at a density of 60 000 cells/well. EC networks were photographed either with an Olympus CK2 inverted microscope under a 4×/0.10 NA objective connected with a DCM500 microscope digital camera at different time points or recorded by time-lapse video microscopy. For some experiments, the assay was performed in the presence of various inhibitors or Abs. The numbers of cable-enclosed regions per field of view were counted visually.
GST pulldown assays
The activation of RhoA, Rac1, p190RhoGAP, and p115RhoGEF was detected by the GST pulldown method with recombinant proteins: glutathione-S-transferase–Rhotekin Rho-binding domain, glutathione-S-transferase–PAK-1 Rac1-binding domain, GST-RhoA(Q63L), and GST-RhoA(G17A), respectively, as described previously.22,24 Briefly, the recombinant GST protein was purified and collected with glutathione-Sepharose 4B beads immediately before the experiments. HMECs seeded on diluted Matrigel (1:10) were lysed in RIPA buffer containing a proteinase inhibitor cocktail. The insoluble fraction was removed by centrifugation, and supernatants were incubated with the GST fusion protein beads at 4°C for 30 minutes. After the beads were washed and separated by SDS-PAGE, the precipitated proteins were detected via immunoblotting with appropriate mAbs.
Assays for ROCK and cAMP
Rho-associated kinase (ROCK) activity and cAMP concentration were measured with commercially available ELISA-based ROCK Activity Kit (MBL International) and cAMP EIA Kit (Cayman), respectively, according to the manufacturer's instructions.
Immunoprecipitation and immunobloting
Immunoprecipitation and immunoblotting were performed as described previously23 with modifications (supplemental Methods).
Cell adhesion assays
Traction force microscopy
Cellular traction forces were measured by traction force microscopy as described previously26,27 with modifications. Briefly, HMECs were plated on fluorescent latex bead-embedded polyacrylamide gel conjugated with fibronectin (FN, 10 μg/mL), laminin 111 (LN 111, 10 μg/mL), or LN 332 (1 μg/mL) and cultured 12 to 24 hours. Individual cells were imaged with an Olympus IX81 confocal microscopy under a 40×/0.6 NA objective equipped with a SensiCamQE CCD camera (Cooke) to capture the positions of the embedded fluorescent beads resulting from the cellular traction forces on cell attaching and spreading. The cells were then trypsinized, and a second confocal image of the bead-embedded substrate was taken to capture the positions of the beads in the absence of cellular traction forces. Then the displacement field caused by cellular traction forces was obtained from the High Density Mapping software analysis26,27 of the 2 images by using the phase correlation method, which is based on the Fourier shift principle. Finally, the displacement data were converted into the traction field using the Fourier-transform traction cytometry method as previously described.26,27 The traction force contour plots were generated from the traction field data for each cell area. The maximum traction force within each cell area was calculated as the maximum traction force magnitude value averaged by the surrounding vector magnitudes after a peak smoothing algorithm was applied. The average perimeter stress was calculated as the average of the traction force values found along the perimeter of the cell. For each transductant, 11-19 cells were analyzed.
Fluorescent microscopy
ECs were cultured on glass-bottom dishes either overnight or until confluent and processed for confocal fluorescent or total internal reflection fluorescence (TIRF) microscopy as described in supplemental Methods.
Transmission electron microscopy
For electron microscopy, 12-week-old male mice were perfused and fixed with 2.5% glutaraldehyde. The lung and aorta tissue samples were post-fixed in 1% osmium tetroxide, dehydrated in dimethoxypropane, and embedded in epoxyresin LX-112. Thin sections were stained with 0.3% potassium ferrocyanide in 2.0% osmium tetroxide and 4.0% uranyl acetate and then examined on a JEOL JEM-1200EX II transmission electron microscope.
Miles assay
The Miles assay was performed as described28 with modifications. Briefly, Evans blue dye was injected into mice intravenously. Oil was administered on ears. After 15 minutes, the ears were photographed, excised, dried, and weighed. The dye was extracted, and its concentration was quantified by measuring the absorbance at 610 nm.
Results
CD151 promotes vascular stability
Using a retrovirus-delivered shRNA system reported previously,4,23 we established the stable transductants of CD151 KD and control MOCK in HMECs (supplemental Figure 1) and HUVECs (data not shown). Compared with the parental HMECs and MOCK cells, CD151 KD cells displayed a total loss of cell surface expression of CD151 (supplemental Figure 1A) and remarkable down-regulation in total cellular CD151 expression (supplemental Figure 1B).
We analyzed the cell surface expressions of CD151-relevant TEM proteins and found no significant differences in the expression of integrins α3, α5, α6, αV, and β1 and tetraspanins CD9 and CD81 in HMEC transductants (supplemental Table 1). These results indicate the expressions of these proteins in HMECs and underscore that neither retroviral transductions nor CD151 knockdown alters the cell surface expressions of these CD151-relevant proteins.
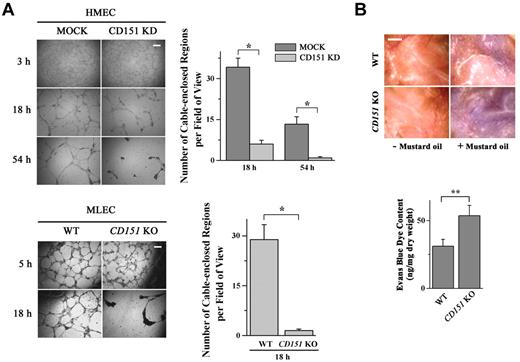
ECs cultured in or on 3-dimensional collagen gel or Matrigel typically form an anastomosing network of capillary-like structures, a process largely resembling vascular morphogenesis.10 We used this model to evaluate the effect of CD151 loss on vascular morphogenesis. As shown in Figure 1A, HMECs or MLECs initially formed similar network structures at 3 to 5 hours on Matrigel regardless of the presence or absence of CD151 expression. Compared with the network structure formed by control ECs (HMEC-MOCK and MLEC-WT), the ones formed by CD151-deficienct (HMEC-CD151 KD and MLEC-CD151 KO) ECs were markedly disrupted at 18 hours. In CD151-silenced HMECs, the networks became completely lost at 54 hours.
CD151 reinforces vascular stability and regulates vascular permeability. (A) Loss of CD151 expression disrupted EC capillary-like structures on Matrigel. HMEC-MOCK and -CD151 KD (top) or MLEC-WT and -CD151 KO (bottom) cells were plated on Matrigel and photographed with an Olympus CK2 inverted microscope under a 4×/0.10 NA objective equipped with a microscope digital camera (DCM500) at the indicated time points. The cable-enclosed regions were counted and compared. Bar represents 250 μm. *P < .01. (B) CD151 ablation results in increased vascular permeability in mice. Evans blue dye (30 mg/kg in PBS) was injected intravenously through the retro-orbital sinus into 12-week-old male mice. Mustard oil in mineral oil (5% volume/volume) or mineral oil alone was applied to the dorsal and ventral surfaces of the ears twice, at 0 minutes and 15 minutes later. After 30 minutes of circulation, the dye leakage area at the ventral surface of the ear was imaged with a Nikon SMZ1500 dissecting microscope equipped with a Nikon DXM1200 digital camera and the content of dye in ears was determined after the extraction with formamide overnight at 55°C. Shown are representative images (top) and quantitative results from WT and CD151 KO mice from the Miles assay. n = 15. Bar represents 1 mm. **P < .05.
CD151 reinforces vascular stability and regulates vascular permeability. (A) Loss of CD151 expression disrupted EC capillary-like structures on Matrigel. HMEC-MOCK and -CD151 KD (top) or MLEC-WT and -CD151 KO (bottom) cells were plated on Matrigel and photographed with an Olympus CK2 inverted microscope under a 4×/0.10 NA objective equipped with a microscope digital camera (DCM500) at the indicated time points. The cable-enclosed regions were counted and compared. Bar represents 250 μm. *P < .01. (B) CD151 ablation results in increased vascular permeability in mice. Evans blue dye (30 mg/kg in PBS) was injected intravenously through the retro-orbital sinus into 12-week-old male mice. Mustard oil in mineral oil (5% volume/volume) or mineral oil alone was applied to the dorsal and ventral surfaces of the ears twice, at 0 minutes and 15 minutes later. After 30 minutes of circulation, the dye leakage area at the ventral surface of the ear was imaged with a Nikon SMZ1500 dissecting microscope equipped with a Nikon DXM1200 digital camera and the content of dye in ears was determined after the extraction with formamide overnight at 55°C. Shown are representative images (top) and quantitative results from WT and CD151 KO mice from the Miles assay. n = 15. Bar represents 1 mm. **P < .05.
Using DIC time-lapse video microscopy, we found that HMEC-MOCK and HMEC-CD151 KD cells attached and spread equally well and actively migrated soon after being plated on Matrigel. In the following hours, both cells assembled networks of cable structures to a similar extent, suggesting that CD151 is not required for initial EC patterning into vascular structures (supplemental Videos). In general, MOCK cells can maintain the network structures for several days, although the cables become thicker and denser (supplemental Video 1 and supplemental Figure 2). In contrast, CD151-silenced cells cannot maintain the network structures, with cable networks continually contracting and eventually breaking into disconnected cell clumps (supplemental Video 2 and supplemental Figure 2). Together, these data suggest that CD151 maintains vascular stability without affecting the initial formation of vascular structures.
Decreased stability of CD151-deficient endothelial networks may result from increased apoptosis, reduced cell proliferation rates, and/or altered cell movement. We assessed the effect of CD151 loss on cell migration and survival and found that CD151 silencing did not significantly alter (1) EC motility in Transwell migration (supplemental Figure 3A) and wound healing (data not shown) assays, and (2) EC viability after being plated on Matrigel overnight (supplemental Figure 3B). These observations agree that CD151 removal has no impact on cell proliferation9 and suggest that the vascular instability caused by CD151 loss is not a result of altered EC movement or death.
To substantiate the in vitro findings, we analyzed the vascular stability of CD151-null mice.29 Using the Miles assay, which examines microvascular permeability, we found that the mustard oil-induced vascular permeability is markedly elevated in CD151-null mice compared with that found in WT littermates (Figure 1B).
CD151 is needed for optimal endothelial cell-matrix adhesiveness
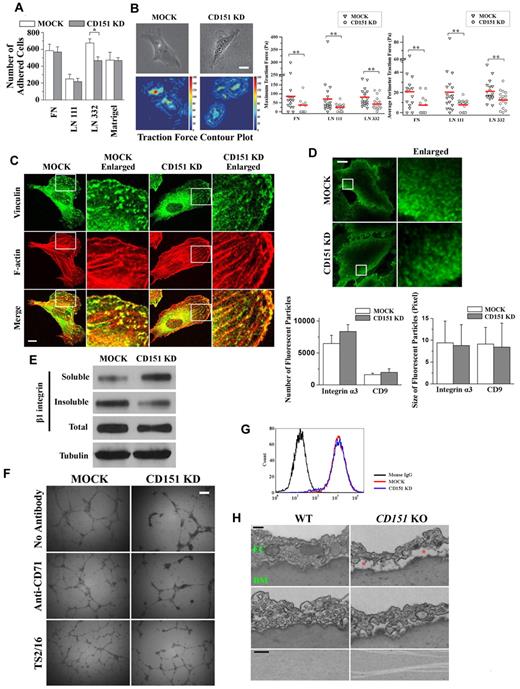
To elucidate the role of CD151 in EC-matrix adhesion, we performed a short-term (35-minute), static cell-matrix adhesion assay. As shown in Figure 2A, CD151 KD cells exhibited diminished adhesion on only LN 332 compared with MOCK cells, but the adhesions on FN, LN 111, and Matrigel were equivalent between MOCK and CD151 KD cells. This result is consistent with earlier observations in other cell types4,9 and with the notion that CD151 markedly attenuates only the static adhesion dependent on LN 332-binding integrins α3β1 and/or α6β4.
CD151 up-regulates endothelial cell-matrix adhesiveness. (A) Cell-matrix adhesion assay. HMEC transductants were seeded on ECM substrate-coated wells in triplicates and allowed to settle at 37°C for 35 minutes. Nonadherent cells were then removed by gentle washing. The adhered cells were counted. *P < .01. (B) Traction force microscopy. Cells were plated on FN-, LN 111-, or LN 332-conjugated fluorescent bead-embedded polyacrylamide gels. Traction forces exerted by the cells were measured as described in “Traction force microscopy.” Left: Phase-contrast images and traction field of cells on FN-conjugated polyarylamide gels. Bar represents 10 μm. Right: Maximum and average perimeter traction forces of the HMEC transductants on FN, LN 111, or LN 332. The magnitudes of maximum and average perimeter traction forces were compared with a nonparametric Mann-Whitney test. **P < .05. (C) CD151 silencing attenuates the formation and maturation of focal adhesions. HMEC transductants, after 2-day culture, were fixed, permeabilized, and incubated with vinculin mAb, followed by AlexaFluor-488–conjugated secondary Ab and phalloidin–AlexaFluor-594 staining. Staining was imaged with a Zeiss LSM510 confocal fluorescence microscope under a 100×/1.4 NA oil objective. Bar represents 10 μm. (D) TIRF microscopy. HMEC transductants were fixed without permeabilization, probed with α3 integrin mAb and AlexaFluro-488–conjugated secondary Ab, and visualized by TIRF microscopy. Bar represents 15 μm. The size and numbers of fluorescent particles of α3 integrin and CD9 from the individual transductants (n = 20) were analyzed and compared. (E) Silencing of CD151 expression elevates detergent solubility of β1 integrin. HMEC transductants were lysed with 0.05% Triton X-100 in HEPES buffer. After ultracentrifugation, supernatants were used as soluble fractions. Pellets were solubilized in 1× Laemmli sample buffer and used as the insoluble fractions. Integrin β1 in both fractions as well as whole cell lysates were subjected to SDS-PAGE and then detected by immunoblotting using TS2/16 mAb. Tubulin from whole cell lysates was used as loading control. (F) TS2/16 partially rescues the defects in maintenance of capillary structures in CD151-silenced ECs. HMEC transductants were incubated with 1 μg/mL β1 integrin-activating mAb TS2/16 on ice for 1 hour and were plated on Matrigel in the presence of TS2/16. The capillary-like structures were imaged and quantified. An isotype-matched antihuman CD71 mAb served as the negative control. Bar represents 250 μm. (G) Active β1 integrins were unaltered on CD151 silencing. The levels of activated β1 integrins on HMECs were measured by flow cytometry using mAb AG89.30 (H) The dissociation of ECs from the basement membrane (BM) and splitting of BM in the absence of CD151. Twelve-week-old male CD151 KO (n = 4) and littermate WT (n = 4) mice were perfused and fixed with 2.5% glutaraldehyde. The aortas were isolated, sectioned transversely, and processed for transmission electron microscopy. The red asterisks indicate the space where ECs were detached from BM. Bar represents 250 nm.
CD151 up-regulates endothelial cell-matrix adhesiveness. (A) Cell-matrix adhesion assay. HMEC transductants were seeded on ECM substrate-coated wells in triplicates and allowed to settle at 37°C for 35 minutes. Nonadherent cells were then removed by gentle washing. The adhered cells were counted. *P < .01. (B) Traction force microscopy. Cells were plated on FN-, LN 111-, or LN 332-conjugated fluorescent bead-embedded polyacrylamide gels. Traction forces exerted by the cells were measured as described in “Traction force microscopy.” Left: Phase-contrast images and traction field of cells on FN-conjugated polyarylamide gels. Bar represents 10 μm. Right: Maximum and average perimeter traction forces of the HMEC transductants on FN, LN 111, or LN 332. The magnitudes of maximum and average perimeter traction forces were compared with a nonparametric Mann-Whitney test. **P < .05. (C) CD151 silencing attenuates the formation and maturation of focal adhesions. HMEC transductants, after 2-day culture, were fixed, permeabilized, and incubated with vinculin mAb, followed by AlexaFluor-488–conjugated secondary Ab and phalloidin–AlexaFluor-594 staining. Staining was imaged with a Zeiss LSM510 confocal fluorescence microscope under a 100×/1.4 NA oil objective. Bar represents 10 μm. (D) TIRF microscopy. HMEC transductants were fixed without permeabilization, probed with α3 integrin mAb and AlexaFluro-488–conjugated secondary Ab, and visualized by TIRF microscopy. Bar represents 15 μm. The size and numbers of fluorescent particles of α3 integrin and CD9 from the individual transductants (n = 20) were analyzed and compared. (E) Silencing of CD151 expression elevates detergent solubility of β1 integrin. HMEC transductants were lysed with 0.05% Triton X-100 in HEPES buffer. After ultracentrifugation, supernatants were used as soluble fractions. Pellets were solubilized in 1× Laemmli sample buffer and used as the insoluble fractions. Integrin β1 in both fractions as well as whole cell lysates were subjected to SDS-PAGE and then detected by immunoblotting using TS2/16 mAb. Tubulin from whole cell lysates was used as loading control. (F) TS2/16 partially rescues the defects in maintenance of capillary structures in CD151-silenced ECs. HMEC transductants were incubated with 1 μg/mL β1 integrin-activating mAb TS2/16 on ice for 1 hour and were plated on Matrigel in the presence of TS2/16. The capillary-like structures were imaged and quantified. An isotype-matched antihuman CD71 mAb served as the negative control. Bar represents 250 μm. (G) Active β1 integrins were unaltered on CD151 silencing. The levels of activated β1 integrins on HMECs were measured by flow cytometry using mAb AG89.30 (H) The dissociation of ECs from the basement membrane (BM) and splitting of BM in the absence of CD151. Twelve-week-old male CD151 KO (n = 4) and littermate WT (n = 4) mice were perfused and fixed with 2.5% glutaraldehyde. The aortas were isolated, sectioned transversely, and processed for transmission electron microscopy. The red asterisks indicate the space where ECs were detached from BM. Bar represents 250 nm.
Cell traction force is generated by the actomyosin cytoskeleton and transmitted to the extracellular matrix through cell adhesion molecules and may reflect the cell adhesion strengthening process.26,27 We next measured EC traction force at the basal surface using traction force microscopy.27 Compared with MOCK cells, CD151-silenced ECs on both FN and LN 111 exhibited significantly diminished average perimeter and maximum traction forces (Figure 2B). As expected, the traction forces exerted on LN 332 were markedly reduced on CD151 silencing. Hence, CD151 is needed for strengthening cell-matrix adhesion on FN, LN 111, and LN 332.
Because the size, number, and maturation of cell-matrix adhesion structures reflect cell-matrix adhesiveness, we next compared the formation of focal adhesion using vinculin as a marker. As shown in Figure 2C, fewer and smaller focal adhesions were typically found in HMEC-CD151 KD cells compared with HMEC-MOCK cells, suggesting that CD151 is needed for the maturation of focal adhesions. We also observed fewer focal complexes, as revealed by paxillin staining, in CD151-silenced ECs (data not shown), underlining that CD151 promotes the maturation and/or stabilization of cell-matrix adhesion structures.
CD151 associates with integrins, such as integrin α3β1, and tetraspanins, such as CD9, in TEM for proper cell adhesion functions.7 Using TIRF microscopy, we next investigated the distribution and organization of integrin α3β1 and CD9 at the EC-matrix interface and observed that integrin α3β1 was localized at the pericentrolar and peripheral regions (Figure 2D), whereas CD9 was largely enriched at the periphery of basal cell surfaces (Figure 3C). The fluorescent particles of integrin α3β1 and CD9 in HMEC-MOCK and HMEC-CD151 KD cells exhibited no distinguishable difference in numbers, size, morphology, or distribution (Figure 2D), suggesting that CD151 modulates cell-matrix adhesion without altering these biophysical properties of TEM and the clustering of integrin α3β1.
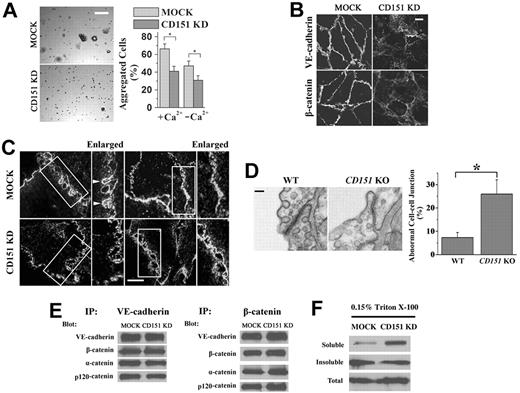
CD151 is needed for proper endothelial cell-cell adhesion. (A) Cell aggregation assay. A total of 2 × 104 cells were seeded into 30-μL hanging drop cultures in either complete or Ca2+-depleted media and allowed to aggregate at 37°C overnight. After passing the cell cluster 10 times through a 200-μL pipette tip, cell clusters were imaged, and the degree of dissociation of the aggregates was quantified using ImageJ 1.42q (published by Wayne Rasband, National Institutes of Health) software analysis. Representative images of aggregation in untreated cells (left panel) and the quantitative results (right panel). Bar represents 100 μm. *P < .01. (B) AJ complexes are mislocalized in CD151-silenced ECs. HUVEC transductants were cultured for 4 days to confluence. After 6-hour starvation, HUVEC monolayers were fixed, permeabilized, and stained with specific Abs. The distributions of VE-cadherin and β-catenin were visualized using a Zeiss LSM510 confocal fluorescence microscope under a 100×/1.4 NA oil objective. Bar represents 10 μm. (C) Deficient formation and maturation of adhesion zipper on CD151 silencing. HMEC transductants were fixed without permeabilization, probed with CD9 mAb and AlexaFluro-488–conjugated secondary Ab, and visualized by TIRF microscopy. Bar represents 15 μm. (D) Abnormal endothelial cell-cell adhesion in lung vessels of CD151 KO mice. Twelve-week-old male CD151 KO (n = 4) and littermate WT (n = 4) mice were perfused and fixed with 2.5% glutaraldehyde. Mouse lung tissue was excised and processed for transmission electron microscopy. Bar represents 100 nm. Right panel: Abnormal EC junctions were counted visually. *P < .01. (E) The protein association in AJ complexes is not affected by the loss of CD151. HMEC-MOCK or HMEC-CD151 cells were lysed in coimmunoprecipitated buffer. The indicated proteins were immunoprecipitated, followed by immunoblotting with specific antibodies. (F) Detergent solubility assay of VE-cadherin. HMEC-MOCK or HMEC-CD151 KD cells were solubilized with 0.15% Triton X-100. Soluble and insoluble fractions were obtained after ultracentrifugation, subjected to SDS-PAGE, followed by immunoblotting with anti–VE-cadherin mAb.
CD151 is needed for proper endothelial cell-cell adhesion. (A) Cell aggregation assay. A total of 2 × 104 cells were seeded into 30-μL hanging drop cultures in either complete or Ca2+-depleted media and allowed to aggregate at 37°C overnight. After passing the cell cluster 10 times through a 200-μL pipette tip, cell clusters were imaged, and the degree of dissociation of the aggregates was quantified using ImageJ 1.42q (published by Wayne Rasband, National Institutes of Health) software analysis. Representative images of aggregation in untreated cells (left panel) and the quantitative results (right panel). Bar represents 100 μm. *P < .01. (B) AJ complexes are mislocalized in CD151-silenced ECs. HUVEC transductants were cultured for 4 days to confluence. After 6-hour starvation, HUVEC monolayers were fixed, permeabilized, and stained with specific Abs. The distributions of VE-cadherin and β-catenin were visualized using a Zeiss LSM510 confocal fluorescence microscope under a 100×/1.4 NA oil objective. Bar represents 10 μm. (C) Deficient formation and maturation of adhesion zipper on CD151 silencing. HMEC transductants were fixed without permeabilization, probed with CD9 mAb and AlexaFluro-488–conjugated secondary Ab, and visualized by TIRF microscopy. Bar represents 15 μm. (D) Abnormal endothelial cell-cell adhesion in lung vessels of CD151 KO mice. Twelve-week-old male CD151 KO (n = 4) and littermate WT (n = 4) mice were perfused and fixed with 2.5% glutaraldehyde. Mouse lung tissue was excised and processed for transmission electron microscopy. Bar represents 100 nm. Right panel: Abnormal EC junctions were counted visually. *P < .01. (E) The protein association in AJ complexes is not affected by the loss of CD151. HMEC-MOCK or HMEC-CD151 cells were lysed in coimmunoprecipitated buffer. The indicated proteins were immunoprecipitated, followed by immunoblotting with specific antibodies. (F) Detergent solubility assay of VE-cadherin. HMEC-MOCK or HMEC-CD151 KD cells were solubilized with 0.15% Triton X-100. Soluble and insoluble fractions were obtained after ultracentrifugation, subjected to SDS-PAGE, followed by immunoblotting with anti–VE-cadherin mAb.
To evaluate the role of CD151 in the integrin-cytoskeleton connection, we compared the detergent solubility of β1 integrins by lysing cells with a low concentration of detergent. MOCK cells contained more insoluble (cytoskeletonassociated) β1 integrins than did CD151 KD cells (Figure 2E), suggesting that CD151 stabilizes the cytoskeletal connection or membrane partition of β1 integrins.
We next examined whether the reinforcement of cell-matrix adhesion can stabilize the endothelial network formed by CD151 KD cells. Indeed, the β1 integrin-activating mAb TS2/16 stabilized the network structure of CD151 KD cells to nearly the levels of untreated and control Ab-treated MOCK cells (Figure 2F; supplemental Table 2), suggesting that β1 integrin activation is sufficient to maintain the endothelial network when CD151 is silenced. Notably, TS2/16-stimulated MOCK cells form more anastomosing and more stable endothelial networks, suggesting that integrin β1 activation cannot fully rescue the defects of CD151 silencing or optimize the network maintenance to the MOCK level.
To further determine whether CD151 regulates integrin activation, we examined active β1 integrins on the surface of ECs using β1 integrin mAb AG89, which specifically binds to the active form of β1 integrins.30 The level of AG89 staining in CD151-silenced ECs was almost identical to that in MOCK ECs (Figure 2G), and the total level of β1 integrins also remained the same (supplemental Table 1), suggesting that CD151 is not required for maintaining β1 integrins in active state.
In addition, CD151 may modulate integrin-independent cell-matrix adhesion in ECs. The expression of adhesion molecule CD44 was down-regulated at both the RNA and protein levels (supplemental Figure 4A). Consistently, CD151 silencing markedly reduced EC adhesion on hyaluronan (supplemental Figure 4B). CD44 is a major cellular receptor of hyaluronan and binds the membrane-cytoskeleton linkers ezrin-radixin-moesin complex and ankyrin.31 These results also suggest that CD151 regulates the connection between the plasma membrane and cytoskeleton. However, the expression levels of CD44 in CD151-null mice remained unchanged (supplemental Figure 4C), suggesting that CD44 reduction can be compensated in CD151-null mice during development.
Furthermore, the endothelia of aortas are frequently dissociated from the underlying basement membrane to different extents in CD151-null mice after being processed for electron microscopy (Figure 2H), reflecting a global reduction of endothelium-matrix adhesion. We also found that the basement membrane of aorta endothelium became split in some CD151-null mice (Figure 2H), consistent with weakened cell-matrix adhesiveness.
Collectively, these data indicate that EC-matrix adhesiveness becomes reduced in the absence of CD151 and that impaired EC-matrix adhesiveness partially contributes to the vascular instability caused by CD151 removal.
CD151 reinforces endothelial cell-cell adhesiveness
Endothelial cell-cell adhesion is critical for blood vessel formation and stability. Because CD151 promotes epithelial cell-cell adhesion,2,12,13 we predicted that endothelial cell-cell adhesion supports CD151-dependent vascular stability. Indeed, using cell aggregation assay, a standard approach to quantify cell-cell adhesiveness, we observed that loss of CD151 expression compromised endothelial cell-cell adhesion, as indicated by significantly fewer aggregates formed in CD151 KD cells after applying shear stress than in MOCK cells (Figure 3A).
In ECs, CD151 colocalized with VE-cadherin at cell-cell contacts.6 We next determined whether CD151 promotes endothelial cell-cell adhesion by stabilizing AJs. As shown in Figure 3B, VE-cadherin and β-catenin were uniformly and continuously localized at cell-cell contacts in HUVEC-MOCK monolayers, indicating the structural soundness of endothelial AJs. In HUVEC-CD151 KD cells, VE-cadherin and β-catenin distribution was irregular and discontinuous at the cell-cell contacts and partially localized to the cytoplasm probably because of diminished structural integrity in the endothelial AJs.
Moreover, the formation and maturation of adhesion zipper, the interdigitating and transition cell-cell adhesive structure that was observed in epithelial cells before the AJs appear and stabilize cell-cell contacts,32 become deficient in ECs when CD151 is silenced. Using TIRF microscopy, we examined the basal engagement of adjacent cells and found that CD151-silenced cells lack either closely interdigitated (Figure 3C left panel) or fully sealed cell-cell contacts (right panel) compared with MOCK cells.
To validate the in vitro observations, we examined endothelial cell-cell junctions in CD151-null mice. Using electron microscopy, we found that EC junctions were shortened, loose, and/or less electronically dense in CD151-null mice compared with WT littermates (Figure 3D). Adjacent plasma membranes in EC junctions tended to dissociate from each other and form open gaps between them when CD151 was ablated. Approximately 26.0% lung EC junctions were abnormal in CD151-null mice versus 7.3% in WT mice (Figure 3D right panel). This phenotype confirms the in vitro observations of decreased cell-cell adhesiveness and increased permeability.
To explore the in-depth mechanism by which CD151 strengthens AJs, we examined whether CD151 enhances cell-cell adhesion by stabilizing the AJ complex and/or the connection of this complex to cytoskeleton. Using coimmunoprecipitation, we found that the levels of α-, β-, and p120-catenin associated with VE-cadherin and the levels of VE-cadherin, α- and p120-catenin associated with β-catenin remained unchanged in HMEC-MOCK and HMEC-CD151 KD cells (Figure 3E), suggesting that CD151 is not required for and also unlike to modulate the assembly of AJ complex. However, more VE-cadherin proteins from confluent CD151 KD cells were soluble in 0.03% (data not shown) and 0.15% (Figure 3F) Triton X-100 than those from MOCK cells, suggesting that CD151 reinforces anchoring AJs to cytoskeleton or alter the membrane partition of VE-cadherin.
Finally, by analyzing cell aggregation in Ca2+-depleted medium, we observed that CD151 silencing markedly reduced EC adhesion (Figure 3A), indicating that CD151 also modulates cadherin-independent cell-cell adhesion. Indeed, we found that the transcription level of junctional adhesion molecule-3 (JAM-3) was significantly down-regulated (∼ 4-fold change) in CD151 KD cells compared with MOCK cells, whereas the mRNA levels of other cell-cell adhesion proteins, such as JAM-1, JAM-2, and PECAM-1, remained unchanged (data not shown), indicating that CD151 may regulate JAM-3–dependent cell adhesions.33
CD151 balances the levels of Rac1 and RhoA signaling
Observing altered EC adhesiveness drove us to analyze the effect of CD151 removal on cytoskeleton organization. CD151-silenced ECs typically exhibit fibroblast-like morphology with long and thick F-actin bundles that span the cell (Figure 4A), indicating that CD151 is needed for inhibiting the formation of robust stress fibers. In contrast, CD151-expressing ECs often display relatively thin, short, and intersected F-actin filaments that frequently form the cortical meshwork, implying the existence of lamellipodia (Figure 4A).
CD151 silencing deregulates RhoA and Rac1 signaling. (A) Loss of CD151 expression results in increased stress fiber formation. After spreading overnight, HMEC transductants were stained with AlexaFluor-488-conjugated phalloidin. F-actin staining was visualized with a Zeiss LSM510 confocal fluorescence microscope under a 100×/1.4 NA oil objective. Bar represents 10 μm. (B) HMEC transductants were seeded in diluted Matrigel-coated dishes and cultured overnight. Cell lysates were incubated with glutathione-S-transferase-Rhotekin Rho-binding domain beads to pull down GTP-bound RhoA. RhoA levels in pull-down precipitates, and whole-cell lysates were determined by SDS-PAGE and immunoblotting using anti–human RhoA mAb. RhoAGTP/total RhoA ratio (right panel) was calculated from the density of 4 blots and normalized to the MOCK group. *P < .01. (C) Cellular ROCK enzymatic activities were measured using a commercially available immunoassay kit. Data from 4 independent experiments were normalized to the MOCK group. **P < .05. (D) HMEC cells were seeded as in the RhoA pulldown assay and lysed in RIPA buffer for 20 minutes at 4°C. After centrifugation, cell lysates were obtained and processed for SDS-PAGE and immunoblotting to detect total MLC and 2P-MLC. (E) GTP-bound Rac1 was precipitated from HMEC cell lysates using glutathione-S-transferase-PAK-1 Rac1-binding domain beads. Levels of Rac1GTP and total cellular Rac1 were determined by immunoblotting using Rac1 mAb (left panel). Rac1GTP/total Rac1 ratio was then calculated, normalized, and compared between MOCK and CD151 KD cells (right panel, n = 4). *P < .01. (F) RhoA signaling inhibitors rescue the defects in cell-cell adhesion and angiogenesis resulted from the loss of CD151. For the in vitro capillary-like network formation assay, various inhibitors (C3 transferase, 2.5 μg/mL; Y27632, 10μM; blebbistatin, 5μM; and ML-7, 5μM) were added at 4 hours after HMEC transductants (top) or MLECs (bottom) were plated on Matrigel. The capillary-like structures were photographed 14 hours later. Bar represents 250 μm.
CD151 silencing deregulates RhoA and Rac1 signaling. (A) Loss of CD151 expression results in increased stress fiber formation. After spreading overnight, HMEC transductants were stained with AlexaFluor-488-conjugated phalloidin. F-actin staining was visualized with a Zeiss LSM510 confocal fluorescence microscope under a 100×/1.4 NA oil objective. Bar represents 10 μm. (B) HMEC transductants were seeded in diluted Matrigel-coated dishes and cultured overnight. Cell lysates were incubated with glutathione-S-transferase-Rhotekin Rho-binding domain beads to pull down GTP-bound RhoA. RhoA levels in pull-down precipitates, and whole-cell lysates were determined by SDS-PAGE and immunoblotting using anti–human RhoA mAb. RhoAGTP/total RhoA ratio (right panel) was calculated from the density of 4 blots and normalized to the MOCK group. *P < .01. (C) Cellular ROCK enzymatic activities were measured using a commercially available immunoassay kit. Data from 4 independent experiments were normalized to the MOCK group. **P < .05. (D) HMEC cells were seeded as in the RhoA pulldown assay and lysed in RIPA buffer for 20 minutes at 4°C. After centrifugation, cell lysates were obtained and processed for SDS-PAGE and immunoblotting to detect total MLC and 2P-MLC. (E) GTP-bound Rac1 was precipitated from HMEC cell lysates using glutathione-S-transferase-PAK-1 Rac1-binding domain beads. Levels of Rac1GTP and total cellular Rac1 were determined by immunoblotting using Rac1 mAb (left panel). Rac1GTP/total Rac1 ratio was then calculated, normalized, and compared between MOCK and CD151 KD cells (right panel, n = 4). *P < .01. (F) RhoA signaling inhibitors rescue the defects in cell-cell adhesion and angiogenesis resulted from the loss of CD151. For the in vitro capillary-like network formation assay, various inhibitors (C3 transferase, 2.5 μg/mL; Y27632, 10μM; blebbistatin, 5μM; and ML-7, 5μM) were added at 4 hours after HMEC transductants (top) or MLECs (bottom) were plated on Matrigel. The capillary-like structures were photographed 14 hours later. Bar represents 250 μm.
Stress fiber and lamellipodia formations are mediated by RhoA and Rac1 signalings, respectively. We observed that CD151 silencing results in significant up-regulation of RhoA activity (Figure 4B), demonstrating that CD151 is needed for restraining RhoA activity in ECs. In accordance with elevated RhoA activation, the downstream ROCK activity and dual-phosphorylated levels of myosin light chain (MLC) at Thr18 and Ser19 (2P-MLC), which is the active form of MLC essential for actomyosin assembly and stress fiber formation, were also significantly higher in CD151 KD cells than in MOCK cells (Figure 4C-D). In contrast, active Rac1 is largely reduced in CD151 KD ECs (Figure 4E). Hence, CD151 silencing deregulates Rho GTPases signaling and leads to the up-regulation of RhoA and down-regulation of Rac1 activity, reflecting the antagonism between RhoA and Rac1 described earlier.22
To determine whether RhoA signaling is needed for CD151-dependent vascular stabilization, we applied C3 transferase, Y27632, and blebbistatin, specific inhibitors for RhoA, ROCK, and myosin II, respectively, in the vascular morphogenesis assay. As shown in Figure 4F, all the inhibitors stabilized the endothelial network structures formed by CD151 KD cells so that both MOCK and CD151-silenced ECs exhibited similar levels of morphogenesis. Consistently, Y27632 also overrode the difference in vascular morphogenesis of primary lung ECs from WT and CD151-null mice (Figure 4F). ML-7, a specific inhibitor of MLC kinase that is exclusively involved in Ca2+-mediated and RhoA-independent muscle cell contraction, exerted no significant influence on the formation and maintenance of endothelial networks (Figure 4F). These results underscore that CD151 maintains vascular stability by, at least partially, restraining RhoA signaling. We also tested the PI3 kinase inhibitors wortmannin and LY294002 and found that these inhibitors have no effect on the formation and maintenance of the cable networks (data not shown).
Mechanism of CD151 deregulation of Rac1 and RhoA signaling
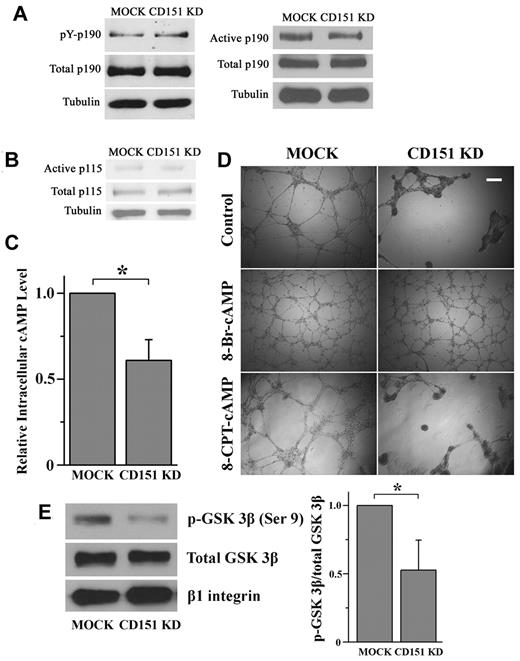
As observed previously, Rac1 and RhoA signaling exhibit constant antagonism coordinated by p190RhoGAP, a RhoA-specific GAP.22 To determine whether CD151 confines RhoA activity in a p190RhoGAP-dependent manner, we examined the tyrosine phosphorylation levels of p190RhoGAP in HMEC transductants and found no significant difference between HMEC-CD151 KD and HMEC-MOCK cells (Figure 5A). Consistently, the amount of functionally activated p190RhoGAP, which could selectively coprecipitate with constitutively active RhoA mutant (Q63L),24 remained unchanged in CD151 KD cells compared with MOCK cells (Figure 5A). Collectively, p190RhoGAP activation is not affected by CD151 silencing, and p190RhoGAP does not appear to be crucial for the CD151-dependent restraint of RhoA signaling.
How CD151 maintains the balance of RhoA and Rac1. (A-B) CD151 does not affect p190RhoGAP and p115RhoGEF activation. (A) Cellular p190RhoGAP was immunoprecipitated with p190RhoGAP mAb. The amount of tyrosine-phosphorylated p190RhoGAP was determined by immunoblotting with the phosphotyrosine mAb PY99. After stripping, total precipitated p190RhoGAP was detected by p190RhoGAP mAb. The active form of p190RhoGAP (A) or p115RhoGEF (B) was detected by a GST pulldown assay using a GST-tagged, constitutively active RhoA mutant (Q63L) or GST-tagged, nucleotide-empty RhoA mutant (G17A), respectively. (C) CD151 silencing reduces the cAMP level in ECs. HMEC transductants were lysed in 0.1N HCl. cAMP contents in lysates were measured using a cAMP EIA kit. *P < .05. (D) 8-Br-cAMP, but not 8-CPT-cAMP, stabilizes the network structures of CD151-silenced ECs. HMEC transductant cells were incubated with 8-Br-cAMP (500μM) or 8-CPT-cAMP (500μM) for the endothelial network formation assay. The images were taken 18 hours after incubation. Bar represents 250 μm. (E) CD151 silencing reduced PKA-phosphorylated GSK-3β in ECs. The phosphorylated GSK-3β at Ser9 residue in HMEC lysates was detected by Western blot using an anti–phospho-GSK-3β (Ser9) Ab and quantified by densitometry (mean ± SE, n = 4). *P < .05. The treatment of PI3K inhibitor LY294002 did not alter the difference in GSK-3β (Ser9) phosphorylation between MOCK and CD151 KD groups.
How CD151 maintains the balance of RhoA and Rac1. (A-B) CD151 does not affect p190RhoGAP and p115RhoGEF activation. (A) Cellular p190RhoGAP was immunoprecipitated with p190RhoGAP mAb. The amount of tyrosine-phosphorylated p190RhoGAP was determined by immunoblotting with the phosphotyrosine mAb PY99. After stripping, total precipitated p190RhoGAP was detected by p190RhoGAP mAb. The active form of p190RhoGAP (A) or p115RhoGEF (B) was detected by a GST pulldown assay using a GST-tagged, constitutively active RhoA mutant (Q63L) or GST-tagged, nucleotide-empty RhoA mutant (G17A), respectively. (C) CD151 silencing reduces the cAMP level in ECs. HMEC transductants were lysed in 0.1N HCl. cAMP contents in lysates were measured using a cAMP EIA kit. *P < .05. (D) 8-Br-cAMP, but not 8-CPT-cAMP, stabilizes the network structures of CD151-silenced ECs. HMEC transductant cells were incubated with 8-Br-cAMP (500μM) or 8-CPT-cAMP (500μM) for the endothelial network formation assay. The images were taken 18 hours after incubation. Bar represents 250 μm. (E) CD151 silencing reduced PKA-phosphorylated GSK-3β in ECs. The phosphorylated GSK-3β at Ser9 residue in HMEC lysates was detected by Western blot using an anti–phospho-GSK-3β (Ser9) Ab and quantified by densitometry (mean ± SE, n = 4). *P < .05. The treatment of PI3K inhibitor LY294002 did not alter the difference in GSK-3β (Ser9) phosphorylation between MOCK and CD151 KD groups.
Next, we analyzed levels of active p115RhoGEF, another major upstream regulator for RhoA, in HMEC transductants by using a GST fusion of a nucleotide binding-deficient RhoA mutant (G17A).24 The amount of the RhoA G17A-bound p115RhoGEF, which represents the active pool of p115RhoGEF, was similar in MOCK and CD151 KD cells (Figure 5B), indicating that p115RhoGEF is unlikely to be the upstream mediator in CD151-induced constraining of RhoA.
cAMP signaling protects the endothelial barrier by inducing Rac1 and/or inhibiting RhoA activity.34,35 We measured the level of cAMP in HMEC transductants and found that cellular cAMP is markedly reduced on CD151 silencing (Figure 5C). Furthermore, elevation of intracellular cAMP by 500μM 8-Br-cAMP, a long-acting cAMP analog, completely stabilized the vascular structures of CD151 KD cells (Figure 5D). Moreover, the rescuing effect 8-Br-cAMP is dose-dependent (supplemental Figure 5), suggesting a close functional relationship between cAMP and CD151 in vascular stabilization. Because 8-Br-cAMP activates both PKA and Epac pathways, we further treated the ECs with 8-CPT-cAMP, an Epac-selective activator, and demonstrated that 8-CPT-cAMP could not rescue the phenotype of CD151-silenced ECs (Figure 5D), strongly suggesting that CD151 maintains vascular stability by sustaining cAMP-PKA signaling. To confirm this notion, we analyzed the PKA-phosphorylated glycogen synthase kinase-3β (GSK-3β) at Ser9 residue as the readout of PKA activity.36,37 Consistent with reduced cAMP content, CD151-silenced ECs displayed significantly decreased phosphorylation of GSK-3β at Ser9 (Figure 5E), indicating that CD151 silencing inhibits cAMP/PKA signaling.
CD151 maintains vascular stability by mainly reinforcing cell-cell adhesion
Because β1 integrin activation, cAMP/PKA signaling activation, and Rho/ROCK signaling inhibition can stabilize CD151-negative endothelial network, we analyzed their roles in regulating the forces of cell-matrix adhesion, cell-cell adhesion, and cytoskeletal tension to determine the contributions of these forces to CD151-dependent vascular stability.
For cell-matrix adhesion, we found that TS2/16 promoted but 8-Br-cAMP and Y27632 inhibited static adhesion on LN 332 (Figure 6A) and focal adhesion formation (supplemental Figure 6) of both MOCK and CD151 KD ECs, suggesting that cell-matrix adhesion is sufficient, but not necessary, for stabilizing CD151-negative endothelial network.
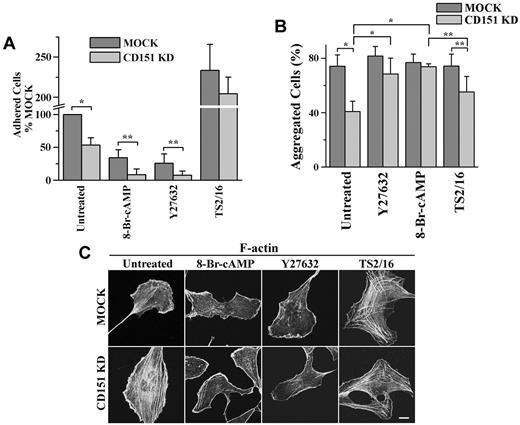
The effects of Y27632, 8-Br-cAMP, and TS2/16 on cell-matrix adhesion, cell-cell adhesion, and stress fiber formation. HMEC transductants were treated with Y27632 (10μM), 8-Br-cAMP (500μM), or TS2/16 (1 μg/mL) overnight either before (A) or during (B-C) experiments. (A) The 35-minute, static cell-matrix adhesion assay was performed on LN 332 (1 μg/mL) as described in supplemental Methods. *P < .01. **P < .05. (B) Cell-cell adhesion assay was performed in complete media. *P < .01. **P < .05. (C) After ECs were spread overnight in the presence or absence of treatment, the IF staining of F-actin was performed as described in supplemental Methods. Images were taken on a Zeiss LSM510 confocal fluorescence microscope under a 100×/1.4 NA oil objective. Bar represents 10 μm. See supplemental Figure 6 for the codistribution of F-actin and vinculin.
The effects of Y27632, 8-Br-cAMP, and TS2/16 on cell-matrix adhesion, cell-cell adhesion, and stress fiber formation. HMEC transductants were treated with Y27632 (10μM), 8-Br-cAMP (500μM), or TS2/16 (1 μg/mL) overnight either before (A) or during (B-C) experiments. (A) The 35-minute, static cell-matrix adhesion assay was performed on LN 332 (1 μg/mL) as described in supplemental Methods. *P < .01. **P < .05. (B) Cell-cell adhesion assay was performed in complete media. *P < .01. **P < .05. (C) After ECs were spread overnight in the presence or absence of treatment, the IF staining of F-actin was performed as described in supplemental Methods. Images were taken on a Zeiss LSM510 confocal fluorescence microscope under a 100×/1.4 NA oil objective. Bar represents 10 μm. See supplemental Figure 6 for the codistribution of F-actin and vinculin.
For cell-cell adhesion, 8-Br-cAMP and Y27632 up-regulated the diminished cell-cell adhesion in CD151-slienced ECs to the MOCK level, but TS2/16 had no effect on the diminution (Figure 6B). Interestingly, 8-Br-cAMP and Y27632 down-regulated Ca2+-independent cell-cell adhesion in MOCK ECs to the CD151 KD level, whereas TS2/16 had no effect (supplemental Figure 7). These results suggest that CD151 maintains vascular stability primarily through reinforcing calcium-dependent cell-cell adhesion.
For cytoskeletal tension, 8-Br-cAMP and Y27632 reduced stress fibers and generated actin bundles at the cell periphery, whereas TS2/16 promoted stress fiber formation in both HMEC transductants (Figure 6C), suggesting that increased cytoskeletal tension in CD151-negative ECs disrupts vascular stability by mainly reducing cell-cell adhesion.
Discussion
Earlier studies revealed that CD151 is needed for pathologic angiogenesis and for in vitro endothelial network formation that mimics vasculogenesis.5,9-11 However, the mechanism by which CD151 facilitates vascular morphogenesis remains to be delineated. Our study demonstrated that CD151 is not essential for neovascularization per se but is required for the stabilization of already formed endothelial structures. Unstabilized vascular structures resulting from CD151 removal cause regression of vessels, especially pathologic vessels that typically contain only one layer of endothelium with hyperpermeability,38 probably leading to the deficient pathologic angiogenesis in CD151-null mice.9 Equally important, our study demonstrated that CD151 is required for proper maintenance of endothelial stability after development and also regulates vascular permeability.
The stability of blood vessels, especially capillaries, depends on proper cell-cell and cell-matrix adhesions of the endothelia.15 Our data indicate that the loss of CD151 expression results in (1) diminished cell-cell and cell-matrix adhesions and (2) increased actomyosin traction signaling and subsequently elevated cytoskeletal tension. Consequently, the imbalance between cell adhesiveness and intrinsic tension directly leads to the instability of both newly formed vascular structure and matured microvessels and disrupts vessel barrier functions. Hence, CD151 potentiates the force that anchors ECs to their environment and mitigates the force of cytoskeletal tension generated within ECs.
CD151 colocalizes with cell adhesion molecules at the basolateral surfaces of ECs5,6,14 and regulates both cell-cell and cell-matrix adhesions. Because CD151 promotes integrin α6β1-mediated cell adhesion strengthening and sustains integrin α3β1 in activation,1,30 CD151 dysfunction reflects the functional deficiency of these laminin-binding integrins. We found that CD151 supports not only EC adhesiveness and adhesion strengthening on LN but also adhesion strengthening on FN. In addition, CD151 appears to potentiate integrin-independent cell-matrix adhesion, such as CD44-hyaluronan interaction. On the other hand, CD151 reinforces endothelial cell-cell adhesion and is required for optimal stability and maintenance of intercellular adhesion structures. Integrin α3β1 modulates cell-cell adhesion and related cytoskeletal organization in epithelial cells probably by interacting with the E-cadherin–β-catenin complex and altering pSmad2 signaling.39 CD151 plays a similar role in epithelial cells.2,12,13 In ECs, CD151 probably facilitates the cytoskeletal anchorage of VE-cadherin complex at AJs because CD151 sustains Rac1 signaling, which is required for the assembly of cortical actin meshwork. In addition, CD151 is needed for sustaining cadherin-independent cell-cell adhesion, evidenced by diminished endothelial cell-cell adhesiveness, aberrant tight junction formation in pulmonary endothelium, and down-regulated JAM-3 expression.
The global effects of CD151 on cell-cell and cell-matrix adhesions unlikely result entirely from the physical association of CD151 with laminin-binding integrins but are more likely derived from general alterations in adhesion molecule dynamics at the plasma membrane and/or the plasma membrane-cytoskeleton connection. Moreover, Ca2+-dependent cell-cell adhesion apparently plays a more important role in CD151-mediated vascular stability than EC-LN 332 adhesion does.
CD151 confines cytoskeletal tension by balancing Rac1 and RhoA activities. Because the activation of RhoA-ROCK-MLC signaling axis induces stress fiber formation and cell contractility, excessive RhoA activity leads to vascular destabilization and vessel regression, whereas suppression of RhoA signaling promotes angiogenesis and vessel stability.19,20 CD151 removal results in excessive activation of RhoA-ROCK-myosin signaling and disrupts the capillary structures without affecting their initial formation. Suppression of RhoA signaling by specific inhibitors sustained the capillary structures formed by CD151-deficienct ECs. Hence, CD151 stabilizes newly formed vessels largely by confining RhoA signaling, and the pathologic neovascularization in CD151-null mice probably undergoes accelerated regression largely because of elevated cytoskeletal tension. In addition, RhoA signaling regulates endothelial intercellular adhesion.21,40 Indeed, ROCK inhibitor restored only Ca2+-dependent cell-cell adhesiveness in CD151-silenced ECs, suggesting that RhoA signaling attenuates CD151-dependent endothelial stability mainly through cell-cell contacts. The reduced Rac1 activity also contributes to deficient cell-cell adhesiveness in CD151-deficient ECs because cAMP/PKA enhances Rac1 activity and Rac1 facilitates the assembly of cortical actin meshwork and subsequent formation of the cadherin-catenin complex.41,42
The tendency of laminin-binding integrins to activate Rac143,44 strongly suggests that CD151 sustains Rac1 activity by reinforcing the function of laminin-binding integrins. Laminin-binding integrins induce cAMP signaling45,46 ; and in microvascular ECs, cAMP primarily enhances Rac1 and subsequently reduces RhoA activity.47 When CD151 is absent, the reduced cAMP/PKA signaling causes the down-regulation of Rac1 and then indirectly leads to the up-regulation of RhoA, given the antagonism between Rac1 and RhoA signaling.48 However, the direct upstream regulator of RhoA is still unknown because CD151-mediated restraint of RhoA is independent of p190RhoGAP and p115RhoGEF. Future studies will focus on other Rho regulators. For example, p73RhoGAP, a splicing variant of FilGAP, is a RhoA-specific GAP that is specifically expressed in endothelium and promotes angiogenesis.49 CD151 may restrain RhoA activity through p73RhoGAP.
In integrin α3-null cells, cell-matrix adhesions mediated by collagen- and FN-binding integrins are up-regulated, accompanied by robust formation of stress fibers.50 However, the adhesion of CD151-silenced ECs on FN was not altered, but the traction forces exerted on FN and other matrices become weakened. Static cell-matrix adhesion reflects integrin-linked interaction between its specific matrix ligand and cytoskeleton, whereas traction force reflects the reinforcement of cytoskeleton and/or adhesion receptors at adhesion sites in addition to the matrix-integrin-cytoskeleton linkage. On FN and LN 111, the unaltered static adhesion, but reduced traction forces in CD151 KD ECs, suggests less reinforcement of cytoskeleton at and/or recruitment of integrins to cell-matrix contacts. In this case, reduced traction forces actually signify less adhesion strengthening. In addition, the increased solubility of adhesion proteins on CD151 silencing implies less stable and/or fewer connections of cytoskeleton to the plasma membrane. Thus, although excessive RhoA activation elevates cytoskeletal tension, the intracellular tension/tugging force cannot be fully transmitted to and through the plasma membrane, reflected by the less mature and fewer focal adhesions.
If CD151 is needed for the cytoskeletal connection to the plasma membrane, why can the enhanced cytoskeletal tension destabilize but ROCK inhibitor or β1 integrin activation stabilize the network structure of CD151-absent ECs? Hence, instead of or in addition to facilitating the cytoskeleton-plasma membrane connections, CD151 is more likely required for the proper distribution and dynamics of lipids and proteins in the plasma membrane.
In conclusion, we demonstrated that CD151 is needed for the endothelial stability of blood vessels. We also revealed that CD151 facilitates vascular morphogenesis, strengthens vascular stability, and reduces vascular permeability by reinforcing endothelial cell-cell and cell-matrix adhesions and alleviating cytoskeletal tension/tugging force (supplemental Figure 8). Our findings highlight a novel paradigm in which CD151 functions as a signaling switch to balance Rac1 and RhoA activities, leading to endothelial stability. It is noteworthy that cell-matrix and cell-cell adhesions contribute at different extents but are coordinated for maintaining vascular stability. Our observations suggest an attractive strategy to develop therapeutics for vascular diseases by modulating the level and functional status of CD151.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs Samuel Connell, Jamshid Temirov, and Jennifer Peters for imaging analysis; Drs A. P. Naren, Lawrence Pfeffer, and Suzanne Baker for helpful discussions; Mr Yuanjian Chen for technical support; and Drs Christopher Stipp and Keith Burridge for constructs.
This work was supported by the National Institutes of Health and American Heart Association (NIH grant CA096991 and AHA grant 0855307E, X.A.Z.; and AHA Predoctoral Fellowship 09PRE2260283, F.Z.).
National Institutes of Health
Authorship
Contribution: F.Z. and X.A.Z. designed the studies and wrote the paper; F.Z., J.E.M., S.M., N.S., and W.Z. performed experiments; Y.S., A.S., J.M.L., and H.H. provided expertise; and F.Z., J.E.M., and X.A.Z. analyzed data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Xin A. Zhang, Vascular Biology Center, 956 Court Ave, Memphis, TN 38163; e-mail: xzhang@uthsc.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal