Abstract
Plexin-A4 is a receptor for sema6A and sema6B and associates with neuropilins to transduce signals of class-3 semaphorins. We observed that plexin-A1 and plexin-A4 are required simultaneously for transduction of inhibitory sema3A signals and that they form complexes. Unexpectedly, inhibition of plexin-A1 or plexin-A4 expression in endothelial cells using specific shRNAs resulted in prominent plexin type specific rearrangements of the actin cytoskeleton that were accompanied by inhibition of bFGF and VEGF-induced cell proliferation. The two responses were not interdependent since silencing plexin-A4 in U87MG glioblastoma cells inhibited cell proliferation and strongly inhibited the formation of tumors from these cells without affecting cytoskeletal organization. Plexin-A4 formed stable complexes with the FGFR1 and VEGFR-2 tyrosine-kinase receptors and enhanced VEGF-induced VEGFR-2 phosphorylation in endothelial cells as well as bFGF-induced cell proliferation. We also obtained evidence suggesting that some of the pro-proliferative effects of plexin-A4 are due to transduction of autocrine sema6B-induced pro-proliferative signals, since silencing sema6B expression in endothelial cells and in U87MG cells mimicked the effects of plexin-A4 silencing and also inhibited tumor formation from the U87MG cells. Our results suggest that plexin-A4 may represent a target for the development of novel anti-angiogenic and anti-tumorigenic drugs.
Introduction
The plexin family of receptors includes 9 members divided into 4 subfamilies. They are single pass transmembrane receptors characterized by an intracellular GTPase activating (GAP) domain. The 4 type-A plexins function as direct receptors for class-6 semaphorins. Plexin-A1 is a receptor for sema6D1 while plexin-A2 and plexin-A4 are receptors for sema6A and sema6B.2-5 Class-6 semaphorins are single pass membrane bound semaphorins that were initially found to function as axon guidance factors3,6 but have recently been found to function outside of the central nervous system too. Sema6D is the best characterized factor of this semaphorin subfamily. It functions as a promoter of tumorigenesis,7,8 immune responses,9 and tissue remodeling.1,10 Interestingly, the sema6D receptor plexin-A1 forms complexes with the VEGF receptor VEGFR-2 which undergoes phosphorylation on stimulation with sema6D.1,7 In contrast, Sema6A was characterized as an inhibitor of angiogenesis.11
Type-A plexins also form complexes with neuropilins to form functional class-3 semaphorin receptors in which neuropilins function as semaphorin binding elements and the plexins as signal transducers.12,13 Class-3 semaphorins function as axon guidance factors during embryonic development, and as anti-angiogenic and anti-tumorigenic agents.14-16 More information about the properties of the semaphorins and their receptors can be found in several recent reviews.17,18 Little is known about the specific roles of individual type-A plexins but it is likely that they posses some specific and some redundant roles. Thus it was observed that plexin-A4 associates primarily with neuropilin-1 while plexin-A3 associates primarily with neuropilin-2 and that activation of these plexins can result in different biologic outcomes.19-21 It was also observed that different plexins can modulate the affinity of neuropilins to given semaphorins.22,23 However, aside from these studies, the similarities and differences between the functions of the 4 type-A plexins have not been well characterized.
Most of the class-3 semaphorins have been found to function as inhibitors of tumor angiogenesis and tumor progression (reviewed in Neufeld et al17 and Capparuccia et al18 ). The roles of the class-6 semaphorins in cancer aside from those of sema6D are less characterized. To gain a better understanding of the contribution of type-A plexins and their various ligands to angiogenesis and tumor progression, we modulated their expression in endothelial cells and cancer cells and determined how these manipulations affect cell behavior.
Methods
Antibodies and reagents
Antibodies directed against the following antigens were used: Neuropilin-1 (Santa Cruz Biotechnology; sc-7239), neuropilin-2 (Santa Cruz Biotechnology; sc-13 117), HA (Cell Signaling; 2362), c-myc (Santa Cruz Biotechnology; sc-40), V5 (Invitrogen; R960-25), R-Ras (Abcam; ab-57650), integrin-β1 (Millipore; MAB2079Z), Active integrin β1 (Chemicon; 1952), vinculin (Chemicon; 3574), p-VEGFR-2 (Cell Signaling; 2478), VEGFR2 (Santa Cruz Biotechnology; sc-504), CD31 (Pharmingen; 557355), and NG2 (Chemicon; 5320). An expression vector directing expression of an extracellular domain of mouse Sema6A fused in frame with FC was kindly provided by Dr Oded Behar (Hebrew University of Jerasalem). Lentiviral ShRNA vectors were purchased from Sigma-Aldrich. BrdU Labeling and detection kit I was purchased from Roche (11296736001). Fibronectin was purchased from Biologic Industries (03-090-1-05). Alexa-conjugated phalloidin was from Molecular Probes. A partial human plexin-A4 cDNA was kindly provided by Dr Luca Tamagnone (University of Turin). The missing C-terminal part of the cDNA was cloned from HUVECs and ligated in frame to this partial cDNA. The FGFR-1, FGFR-2, and sema6B cDNAs were purchased from Open Biosystems. Recombinant extracellular part of human plexin-A4 (5856-PA-050) and mouse plexin-A1 (4309-PA-050) were purchased from R&D systems. Recombinant VEGF165 and bFGF were produced and purified as described.24,25
Cell lines
HUVECs were isolated and cultured as previously described.26 BHK-21 cells were cultured in serum containing or serum free medium as previously described.27 All other cell types used were cultured in DMEM medium containing 10% FCS and antibiotics in a humidified incubator at 5% CO2. The WW94, YU-PAC, and YU-ZAZ6 malignant melanoma cells were obtained from Dr Ruth Halaban (Yale University).28 The rest of the cell lines were from the ATCC.
Quantification of plexin expression levels by real-time PCR
Primers for RT-PCR of various class-3 semaphorins were used as described previously.15 Real-time PCR was performed using Absolute Blue QPCR SYBR green mix according to the instructions of the manufacturer (Thermo Scientific). The primers used are listed in Table 1.
Primers used in quantitative RT-PCR experiments
| Gene . | Forward . | Reverse . |
|---|---|---|
| Plexin-A1 | TCCTGGTGGACTCTCAAAC | ACTGCACACAGCTCTCCACA |
| Plexin-A2 | CATCTCGTACTGGACCCCAC | TTTACAACGGCTACAGCGTG |
| Plexin-A3 | ACCACGAAGGCACGGAAG | AGCCAGCGGAGGGACAG |
| Plexin-A4 | TCTCAGTACAACGTGCTG | TAGCACTGGATCTGATTGC |
| Sema3A | GGTTAACTAGGATTGTCTGTC | GTGATCACATTGTTGGATTC |
| Sema6B | CTTACTTTGTCCATGCGGTG | CACGTCGTTCTTGCACACTC |
| Actin | TTGCCGACAGGATGCAGAAGGA | AGGTGGACAGCGAGGCCAGGAT |
| Gene . | Forward . | Reverse . |
|---|---|---|
| Plexin-A1 | TCCTGGTGGACTCTCAAAC | ACTGCACACAGCTCTCCACA |
| Plexin-A2 | CATCTCGTACTGGACCCCAC | TTTACAACGGCTACAGCGTG |
| Plexin-A3 | ACCACGAAGGCACGGAAG | AGCCAGCGGAGGGACAG |
| Plexin-A4 | TCTCAGTACAACGTGCTG | TAGCACTGGATCTGATTGC |
| Sema3A | GGTTAACTAGGATTGTCTGTC | GTGATCACATTGTTGGATTC |
| Sema6B | CTTACTTTGTCCATGCGGTG | CACGTCGTTCTTGCACACTC |
| Actin | TTGCCGACAGGATGCAGAAGGA | AGGTGGACAGCGAGGCCAGGAT |
Inhibition of plexin and semaphorin expression with shRNA- expressing lentiviruses
Lentiviral shRNA vectors carrying the shRNA sequences shown in Table 2 were purchased from Sigma-Aldrich.
shRNA sequences used to inhibit gene expression
| Gene . | Sh-RNA sequence . |
|---|---|
| Plexin-A1 | CCGGGCACTTCTTCACGTCCAAGATCTCGAGATCTTGGACGTGAAGAAGTGCTTTTTG |
| Plexin-A2 | CCGGCGGCAATTTCATCATTGACAACTCGAGTTGTCAATGATGAAATTGCCGTTTTTG |
| Plexin-A3 | CCGGGCTGTATTTCTATGTCACCAACTCGAGTTGGTGACATAGAAATACAGCTTTTTG |
| Plexin-A4 | #1:CCGGGCAGATAAATGACCGCATTAACTCGAGTTAATGCGGTCATTTATCTGCTTTTTG |
| #2: CCGGCCTGACTTTGATATCTACTATCTCGAGATAGTAGATATCAAAGTCAGGTTTTTG | |
| Sema3A | CCGGCCCAATCTCAACACGATGGATCTCGAGATCCATCGTGTTGAGATTGGGTTTTTG |
| Sema6B | #1:CCGGTGGTTCAAAGAGCCTTACTTTCTCGAGAAAGTAAGGCTCTTTGAACCATTTTTG |
| #2:CCGGCGGAGACAACATCAGCGGTATCTCGAGATACCGCTGATGTTGTCTCCGTTTTTG |
| Gene . | Sh-RNA sequence . |
|---|---|
| Plexin-A1 | CCGGGCACTTCTTCACGTCCAAGATCTCGAGATCTTGGACGTGAAGAAGTGCTTTTTG |
| Plexin-A2 | CCGGCGGCAATTTCATCATTGACAACTCGAGTTGTCAATGATGAAATTGCCGTTTTTG |
| Plexin-A3 | CCGGGCTGTATTTCTATGTCACCAACTCGAGTTGGTGACATAGAAATACAGCTTTTTG |
| Plexin-A4 | #1:CCGGGCAGATAAATGACCGCATTAACTCGAGTTAATGCGGTCATTTATCTGCTTTTTG |
| #2: CCGGCCTGACTTTGATATCTACTATCTCGAGATAGTAGATATCAAAGTCAGGTTTTTG | |
| Sema3A | CCGGCCCAATCTCAACACGATGGATCTCGAGATCCATCGTGTTGAGATTGGGTTTTTG |
| Sema6B | #1:CCGGTGGTTCAAAGAGCCTTACTTTCTCGAGAAAGTAAGGCTCTTTGAACCATTTTTG |
| #2:CCGGCGGAGACAACATCAGCGGTATCTCGAGATACCGCTGATGTTGTCTCCGTTTTTG |
Expression of recombinant proteins
The full-length cDNA of plexin-A4 was cloned into the gateway pDonor221 plasmid and then transferred into the pLenti6/V5-DEST lentiviral expression vector in frame with a c-terminal V5 tag by recombination according to the instructions of the manufacturer (Invitrogen, Cat. # V496-10). An expression vector directing expression of a deletion mutant of plexin-A4 containing the transmembrane and extracellular domains (amino-acids 1-1237) fused to a c-terminal V5 tag was constructed similarly. The cDNAs encoding FGFR1 and FGFR2 fused in frame at their 3′ ends to a VSV epitope tag and a sema6B cDNA fused in frame at the 3′ end to a myc tag were subcloned into the NSPI-CMV plasmid.29 Production of lentiviruses using these plasmids and stable infection of target cells were performed essentially as previously described.30 The expression plasmid containing the sema6A cDNA was transfected into target cells using FuGENE6 transfection reagent (Roche) as previously described.31
HUVEC and cancer cells proliferation assays
HUVECs were seeded at a concentration of 2 × 104 cells/well in 24-well dishes coated with PBS-gelatin. The number of adherent cells was then determined after trypsinization using a coulter counter (time 0). Growth factors were then added or not, and the number of adherent cells in each culture determined after 3 days in culture. The induction of proliferation was calculated as the fold increase in the number of cells relative to time 0. A similar protocol was used for cancer cells which were grown in full growth medium containing 10% FBS without added growth factors. Serum- free proliferation assays using BHK-21 cells were performed using increasing concentrations of bFGF as described.27
Cytoskeletal contraction assay
Cytoskeletal contraction of HUVECs and tumor cells in response to semaphorins was performed as described.32
Tube formation and in vitro angiogenesis assays
Tube formation assays on Matrigel were done and quantified as previously described.32 Briefly, HUVECs were seeded at a concentration of 1.2 × 104 cells/well on top of 150 μL Matrigel in 48 well dishes. After the indicated times the cells were photographed and bifurcations per microscopic field counted. The 3D in vitro angiogenesis assay was performed essentially as described.33,34 Briefly, spheroids containing 400 cells each were prepared by the hanging drop method35 and implanted in collagen gels in the presence or absence of angiogenic factors. The spheroids were photographed after 24 hours and sprout formation assessed as described.33,34
Coimmunoprecipitation
Coimmunoprecipitation assays were performed as previously described.33
ELISA assay
The experiment was performed using standard protocols. In short, 96 wells were coated with 100 ng of the extracellular portion of plexin-A4 or BSA for 1 hour in 37°C. The wells were washed with 0.05% (vol/vol) of PBS-T (Tween 20) and then blocked with 1% BSA solution. Various concentrations of the extracellular portion of plexin-A1 fused to an FC tag were placed for 2 hours at room temperature on the coated wells in the presence of 0.5% BSA. The wells were washed and an anti-human IgG1 HRP antibody was added for 1 hour at room temperature. A TMB substrate-chromagen was added to initiate a colorimetric reaction that was terminated using 0.2M sulphuric acid.
HUVEC repulsion assay
The assay was performed as previously described.15 Essentially, clonal densities of HEK293 cells expressing either class-3 semaphorins or control vector were seeded on a monolayer of HUVECs. After 24 hours an area of no entry zone appeared around HEK293 expressing semaphorins.
Immunocytochemistry
HUVECs were plated on glass coverslips coated with PBS-gelatin. Detection of vinculin and actin was done as described.31 Frozen tumor sections were prepared and stained using antibodies directed against CD-31 and NG2. Quantification of stained areas in microscopic fields was performed using the morphometric Image-Pro plus 5.1 software package essentially as previously described.15
Adhesion assay
Non-adhesive 12-well tissue culture plates were coated with gelatin. HUVECs in which the expression of various type-A plexins was inhibited or control cells were seeded (4 × 104 cells/well) in the wells. Adherent cells were counted after 15 minutes and after 3 hours. The percentage of the adhered cells from the seeded at each time point was then determined.
BrdU incorporation
Detection of BrdU positive cells was done using Roche Labeling and detection kit I according to the instructions of the manufacturer. The percentage of proliferating cells was obtained from the ratio of BrdU positive cells out of the total number of DAPI stained cells.
ERK-1/2 phosphorylation assay
Determination of ERK1/2 phosphorylation in HUVEC after stimulation with various growth factors was done essentially as described.31 In all the biochemical assays including this one, the density of cells at the start of the experiment was ∼ 80% of confluence.
Determination of active Rac1
The concentration of active Rac1 was determined using the G-LISA Rac1 activation kit of Cytoskeleton (Cat. #BK126) according to the instructions of the manufacturer.
R-ras pull down assay
The activated R-ras pull down assay was performed as previously described.36
Tumor formation assay
U87MG cells infected with lentiviruses directing expression of plexin-A4 or sema6B targeting shRNAs or with control nontargeting shRNA were trypsinized, washed twice with PBS, suspended in 100 μL of PBS and injected subcutaneously into the flanks of 4 to 5-week old male athymic nude mice (1 × 106 cells/mouse). Tumor size was monitored once a week for the duration of the experiment as previously described.15 All the animal experiments were approved by the Technion ethics committee.
Statistical analysis
The 1-tailed, unpaired with the Welch correction Student t test was used in most experiments. In cell proliferation experiments in which we normalized the data across several experiments we used the paired Student t test taking care that the differences between the controls do not vary by > 50%. Cell proliferation experiments were performed in triplicates. Variations between replicates within single experiments did not exceed 10%. Error bars represent the SEM. Statistical significance is presented in the following manner: *P < .05, **P < .01, and ***P < .001. All experiments were repeated independently at least 3 times unless otherwise stated.
Results
Silencing type-A plexins in HUVECs results in plexin specific cytoskeletal changes
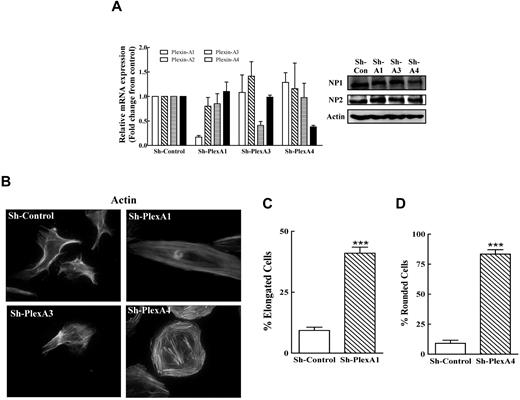
HUVECs express the 4 type-A plexins (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) that associate with neuropilin-1 and neuropilin-2 to form functional class-3 semaphorin receptors.17 We have used shRNA that inhibit specifically the expression of the targeted plexins, but not the expression of other type-A plexins or neuropilins, to silence the expression of plexin-A1, plexin-A3 and plexin-A4 in HUVECs (Figure 1A). Silencing plexin-A3 expression had no effect on cell morphology (Figure 1B). In contrast, ∼ 40% of the plexin-A1 silenced HUVECs acquired an elongated fibroblast-like morphology rich in stress fibers (Figure 1B-C) while ∼ 85% of the plexin-A4 silenced cells displayed a rounded morphology characterized by a circumferential organization of actin fibers (Figure 1B,D and supplemental Videos 1-2). A similar though less prominent change was produced by a different less efficient plexin-A4 targeting shRNA (supplemental Figure 2A). Similar morphologic effects were observed when plexin-A4 was silenced in several additional types of human endothelial cells (supplemental Figure 2D).
Silencing type-A plexins in HUVECs with shRNAs induces plexin specific morphologic effects. (A) The expression levels of the mRNAs encoding type-A plexins were determined in HUVECs infected with nontargeting shRNA (sh-control) and in HUVECs in which the expression of plexin-A1 (sh-plexA1), plexin-A3 (sh-plexA3), or plexin-A4 (sh-plexA4) was silenced with specific shRNAs using real-time quantitative PCR (left panel). Neuropilin levels were also determined in cell lysates using Western blot analysis (right panel). (B) HUVECs expressing sh-control or different sh-plexins as indicated were stained with fluorescent phalloidin as described in “Immunocytochemistry” and photographed. (C-D) The percentage of the HUVECs which changed their morphology after the silencing of Plexin-A1 or Plexin-A4 was determined. Cells in 6 microscopic fields (∼ 300 cells) were photographed and scored using a phase contrast microscope at 20× magnification.
Silencing type-A plexins in HUVECs with shRNAs induces plexin specific morphologic effects. (A) The expression levels of the mRNAs encoding type-A plexins were determined in HUVECs infected with nontargeting shRNA (sh-control) and in HUVECs in which the expression of plexin-A1 (sh-plexA1), plexin-A3 (sh-plexA3), or plexin-A4 (sh-plexA4) was silenced with specific shRNAs using real-time quantitative PCR (left panel). Neuropilin levels were also determined in cell lysates using Western blot analysis (right panel). (B) HUVECs expressing sh-control or different sh-plexins as indicated were stained with fluorescent phalloidin as described in “Immunocytochemistry” and photographed. (C-D) The percentage of the HUVECs which changed their morphology after the silencing of Plexin-A1 or Plexin-A4 was determined. Cells in 6 microscopic fields (∼ 300 cells) were photographed and scored using a phase contrast microscope at 20× magnification.
The morphology of the plexin-A4 silenced HUVECs resembles superficially the morphology produced as a result of the expression of dominant-active Rac1 (supplemental Figure 2C).37 Indeed, the concentration of activated Rac1 in plexin-A4 silenced HUVECs increased (supplemental Figure 3A). The increased Rac1 activity was associated with an increase in the concentration of activated R-ras as determined by a GTP/R-ras pull-down assay. This was because of an increase in the overall concentration of R-ras rather than due to a change in the ratio of R-ras-GTP to total-R-ras (supplemental Figure 3C), and was accompanied by increased adherence to collagen (supplemental Figure 3B).
Both plexin-A1 and plexin-A4 are required for sema3A signal transduction
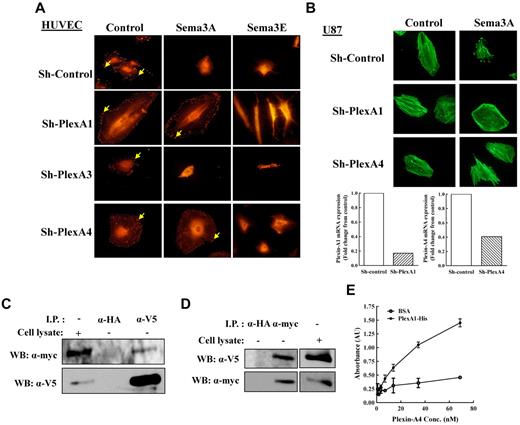
Neuropilins and type-A or type-D plexins form complexes to produce functional class-3 semaphorin receptors in which the neuropilin is the binding element and the plexin is the signal transducer.12,13 Sema3E is unique among class-3 semaphorins since it does not bind to neuropilins and transduces signals directly using the plexin-D1 receptor.38 When we stimulated parental HUVECs, HUVECs expressing a nontargeting shRNA or HUVECs in which plexin-A3 expression was silenced with sema3A or sema3E, the cells rapidly lost vinculin from focal adhesions and subsequently contracted (Figure 2A).15,31 HUVECs in which the expression of either plexin-A1 or plexin-A4 was silenced responded to sema3E like untreated HUVECs but failed to respond to sema3A, suggesting that both plexins are concomitantly required for sema3A signal transduction (Figure 2A). These results were supported by cell repulsion experiments which demonstrated that sema3A, but not sema3E, failed to repel HUVECs in which plexin-A4 was silenced (supplemental Figure 3D). Similar results were observed when U87MG glioblastoma cells in which the expression of plexin-A1 or plexin-A4 was silenced were stimulated with sema3A. Although these cells did not display morphologic changes in response to plexin-A1 or plexin-A4 silencing, the silencing of either plexins completely abrogated the response to sema3A (Figure 2B). The concentration of active Rac-1 and active R-ras in U87MG cells silenced for plexinA4 were not elevated, in agreement with the lack of effect of plexinA4 silencing on their morphology (data not shown).
Both plexin-A1 and plexin-A4 are required for sema3A-induced cell contraction. (A) HUVECs expressing sh-control or sh-plexins were incubated for 15 minutes with conditioned medium derived from HEK293 cells infected with lentivirus expression vector (control) or with lentiviruses directing expression of sema3A (sema3A), or sema3E (sema3E). Cells were fixed and stained for the intracellular distribution of vinculin (arrows). (B) U87MG cells expressing sh-plexA1 or sh-plexA4 (bottom) were incubated with control or sema3A containing conditioned medium for 15 minutes. Actin filaments were then visualized using fluorescent phalloidin (top). (C-D) PAE cells coexpressing myc tagged plexin-A1 and neuropilin-115 were infected with a lentivirus directing expression of V5 tagged plexin-A4. The infected cells were lysed, (C) plexin-A4 was immunoprecipitated using anti-V5 antibody, and the lysates examined for the presence of plexin-A1 using anti-myc antibody. (D) Plexin-A1 was immunoprecipitated from cell lysates using anti-myc antibody and the presence of plexin-A4 in the lysates was examined using an anti-V5 antibody. Immunoprecipitation with an anti-HA antibody was performed as a negative control. Shown is a representative experiment of 3 that gave similar results. (E) ELISA assay using the extra-cellular HIS-tagged human plexin-A4 coated wells and increased concentrations of the extra-cellular FC-tagged mouse plexin-A1.
Both plexin-A1 and plexin-A4 are required for sema3A-induced cell contraction. (A) HUVECs expressing sh-control or sh-plexins were incubated for 15 minutes with conditioned medium derived from HEK293 cells infected with lentivirus expression vector (control) or with lentiviruses directing expression of sema3A (sema3A), or sema3E (sema3E). Cells were fixed and stained for the intracellular distribution of vinculin (arrows). (B) U87MG cells expressing sh-plexA1 or sh-plexA4 (bottom) were incubated with control or sema3A containing conditioned medium for 15 minutes. Actin filaments were then visualized using fluorescent phalloidin (top). (C-D) PAE cells coexpressing myc tagged plexin-A1 and neuropilin-115 were infected with a lentivirus directing expression of V5 tagged plexin-A4. The infected cells were lysed, (C) plexin-A4 was immunoprecipitated using anti-V5 antibody, and the lysates examined for the presence of plexin-A1 using anti-myc antibody. (D) Plexin-A1 was immunoprecipitated from cell lysates using anti-myc antibody and the presence of plexin-A4 in the lysates was examined using an anti-V5 antibody. Immunoprecipitation with an anti-HA antibody was performed as a negative control. Shown is a representative experiment of 3 that gave similar results. (E) ELISA assay using the extra-cellular HIS-tagged human plexin-A4 coated wells and increased concentrations of the extra-cellular FC-tagged mouse plexin-A1.
These experiments imply that plexin-A3 does not play a role in sema3A signaling while plexin-A1 and plexin-A4 are both essential, suggesting that these plexins may physically interact. We therefore expressed both plexins that were tagged with different epitope tags at their C-terminals in porcine aortic endothelial (PAE) cells. We then performed coimmunoprecipitation experiments which revealed that they form complexes (Figure 2C-D). The formation of these complexes was not affected by the presence or absence of neuropilin-1, by the addition of exogenous sema3A, or by the addition of the recombinant soluble extracellular domains of sema6A or sema6B suggesting that the complexes are formed spontaneously (data not shown). The association seemed to be mediated by extracellular domains of the plexins since the isolated soluble extracellular domains of plexin-A1 and plexin-A4 were able to bind to each other (Figure 2E). These experiments imply that functional sema3A receptors may be composed of complexes containing neuropilin-1, plexin-A1, and plexin-A4.
Inhibition of plexin-A4 expression in HUVECs inhibits bFGF-induced cell proliferation
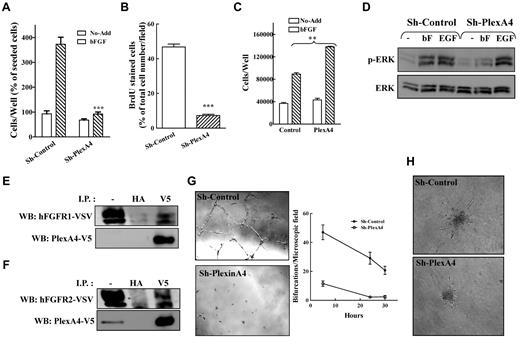
Silencing plexin-A4 in HUVECs, as well as in 3 additional types of endothelial cells, was accompanied by potent inhibition of bFGF-induced proliferation (Figure 3A and supplemental Figure 2E) but did not affect the survival of HUVECs in the absence of added growth factors (Figure 3A). A different somewhat less efficient plexin-A4 targeting shRNA also inhibited the mitogenic response to bFGF although less potently (supplemental Figure 2B). Plexin-A4 silencing inhibited the bFGF-induced entry of the cells into the S-phase of the cell cycle as determined by BrdU incorporation assay (Figure 3B and supplemental Figure 3E), and was not associated with increased apoptosis as revealed by tunnel assay (data not shown). These results suggested, surprisingly, that silencing plexin-A4 specifically interferes with the transduction of pro-proliferative signals. To verify this hypothesis, we over-expressed human recombinant plexin-A4 ectopically in HUVEC. The ectopic over-expression resulted in a significant enhancement of bFGF-induced cell proliferation (Figure 3C). Furthermore, expression of recombinant plexin-A4 in BHK-21 cells, a cell line that is completely dependent on bFGF under serum-free conditions,27 also resulted in a significant 20% enhancement of bFGF-induced cell proliferation (supplemental Figure 3F). In contrast, expression of a truncated plexin-A4 lacking the intracellular domain in BHK-21 cells partially inhibited bFGF-induced proliferation (supplemental Figure 3F).
Plexin-A4 modulates bFGF-induced signal transduction. (A) HUVEC expressing sh-control or sh-plexA4 were seeded in gelatin coated 24 well dishes and the number of attached cells determined as described. BFGF (5 ng/mL) was then added and number of attached cells was determined again after 3 days as described. The average of 3 independent experiments each of which was done in triplicates is shown. (B) Incorporation of BrdU into the DNA of HUVECs infected with lentiviruses directing expression of nontargeting shRNA (Sh-Control) or an shRNA targeting plexin-A4 (sh-plexA4) was measured 24 hours after stimulation with bFGF. The number of microscopic fields photographed and counted was 5 and 7 for sh-control, and sh-plexA4, respectively. Shown is a representative experiment of 2 experiments that produced similar results. (C) HUVECs ectopically expressing plexin-A4 or control cells infected with an empty lentiviral expression vector (Control) were seeded in 24 well dishes (2 × 104 cells/well) in the presence or absence of bFGF (5 ng/mL). After 3 days adherent cells were detached and counted in a coulter counter. Shown is one representative experiment of 3 that gave similar results. (D) HUVECs expressing a nontargeting shRNA (sh-control) or HUVECs expressing a plexin-A4 targeting shRNA (sh-plexA4) were stimulated with bFGF (5 ng/mL) or EGF (50 ng/mL). After 10 minutes at room temperature the cells were lysed and ERK1/2 phosphorylation determined using Western blot analysis. Shown is one representative experiment of 3 that gave similar results. (E-F) A full-length human plexin-A4 was fused to a c-terminal V5 tag and expressed along with FGFR-1/VSV (E) or FGFR-2/VSV (F) in PAE cells. The cells were lysed and immunoprecipitation was performed using anti-V5 or anti-HA antibodies used as negative controls. Precipitates were subjected to Western blot analysis using antibodies directed against VSV. Blots were then stripped and reprobed with an anti-V5 antibody. (G) HUVEC expressing sh-plexA4 or sh-control were seeded (1.2 × 104 cells/well) on top of Matrigel. Tube formation and quantification of bifurcations in the tubular network formed were assessed at various time points. Shown is one representative experiment of 3 that gave similar results. (H) Spheroids (500 cells/spheroid) containing HUVECs expressing control or a plexin-A4 targeting shRNA were seeded in collagen and stimulated to sprout with 5 ng/mL bFGF. Shown are representative pictures of sprouting spheroids taken after 24 hours.
Plexin-A4 modulates bFGF-induced signal transduction. (A) HUVEC expressing sh-control or sh-plexA4 were seeded in gelatin coated 24 well dishes and the number of attached cells determined as described. BFGF (5 ng/mL) was then added and number of attached cells was determined again after 3 days as described. The average of 3 independent experiments each of which was done in triplicates is shown. (B) Incorporation of BrdU into the DNA of HUVECs infected with lentiviruses directing expression of nontargeting shRNA (Sh-Control) or an shRNA targeting plexin-A4 (sh-plexA4) was measured 24 hours after stimulation with bFGF. The number of microscopic fields photographed and counted was 5 and 7 for sh-control, and sh-plexA4, respectively. Shown is a representative experiment of 2 experiments that produced similar results. (C) HUVECs ectopically expressing plexin-A4 or control cells infected with an empty lentiviral expression vector (Control) were seeded in 24 well dishes (2 × 104 cells/well) in the presence or absence of bFGF (5 ng/mL). After 3 days adherent cells were detached and counted in a coulter counter. Shown is one representative experiment of 3 that gave similar results. (D) HUVECs expressing a nontargeting shRNA (sh-control) or HUVECs expressing a plexin-A4 targeting shRNA (sh-plexA4) were stimulated with bFGF (5 ng/mL) or EGF (50 ng/mL). After 10 minutes at room temperature the cells were lysed and ERK1/2 phosphorylation determined using Western blot analysis. Shown is one representative experiment of 3 that gave similar results. (E-F) A full-length human plexin-A4 was fused to a c-terminal V5 tag and expressed along with FGFR-1/VSV (E) or FGFR-2/VSV (F) in PAE cells. The cells were lysed and immunoprecipitation was performed using anti-V5 or anti-HA antibodies used as negative controls. Precipitates were subjected to Western blot analysis using antibodies directed against VSV. Blots were then stripped and reprobed with an anti-V5 antibody. (G) HUVEC expressing sh-plexA4 or sh-control were seeded (1.2 × 104 cells/well) on top of Matrigel. Tube formation and quantification of bifurcations in the tubular network formed were assessed at various time points. Shown is one representative experiment of 3 that gave similar results. (H) Spheroids (500 cells/spheroid) containing HUVECs expressing control or a plexin-A4 targeting shRNA were seeded in collagen and stimulated to sprout with 5 ng/mL bFGF. Shown are representative pictures of sprouting spheroids taken after 24 hours.
To determine whether the inhibition produced by plexin-A4 silencing is specific to bFGF, we stimulated plexin-A4 silenced HUVECs or control cell with bFGF or with epidermal growth factor (EGF). In control cells, both bFGF and EGF induced strong phosphorylation of ERK1/2. In the silenced cells, bFGF-induced phosphorylation was strongly inhibited while EGF-induced phosphorylation of ERK1/2 was not (Figure 3D). These results indicated that plexin-A4 can function as a selective modulator of bFGF signaling and further suggested that plexin-A4 may physically interact with FGF receptors. Indeed, when a V5-tagged plexin-A4 receptor was expressed along with VSV-tagged FGFR1 or FGFR2 receptors in PAE cells, we found that both FGF receptors coimmunoprecipitate with plexin-A4 (Figure 3E-F). In contrast, when a truncated V5-tagged plexin-A4 receptor lacking the extracellular domain was expressed along with the FGF receptors the V5 antibody failed to coimmunoprecipitate the FGF receptors (data not shown).
Because plexin-A4 seems to be important for the transduction of bFGF-induced mitogenic signals in HUVECs, and because bFGF functions as a potent angiogenic factor,39 we also determined if plexin-A4 can modulate in vitro processes crucial for successful angiogenesis. Inhibition of plexin-A4 expression in HUVECs strongly inhibited the spontaneous formation of tubes after the seeding of HUVECs on Matrigel (Figure 3G).40 The silencing also abolished bFGF-induced sprouting from pre-formed spheroids of HUVECs in a 3D in vitro angiogenesis assay, suggesting that plexin-A4 may play a crucial role in bFGF-induced angiogenic sprouting of blood vessels (Figure 3H).34
Plexin-A4 forms complexes with the VEGFR-2 receptor and modulates VEGF signaling
Silencing plexin-A4 in HUVECs seemed to affect processes associated with the induction of angiogenesis and we therefore determined if plexin-A4 can modulate VEGF signaling. Silencing plexin-A4 expression inhibited VEGF-induced proliferation of HUVECs (Figure 4A) and VEGF-induced angiogenesis in an in vitro 3D angiogenesis assay (Figure 4B), but less than the inhibition seen with bFGF stimulation. These results suggest that plexin-A4 may also interact with VEGF receptors. Indeed we found that plexin-A4 and the VEGFR-2 tyrosine-kinase receptor form stable complexes as determined by coimmunoprecipitation experiments using PAE cells expressing recombinant V5 tagged plexin-A4 and VEGFR-2 (Figure 4C). This interaction apparently enhances the responses of VEGFR-2 to stimulation with VEGF since the VEGF-induced phosphorylation of VEGFR-2 was strongly inhibited in the silenced HUVEC (Figure 4D). To verify this observation we also stimulated PAE cells expressing only VEGFR-2 or PAE cells expressing both plexin-A4 and VEGFR-2 with VEGF. Notably, we found that in these cells too the VEGF-induced phosphorylation of VEGFR-2 was strongly enhanced as a result of the presence of plexin-A4 (Figure 4E). Taken together, these experiments suggest that plexin-A4 can also enhance VEGF-induced signal transduction.
Plexin-A4 modulates VEGF-induced signaling mediated by the VEGFR-2 receptor. (A) HUVECs expressing a nontargeting shRNA (sh-control) or HUVECs in which plexin-A4 expression was silenced (sh-plexA4) were stimulated with VEGF (10 ng/mL) as described. Adherent cells were counted after 3 days. The average of 3 independent experiments each of which was done in triplicates is shown. (B) Spheroids (500 cells/spheroid) containing HUVECs expressing a control shRNA (sh-control) or HUVECs silenced for plexin-A4 expression (sh-plexA4) were seeded in collagen and stimulated to sprout with 50 ng/mL VEGF. Shown are representative pictures of sprouting spheroids taken after 24 hours. (C) The full-length human plexin-A4 cDNA was fused to a c-terminal V5 tag and coexpressed with the VEGFR-2 cDNA in PAE cells. The cells were lysed and immunoprecipitation was performed using anti-V5 antibodies or anti-HA antibodies used as a negative control. Precipitates were subjected to Western blot analysis using antibodies directed against VEGFR-2. Blots were then stripped and reprobed with an anti-V5 antibody. (D) HUVECs expressing a nontargeting shRNA (sh-control) or an shRNA targeting plexin-A4 (sh-plexA4) were incubated with or without VEGF (10 ng/mL) at room temperature as indicated. After 10 minutes the cells were lysed, and subjected to Western blot analysis using an antibody against the phosphorylated Y-1175 residue of VEGFR-2. Blots were then stripped and reprobed with an antibody directed against VEGFR-2. (E) Confluent 6-well dishes containing PAE cells expressing VEGFR-2 and either plexin-A4-V5 or control vector were stimulated with increasing concentrations of VEGF as indicated. Cell extracts were analyzed by Western blot using an antibody directed against the phosphorylated Y-1175 residue of VEGFR-2. The membrane was subsequently stripped and probed with an antibody directed against VEGFR-2 to assess total VEGFR-2 levels in the extracts. All the experiments were repeated independently at least 3 times with similar results.
Plexin-A4 modulates VEGF-induced signaling mediated by the VEGFR-2 receptor. (A) HUVECs expressing a nontargeting shRNA (sh-control) or HUVECs in which plexin-A4 expression was silenced (sh-plexA4) were stimulated with VEGF (10 ng/mL) as described. Adherent cells were counted after 3 days. The average of 3 independent experiments each of which was done in triplicates is shown. (B) Spheroids (500 cells/spheroid) containing HUVECs expressing a control shRNA (sh-control) or HUVECs silenced for plexin-A4 expression (sh-plexA4) were seeded in collagen and stimulated to sprout with 50 ng/mL VEGF. Shown are representative pictures of sprouting spheroids taken after 24 hours. (C) The full-length human plexin-A4 cDNA was fused to a c-terminal V5 tag and coexpressed with the VEGFR-2 cDNA in PAE cells. The cells were lysed and immunoprecipitation was performed using anti-V5 antibodies or anti-HA antibodies used as a negative control. Precipitates were subjected to Western blot analysis using antibodies directed against VEGFR-2. Blots were then stripped and reprobed with an anti-V5 antibody. (D) HUVECs expressing a nontargeting shRNA (sh-control) or an shRNA targeting plexin-A4 (sh-plexA4) were incubated with or without VEGF (10 ng/mL) at room temperature as indicated. After 10 minutes the cells were lysed, and subjected to Western blot analysis using an antibody against the phosphorylated Y-1175 residue of VEGFR-2. Blots were then stripped and reprobed with an antibody directed against VEGFR-2. (E) Confluent 6-well dishes containing PAE cells expressing VEGFR-2 and either plexin-A4-V5 or control vector were stimulated with increasing concentrations of VEGF as indicated. Cell extracts were analyzed by Western blot using an antibody directed against the phosphorylated Y-1175 residue of VEGFR-2. The membrane was subsequently stripped and probed with an antibody directed against VEGFR-2 to assess total VEGFR-2 levels in the extracts. All the experiments were repeated independently at least 3 times with similar results.
We have observed that plexin-A4 and plexin-A1 can form complexes (Figure 2), and we therefore determined if silencing plexin-A1 expression in HUVECs can also inhibit their proliferation in response to bFGF and VEGF. Silencing plexin-A1 expression inhibited significantly both the bFGF and the VEGF-induced proliferation of HUVECs although the inhibition was considerably less potent than the inhibition observed after plexin-A4 silencing (supplemental Figure 4A-B). In this case too the inhibition was because of inhibition of cell cycle entry (supplemental Figure 4C). The tube forming ability of plexin-A1 silenced HUVECs was also inhibited, although the inhibition was much weaker than the inhibition produced by plexin-A4 silencing (supplemental Figure 4D).
Inhibition of sema6B expression in HUVECs mimics the effects of plexin-A4 silencing
Class-6 semaphorins such as sema6A and sema6B bind directly to plexin-A45 and sema6D binding to plexin-A1 was recently found to induce pro-proliferative responses.7 In contrast, class-3 semaphorins that bind to neuropilins but signal via associated type-A plexins usually inhibit the proliferation of HUVEC.31 When we silenced the expression of sema3A in HUVEC, we found that the silencing did not significantly affect bFGF-induced proliferation or bFGF-induced phosphorylation of ERK1/2 (supplemental Figure 5A-C). Therefore, the inhibition of cell proliferation observed in plexin-A4 silenced HUVEC is not likely to be caused by the disruption of an autocrine sema3A signaling loop. However, HUVEC also express sema6A and sema6B (supplemental Figure 1).2,5 We therefore determined if the inhibition of cell proliferation observed after plexin-A4 silencing may also be caused in part by the disruption of sema6A or sema6B signal transduction.
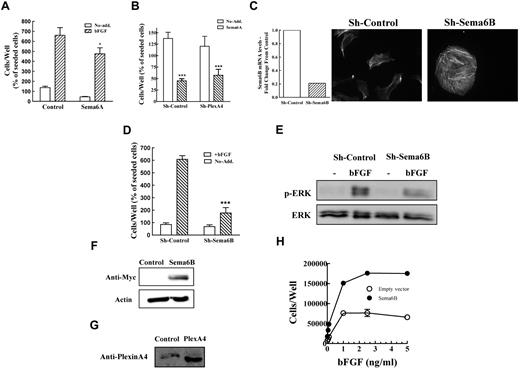
Exposure of HUVECs to an exogenous soluble extracellular domain of sema6A inhibited bFGF-induced proliferation of HUVECs by ∼ 20%, suggesting that sema6A does not convey pro-proliferative signals (Figure 5A). Furthermore, our results suggest that in HUVECs, plexin-A4 is not likely to transduce sema6A signals since sema6A was able to inhibit the survival of plexin-A4 silenced HUVECs almost as efficiently as it inhibited the survival of nonsilenced cells (Figure 5B).
Inhibition of sema6B expression in HUVECs mimics the effects of plexin-A4 silencing. (A) HUVECs were seeded and after 4 hours the medium was exchanged with conditioned medium derived from HEK293 cells transfected with empty expression vector (control) or conditioned medium from HEK293 cells expressing the sema6A extracellular domain tagged with an Fc tag (sema6A-Fc). bFGF (5ng/mL) was then added or not. The number of adherent cells was then determined as described. The average of 2 independent experiments each of which was done in triplicates is shown. (B) The effect of sema6A-Fc on the survival of HUVECs expressing the sh-control or sh-plexA4 was determined as described without the stimulation of bFGF. (C) The expression of sema6B mRNA in HUVECs infected with lentiviruses expressing control shRNA or a sema6B targeting shRNA was determined using real time PCR (left). The actin cytoskeleton of control and sema6B silenced cells was visualized with fluorescent phalloidin (right). (D) HUVECs expressing control shRNA or sema6B shRNA were seeded in 24 well dishes in the presence or absence of bFGF (5 ng/mL). After 3 days adherent cells were detached and counted. Hundred percent represents the number of adherent cells/well as counted 4 hours after seeding. The average of 2 independent experiments each of which was done in triplicates is shown. (E) HUVECs expressing sh-control or sh-sema6B were stimulated with bFGF (5 ng/mL) for 10 minutes at room temperature. The cells were lysed and ERK1/2 phosphorylation was determined using Western blot analysis. Shown is a representative experiment of 3 performed with similar results. (F) Expression of sema6B was determined in western blots prepared from cell lysates of BHK-21 cells expressing a control plasmid or cells over-expressing myc-tagged sema6B using an antibody directed against myc. (G) Expression of plexin-A4 was determined in western blots prepared from cell lysates of parental BHK-21 cells or cells over-expressing recombinant plexin-A4 using an antibody directed against plexin-A4. (H) BHK-21 cells over-expressing recombinant sema6B or control cells infected with an empty expression vector were seeded in triplicates in 24-well dishes (2 × 104 cells/well) in the presence or absence of increasing concentrations of bFGF. After 3 days adherent cells were detached and counted in a coulter counter. Shown is a representative experiment of 3 performed with similar results.
Inhibition of sema6B expression in HUVECs mimics the effects of plexin-A4 silencing. (A) HUVECs were seeded and after 4 hours the medium was exchanged with conditioned medium derived from HEK293 cells transfected with empty expression vector (control) or conditioned medium from HEK293 cells expressing the sema6A extracellular domain tagged with an Fc tag (sema6A-Fc). bFGF (5ng/mL) was then added or not. The number of adherent cells was then determined as described. The average of 2 independent experiments each of which was done in triplicates is shown. (B) The effect of sema6A-Fc on the survival of HUVECs expressing the sh-control or sh-plexA4 was determined as described without the stimulation of bFGF. (C) The expression of sema6B mRNA in HUVECs infected with lentiviruses expressing control shRNA or a sema6B targeting shRNA was determined using real time PCR (left). The actin cytoskeleton of control and sema6B silenced cells was visualized with fluorescent phalloidin (right). (D) HUVECs expressing control shRNA or sema6B shRNA were seeded in 24 well dishes in the presence or absence of bFGF (5 ng/mL). After 3 days adherent cells were detached and counted. Hundred percent represents the number of adherent cells/well as counted 4 hours after seeding. The average of 2 independent experiments each of which was done in triplicates is shown. (E) HUVECs expressing sh-control or sh-sema6B were stimulated with bFGF (5 ng/mL) for 10 minutes at room temperature. The cells were lysed and ERK1/2 phosphorylation was determined using Western blot analysis. Shown is a representative experiment of 3 performed with similar results. (F) Expression of sema6B was determined in western blots prepared from cell lysates of BHK-21 cells expressing a control plasmid or cells over-expressing myc-tagged sema6B using an antibody directed against myc. (G) Expression of plexin-A4 was determined in western blots prepared from cell lysates of parental BHK-21 cells or cells over-expressing recombinant plexin-A4 using an antibody directed against plexin-A4. (H) BHK-21 cells over-expressing recombinant sema6B or control cells infected with an empty expression vector were seeded in triplicates in 24-well dishes (2 × 104 cells/well) in the presence or absence of increasing concentrations of bFGF. After 3 days adherent cells were detached and counted in a coulter counter. Shown is a representative experiment of 3 performed with similar results.
To find out if sema6B can function as an autocrine inducer of HUVEC proliferation, we silenced its expression in HUVECs. The silencing produced morphologic and cytoskeletal changes that resembled those observed after the silencing of plexin-A4 (Figure 5C). Furthermore, bFGF-induced proliferation of HUVECs was strongly inhibited by 2 different sema6B targeting shRNAs (Figures 5D and supplemental Figure 5D) as was bFGF-induced phosphorylation of ERK1/2 (Figure 5E). To verify that sema6B is able to induce mitogenic responses we tried to over-express sema6B ectopically in HUVECs. However, we failed in this attempt because of technical reasons. We also over-expressed recombinant full-length sema6B in BHK-21 cells (Figure 5F). These cells express endogenous plexin-A4 receptors (Figure 5G) and their proliferation is completely dependent on bFGF in serum free medium.27,41 When cultured in serum free medium the expression of recombinant sema6B significantly enhanced the mitogenic response to bFGF and even produced a ∼ 2-fold stimulation of cell proliferation on its own (Figure 6I). These results argue that silencing plexin-A4 expression in HUVEC may also disrupt sema6B-induced autocrine pro-proliferative signals thereby adding to the anti-proliferative effect that is observed as a result of plexin-A4 silencing.
Inhibition of plexin-A4 expression in cancer cells inhibits their proliferation and the tumor forming ability of U87MG glioma cells. (A-B) U87MG or A549 cells were infected with lentiviruses directing expression of sh-control or sh-plexA4. The cells were then seeded at a concentration of 2 × 104 cells/well in 24-well dishes. The number of adherent cells in each well was determined after 3 days as described. The average of 3 independent experiments each of which was done in triplicates is shown. (C) MDA-MB-435 or A549 tumor cells were infected with an empty lentiviral expression vector or with lentiviruses directing expression of plexin-A4. The proliferation of the cells was then determined as described. The average ratio between cell density on days 3 and 0 of control cells determined in 3 independent experiments was designated as 100% and compared with the same ratio determined in silenced cells. Each column represents the average of 3 independent experiments. (D) The development of tumors derived from U87MG cells silenced for plexin-A4 expression (sh-plexA4) was compared with the development of tumors derived from control cells expressing a nontargeting shRNA (sh-control). (E) At the end of the experiment tumors were excised and weighed. Shown is a representative experiment. The experiment was repeated twice with similar results. (F) U87MG cells expressing a control shRNA (sh-control) or shRNAs targeting sema3A (sh-sema3A) or sema6B (sh-sema6B) were seeded in 24 well dishes and their proliferation examined as described in “Methods.” The average of 2 independent experiments each of which was done in triplicates is shown. (G) The development of tumors derived from U87MG cells silenced for sema-6B expression (sh-sema6B) was compared with the development of tumors derived from control cells expressing a nontargeting shRNA (sh-control). (H) At the end of the experiment tumors were excised and weighed.
Inhibition of plexin-A4 expression in cancer cells inhibits their proliferation and the tumor forming ability of U87MG glioma cells. (A-B) U87MG or A549 cells were infected with lentiviruses directing expression of sh-control or sh-plexA4. The cells were then seeded at a concentration of 2 × 104 cells/well in 24-well dishes. The number of adherent cells in each well was determined after 3 days as described. The average of 3 independent experiments each of which was done in triplicates is shown. (C) MDA-MB-435 or A549 tumor cells were infected with an empty lentiviral expression vector or with lentiviruses directing expression of plexin-A4. The proliferation of the cells was then determined as described. The average ratio between cell density on days 3 and 0 of control cells determined in 3 independent experiments was designated as 100% and compared with the same ratio determined in silenced cells. Each column represents the average of 3 independent experiments. (D) The development of tumors derived from U87MG cells silenced for plexin-A4 expression (sh-plexA4) was compared with the development of tumors derived from control cells expressing a nontargeting shRNA (sh-control). (E) At the end of the experiment tumors were excised and weighed. Shown is a representative experiment. The experiment was repeated twice with similar results. (F) U87MG cells expressing a control shRNA (sh-control) or shRNAs targeting sema3A (sh-sema3A) or sema6B (sh-sema6B) were seeded in 24 well dishes and their proliferation examined as described in “Methods.” The average of 2 independent experiments each of which was done in triplicates is shown. (G) The development of tumors derived from U87MG cells silenced for sema-6B expression (sh-sema6B) was compared with the development of tumors derived from control cells expressing a nontargeting shRNA (sh-control). (H) At the end of the experiment tumors were excised and weighed.
Silencing plexin-A4 and sema6B expression in tumor cells inhibits their proliferation without affecting cytoskeletal organization
Many types of tumor cells express type-A plexins (supplemental Figure 1). We therefore examined the effects of plexin-A4 silencing on the proliferation and morphology of plexin expressing tumor cells. When plexin-A4 expression was silenced in U87MG glioblastoma cells (Figure 2B) or in A549 lung cancer cells (not shown), the morphology of the cells and the organization of their actin cytoskeleton remained unchanged. Nevertheless, silencing plexin-A4 expression significantly inhibited the proliferation of these malignant cells (Figure 6A-B). Furthermore, silencing the expression of endogenous sema6B in U87MG cells, but not of sema3A, also resulted in significant inhibition of cell proliferation (Figure 6F). Inhibition of plexin-A4 expression also inhibited the proliferation of several additional plexin-A4 expressing tumor cells derived from malignant melanoma and lung cancer (supplemental Figure 6A-B). However, the proliferation of some additional types of plexin-A4 expressing tumor cells such as the PC3 prostate cancer cells, was not inhibited as a result of plexin-A4 silencing (supplemental Figure 6C). To verify that plexin-A4 can indeed exert a pro-proliferative effect on tumor cells, we over-expressed the plexin-A4 cDNA in MDA-MB-435 and in A549 tumor cells. This over-expression resulted in a small but statistically significant ∼ 20%-30% increase in their proliferation (Figure 6C).
To determine whether plexin-A1 can affect the proliferation of tumor cells too, we silenced its expression in U87MG glioblastoma cells and in A549 lung cancer cells. As in the case of the HUVEC, the silencing inhibited their proliferation, although not as potently as the silencing of plexin-A4. Interestingly, when both plexin-A1 and plexin-A4 were silenced in these cells the inhibitory effect seemed to be additive (supplemental Figure 4E-F).
Silencing plexin-A4 or sema6B expression in U87MG glioblastoma cells strongly inhibits tumor development from these cells
The observations described in the previous paragraph suggested that inhibition of plexin-A4 or sema6B expression in some tumor cells could potentially inhibit their tumor forming ability. Indeed, the development of tumors from U87MG glioblastoma cells silenced for either plexin-A4 or sema6B was strongly inhibited (Figure 6D-H). The small subcutaneous tumors that did develop from the plexin-A4 silenced cells contained the same blood vessel density and the pericyte coverage of the blood vessels was similar to that found in tumors arising from control cells, arguing against an anti-angiogenic effect in trans (supplemental Figure 6D). These results strongly suggest that plexin-A4 and possibly sema6B may be regarded as novel targets for the development of anti-tumorigenic drugs.
Discussion
The best studied type-A plexin is plexin-A1, a receptor which forms stable complexes with the neuropilin-1 coreceptor to transduce repulsive signals of several neuropilin-1 binding class-3 semaphorins such as sema3A.12,13,42 Additional type-A plexins as well as plexin-D1 were also reported to associate with neuropilins and to replace plexin-A1 in such complexes.12,43 There is some evidence suggesting that the plexins found in these complexes can restrict the binding specificity of semaphorins.22 It was also found that plexin-A3 differs from other type-A plexins in that it preferentially associates with the neuropilin-2 receptor rather than with neuropilin-1, and thus transduces preferentially signals of neuropilin-2 binding class-3 semaphorins.19,21,44
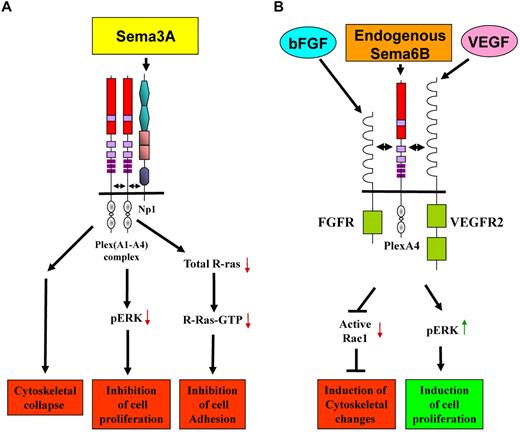
We inhibited the expression of 3 of the 4 type-A plexins in HUVECs to determine whether their function in endothelial cells is redundant. Surprisingly, silencing plexin-A1 or plexin-A4, but not plexin-A3, induced distinctive plexin-type specific morphologic changes. The silencing of plexin-A1 induced a fibroblast like morphology rich in stress fibers while silencing plexin-A4 produced an epithelial like morphology and a different arrangement of actin fibers that was accompanied by increased adhesion to substrate. These observations suggest that in endothelial cells plexin-A1, plexin-A3 and plexin-A4 fulfill distinct, nonredundant roles. Despite the different morphologic effects induced by the silencing of plexin-A1 or plexin-A4, we found that the presence of both plexins was required for the transduction of sema3A signals in HUVECs as well as in U87MG glioblastoma cells. When the expression of either of these plexins was inhibited it caused complete loss of the sema3A induced collapse of the actin cytoskeleton despite the presence of the other plexin in the cells. This loss of responsiveness was specific since HUVECs in which the expression of either of these plexins was silenced still responded fully to sema3E, a semaphorin that transduces signals using the plexin-D1 receptor.38 These results suggested that plexin-A1 and plexin-A4 may form stable complexes to transduce sema3A signals and we have found evidence supporting the existence of such complexes. Our results imply that in endothelial cells, functional sema3A receptors may consist of tripartite neuropilin-1/plexin-A1/plexin-A4 complexes (Figure 7A). Our results also support previous observations which indicated that plexin-A3 does not transduce neuropilin-1 mediated signaling induced by neuropilin-1 binding semaphorins such as sema3A.19,21,44
A model describing the suggested effect of plexin-A4 on signal transduction induced by selected semaphorins and growth factors in endothelial cells. Our results suggest that plexin-A4 transduces or modulates the transduction of diverse signals. (A) Plexin-A4 forms a tripartite complex with plexin-A1 and neuropilin-1. This complex transduces growth inhibitory signals of sema3A that inhibit ERK1/2 phosphorylation as well as signals that result in inhibition of cell adhesion and affect the organization of the cytoskeleton as a result of the activation of the GAP domain of these plexins (reviewed in Neufeld and Kessler17 ). (B) Plexin-A4 is also able to form complexes with the FGF receptors FGFR1 and FGFR2 and with the VEGF receptor VEGFR-2. This association enhances bFGF and VEGF-induced signal transduction mediated by their respective tyrosine-kinase receptors. In addition, our results suggest that plexin-A4 transduces pro-proliferative signals induced by sema6B. Lastly, when the expression of plexin-A4 in endothelial cells is inhibited we find that the cells undergo profound changes in the organization of their cytoskeleton that are accompanied by changes in the activation state of Rac-1, suggesting that signals transduced by plexin-A4 are important for the maintenance of the correct organization of the cytoskeleton of the endothelial cells.
A model describing the suggested effect of plexin-A4 on signal transduction induced by selected semaphorins and growth factors in endothelial cells. Our results suggest that plexin-A4 transduces or modulates the transduction of diverse signals. (A) Plexin-A4 forms a tripartite complex with plexin-A1 and neuropilin-1. This complex transduces growth inhibitory signals of sema3A that inhibit ERK1/2 phosphorylation as well as signals that result in inhibition of cell adhesion and affect the organization of the cytoskeleton as a result of the activation of the GAP domain of these plexins (reviewed in Neufeld and Kessler17 ). (B) Plexin-A4 is also able to form complexes with the FGF receptors FGFR1 and FGFR2 and with the VEGF receptor VEGFR-2. This association enhances bFGF and VEGF-induced signal transduction mediated by their respective tyrosine-kinase receptors. In addition, our results suggest that plexin-A4 transduces pro-proliferative signals induced by sema6B. Lastly, when the expression of plexin-A4 in endothelial cells is inhibited we find that the cells undergo profound changes in the organization of their cytoskeleton that are accompanied by changes in the activation state of Rac-1, suggesting that signals transduced by plexin-A4 are important for the maintenance of the correct organization of the cytoskeleton of the endothelial cells.
Silencing plexin-A4 expression in HUVECs also resulted in reduced mitogenic responses to bFGF as well as inhibition of bFGF-induced angiogenesis in “in vitro” assays. A similar inhibition of VEGF-induced cell proliferation was also observed in silenced cells. This was a surprising result as we expected to see opposite effects because of inhibition of the effects of inhibitory class-3 semaphorins that signal via plexin-A4, and further suggested that plexin-A4 also transduces pro-proliferative signals. Silencing plexin-A4 expression also inhibited the proliferation of several types of tumor cells. Inhibition of cell proliferation as a result of plexin-A4 silencing did not depend on concomitant changes in cytoskeletal organization, since in the tumor cells we studied the silencing of plexin-A4 had no effect on the cytoskeleton but nevertheless inhibited cell proliferation. These results were supported by experiments that demonstrated that silencing plexin-A4 expression in endothelial cells inhibits bFGF-induced phosphorylation of ERK1/2, a key mediator of mitogenic signaling, and by experiments demonstrating that ectopic over-expression of plexin-A4 in HUVEC and tumor cells enhances their proliferation.
Since the silencing of plexin-A4 expression seemed to interfere with bFGF and VEGF signal transduction in endothelial cells, and because the related plexin-A1 receptor was previously observed to form complexes with the VEGFR-2 receptor,1,7 we determined if plexin-A4 forms complexes with FGF and VEGF tyrosine-kinase receptors. Indeed, we found that plexin-A4 associates with FGFR1 and FGFR2 as well as with VEGFR-2 suggesting that plexin-A4 may modulate bFGF and VEGF signal transduction (Figure 7B). Indeed, plexin-A4 silencing in endothelial cells inhibited strongly VEGF-induced phosphorylation of VEGFR-2, the VEGF receptor responsible for transduction of pro-angiogenic signals,45 while expression of recombinant plexin-A4 in PAE cells that also express recombinant VEGFR-2 strongly potentiated VEGF-induced phosphorylation of VEGFR-2.
Several semaphorins were found to induce signal transduction via tyrosine-kinase receptors after their binding to plexins that associate with such receptors. The best studied examples are those of sema4D and sema6D.7,46 We therefore hypothesized that one of the semaphorins that uses plexin-A4 as a receptor may induce cell proliferation by a similar mechanism. Class-3 semaphorins are usually inhibitory31 and not expected to transduce pro-mitogenic signals. Sema6A was previously found to function as an inhibitory factor11 and our results agree with this conclusion. However, inhibition of sema6B expression in HUVECs as well as in several types of tumor cells closely mimicked the effects of plexin-A4 silencing, suggesting that inhibition of plexin-A4 may disrupt a pro-proliferative autocrine loop driven by endogenous sema6B. Furthermore, over-expression of sema6B in BHK-21 cell-induced cell proliferation by itself and enhanced the mitogenic effects of bFGF suggesting that the anti-proliferative effects observed in cells silenced for plexin-A4 expression may be due in part to inhibition of autocrine sema6B signaling and in part to inhibition of bFGF and VEGF signaling (Figure 7B). These results imply a possible role for sema6B and plexin-A4 in tumor progression. Indeed, silencing plexin-A4 or sema6B expression in U87MG glioblastoma cells strongly inhibited their tumor forming ability suggesting that targeting the sema6B/plexin-A4 signaling loop may represent a novel anti-tumorigenic strategy. This last conclusion is supported by prior observations which have revealed that the plexin-A4 gene is amplified in melanomas as well as in several other types of tumors.47,48 These results suggest that inhibitors of plexin-A4 may function as anti-tumorigenic agents although it should be remembered that plexin-A4 also transduces anti-proliferative signals of class-3 semaphorins and thus the final outcome would depend on the initial contribution of these two semaphorin types to the proliferative state of the tumor cells and of tumor associated endothelial cells.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Luca Tamagnone from the Institute for Cancer Research (IRCC) of the University of Torino, Italy, for the partial plexin-A4 cDNA clone; Dr Oded Behar from the Hebrew University of Jerusalem for the sema6A expression vector; Dr Yoel Klug from Tel-Aviv University for advice and reagents used in R-ras pulldown assays; and Mr Yaron Gruper for the help in performing several real-time PCR experiments. They also thank Roni Zidon for her help in experiments designed to express recombinant RAC-1 in endothelial cells.
This work was supported by grants from the Israel Science Foundation (ISF); by the Israel Ministry of Science, joint program with the Deutsches Krebsforschungszentrum (DKFZ) in Germany; by a grant from the McDonnel Foundation; by a grant from the Niedersachsen Foundation; and by a grant from the Rappaport Family Institute for Research in the Medical Sciences of the Faculty of Medicine at the Technion, Israel Institute of Technology (to G.N.).
Authorship
Contribution: B.K. and N.R. conducted most of the experiments and contributed to the design of the experiments as well as to the writing of the manuscript; A.V. and O.K. designed and conducted some of the experiments; and G.N. contributed to the design of the experiments, data analysis and interpretation, and to the writing of the manuscript.
Conflict-of-interest disclosure: B.K. now works at PlexiCure, a company established based on results presented in this manuscript. The remaining authors have rights in a patent application based on results presented in this manuscript.
Correspondence: Gera Neufeld, The Bruce Rappaport Faculty of Medicine, Technion, Israel Institute of Technology, 1 Efron Street, Haifa 31096, Israel; e-mail: gera@tx.technion.ac.il.
References
Author notes
B.K. and N.R. contributed equally to this article.