Abstract
A key event and potential therapeutic target in allergic and asthmatic diseases is signaling by the IgE receptor FcϵRI, which depends on its interactions with Src family kinases (SFK). Here we tested the hypothesis that glycosylphosphatidylinositiol-anchored proteins (GPI-AP) are involved in FcϵRI signaling, based on previous observations that GPI-AP colocalize with and mediate activation of SFK. We generated mice with a hematopoietic cell-specific GPI-AP deficiency by targeted disruption of the GPI biosynthesis gene PigA. In these mice, IgE-mediated passive cutaneous anaphylaxis was largely abolished. PigA-deficient mast cells cultured from these mice showed impaired degranulation in response to stimulation with IgE and antigen in vitro, despite normal IgE binding and antigen-induced FcϵRI aggregation. On stimulation of these cells with IgE and antigen, coprecipitation of the FcϵRI α-chain with the γ-chain and β-chain was markedly reduced. As a result, IgE/antigen–induced FcϵRI-Lyn association and γ-chain tyrosine phosphorylation were both impaired in PigA-deficient cells. These data provide genetic evidence for an unanticipated key role of GPI-AP in FcϵRI interchain interactions and early FcϵRI signaling events, necessary for antigen-induced mast cell degranulation.
Introduction
Central to the pathophysiology of allergy and asthma is antigen-induced aggregation of cell surface-associated IgE, which initiates signal transduction through high-affinity IgE-Fc receptor type I (FcϵRI) on mast cells and basophils. This signaling step involves activation of Src family kinases (SFK), especially Lyn and Fyn, which phosphorylate tyrosine residues on key intermediates, including the receptor itself. These phosphorylation events lead to assembly of multiprotein complexes and activation of additional kinases that ultimately induce the critical transcriptional and secretory effector mechanisms of the allergic response. The final effectors of FcϵRI signaling include the release of vasoactive compounds, such as histamine and serotonin, and proinflammatory mediators, such as TNF-α and IL-6.1,2
How aggregation of FcϵRI leads to activation of SFK remains unclear. A significant body of literature has proposed that antigen-mediated FcϵRI aggregation brings FcϵRI into physical proximity to acylated, membrane-associated SFK, perhaps via transient translocation to specialized membrane domains called lipid rafts, allowing phosphorylation.3-5 However, there is no obvious molecular mechanism for translocation of aggregated FcϵRI to lipid rafts. Both GPI-anchored proteins (GPI-AP) and SFK are concentrated in lipid rafts,6,7 and aggregation of GPI-AP can activate SFK.8,9 Activating IgG-Fc receptors (FcγR), which are structurally and functionally related to FcϵRI, have been reported to interact physically with GPI-AP and to depend on their presence for some effector functions.10-12 Thus, one possible mechanism for FcϵRI activation of SFK is through the intermediation of GPI-AP. One prediction of this hypothesis is that FcϵRI function would be defective in the absence of GPI-AP.
A genetic test of this hypothesis was initiated by analyzing the effects of GPI-AP deficiency on FcϵRI signaling events and effector functions. We generated a new mouse strain with a hematopoietic cell-specific GPI-AP deficiency resulting from targeted disruption of PigA, which is the first enzyme committed to the GPI biosynthetic pathway.13 These mice showed attenuated IgE-mediated passive cutaneous anaphylaxis. On stimulation with IgE and antigen, PigA-deficient mast cells cultured from these mice showed impaired degranulation, coprecipitation of the FcϵRI β and γ-chains with the α-chain, FcϵRI-Lyn association, and γ-chain tyrosine phosphorylation. These data demonstrate that GPI-AP are essential for early FcϵRI signaling steps.
Methods
Generation of PigA-deficient BMMC
The X-linked gene PigA, which is essential for the biosynthesis of the GPI lipid anchor,13 was targeted in hematopoietic cells by crossing mice expressing PigALoxP14 with mice expressing hematopoietic-specific Vav1Cre15 (VavCrePigALoxP mice). Disruption of PigA was confirmed by polymerase chain reaction.14 Both mouse strains were backcrossed in a C58Bl/6 background for at least 10 generations. Male VavCrePigALoxP mice were normally fertile, showed no abnormalities in gross appearance or hematopoietic cell numbers, and were used for experiments at 2-3 months of age. Littermate male mice expressing only VavCre were used as controls. Flow cytometry analysis of peripheral blood showed grossly normal total numbers of T and B lymphocytes, monocytes, neutrophils, and erythrocytes in VavCrePigALoxP mice. All hematopoietic cells from male VavCrePigALoxP mice tested, that is, total bone marrow, erythrocytes, blood monocytes, neutrophils, blood T and B lymphocytes, splenocyte-derived dendritic cells, and bone marrow–derived macrophages, showed a completely GPI-AP deficient phenotype (not shown).
Bone marrow–derived mast cells (BMMCs) were generated from male VavCrePigALoxP or VavCre littermate control mice by culturing bone marrow cell suspensions in RPMI medium supplemented with 10% FBS (Gibco), sodium pyruvate at 0.11 mg/mL, 10mM HEPES, and 25% culture supernatant of WEHI-3 cells (ATCC) for at least 4 weeks. The phenotype of BMMC was analyzed by flow cytometry. For phenotype analysis of peritoneal mast cells, c-Kit positive peritoneal cells from male VavCrePigALoxP or VavCre littermate control mice were gated from forward/sideward scatter flow cytometry plots before analysis of staining with GPI-AP markers. All mouse experiments have been approved by the Institutional Review Board of the University of California San Franciso and the Institutional Animal Care and Use Committee of Genentech Inc.
Antibodies and reagents
Antibodies used were anti-CD48, anti-HSA, and anti–c-Kit (BD Biosciences); 2.4G2 anti-FcγRII/III (BD Biosciences); anti–FcϵRI α-chain (Santa Cruz Biotechnology), JRK anti–FcϵRI β-chain16 (generously provided by Dr Juan Rivera, National Institutes of Health); anti–FcϵRI γ-chain (Upstate Biotechnology); 4G10 anti-phosphotyrosine (Upstate Biotechnology); anti–phospho-Src(Tyr416; pSrc) specific for activated SFK (Cell Signaling), anti-Lyn (Santa Cruz Biotechnology); Ly17.2 anti-FcγRII (Caltag); goat anti–mouse IgE (Southern Biotech); goat anti–mouse IgG(H+L) (Molecular Probes); and goat anti–mouse IgE (Southern Biotech). Proaerolysin from Aeromonas hydrophila (Protox Biotech) was used as a marker for GPI17 ; and ganglioside GM1-specific cholera toxin subunit B (Molecular Probes) as a general marker for lipid rafts. To stimulate mast cells in vitro, supernatant of the IgE anti-DNP producing hybridoma IGEL-A2 (ATCC) in combination with DNP-BSA (Calbiochem), or compound 48/80 (Sigma-Aldrich)18 was used. Dinitrophenyl (DNP)–conjugated human serum albumin (Sigma-Aldrich) was used for in vivo experiments. To detect IgE binding to BMMC, IgE-sensitized BMMC were stained with goat anti–mouse IgG(H+L) antibodies and analyzed by flow cytometry.
Passive cutaneous anaphylaxis
Mice were injected intradermally with 25 μL of hybridoma supernatant of IgE anti-DNP (IGEL-A2) in one ear, and as control with 25 μL of hybridoma culture medium in the other ear. On the next day, mice were injected intravenously with 100 μL of saline containing 5 mg of DNP-conjugated human serum albumin per milliliter and 1% Evans Blue. Thirty minutes later, blue staining of the ears was visually examined, mice were euthanized and Evans blue was extracted from the ears by overnight incubation at 65°C in 500 μL formamide and extravasation was quantified by measurement of the absorbance at 620 nm using a standard curve.
For quantification of skin mast cells in the ears of mice, 4-5 micron tissue sections were stained with Alcian blue as described.19 Stained slides were scanned by the Olympus Nanozoomer (Olympus) automated slide scanning platform, and analyzed using the Matlab R2010b software (Mathworks) as 24-bit red, green, blue (RGB) images. Individual mast cells were identified by their distinct blue hue through the training of Support Vector Machine (SVM) to segment the RGB colorspace.
Mast cell degranulation
Degranulation of mast cells was determined by measuring the release of the granule enzyme β-hexosaminidase. Briefly, BMMC were suspended at 106/mL of Tyrode buffer (Sigma-Aldrich) containing 0.1% BSA certified for low endotoxin and IgG levels (Sigma-Aldrich), and seeded in 96-well tissue culture plates. The cells were sensitized by incubation for 3 hours at 37°C with supernatant of the IgE anti-DNP producing hybridoma IGEL-A2 at a dilution of 1:100. The cells were challenged by incubation for 1 hour at 37°C with various concentrations of DNP-BSA. As control, cells were stimulated with the nonspecific degranulation stimulus compound 48/8020 for 1 hour at 37°C. Cell supernatants and cell pellets lysed for 5 minutes at room temperature in 2% NP-40, were incubated with the substrate 1mM 4-methylumbelliferyl N-acetyl-b-D-glucosaminide (Sigma-Aldrich) in 0.1M sodium citrate (pH 4.8) for 1 hour at 37°C, and absorbance at 460 nm was measured. Degranulation was expressed as the percentage specific release of β-hexosaminidase relative to the total cell content. This percentage was calculated by dividing the absorbance of the supernatant by the absorbance of the supernatant plus that of the lysate, after both were corrected for basal release by subtraction of the absorbance of supernatants of nonstimulated cells. The IgE anti-DNP hybridoma supernatant IGEL-A2, DNP-BSA, as well as BSA-supplemented Tyrode buffer, all tested endotoxin negative by Limulus amebocyte lysate assay (Sigma-Aldrich).
Quantification of FcϵRI aggregation
BMMCs were sensitized with IgE anti-DNP, and next challenged for 3 minutes at 37°C with 0.5 μg DNP-BSA/mL or left in medium, as previously described for the degranulation assay. The cells were immediately fixed in 2% paraformaldehyde, blocked with 2% BSA in PBS, and stained with Alexa488-conjugated goat anti–mouse IgG(H+L; Molecular Probes). Control flow cytometry experiments confirmed that binding of the latter antibody to BMMC is not detectable without prior IgE-sensitization (not shown). After fixing and staining, the cells were immediately mounted onto glass slides using ProLong Antifade mounting solution (Invitrogen). The fluorescence of the cells was visualized using an LSM510 Pascal confocal scanning microscope and camera (Zeiss) with a 63× oil objective and a numeric aperature of 1.4, and images were acquired by LSM510 software. The formation of FcϵRI aggregates was quantified using the Particle Analysis of the ImageJ 1.440 software (National Institutes of Health). Standard arbitrary thresholds for both pixel intensity and particle size were set using Threshold/Particle Size. These settings were kept consistent for all conditions and cells tested, and the numbers of particles per cell, as a measure for numbers of FcϵRI aggregates, were determined. At least 15 cells per group were analyzed.
Immunoblot analysis of FcϵRI subunit assembly and intracellular signals
After stimulation with IgE anti-DNP (IGEL-A2) and DNP-BSA, BMMC were lysed by incubation for 30 minutes on ice in lysis buffer (20mM Tris-HCl, at pH 7.4 containing 0.5% Triton X-100, 150mM NaCl, 2mM EDTA, 50mM NaF, 1mM Na3VO4, 1mM PMSF, 10 μg aprotinin/mL, and 10 μg leupeptin/mL). Lysates corresponding to 1 × 106 to 3 × 106 BMMC per sample were used, depending on the application. For immunoprecipitation of the FcϵRI complex, cell lysates were incubated with protein G Sepharose (GE Healthcare) coated with goat anti–mouse IgE antibodies (Southern Biotech). To minimize the appearance of antibody protein on the blots, these antibodies were crosslinked to the Sepharose for 30 minutes at RT in 10mM Dimethyl pimelimidate-2HCl (Pierce) and 0.1M HEPES before immunoprecipitation. Proteins were separated by SDS-PAGE electrophoresis and transferred to Immobilon PVDF membranes (Millipore). Immunoblotting was performed using antibodies against anti-FcϵRI subunits, anti-phosphotyrosine, anti–phospho-Src(Tyr416), or anti-Lyn. For quantification of immunoblot signal intensities, films were scanned using a CanoScan LiDE 200 scanner (Canon), and pixel intensities of individual bands were determined using Quantity One 4.6.9 software (Bio-Rad).
Statistical analysis
Data were assessed for significance using the unpaired Student t test.
Results
Generation of PigA-deficient BMMCs
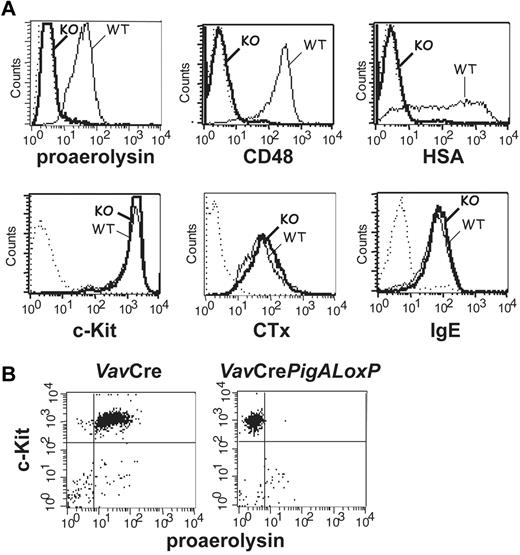
Mice with complete GPI-AP deficiency in hematopoietic cells were generated by crossing mice expressing floxed PigA, an X-linked gene essential for GPI-anchor biosynthesis,13 with mice expressing the Cre recombinase under the control of the Vav1 promoter.15 The specificity of PigA disruption was previously verified by a complete GPI-AP deficiency in PigA-depleted T lymphocytes and restoration of GPI-AP expression using retroviral transduction of the PigA gene.21 BMMCs from male VavCrePigALoxP mice expressed no GPI anchor at the plasma membrane, as demonstrated by a lack of staining with proaerolysin, a bacterial protein that binds to the GPI lipid anchor itself17 (Figure 1A). As a result of failure to synthesize the GPI lipid anchor, these cells did not express GPI-AP, as confirmed by the lack of staining with antibodies against individual GPI-AP CD48 and heat-stable antigen (HSA; Figure 1A).
Phenotype analysis of PigA-deficient mast cells by flow cytometry. (A) PigA-deficient BMMCs cultured from VavCrePigALoxP (KO; bold lines) or littermate control VavCre (WT; filled lines) mice were analyzed by flow cytometry. Complete GPI-deficient phenotype was evident from a lack of staining with proaerolysin, a general marker for the GPI lipid anchor, and with antibodies against the GPI-AP CD48 and HSA. PigA-deficient BMMCs demonstrated normal staining with antibodies against the mast cell marker c-Kit, and with the lipid raft marker cholera toxin subunit B (CTx); and normal binding of monomeric mouse IgE. Dotted lines: staining controls. (B) Peritoneal mast cells from littermate control VavCre or VavCrePigALoxP mice were stained with anti–c-Kit and proaerolysin; c-Kit positive cells were gated in forward/side scatter. Negative proaerolysin staining demonstrates in vivo mast cell GPI-deficiency; this was confirmed using antibodies against CD48 and HSA (not shown).
Phenotype analysis of PigA-deficient mast cells by flow cytometry. (A) PigA-deficient BMMCs cultured from VavCrePigALoxP (KO; bold lines) or littermate control VavCre (WT; filled lines) mice were analyzed by flow cytometry. Complete GPI-deficient phenotype was evident from a lack of staining with proaerolysin, a general marker for the GPI lipid anchor, and with antibodies against the GPI-AP CD48 and HSA. PigA-deficient BMMCs demonstrated normal staining with antibodies against the mast cell marker c-Kit, and with the lipid raft marker cholera toxin subunit B (CTx); and normal binding of monomeric mouse IgE. Dotted lines: staining controls. (B) Peritoneal mast cells from littermate control VavCre or VavCrePigALoxP mice were stained with anti–c-Kit and proaerolysin; c-Kit positive cells were gated in forward/side scatter. Negative proaerolysin staining demonstrates in vivo mast cell GPI-deficiency; this was confirmed using antibodies against CD48 and HSA (not shown).
PigA-deficient BMMCs expressed normal amounts of the mast cell marker c-Kit (Figure 1A). In addition, binding of monomeric IgE to PigA-deficient BMMCs was not affected (Figure 1A), demonstrating that these cells expressed normal levels of the FcϵRI ligand-binding α-chain. In mouse cells, membrane expression of the FcϵRI α subunit requires association with the receptor β and γ subunits22 ; therefore, this result suggests that FcϵRI synthesis and assembly is unaffected by the absence of GPI-AP. BMMCs deficient in GPI-AP had normal expression of FcγRs, as demonstrated by staining with antibodies against FcγRII (Ly17.2) and antibodies against FcγRII/III (2.4G2; not shown). The cholera toxin B subunit, which binds to the ganglioside GM1, a membrane lipid concentrated in raft microdomains, bound normally to PigA-deficient BMMCs (Figure 1A), which is in agreement with previous observations that lipid rafts are intact in a GPI-deficient cell line.23
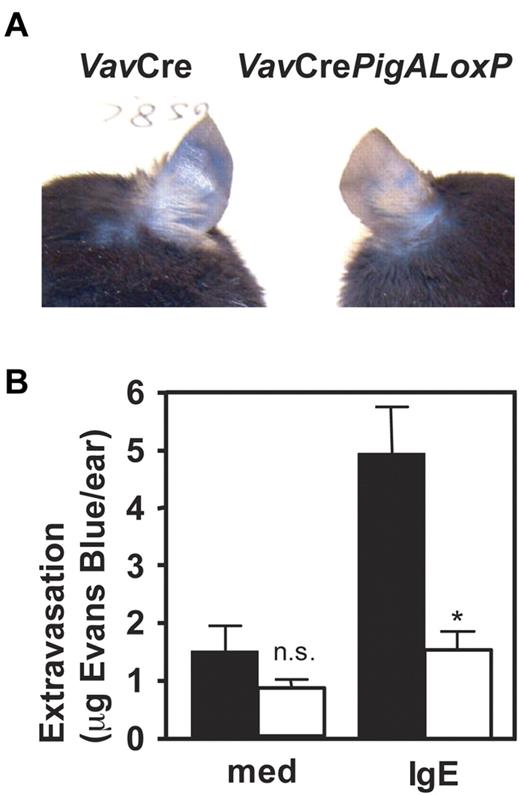
Reduced IgE/antigen-mediated passive cutaneous anaphylaxis in mice with PigA-deficient mast cells
To determine the role of GPI-AP in mast cell function in vivo, IgE-mediated passive cutaneous anaphylaxis was studied in VavCrePigALoxP mice. Peritoneal mast cells from these mice failed to bind proaerolysin (Figure 1B) or antibodies against CD48 or HSA (not shown), confirming their GPI-AP deficient phenotype in vivo, although their total numbers in peritoneal lavages were normal (not shown). Passive cutaneous anaphylaxis was induced by intradermal injections of IgE anti-DNP in one ear, followed by intravenous injections of DNP-conjugated human serum albumin mixed with Evans Blue. In VavCre littermate control mice, IgE-mediated extravasation was evident by blue staining of the ears. In contrast, blue staining was strikingly diminished in IgE-injected ears of VavCrePigALoxP mice (Figure 2A). Quantification of Evans Blue extracted from the ears revealed a significant reduction of extravasation in the IgE-injected ears of VavCrePigALoxP mice compared with littermate controls (Figure 2B). Ear sections contained 28.3 ± 2.3 mast cells per mm2 in VavCrePigALoxP mice (n = 3) and 38.4 ± 8.4 cell per mm2 (mean ± SEM) in VavCre littermate controls (n = 5). This difference was not statistically significant (P = .06) and cannot explain the much more dramatic reduction in extravasation. Thus, GPI-AP are required for normal FcϵRI-induced mast cell degranulation in vivo.
Resistance to IgE-mediated passive cutaneous anaphylaxis in mice with PigA-deficient mast cells. Mice were given injections with IgE anti-DNP in one ear and as control with medium in the other. On the next day, intravenous injections of DNP-BSA mixed with Evans Blue were given. Extravasation was visualized by blue staining of the ears (top panel). After extraction from the ears, extravasated Evans blue was quantified (bottom panel). Filled bars: VavCre littermate control mice; open bars: VavCrePigALoxP mice. Results represent mean ± SEM of 3 mice per group. Asterisk indicates significant difference (P < .01) with control mice; n.s. indicates not significant.
Resistance to IgE-mediated passive cutaneous anaphylaxis in mice with PigA-deficient mast cells. Mice were given injections with IgE anti-DNP in one ear and as control with medium in the other. On the next day, intravenous injections of DNP-BSA mixed with Evans Blue were given. Extravasation was visualized by blue staining of the ears (top panel). After extraction from the ears, extravasated Evans blue was quantified (bottom panel). Filled bars: VavCre littermate control mice; open bars: VavCrePigALoxP mice. Results represent mean ± SEM of 3 mice per group. Asterisk indicates significant difference (P < .01) with control mice; n.s. indicates not significant.
Requirement for GPI-AP in FcϵRI-mediated degranulation of BMMCs
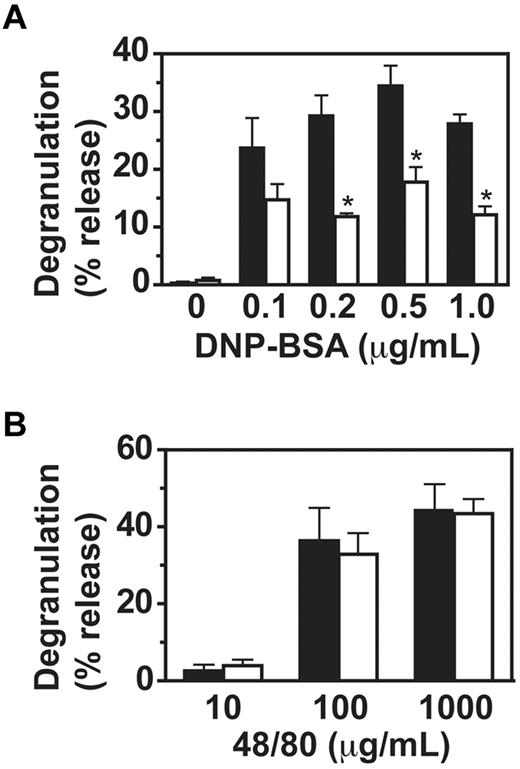
To investigate the mechanism for the observed impaired passive cutaneous anaphylaxis in VavCrePigALoxP mice, we analyzed IgE-mediated mast cell functions in vitro. Binding of IgE to PigA-deficient BMMC in vitro was normal (Figure 1A). In contrast, degranulation of PigA-deficient cells was impaired two- to three-fold in response to IgE and multiple antigen concentrations (Figure 3A). As control, the compound 48/80, which activates mast cells through trimeric G-proteins and independent of FcϵRI,18 induced normal degranulation in PigA-deficient cells (Figure 3B), confirming that the intrinsic machinery for degranulation was intact in PigA-deficient BMMCs. These data demonstrate that PigA-deficient mast cells have a specific defect in FcϵRI-induced degranulation.
Impaired IgE/antigen-mediated degranulation of PigA-deficient BMMCs. BMMCs were (A) sensitized with IgE anti-DNP, followed by challenge with various concentrations of DNP-BSA for 1 hour, or (B) activated with the nonspecific degranulation stimulus compound 48/80. Degranulation of the cells was expressed as the percentage specific release of β-hexosaminidase into the supernatant relative to the total cell content. In the absence of IgE sensitization, the mean specific β-hexosaminidase release was below 3% for both control and PigA-deficient BMMC at either concentration of DNP-BSA tested (not shown). Filled bars: littermate control BMMC; open bars: PigA-deficient BMMC. Results represent mean ± SEM of at least 3 experiments; asterisks indicate significant difference (P < .05) with control cells.
Impaired IgE/antigen-mediated degranulation of PigA-deficient BMMCs. BMMCs were (A) sensitized with IgE anti-DNP, followed by challenge with various concentrations of DNP-BSA for 1 hour, or (B) activated with the nonspecific degranulation stimulus compound 48/80. Degranulation of the cells was expressed as the percentage specific release of β-hexosaminidase into the supernatant relative to the total cell content. In the absence of IgE sensitization, the mean specific β-hexosaminidase release was below 3% for both control and PigA-deficient BMMC at either concentration of DNP-BSA tested (not shown). Filled bars: littermate control BMMC; open bars: PigA-deficient BMMC. Results represent mean ± SEM of at least 3 experiments; asterisks indicate significant difference (P < .05) with control cells.
Normal FcϵRI aggregation in PigA-deficient BMMCs
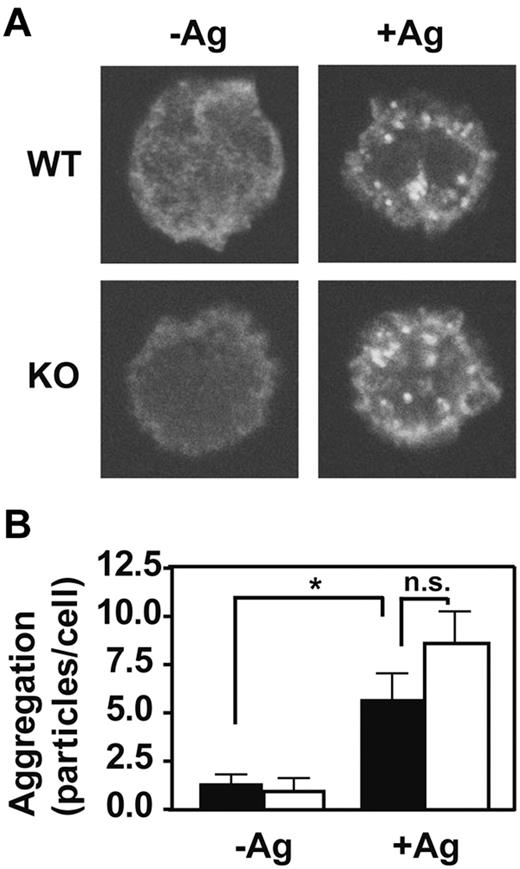
The FcϵRI signaling cascade is initiated by aggregation of the IgE-bound receptor in response to multivalent antigen. To determine whether the presence of GPI-AP is required for this first step, we examined the effects of PigA-deficiency on the extent of antigen-induced FcϵRI aggregation using confocal microscopy. FcϵRI aggregates were visually indistinguishable between control and PigA-deficient BMMCs (Figure 4A). Quantitative determination of the numbers of FcϵRI aggregates per cell confirmed that PigA-deficiency did not alter the extent of FcϵRI aggregation induced by IgE and antigen (Figure 4B). Thus, the effects of PigA-deficiency on FcϵRI-induced degranulation must be attributed to events downstream of receptor aggregation.
Normal IgE/antigen-induced FcϵRI aggregation in PigA-deficient BMMCs. BMMCs were sensitized with IgE anti-DNP, and either left in medium (-Ag) or challenged with DNP-BSA antigen (+Ag) for 3 minutes, fixed and stained with fluorescent anti–mouse IgG(H+L). (A) Confocal microscope images show the appearance of FcϵRI aggregates at the upper cell surface after antigen challenge (+Ag) in both control (WT; top panels) and PigA-deficient BMMCs (KO; bottom panels). (B) Quantification of the number of FcϵRI aggregates per cell by the Particle Analysis software. Shown are the mean numbers ± SEM particles per cell, as a measure for FcϵRI aggregation, of at least 15 cells per group. Filled bars: littermate control BMMC; open bars: PigA-deficient BMMCs. Asterisk indicates significant difference (P < .05) with control cells; n.s. indicates not significant.
Normal IgE/antigen-induced FcϵRI aggregation in PigA-deficient BMMCs. BMMCs were sensitized with IgE anti-DNP, and either left in medium (-Ag) or challenged with DNP-BSA antigen (+Ag) for 3 minutes, fixed and stained with fluorescent anti–mouse IgG(H+L). (A) Confocal microscope images show the appearance of FcϵRI aggregates at the upper cell surface after antigen challenge (+Ag) in both control (WT; top panels) and PigA-deficient BMMCs (KO; bottom panels). (B) Quantification of the number of FcϵRI aggregates per cell by the Particle Analysis software. Shown are the mean numbers ± SEM particles per cell, as a measure for FcϵRI aggregation, of at least 15 cells per group. Filled bars: littermate control BMMC; open bars: PigA-deficient BMMCs. Asterisk indicates significant difference (P < .05) with control cells; n.s. indicates not significant.
Requirement for GPI-AP in FcϵRI interchain interactions after IgE/antigen stimulation
Approximately two decades ago, Metzger's group showed the stability of interchain interactions within the FcϵRI receptor complex to be sensitive to their lipid environment.24 Therefore, we investigated the possibility that GPI-AP, being concentrated in specialized lipid microdomains,25,26 are involved in the dynamics of noncovalent interactions between FcϵRI α, β, and γ-chains. Disruption of these interchain interactions would probably affect the availability of immunoreceptor tyrosine-based activation motif (ITAM)–containing chains as substrates for SFK on antigen stimulation and subsequent phosphorylation events.
In nonstimulated BMMCs, regardless of whether or not the cells expressed GPI-AP, both γ and β-chains could be normally coprecipitated with the α-chain through immunoprecipitation of FcϵRI-bound IgE (Figure 5A). This shows that the FcϵRI interchain interactions in PigA-deficient cells are stable under resting conditions. After IgE/antigen stimulation of littermate control BMMCs, γ and β-chains could still be coprecipitated with the α-chain (Figure 5A). In contrast, on IgE/antigen stimulation of PigA-deficient cells, coprecipitation of both the γ and the β-chain with the α-chain was markedly impaired (Figure 5A). Quantitative scanning of multiple experiments confirmed a significant reduction in coprecipitation of both the γ and β-chain, showing an average reduction of at least 75% for both chains at 3 minutes after stimulation (Figure 5B). This did not reflect destruction of these subunits, or alterations in sensitivity of FcϵRI to detergent solubilization, because whole cell lysates from antigen-stimulated PigA-deficient BMMCs, prepared under identical detergent conditions, showed normal and unchanged total amounts of α-chain, β-chain, and γ-chain (Figure 5A). Flow cytometry experiments confirmed that throughout the course of antigen stimulation, IgE binding to PigA-deficient BMMCs remained similar to binding to control cells (Figure 5C). These results demonstrate that the presence of GPI-AP is essential for the stability of the interactions between the FcϵRI α, β, and γ-chains after antigen-mediated receptor aggregation.
Reduced IgE/antigen induced FcϵRI-SFK assoctiation and FcϵRI interchain interactions in PigA-deficient BMMCs. (A) Littermate control (WT) or PigA-deficient (KO) BMMCs were sensitized with IgE anti-DNP for 3 hours at 37°C, and either mixed with DNP-BSA on ice (0 minutes), or further incubated with prewarmed DNP-BSA at 37°C for 1 or 3 minutes before transfer to lysis buffer on ice. Top panels: FcϵRI receptor complexes were immunoprecipitated from lysates by anti-IgE (IP: IgE), and immunoblotted with antibodies against FcϵRI α-chain (α), γ-chain (γ), or β-chain (β); or against Lyn (Lyn) or activated SFK (pSrc). In the pSrc blot, the two bottom bands presumably represent Lyn, the upper band Fyn, based on their molecular weight values (53/56 kDa and 59 kDa, respectively). Bottom panels: whole cell lysates (WCL) were immunoblotted with antibodies against FcϵRI α-chain (α), γ-chain (γ), or β-chain (β); data representative of at least two experiments are shown. (B) Quantitative scanning of multiple coimmunoprecipitation experiments specified in panel A, confirming significantly reduced coprecipitation of the γ-chain and β-chain with the receptor complex on stimulation. Shown are signal intensities of anti-IgE immunoprecipitates which were immunoblotted with antibodies against FcϵRI γ-chain (middle panel; n = 4) or β-chain (bottom panel; n = 3); or against α-chain as control (top panel; n = 3). Data are expressed as mean percentages of pixel intensities ± SEM, compared with nonstimulated cells (T = 0). Asterisks indicate significant differences (P < .05) compared with WT cells. (C) IgE binding to control (filled lines) or PigA-deficient (bold lines) BMMCs after IgE sensitization followed by 1 or 3 minutes antigen stimulation.
Reduced IgE/antigen induced FcϵRI-SFK assoctiation and FcϵRI interchain interactions in PigA-deficient BMMCs. (A) Littermate control (WT) or PigA-deficient (KO) BMMCs were sensitized with IgE anti-DNP for 3 hours at 37°C, and either mixed with DNP-BSA on ice (0 minutes), or further incubated with prewarmed DNP-BSA at 37°C for 1 or 3 minutes before transfer to lysis buffer on ice. Top panels: FcϵRI receptor complexes were immunoprecipitated from lysates by anti-IgE (IP: IgE), and immunoblotted with antibodies against FcϵRI α-chain (α), γ-chain (γ), or β-chain (β); or against Lyn (Lyn) or activated SFK (pSrc). In the pSrc blot, the two bottom bands presumably represent Lyn, the upper band Fyn, based on their molecular weight values (53/56 kDa and 59 kDa, respectively). Bottom panels: whole cell lysates (WCL) were immunoblotted with antibodies against FcϵRI α-chain (α), γ-chain (γ), or β-chain (β); data representative of at least two experiments are shown. (B) Quantitative scanning of multiple coimmunoprecipitation experiments specified in panel A, confirming significantly reduced coprecipitation of the γ-chain and β-chain with the receptor complex on stimulation. Shown are signal intensities of anti-IgE immunoprecipitates which were immunoblotted with antibodies against FcϵRI γ-chain (middle panel; n = 4) or β-chain (bottom panel; n = 3); or against α-chain as control (top panel; n = 3). Data are expressed as mean percentages of pixel intensities ± SEM, compared with nonstimulated cells (T = 0). Asterisks indicate significant differences (P < .05) compared with WT cells. (C) IgE binding to control (filled lines) or PigA-deficient (bold lines) BMMCs after IgE sensitization followed by 1 or 3 minutes antigen stimulation.
Reduced IgE/antigen-induced FcϵRI-Lyn association and FcϵRI γ-chain phosphorylation in PigA-deficient BMMCs
After FcϵRI aggregation, the first biochemical event in the FcϵRI signaling pathway is phosphorylation of tyrosines within ITAMs of the FcϵRI-associated γ-chain and β-chain by SFK, in particular Lyn.2,22,27 A number of studies have suggested that these events occur in lipid rafts, which concentrate both GPI-AP and acylated SFK.5 Because we observed a requirement for GPI-AP in antigen-induced FcϵRI interchain interactions and degranulation, next we tested the effects of PigA-deficiency on kinase activation and phosphorylation events. We hypothesized that the attenuated FcϵRI interchain interactions in the absence of GPI-AP could affect activation of Lyn. We first examined physical association of Lyn with FcϵRI on antigen stimulation. In IgE-sensitized control BMMCs, stimulation with DNP-BSA for 1 minute induced FcϵRI-Lyn association, which was further enhanced after 3 minutes (Figure 5A). In contrast, PigA-deficient cells showed a higher basal level of FcϵRI-Lyn association, which decreased to an almost undetectable level throughout the course of antigen stimulation (Figure 5A).
To determine whether there also was an effect of PigA-deficiency on association of activated kinase with FcϵRI, we used an antibody that recognizes a conserved phosphotyrosine in the activation loop of all SFK family members, including Lyn and Fyn. As was the case for total Lyn, association of FcϵRI with activated SFK was enhanced in control cells after antigen activation, but reduced in PigA-deficient BMMCs (Figure 5A). These data suggest that GPI-AP are required for stable association of activated SFK with FcϵRI on antigen-receptor ligation.
FcϵRI itself is a substrate for receptor associated SFK during IgE-mediated signaling. To test whether the decreased association of activated Lyn with FcϵRI affected this step in signal transduction, γ and β-chain phosphorylation was examined. On stimulation with IgE and antigen, tyrosine phosphorylation of the γ-chain was markedly impaired in PigA-deficient BMMCs, compared with control cells (Figure 6). In contrast, tyrosine phosphorylation of the β-chain was normal (Figure 6). Thus, the presence of GPI-AP is required for γ-chain tyrosine phosphorylation in response to FcϵRI aggregation, which is probably explained by the observed attenuated α-γ chain and FcϵRI-Lyn association in PigA-deficient BMMCs.
Reduced IgE/antigen-induced FcR γ-chain tyrosine phosphorylation in PigA-deficient BMMCs. After sensitization of BMMCs with IgE anti-DNP for 3 hours at 37°C, DNP-BSA (Ag) was added and the cells were challenged for 3 minutes at 37°C, or left on ice (0 minutes), and lysed. Top panel: immunoprecipitates prepared using anti–γ-chain antibodies were immunoblotted with anti-phosphotyrosine antibody 4G10 (γ-pY), or as loading control with anti–γ-chain (γ). Bottom panel: lysates were immunoprecipitated using anti-phosphotyrosine antibody 4G10 and immunoblotted with anti–β-chain antibodies (β-pY); loading controls are lysates immunoblotted with anti–β-chain antibodies (β). WT: littermate control BMMC; KO: PigA-deficient BMMCs; data representative of at least 2 experiments are shown.
Reduced IgE/antigen-induced FcR γ-chain tyrosine phosphorylation in PigA-deficient BMMCs. After sensitization of BMMCs with IgE anti-DNP for 3 hours at 37°C, DNP-BSA (Ag) was added and the cells were challenged for 3 minutes at 37°C, or left on ice (0 minutes), and lysed. Top panel: immunoprecipitates prepared using anti–γ-chain antibodies were immunoblotted with anti-phosphotyrosine antibody 4G10 (γ-pY), or as loading control with anti–γ-chain (γ). Bottom panel: lysates were immunoprecipitated using anti-phosphotyrosine antibody 4G10 and immunoblotted with anti–β-chain antibodies (β-pY); loading controls are lysates immunoblotted with anti–β-chain antibodies (β). WT: littermate control BMMC; KO: PigA-deficient BMMCs; data representative of at least 2 experiments are shown.
Discussion
The present data reveal an unexpected requirement for GPI-AP in the early FcϵRI signaling events and subsequent downstream function in mast cells. GPI-AP deficiency in mast cells led to attenuated passive cutaneous anaphylaxis in vivo and IgE-mediated degranulation in vitro. These functional defects correlated with a marked reduction in (1) FcϵRI receptor complex stability, (2) FcϵRI-Lyn association, and (3) FcϵRI γ-chain tyrosine phosphorylation, in response to stimulation by IgE and antigen.
The observation that receptor binding of IgE and the extent of FcϵRI aggregation in response to IgE and antigen were normal supports the hypothesis that GPI-AP deficiency specifically affects FcϵRI signaling events downstream of this first step. This suggests that the simplest model to explain the GPI-AP requirement, ie that FcϵRI clustering results from interaction of ligated receptor with one or more GPI-AP, which often appear preclustered,25 is not correct. Instead, reduced coprecipitation of the FcϵRI α-chain with the γ and the β-chain on IgE/antigen stimulation suggests that the presence of GPI-AP is important for receptor stability, and presumably conformation, after FcϵRI aggregation. Receptor aggregation triggers association of FcϵRI with the SFK necessary for signaling.28 Thus, the reduced γ-chain phosphorylation in PigA-deficient cells can be explained by decreased availability of the ITAM-containing γ-chain as substrate for activated SFK.
In addition to a decreased FcϵRI-Lyn association on antigen stimulation in PigA-deficient cells, we observed an enhanced basal level of FcϵRI association with Lyn and activated SFK without stimulation. The reason for the enhanced baseline activity is presently unknown, but we speculate that this difference is caused by altered interactions of the receptor with phosphatases. A recent study demonstrated the presence of pre-existing complexes of FcϵRI with both SFK and tyrosine phosphatases in nonstimulated cells.29 This correlated with a weak baseline phosphorylation state of FcϵRI in the absence of stimulation, which could be enhanced by addition of phosphatase inhibitors.29 It is possible that GPI-AP influence the topology of phosphatases at resting conditions. Nonetheless, on antigen stimulation, PigA-deficiency resulted in decreased FcϵRI-Lyn association and the inability to trigger γ-chain phosphorylation, which supports the requirement of GPI-AP for a productive aggregation-induced FcϵRI-SFK interaction.
In contrast to the γ-chain, β-chain phosphorylation was unaffected by GPI-AP deficiency. It is known that γ- and β-chains have different affinities for individual kinases, because of slight differences in ITAM structure. Lyn has a higher affinity for the β-chain than for the γ-chain, and weakly but constitutively interacts with the β-chain, even under nonstimulated conditions. FcϵRI aggregation activates β chain-associated Lyn, which in turn phosphorylates the γ-chain.30 This constitutive association may explain why GPI-AP are not required for β-chain phosphorylation. It is also possible that even though both chains dissociate from the FcϵRI complex on stimulation, they do so with different kinetics. If the β-chain dissociates after the γ-chain, a difference in antigen-stimulated phosphorylation might result. Our quantitative analysis of chain dissociation suggests this possibility, because at 1 minute after antigen activation more γ-chain than β-chain was lost from the complex, but a more thorough analysis is needed to test this hypothesis.
We hypothesize that the conformation of the aggregated hetero-multimeric receptor is at least subtly distinct from its nonaggregated state, and that GPI-AP are required for a signaling-competent aggregated FcϵRI. It may be that the as yet unidentified GPI-AP necessary for this signaling-competent state should be thought of as additional chains of the aggregated, but not of the nonaggregated FcϵRI. However, whether direct physical interactions between GPI-AP and aggregated FcϵRI exist is currently unknown, nor is it clear whether specific GPI-AP, or merely the GPI glycolipid anchor itself, are responsible for the effects on FcϵRI signaling in PigA-deficient cells. One potential candidate GPI-AP is CD48, which has recently been proposed to facilitate eosinophil activation during ovalbumin-induced airway inflammation in mice31 ; more studies are warranted to determine its possible role in FcϵRI signaling.
In the 1980s, Metzger's group showed that lipid environment had a major effect on association of the FcϵRI α-chain with the γ and β-chain.24 Although they did not investigate differences between antigen-ligated and nonaggregated receptors, our data suggest the possibility of an alteration of the lipid environment on aggregation, leading to chain dissociation in the absence of GPI-AP. This alteration may reflect movement of aggregated receptor into lipid rafts, but more work is required to determine whether this is the case.
It is unlikely that the absence of GPI caused a general disruption of membrane organization, lipid raft formation, or transmembrane signaling for several reasons. First, lipid rafts were found to be intact in a GPI-deficient mutant CHO cell line.23 Second, we observed normal staining of PigA-deficient BMMCs with the common lipid raft marker cholera toxin subunit B. Third, cholesterol and sphingolipid rich lipid rafts can form in artificial membranes without GPI.32 Thus, the data indicate that GPI-AP are required specifically for communication of FcϵRI with intracellular signaling pathways mediating mast cell activation. Furthermore, despite the observed reduced degranulation in response to IgE and antigen, PigA-deficient BMMCs degranulated normally in response to compound 48/80, which triggers degranulation through trimeric G-proteins.18 This indicates that an FcϵRI-independent signaling pathway is intact in PigA-deficient BMMCs, and also that these cells have no general defect in the degranulation machinery. Because heterotrimeric G proteins also concentrate in lipid rafts, this is further evidence that there is no general disruption of raft architecture or function in the absence of GPI. In addition, we observed previously that PigA-deficient T lymphocytes respond normally to T-cell receptor (TCR) stimulation by phytohemagglutinin or protein antigen,21 confirming that PigA-deficiency does not generally affect all receptor function.
The lipid raft hypothesis proposes that FcϵRI aggregation induces coalescence with lipid microdomains containing SFK including Lyn, which in turn stabilizes a functional signaling FcϵRI complex.2,5 Although many concerns have been raised regarding the interpretation of studies on lipid rafts,33 functional support for the involvement of lipids in mast cell FcϵRI signaling was recently provided using a mouse with defective cholesterol production, which showed partially impaired Lyn-raft association and FcϵRI phosphorylation.34 Our genetic evidence, showing an essential role of GPI-AP in FcϵRI-SFK interaction required for signaling on FcϵRI aggregation, is compatible with the lipid raft hypothesis. We propose a model in which GPI-AP, present in lipid microdomains, are key factors to stabilize the noncovalent interactions between the FcϵRI chains on receptor aggregation. Stabilization of these interchain interactions in turn is prerequisite for a productive, and perhaps sustained, association between FcϵRI and SFK, and subsequent γ-chain phosphorylation. GPI-AP may be essential to keep aggregated FcϵRI in a specific lipid microenvironment that is optimal for signaling.
In conclusion, the present data demonstrate that GPI-AP represent an important and previously unrecognized component of early FcϵRI signaling steps. The presence of GPI-AP is crucial for FcϵRI-aggregation induced receptor stability, phosphorylation, and effector function. These findings shed new light on the molecular mechanisms underlying the pathophysiology of allergy and asthma.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by a grant from the Sandler Awards in Basic Biology of Asthma and by National Institutes of Health RO1 grant AI24674.
The authors thank Dmitri Kioussis (MRC, London) for providing VavCre mice; Juan Rivera (National Institutes of Health) for providing anti-β chain antibody JRK; Chen Wang, Shilpa Joshi, Marlea Lamoureux, Nicolas Lewin-Kohk, Charles Jones, Lauri Diehl, and Delu Zhou (Genentech) for technical assistance; Tomoko Jippo (Osaka University) and Marc Daëron (Pasteur Institute, Paris) for helpful suggestions; and Lawren Wu, Menno van Lookeren-Campagne, and Flavius Martin (Genentech) for critical review.
National Institutes of Health
Authorship
Contribution: W.L.W.H. designed and performed experiments and wrote the paper; P.W. performed experiments; J.E.-A. quantified skin mast cells; T.K. gave essential advice and provided PigALoxP mice; and E.B. supervised the project and helped write the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for W.L.W.H. and E.J.B is Department of Microbial Pathogenesis, Genentech Inc, South San Francisco, CA.
Correspondence: Wouter L. W. Hazenbos, Genentech Inc, 1 DNA Way, South San Francisco, CA 94080; e-mail: wouterh@gene.com.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal