Abstract
In Eμ-myc transgenic animals lymphoma formation requires additional genetic alterations, which frequently comprise loss of p53 or overexpression of BCL-2. We describe that the nature of the “second hit” affects the ability of the immune system to contain lymphoma development. Tumors with disrupted p53 signaling killed the host more rapidly than BCL-2 overexpressing ones. Relaxing immunologic control, using Tyk2−/− mice or by Ab-mediated depletion of CD8+ T or natural killer (NK) cells accelerated formation of BCL-2–overexpressing lymphomas but not of those lacking p53. Most strikingly, enforced expression of BCL-2 prolonged disease latency in the absence of p53, whereas blocking p53 function in BCL-2–overexpressing tumors failed to accelerate disease. This shows that blocking apoptosis in p53-deficient cells by enforcing BCL-2 expression can mitigate disease progression increasing the “immunologic visibility.” In vitro cytotoxicity assays confirmed that high expression of BCL-2 protein facilitates NK and T cell–mediated killing. Moreover, we found that high BCL-2 expression is accompanied by significantly increased levels of the NKG2D ligand MULT1, which may account for the enhanced killing. Our findings provide first evidence that the nature of the second hit affects tumor immunosurveillance in c-MYC–driven lymphomas and define a potential shortcoming of antitumor therapies targeting BCL-2.
Introduction
Enhanced expression of the proto-oncogene MYC has been implicated in disease induction or progression of different tumors and is considered one of the most frequent genetic alterations found in human cancers.1 Because c-MYC induces apoptosis on enforced expression, c-MYC–driven tumor formation requires a cooperating mutation counterbalancing the proapoptotic activity, the so-called second hit.
It is a common feature of proto-oncogenes that their oncogenic potential is accompanied by the activation of pathways, which sensitize cells to apoptotic stimuli.2-4 c-MYC is known to activate 2 pathways that are supposed to provide a safety guard against c-MYC–induced transformation. First, p53 accumulates as a result of p19ARF induction, which relieves MDM2-dependent feedback5 and triggers apoptosis and senescence.6-10 Second, high levels of c-MYC suppress the expression of the antiapoptotic genes BCL-2 and BCL-xL.11,12 This shifts the balance between proapoptotic and antiapoptotic proteins and sensitizes the cells to apoptotic stimuli. Thus, efficient transformation by c-MYC requires concomitant second hits to counterbalance apoptosis. This has been well studied in Eμ-myc mice, whereby Myc is placed under the control of the intronic enhancer of the μ-immunoglobulin heavy chain locus.13 These mice develop B-cell lymphomas with a mean latency of 12-16 weeks of age.13-17 The phenotype of these mice reflects to a certain extent human Burkitt lymphoma. The cooperating second hits have been well defined for this tumor model.13 Most tumors found in Eμ-myc mice display either a disruption of the p19ARF-MDM2-p53 pathway or overexpress the antiapoptotic protein BCL-2 or BCL-xL.5,11,12,18-20 Double transgenic animals have confirmed the cooperation between c-MYC and BCL-2 and defined proapoptotic BH3-only BCL-2 family members such as BIM, BMF, and PUMA as suppressors of Eμ-myc–induced lymphomagenesis.20,21 In mice and men a selection against BH3-only protein gene expression was found to associate with accelerated disease.21,22 It is well established that additional genetic alterations that occur in a transformed cell may influence and shape cell autonomous properties such as cell proliferation, apoptosis, or differentiation. The consequences for the interaction with the tumor environment are less well understood. One factor that determines disease progression in vivo is the interaction of the tumor with the immune system. The innate and the adaptive arms of the immune system are capable to recognize and eliminate transformed cells. Thereby, the immune system exerts an evolutionary pressure onto a genetically unstable cell population and contributes to the consistent changes that are on-going with an evolving malignancy23 (and references therein). This process is also referred to as sculpting or editing of the tumor. In later phases the effect of the immune system may be converted because immune cells can foster tumor growth and provide a supporting environment.
In this report we provide the first evidence that the nature of the second hit determines and sculptures the way a tumor communicates and interacts with the immune system. We show that the immunologic visibility of Eμ-myc–induced lymphoma critically depends on the expression of the antiapoptotic protein BCL-2.
Methods
Mice
Eμ-myc,13 Vav-bcl-2,24 p53−/−,25 and Tyk2−/−26 Rag2−/−cγ−/−27 mice were described previously. Animal experiments were performed in accordance with protocols approved by the Animal Welfare Committee of the Medical University of Vienna.
Eμ-myc as well as all primary and secondary mice that received a transplant were killed when they became ill. Peripheral blood, lymphoid organs, and, if available, tumors were analyzed for leukemic cells by FACS or histopathology or both. Offspring from Eμ-myc/p53+/− and Eμ-myc/vav-bcl-2 mice were killed at 3 weeks of age. Splenocytes were both taken in culture to obtain cell lines and frozen in liquid nitrogen for further transplantation experiments. For analysis of expression levels (by immunoblots; Figure 1), only freshly isolated tissues were used; in several instances and despite daily inspection mice were found dead and subjected to analysis by macroscopic anatomical dissection. However, because the time point of death was unknown and proteolysis was thus a confounding variable, tumor samples were neither used for microscopic nor biochemical analysis.
Cell culture, infection, and expression vectors
Tissue culture conditions, virus preparation, and infections of cell lines were performed as described previously.28 To establish tumor cell lines, splenocytes or BM from diseased mice were propagated in RPMI 1640 growth medium containing 10% FCS, 100 U/mL penicillin/streptomycin, and 50μM 2-mercaptoethanol, until the dish contained a visibly homogeneous population of transformed cells. Expression vectors used for the experiments were pMSCV-IRES-GFP, pMSCV-IRES-BCL-2, and pMSCV-IRES-p53DD.29 All cell lines were sorted with the use of FACS for green fluorescent protein–positive (GFP+) cells 48 hours after infection.
Transplantation of tumor cells or primary splenocytes into C57BL/6, Tyk2−/−, and Rag2−/−cγ−/− mice
Cells (104) were suspended in 100 μL of PBS and injected into the tail vain. Thereafter, mice were controlled daily for signs of illness. When mice became ill, they were killed, and organs were analyzed as described in “Mice.”
[3H]-thymidine incorporation
Cells (105) were plated in triplicates in 96-well plates, and [3H]-thymidine (0.1 μCi/well [0.0037 MBq/well]) was added. After 24 hours of incubation, analysis was performed with Ultima Gold MV scintillation fluid (Packard Instruments) by a scintillation counter.
Flow cytometric analysis
FACS analysis was done by a FACSCantoII flow cytometer with the use of FACSDiva software.
B-cell development staining.
We used different Abs to determine the specific B-lineage maturation stages: CD43 (1B11), CD19 (1D3), and IgM (R6-60.2; all BD PharMingen). Calreticulin surface expression was accessed with the use of calreticulin Ab (ab4 from Abcam) and appropriate secondary Ab.
Cell cycle analysis.
Cells (5 × 106) were stained with PI (50 μg/mL) in a hypotonic lysis solution (0.1% sodium citrate, 0.1% Triton X-100, 100 μg/mL RNAse) and incubated at 37°C for 30 minutes.
To monitor the success of depletion we stained whole blood with different natural killer (NK) and T cell–specific Abs: NK1.1 (Clone PK136), DX5 (HMα2), CD8 (53-6.7), CD4 (GK1.5; all BD PharMingen). Staining of whole blood was followed by lysis of erythrocytes with FACS Lysing Solution (BD Biosciences) according to the manufacturer's instructions.
Protein analysis and immunoblotting
Cells were lysed in a buffer containing protease and phosphatase inhibitors (50mM HEPES, pH 7.5, 0.1% Tween-20, 150mM NaCl, 1mM EDTA, 20mM β-glycero-phosphate, 0.1mM sodium vanadate, 1mM sodium fluoride, 10 μg/mL aprotinin, leupeptin, and 1mM PMSF, respectively). Protein concentrations were determined with a BCA-kit (Pierce). Proteins (70 μg) were separated on polyacrylamide gels and transferred onto Immobilon membranes. Membranes were probed with Abs directed against c-MYC (sc-788), β-actin (A-4700; Sigma-Aldrich), p53 (sc-6243), BCL-2 (sc-7382), p19 (sc-1063), p21 (sc-397), MDM2 (sc-812), and α-tubulin (sc-53 029; Santa Cruz Biotechnology Inc). Immunoreactive bands were visualized by chemiluminescent detection (ECL detection kit; Amersham).
Ab-mediated depletion of NK cells and CD8+ cells
Abs against the NK1.1 receptor expressed on NK cells were isolated from supernatant of the hybridoma cell line PK136 and purified with ammonium-sulfate precipitation and protein G–based column affinity purification. Four days before transplantation mice were treated with 150 μg of Ab administrated intraperitoneally. Thereafter, 150 μg of Ab was administered each third day. Success of NK-cell depletion was determined by FACS analysis after killing mice. CD8+ T cells were depleted by intraperitoneal injections of 100 μg of 53-6.72 anti-CD8+–specific Ab (BioXCell) twice a week, starting 2 days before tumor cell injection.
CFSE-based proliferation assay
Eμ-myc/p53+/− (± BCL-2) and Eμ-myc/vav-bcl-2 (± dnp53) tumor cells (107) were stained with CFSE (1.66μM) according to manufacturer's instructions (Molecular Probes; CellTrace CFSE Cell Proliferation Kit). The CFSE-labeled cells were propagated in RPMI 1640 growth medium as described in “Cell culture infection, and expression vectors,” and the decrease in mean fluorescence intensities (MFIs) of CFSE was investigated 4, 20, 28, 44, 52 and, 68 hours after the staining procedure. As a control unstained tumor cells were analyzed, and MFI values were subtracted. In addition, we stained all samples with 7-aminoactinomycin D (7-AAD; 0.1 μg; eBioscience) to exclude possible dead cells. The relative percentage of fluorescence relative to the starting point was calculated. The cells were always kept in a proliferative condition and were split regularly.
NK-cell isolation and cytotoxicity assay
NK cells were isolated from splenocytes of C57BL/6 mice with the use of the MACS separation kit (Miltenyi Biotec; positive selection of DX5+ NK cells). Purified NK cells were cultured in RPMI 1640 medium containing 10% FCS, 100 U/mL penicillin/streptomycin, 50μM 2-mercaptoethanol, and 5000 U/mL rhIL-2 (Proleukin; Novartis) for 7 days in 24-well plates.
NK-cell cytotoxicity toward Eμ-myc/p53+/− (± BCL-2) and Eμ-myc/vav-bcl-2 (± dnp53) tumor cells was quantified in a FACS-based assay. The NK-target cell line YAC-1 was included as a positive control for NK cell–mediated lysis. Tumor cells were harvested and stained with CFSE as stated in “CFSE-based proliferation assay.” CFSE-labeled tumor cells (5 × 104) were seeded in 96-well round bottom plates, and NK cells were added in effector-to-target ratios (E:T) of 15:1, 7.5:1, and 3.75:1 in triplicates. After 4 hours of coincubation all samples were subjected to 7-AAD for 5 minutes and analyzed by FACS. Percentage of specific NK-cell lysis was calculated as follows: percentage of specific lysis = (percentage of 7-AAD+ CFSE+ cells after coincubation with NK cells) − (percentage of 7-AAD+ CFSE+ cells without addition of NK cells).
T cell–mediated lysis of Eμ-myc tumor cells
To generate aCD3-activated T cells, splenocytes from untreated C57BL/6 mice were stimulated with aCD3e-Ab (0.5 μg/μL; BD PharMingen) and cultured in T-cell medium (RPMI 1640 medium containing 10% FCS, 50μM 2-mercaptoethanol, nonessential amino acids [PAA], 1mM sodium-pyruvate [Gibco], and 100 U/mL rhIL-2) for 3 days. To generate tumor cell Ag-reactive T cells, C57BL/6 mice were immunized intraperitoneally with 105 tumor cells/100 μL PBS, either with Eμ-myc/vav-bcl-2 or Eμ-myc/p53+/− cells, respectively. Seven days later mice were boosted, and splenocytes were isolated at day 14. In parallel Eμ-myc/vav-bcl-2 and Eμ-myc/p53+/− cells were irradiated (30 Gy γ ray). Splenocytes (5 × 106) of immunized or control mice were cocultured with 2 × 106 irradiated tumor cells in 1 mL of RPMI 1640 supplemented with 10% FCS, 50μM 2-mercaptoethanol, 100 U/mL penicillin/streptomycin, and 100 U/mL rhIL-2 for 4 days. After 4 days, splenocytes of control mice and tumor Ag-reactive T cells were collected and cocultured with 5 × 104 CFSE-labeled (2.5μM) Eμ-myc/vav-bcl-2 and Eμ-myc/p53+/− target cells at E:Ts of 60:1, 30:1, 15:1, and 5:1 in duplicates in 96-well plates. To eliminate the possibility of false-positive spontaneously occurring apoptosis, tumor cells only were incubated. Peptide-specific killing was quantified with FACS analysis 20 hours after incubation start. Dead target cells were discriminated from living cells with staining with 7-AAD. In addition, we stained aCD3-activated T cells, tumor Ag-reactive T cells, and control splenocytes for the expression of CD8+ T-cell markers (CD3, CD8) to assess the real effector ratio depicted in the figures. CFSE+ tumor cells (10 000) were counted, and the percentage of specific lysis was calculated as (percentage of 7-AAD+ CFSE+ target cells after coincubation) − (percentage of 7-AAD+ CFSE+ cells without coincubation).
Patient characteristics and patient data analysis
Fifty-nine tumors that had been initially diagnosed either as Burkitt lymphoma or diffuse large B-cell lymphoma (DLBCL) were included in the study. All patients had been admitted to the Medical University of Vienna during 1999-2010. The procedure concerning patient's data protection was according to regulations of the Medical University of Vienna that accord to the principles expressed in the Declaration of Helsinki. First, rearrangements of MYC and BCL2 as well as TP53 deletions were screened in 22 fresh tumor samples and 37 paraffin-embedded tissues by FISH as described previously.30,31 Patients were treated with the B-cell acute lymphoblastic leukemia protocol for Burkitt lymphoma of the German Multicenter Study Group for the treatment of adult acute lymphoblastic leukemia or rituximab plus cyclophosphamide doxorubicin vincristine and prednisone.
IHC was performed with Abs directed against p53 (Clone SP5; Labvision; 1:50) and BCL-2 (Clone 124 (1); Dako; 1:80). IHC was done on formalin-fixed (7.5% buffered formalin) paraffin-embedded tissue sections. Four-micrometer sections were mounted on silicon-coated slides, deparaffinized, and heated in 10mM citrate buffer with the use of microwave radiation. Tissue sections were incubated with anti-p53 and anti–BCL-2 Ab for 1 hour at room temperature. Visualization was performed with the ABC kit (Vector Laboratories, DAB) according to the manufacturer's recommendations. Pictures were taken at room temperature with a Nikon Microphot-FXA microscope (objective lens, numerical aperture: Plan Apo40/0.95; magnification 400×; imaging medium: air), a Nikon Digital Net Camera DN100 camera and Nikon Digital Net Camera DN100 software.
TP53 sequencing included exons 4-11. DNA was extracted from paraffin-embedded tissue with the use of the QIAamp Tissue kit (QIAGEN GmbH). Exons were amplified by PCR with the use of Jump Start PCR mastermix REDtaq (Sigma-Aldrich). Primer concentration was 1 pmol/μL in a 30-μL PCR reaction mix. Primer sequences are included in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Annealing temperature was 60°C for exons 4-9; and 57°C for exon 10. PCR products were checked by product length with a 2% agarose gel (349 bp exon 4, 248 bp exon 5, 224 bp exon 6, 192 bp exon 7, 232 bp exon 8, 175 bp exon 9, 259 bp exon 10, 145 bp exon 11). PCR products (5 μL) were then treated with 2 μL of Exo-SAP (USB; no. 78 201) for purification. Capillary sequencing of PCR products was done with ABI Big Dye Terminator Version 1.1 cycle sequencing kit according to the manufacturer's recommendations. Ten microliters of mastermix (2 μL of Big Dye Ready Reaction Mix, 1 μL of primer forward/reverse, 1 μL of purified PCR product, and 6 μL Abd) were run for 24 cycles of sequencing reaction. DNA fragments were purified with QIA Dye Ex 96 kit (Qiagen GmbH) and processed to ABI 3130 Genetic Analyzer. Sequences were analyzed with the SeqScape Version 2.5 analysis software program (Applied Biosystem).
Statistical analysis
P values for data were calculated with the χ2 test, Student t test, and log-rank test as appropriate. P values < .05 were considered statistically significant.
Results
Eμ-myc–induced lymphoma expressing high levels of BCL-2 evolve later than lymphomas with a disrupted p53 pathway
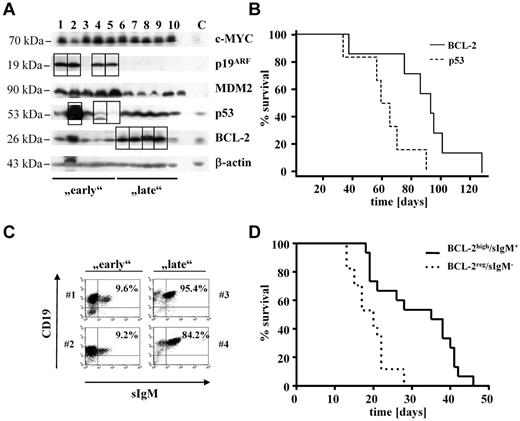
Eμ-myc transgenic mice develop B-cell lymphoma with strongly enlarged inguinal, axillar, and peritoneal lymph nodes; splenomegaly; and occasionally ascites formation.13,15,17 Interestingly, disease latency significantly varies from 1 month to ≤1 year of age in C57BL/6-Eμ-myc transgenic mice. Lymphoma formation in Eμ-myc transgenic mice requires additional genetic alterations to counterbalance the apoptotic effects of c-MYC overexpression. The most frequent second hits are disruption of the p53 signaling pathway or alterations resulting in BCL-2 overexpression. We analyzed the p53 status by immunoblot analysis of those animals in which tissues were available. A strong accumulation of p53 or p19ARF protein indicates the loss of a functional pathway, as does a complete lack of p53 expression in the presence of high levels of ARF.5 Similarly, we quantified levels of BCL-2 (Figure 1A). Thus, we identified animals that either had a disrupted p53 pathway or high BCL-2 levels. If their survival was plotted, it became evident that the Kaplan-Meier plots differed significantly (P = .0183, log-rank test). After 75 days > 80% of the animals harboring a disruption in p53-dependent pathways had succumbed to disease (Figure 1B). In contrast, > 80% of mice with BCL-2 overexpression were still alive. Thus, we used 75 days as an operational cutoff to define “early” and “late” tumors (Table 1).
Eμ-myc–induced lymphoma expressing high levels of BCL-2 evolve later than lymphomas with a disrupted p53 pathway. (A) Immunoblot of primary tumors found in a population of Eμ-myc mice (n = 23). Early and late indicate mice that survived shorter or longer than 75 days, respectively. Squares are highlighting protein alterations associated with respective second hit. Splenocytes from wild-type mice were used as control (lane C). (B) Kaplan-Meier plot of mice from before mentioned population subdivided into 2 groups on the basis of the observed second hit (PBCL-2 vs p53 = .0183; nBCL-2 = 7; np53 = 6). (C) All lymphomas (n = 23) were analyzed by FACS for the presence of surface IgM (sIgM). Dot blots show 2 representative tumors from each subpopulation (early lymphomas: #1 and #2; late lymphomas: #3 and #4). Numbers indicate percentages of sIgM+ B cells. (D) Kaplan-Meier plot of mice that received a transplant with primary tumor cells obtained from diseased Eμ-myc mice. (sIgM−, n = 17; sIgM+, n = 15). Mice receiving sIgM+ tumor cells show significant longer survival time than mice that received a transplant with cells negative for IgM surface expression (PsIgM−vs sIgM+ < .001).
Eμ-myc–induced lymphoma expressing high levels of BCL-2 evolve later than lymphomas with a disrupted p53 pathway. (A) Immunoblot of primary tumors found in a population of Eμ-myc mice (n = 23). Early and late indicate mice that survived shorter or longer than 75 days, respectively. Squares are highlighting protein alterations associated with respective second hit. Splenocytes from wild-type mice were used as control (lane C). (B) Kaplan-Meier plot of mice from before mentioned population subdivided into 2 groups on the basis of the observed second hit (PBCL-2 vs p53 = .0183; nBCL-2 = 7; np53 = 6). (C) All lymphomas (n = 23) were analyzed by FACS for the presence of surface IgM (sIgM). Dot blots show 2 representative tumors from each subpopulation (early lymphomas: #1 and #2; late lymphomas: #3 and #4). Numbers indicate percentages of sIgM+ B cells. (D) Kaplan-Meier plot of mice that received a transplant with primary tumor cells obtained from diseased Eμ-myc mice. (sIgM−, n = 17; sIgM+, n = 15). Mice receiving sIgM+ tumor cells show significant longer survival time than mice that received a transplant with cells negative for IgM surface expression (PsIgM−vs sIgM+ < .001).
Second hits found in primary Eμ-myc lymphomas
| Mouse no. . | Second hit . | |
|---|---|---|
| p53 disruption . | BCL-2high . | |
| Eμ-myc early | ||
| E1 | + | − |
| E2 | − | + |
| E6 | + | − |
| E7 | + | − |
| E9 | − | − |
| E12 | + | − |
| E4 | ND | ND |
| E5 | ND | ND |
| E8 | ND | ND |
| E11 | ND | ND |
| E3 | ND | ND |
| E10 | ND | ND |
| Eμ-myc late | ||
| L1 | − | + |
| L4 | − | + |
| L5 | − | + |
| L6 | − | + |
| L7 | − | + |
| L8 | − | + |
| L10 | + | − |
| L2 | ND | ND |
| L3 | ND | ND |
| L9 | ND | ND |
| L11 | ND | ND |
| Mouse no. . | Second hit . | |
|---|---|---|
| p53 disruption . | BCL-2high . | |
| Eμ-myc early | ||
| E1 | + | − |
| E2 | − | + |
| E6 | + | − |
| E7 | + | − |
| E9 | − | − |
| E12 | + | − |
| E4 | ND | ND |
| E5 | ND | ND |
| E8 | ND | ND |
| E11 | ND | ND |
| E3 | ND | ND |
| E10 | ND | ND |
| Eμ-myc late | ||
| L1 | − | + |
| L4 | − | + |
| L5 | − | + |
| L6 | − | + |
| L7 | − | + |
| L8 | − | + |
| L10 | + | − |
| L2 | ND | ND |
| L3 | ND | ND |
| L9 | ND | ND |
| L11 | ND | ND |
+ or − indicates presence or absence, respectively, of indicated second hit; and ND, absence of appropriate tumor material for immunoblotting.
Differences were also observed when we analyzed the tumors according to the differentiation status with the use of the expression of IgM as a marker (Figure 1C).32,33 Tumors displaying IgM expression were classified “differentiated” and were found overrepresented in the late population (data not shown). Accordingly, a statistically significant correlation between the IgM expression and BCL-2 expression exists: most IgM+ tumors, which evolve late, expressed elevated levels of BCL-2 (P = .01). The observation that BCL-2 expressing and IgM+ tumors arose late could be recapitulated by transplanting lymphoma cells into C57BL/6 recipient wild-type mice. As depicted in Figure 1D, IgM+/BCL-2–overexpressing tumors evolved with a significantly increased latency (P < .001). In contrast, most tumors harboring a disruption in the p53 pathway were IgM− (P = .0485). In summary, these data suggest that Eμ-myc–induced lymphomas with high BCL-2 expression are less aggressive, evolved with an enhanced latency, and are of a higher differentiation status as indicated by IgM surface expression.
Predefining the second hit by crossing Eμ-myc/vav-bcl-2 and Eμ-myc/p53+/− mice confirms a correlation between disease latency and second hit
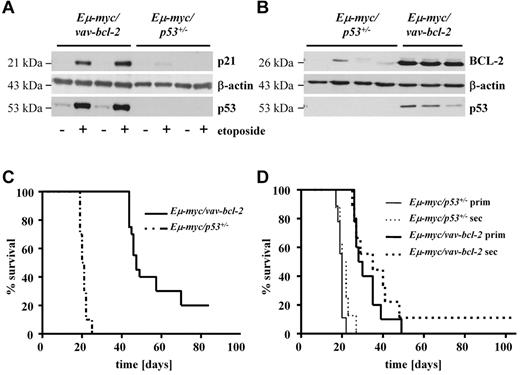
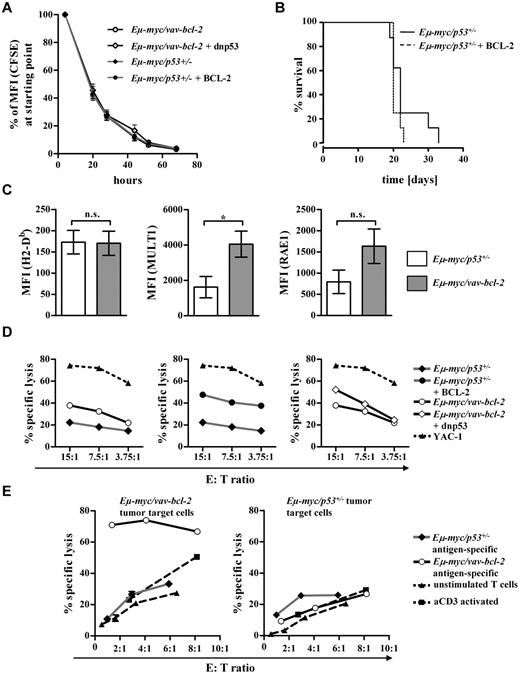
So far our observations suggested a correlation between second hit and/or differentiation status and disease latency. To gain further insight into the relation of these processes, we chose the following approach: Eμ-myc animals were crossed to Vav-bcl-2 transgenic or to p53−/− mice. Thus, we generated a scenario in which the second hit was predetermined, and we obtained lymphoma cells that were either Eμ-myc/vav-bcl-2 or Eμ-myc/p53+/−. All attempts to obtain Eμ-myc/p53−/− animals failed despite intense breeding efforts. This was because that already at the age of 20-25 days, Eμ-myc/vav-bcl-2 and Eμ-myc/p53+/− mice showed clear signs of disease with hepato-splenomegaly, growth retardation, weight loss, and scrubby fur. Thus, the mice were killed at day 21, and the splenocytes prepared. An aliquot of the splenocytes derived from Eμ-myc/vav-bcl-2 and Eμ-myc/p53+/− mice was treated with 50μM etoposide to verify the disruption of the p53 signaling cascade. One typical example is depicted in Figure 2A; as expected etoposide induced a pronounced p53-dependent p21 induction in Eμ-myc/vav-bcl-2–derived cells, indicating an intact p53 response. In contrast, p21 induction and p53 expression were lacking in Eμ-myc/p53+/−–derived cells that are known to loose the second allele.5,34,35 We also verified the high expression of BCL-2 and p53 in Eμ-myc/vav-bcl-2–derived splenocytes (Figure 2B). Most Eμ-myc/p53+/− cells, as well as Eμ-myc/vav-bcl-2 cells, were negative for IgM but expressed CD19 and CD43 corresponding to a pro-B cell stage (data not shown). This is in line with observations made by others.20,35 We normalized the experimental conditions with the use of splenocytes of the diseased mice for further transplantation in nonirradiated C57BL/6 recipient animals. Cells (104) were transplanted via the tail vein. Figure 2C summarizes our efforts. Mice that received splenocytes of Eμ-myc/p53+/− mice succumbed to disease significantly faster than mice that received Eμ-myc/vav-bcl-2 cells. In both groups the tumor cells densely infiltrated BM, spleen, and lymph nodes (data not shown). To test whether the Eμ-myc/vav-bcl-2 cells have to acquire an additional mutation in the p53 pathway, which could explain the increased disease latency, we performed secondary transplantations. In case the cells had acquired a secondary p53-related mutation, differences should level out and disease should evolve with comparable latency. This was not the case as shown in Figure 2D. Moreover, when we challenged Eμ-myc/vav-bcl-2 cells derived from spleens of diseased animals with etoposide, we could again verify p21 induction, indicating an intact p53 pathway (supplemental Figure 1A). In addition, IHC analysis for p53 did not show any accumulation of p53 in Eμ-myc/vav-bcl-2–induced tumors, which we would expect in case of a p53 mutation (supplemental Figure 1B). This experiment verified the dependency of disease latency on the nature of the second hit and excludes a role for the differentiation status of the tumor cells. This led us to conclude that irrespective of the experimental setting Eμ-myc/vav-bcl-2–induced lymphoma consistently displayed increased disease latency (Figure 2D).
Predefining the second hit by crossing Eμ-myc/vav-bcl-2 and Eμ-myc/p53+/− mice confirms a correlation between disease latency and second hit. (A) Representative immunoblots of splenocytes from Eμ-myc/vav-bcl-2 and Eμ-myc/p53+/− mice (n = 2 each). The addition of etoposide is used as functional test of the DNA-damage pathway mediated by p53. After addition of etoposide, splenocytes from Eμ-myc/p53+/− mice did not show enhanced p21 levels, indicating genetic alterations leading to loss of functional p53. Further, we detected increased levels of p53 in splenocytes from Eμ-myc/vav-bcl-2 mice after etoposide treatment, whereas no p53 could be detected in splenocytes derived from Eμ-myc/p53+/− mice. (B) Representative immunoblots of splenocytes from Eμ-myc/p53+/− and Eμ-myc/vav-bcl-2 mice (n = 4 and n = 3, respectively). As expected splenocytes from Eμ-myc/vav-bcl-2 showed higher expression levels of BCL-2. Splenocytes from Eμ-myc/vav-bcl-2 mice displayed regular levels of p53, whereas splenocytes derived from Eμ-myc/p53+/− mice did not show any p53 protein. (C) Transplantation of splenocytes from Eμ-myc/vav-bcl-2 or Eμ-myc/p53+/− mice into C57BL/6 recipients. Mice receiving cells from Eμ-myc/vav-bcl-2 showed massively enhanced survival (Eμ-myc/53+/−, n = 16; Eμ-myc/vav-bcl-2 n = 19; PEμ-myc/p53+/− vs Eμ-myc/vav-bcl-2 < .001). (D) Survival analysis for serially transplanted Eμ-myc/p53+/− and Eμ-myc/vav-bcl-2 splenocytes. Cells from diseased primary mice that received a transplant were used for 1 subsequent transplantation. Serial transplantation did not result in altered survival time of the mice. (Eμ-myc/p53+/− prim, n = 10; Eμ-myc/vav-bcl-2 prim, n = 10; Eμ-myc/p53+/− sec, n = 8; Eμ-myc/vav-bcl-2 sec, n = 9; PEμ-myc/p53+/− prim vs Eμ-myc/p53+/−sec > .05; PEμ-myc/vav-bcl-2 prim vs Eμ-myc/vav-bcl-2 sec > .05.) Prim indicates primary transplantation; and sec, secondary transplantation.
Predefining the second hit by crossing Eμ-myc/vav-bcl-2 and Eμ-myc/p53+/− mice confirms a correlation between disease latency and second hit. (A) Representative immunoblots of splenocytes from Eμ-myc/vav-bcl-2 and Eμ-myc/p53+/− mice (n = 2 each). The addition of etoposide is used as functional test of the DNA-damage pathway mediated by p53. After addition of etoposide, splenocytes from Eμ-myc/p53+/− mice did not show enhanced p21 levels, indicating genetic alterations leading to loss of functional p53. Further, we detected increased levels of p53 in splenocytes from Eμ-myc/vav-bcl-2 mice after etoposide treatment, whereas no p53 could be detected in splenocytes derived from Eμ-myc/p53+/− mice. (B) Representative immunoblots of splenocytes from Eμ-myc/p53+/− and Eμ-myc/vav-bcl-2 mice (n = 4 and n = 3, respectively). As expected splenocytes from Eμ-myc/vav-bcl-2 showed higher expression levels of BCL-2. Splenocytes from Eμ-myc/vav-bcl-2 mice displayed regular levels of p53, whereas splenocytes derived from Eμ-myc/p53+/− mice did not show any p53 protein. (C) Transplantation of splenocytes from Eμ-myc/vav-bcl-2 or Eμ-myc/p53+/− mice into C57BL/6 recipients. Mice receiving cells from Eμ-myc/vav-bcl-2 showed massively enhanced survival (Eμ-myc/53+/−, n = 16; Eμ-myc/vav-bcl-2 n = 19; PEμ-myc/p53+/− vs Eμ-myc/vav-bcl-2 < .001). (D) Survival analysis for serially transplanted Eμ-myc/p53+/− and Eμ-myc/vav-bcl-2 splenocytes. Cells from diseased primary mice that received a transplant were used for 1 subsequent transplantation. Serial transplantation did not result in altered survival time of the mice. (Eμ-myc/p53+/− prim, n = 10; Eμ-myc/vav-bcl-2 prim, n = 10; Eμ-myc/p53+/− sec, n = 8; Eμ-myc/vav-bcl-2 sec, n = 9; PEμ-myc/p53+/− prim vs Eμ-myc/p53+/−sec > .05; PEμ-myc/vav-bcl-2 prim vs Eμ-myc/vav-bcl-2 sec > .05.) Prim indicates primary transplantation; and sec, secondary transplantation.
p53-deficient lymphomas escape immunosurveillance
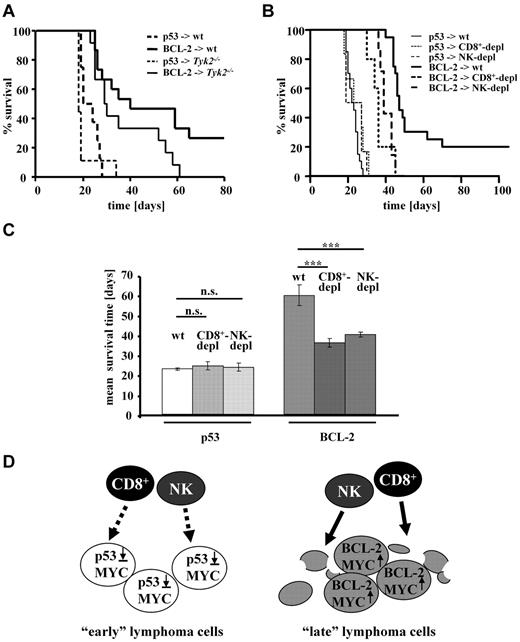
We first reasoned that BCL-2–expressing Eμ-myc tumors differed in certain cell intrinsic properties, such as proliferation or survival, from cells harboring disruptions in the p53 signaling pathway. However, all our attempts to confirm this concept and substantiate this assumption failed. The cells proliferated with comparable kinetics and similar rates of spontaneous apoptotic cells were detected in vitro in established cell lines but also in situ with the use of Ki67 staining on primary tumor sections (data not shown). Therefore, we reasoned that BCL-2–expressing tumors might be differentially recognized and eliminated by the immune system. To test this idea we made use of Tyk2−/− mice. Our recent work had shown that deletion of the Janus kinase TYK2 impairs tumor surveillance.28,36 Eμ-myc–derived cell lines were injected into wild-type or Tyk2−/− mice. As depicted in Figure 3A, injection of wild-type tumor cell lines harboring a disrupted p53 signaling pathway induced disease with a short and comparable latency in wild-type and Tyk2−/− mice (dashed lines). In contrast, tumor cell lines with an intact p53 pathway, but elevated BCL-2 expression, displayed significant differences in disease incidence and latency between wild-type and Tyk2−/− mice (solid lines). Although all Tyk2−/− mice developed lymphomas on transplantation of Eμ-myc/vav-bcl-2 cells, 25% of the wild-type recipients were capable to clear the tumor load and remained disease free for ≤ 4 months. Moreover, disease latency was prolonged in wild-type recipient mice compared with Tyk2−/− animals. Hence, TYK2-expressing mice, with proper immunologic functions, can limit the growth of BCL-2–expressing lymphomas, but they are unable to fight p53-deficient lymphomas. This observation suggests that the immune system suppresses Eμ-MYC–induced lymphomagenesis and that loss of p53 facilitates immune evasion. Hematologic malignancies may be eliminated by both cytotoxic CD8+ T cells and NK cells. Therefore, we next injected Eμ-myc/vav-bcl-2– and Eμ-myc/p53+/− –derived cells into C57BL/6 mice and impaired immune control with the use of CD8+ or NK cell–depleting Abs. The efficiency of our Ab-mediated depletion over this long period was monitored by FACS analysis (supplemental Figure 2). Our efforts are summarized in Figure 3B. Disease latency remained unchanged if we injected cells derived from Eμ-myc/p53+/− mice that are functionally p53 null, irrespective of the presence of immune cells. In contrast, the disease latency significantly decreased when we transplanted tumors with high BCL-2 expression paralleled by CD8+ or NK-cell depletion, respectively (Figure 3C-D; P < .001, in both cases). Hence, Eμ-MYC–induced tumors with elevated BCL-2 expression, but not tumors with disruptions in the p53 pathway, are subject to tumor surveillance by CD8+ and NK cells (Figure 3D). Therefore, early tumors emerge rapidly, at least in part, because p53 deficiency allows escaping from immunosurveillance.
p53-deficient lymphomas escape immunosurveillance. (A) Transplantation of tumor cell lines with indicated second hit, obtained from Eμ-myc mice, in C57BL/6 and Tyk2−/− recipient mice. The TYK2-associated immune defect led to reduced survival time of the recipient mice irrespectively of the second hit found in transplanted tumor cells. (C57BL/6 − p53, n = 8; and Tyk2 − p53, n = 9; C57BL/6 − BCL-2, n = 15; and Tyk2 − BCL-2, n = 12). Only 2 combinations meet the criterion of statistical significance (C57BL/6 − BCL-2 vs Tyk2 − BCL-2; P = .042). (B) Transplantation of Eμ-myc/vav-bcl-2 or Eμ-myc/p53+/− splenocytes into C57BL/6, CD8+, or NK cell–depleted recipient mice. Immune cell depletion does not alter the survival time of mice receiving splenocytes from Eμ-myc/p53+/− mice. In contrast NK-cell depletion as well as CD8+-cell depletion reduces the survival of recipient mice significantly (wt < BCL-2 vs CD8+ < BCL-2, P < .001; wt < BCL-2 vs NK < BCL-2, P < .001). Depl indicates depleted. (C) Mean survival of mice (C57BL/6 wt, CD8+-cell or NK-cell depleted) that received a transplant with Eμ-myc/vav-bcl-2 or Eμ-myc/p53+/− splenocytes. This figure is for better illustration of findings described in panel B. Asterisks indicate level of statistical significance (***P ≤ .001). (D) Schematic drawing of our working hypothesis. Tumor cells overexpressing BCL-2 are recognized or destroyed by the immune system more easily than the ones harboring defects in the p19-p53 signaling pathway.
p53-deficient lymphomas escape immunosurveillance. (A) Transplantation of tumor cell lines with indicated second hit, obtained from Eμ-myc mice, in C57BL/6 and Tyk2−/− recipient mice. The TYK2-associated immune defect led to reduced survival time of the recipient mice irrespectively of the second hit found in transplanted tumor cells. (C57BL/6 − p53, n = 8; and Tyk2 − p53, n = 9; C57BL/6 − BCL-2, n = 15; and Tyk2 − BCL-2, n = 12). Only 2 combinations meet the criterion of statistical significance (C57BL/6 − BCL-2 vs Tyk2 − BCL-2; P = .042). (B) Transplantation of Eμ-myc/vav-bcl-2 or Eμ-myc/p53+/− splenocytes into C57BL/6, CD8+, or NK cell–depleted recipient mice. Immune cell depletion does not alter the survival time of mice receiving splenocytes from Eμ-myc/p53+/− mice. In contrast NK-cell depletion as well as CD8+-cell depletion reduces the survival of recipient mice significantly (wt < BCL-2 vs CD8+ < BCL-2, P < .001; wt < BCL-2 vs NK < BCL-2, P < .001). Depl indicates depleted. (C) Mean survival of mice (C57BL/6 wt, CD8+-cell or NK-cell depleted) that received a transplant with Eμ-myc/vav-bcl-2 or Eμ-myc/p53+/− splenocytes. This figure is for better illustration of findings described in panel B. Asterisks indicate level of statistical significance (***P ≤ .001). (D) Schematic drawing of our working hypothesis. Tumor cells overexpressing BCL-2 are recognized or destroyed by the immune system more easily than the ones harboring defects in the p19-p53 signaling pathway.
BCL-2 enhances disease latency irrespective of p53-dependent signaling
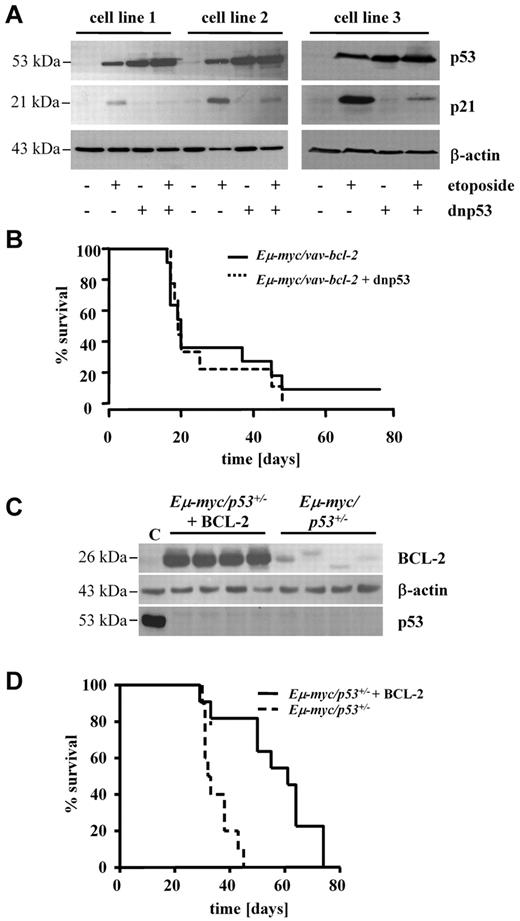
We next asked which of the second hits, BCL-2 overexpression or p53 disruption, is dominant to either induce or escape immunologic surveillance. To address this issue, we established individual clonal cell lines from Eμ-myc/vav-bcl-2 and Eμ-myc/p53+/− splenocytes. Subsequently, the Eμ-myc/vav-bcl-2 cells were infected with either a retrovirus encoding a dnp53 or the empty vector alone.29 The cell lines were then sorted to purity with the use of GFP expression and the efficient disruption of p53-dependent signaling was verified with etoposide treatment (Figure 4A). Three individually derived Eμ-myc/vav-bcl-2 cell lines in which p53 signaling was disrupted by overexpression of dnp53 and their maternal clones were chosen for transplantation into C57BL/6 recipient mice. Interestingly, no difference in disease latency was induced (Figure 4B). The reciprocal experiment was performed by overexpressing BCL-2 in Eμ-myc/p53+/−–derived cell lines, lacking functional p53. BCL-2 and p53 protein expression was verified by immunoblotting (Figure 4C). Again, 4 individually derived cell lines and their maternal counterparts were transplanted. In this experiment we obtained significant changes; the enforced expression of BCL-2 profoundly enhanced the median survival of the recipient mice (Figure 4D).
BCL-2 enhances disease latency irrespective of p53-dependent signaling. (A) Functional analysis of 3 cell lines obtained from Eμ-myc/vav-bcl-2 mice. Cell lines were infected with retrovirus expressing a dnp53. The expression in turn abrogates p21 up-regulation after the addition of etoposide to the cell culture medium for 24 hours. (B) Survival analysis of mice receiving cell lines from Eμ-myc/vav-bcl-2 mice with or without a dnp53. The expression of dnp53 does not alter the survival time (n = 10 mice of each cell type, 3 different cell lines of each genotype). (C) Expression of BCL-2 in cell lines obtained from Eμ-myc/p53+/− mice. Cell lines were infected with a retrovirus expressing BCL-2 and the empty vector (n = 4 each). The immunoblot shows the enhanced levels of BCL-2 in infected cell lines. p53 was not detected in any cell line derived from Eμ-myc/p53+/− mice. (C) The positive control for p53 derived from HEK 293 crude cell lysate. (D) Survival analysis of mice that received a transplant with Eμ-myc/p53+/− cell lines with or without overexpression of BCL-2. As depicted, overexpression of BCL-2 significantly increases the overall survival time (P = .0128; Eμ-myc/p53+/−, n = 10; Eμ-myc/p53+/− + BCL-2, n = 11).
BCL-2 enhances disease latency irrespective of p53-dependent signaling. (A) Functional analysis of 3 cell lines obtained from Eμ-myc/vav-bcl-2 mice. Cell lines were infected with retrovirus expressing a dnp53. The expression in turn abrogates p21 up-regulation after the addition of etoposide to the cell culture medium for 24 hours. (B) Survival analysis of mice receiving cell lines from Eμ-myc/vav-bcl-2 mice with or without a dnp53. The expression of dnp53 does not alter the survival time (n = 10 mice of each cell type, 3 different cell lines of each genotype). (C) Expression of BCL-2 in cell lines obtained from Eμ-myc/p53+/− mice. Cell lines were infected with a retrovirus expressing BCL-2 and the empty vector (n = 4 each). The immunoblot shows the enhanced levels of BCL-2 in infected cell lines. p53 was not detected in any cell line derived from Eμ-myc/p53+/− mice. (C) The positive control for p53 derived from HEK 293 crude cell lysate. (D) Survival analysis of mice that received a transplant with Eμ-myc/p53+/− cell lines with or without overexpression of BCL-2. As depicted, overexpression of BCL-2 significantly increases the overall survival time (P = .0128; Eμ-myc/p53+/−, n = 10; Eμ-myc/p53+/− + BCL-2, n = 11).
NK and T cell–mediated cytotoxicity is enhanced on BCL-2 expression in MYC-transformed cells
To get mechanistic insight why BCL-2 overexpression is associated with a prolonged survival in mice we first tested whether BCL-2 expression alters proliferation. Therefore, we performed [3H]-thymidine incorporation assays, cell cycle analysis, and CFSE-based proliferation assays. 7-AAD staining was used to analyze apoptotic cells (Figure 5A; supplemental Figure 3). The overall viability of the cells ranged between 85% and 95%; no changes were induced on expression of BCL-2 or dnp53 (supplemental Figure 3D). In summary we failed to detect significant changes in growth rates irrespective of the method used; neither BCL-2 expression nor the expression of dnp53 had an effect on proliferation. Moreover, we transplanted Eμ-myc/p53+/− (± BCL-2) cell lines in Rag2−/−γc−/− mice that are devoid of NK, NKT, B, and T cells. In these mice tumor surveillance is severely hampered, and differences in growth rate would manifest themselves as changes in disease latency. Again, we failed to observe any significant difference (Figure 5B). Because NK-cell and CD8+-cell depletion experiments showed a clear effect on disease latency, we next analyzed the expression levels of cell surface molecules known to mediate NK-cell cytotoxicity.37,38 Interestingly, the NKG2D ligands MULT1 and RAE1 were more abundantly expressed on the cell surface of BCL-2–overexpressing cells, whereas H2-Db surface expression levels were unaltered (Figure 5C). Accordingly, NK cell–mediated cytotoxicity was consistently enhanced when the tumor cells displayed high expression of BCL-2. This held true in all experimental settings tested (Figure 5D). In contrast, only minor alterations in NK cell–mediated killing were observed on expression of a dnp53 in Eμ-myc/vav-bcl-2 cells (Figure 5D right). The well-known NK-target cell line YAC-1, which lacks MHCI expression, was used as a positive control in all experimental settings.39
BCL-2 facilitates NK and T cell–mediated killing. (A) CFSE-based proliferation assay of Eμ-myc/p53+/− (± BCL-2) and Eμ-myc/vav-bcl-2 (± dnp53) cells. Eμ-myc/p53+/− (± BCL-2, n = 2 each) and Eμ-myc/vav-bcl-2 (± dnp53, n = 3 each) cell lines were subjected to FACS analysis, no difference in proliferation was detected (values represent mean MFI ± SD). 7-AAD+ dead cells were excluded; no differences between the different groups in 7-ADD+ cells were evident. (B) Transplantation of indicated cell lines into Rag2−/−cγ−/− mice. No statistically significant difference in survival time between the groups analyzed could be detected (Eμ-myc/p53+/−, n = 8; Eμ-myc/p53+/− + BCL-2, n = 8; P = .2). (C) FACS analysis of H2-Db (MHC class I), MULT1, and RAE1 in Eμ-myc/p53+/− (n = 4) and Eμ-myc/vav-bcl-2 (n = 3) cell lines. Eμ-myc/p53+/− cells expressed significantly less MULT1 than with Eμ-myc/vav-bcl-2 cells (P = .0494) and lower levels of RAE1 (P = .1356). (D) NK-cell cytotoxicity assay with the use of different Eμ-myc tumor cell lines as targets as indicated in the figure. Purified and in vitro–expanded NK cells were incubated at different E:T with CFSE-labeled Eμ-myc cells for 4 hours. The percentage of NK cell–specific tumor cell lysis is depicted. (E) T cell–mediated lysis of Eμ-myc tumor cells. CD8+ effectors were coincubated with CFSE-labeled tumor target cells for 20 hours in ascending E:Ts, which were calculated after FACS-based quantification of CD8+ cytotoxic T cells within the effector cell suspensions. The percentage of T cell–specific tumor cell lysis is depicted.
BCL-2 facilitates NK and T cell–mediated killing. (A) CFSE-based proliferation assay of Eμ-myc/p53+/− (± BCL-2) and Eμ-myc/vav-bcl-2 (± dnp53) cells. Eμ-myc/p53+/− (± BCL-2, n = 2 each) and Eμ-myc/vav-bcl-2 (± dnp53, n = 3 each) cell lines were subjected to FACS analysis, no difference in proliferation was detected (values represent mean MFI ± SD). 7-AAD+ dead cells were excluded; no differences between the different groups in 7-ADD+ cells were evident. (B) Transplantation of indicated cell lines into Rag2−/−cγ−/− mice. No statistically significant difference in survival time between the groups analyzed could be detected (Eμ-myc/p53+/−, n = 8; Eμ-myc/p53+/− + BCL-2, n = 8; P = .2). (C) FACS analysis of H2-Db (MHC class I), MULT1, and RAE1 in Eμ-myc/p53+/− (n = 4) and Eμ-myc/vav-bcl-2 (n = 3) cell lines. Eμ-myc/p53+/− cells expressed significantly less MULT1 than with Eμ-myc/vav-bcl-2 cells (P = .0494) and lower levels of RAE1 (P = .1356). (D) NK-cell cytotoxicity assay with the use of different Eμ-myc tumor cell lines as targets as indicated in the figure. Purified and in vitro–expanded NK cells were incubated at different E:T with CFSE-labeled Eμ-myc cells for 4 hours. The percentage of NK cell–specific tumor cell lysis is depicted. (E) T cell–mediated lysis of Eμ-myc tumor cells. CD8+ effectors were coincubated with CFSE-labeled tumor target cells for 20 hours in ascending E:Ts, which were calculated after FACS-based quantification of CD8+ cytotoxic T cells within the effector cell suspensions. The percentage of T cell–specific tumor cell lysis is depicted.
To investigate the effect of T cells, we performed in vitro T-cell cytotoxicity assays. Although no specific killing over the baseline was observed against Eμ-myc/p53+/− tumor targets, Eμ-myc/vav-bcl-2 tumor targets were efficiently recognized and lysed by cytotoxic T lymphocytes (Figure 5E).
Patients with Burkitt lymphoma and with DLBCL with deregulated MYC but high BCL-2 expression show increased disease latency
Our study in mice strongly suggests that c-MYC–driven lymphoma is subject to NK and T cell–mediated immunosurveillance, given that an elevated BCL-2 level represents the cooperating oncogenic event. Unfortunately, there is no perfect human match to this system, so we have relied to test the significance of our findings for human disease in MYC-induced lymphomas, including Burkitt lymphomas and DLBCLs. We analyzed the survival of 59 patients, including 29 cases of Burkitt lymphoma and 30 cases of DLBCL, with MYC translocation. We first performed FISH analysis to test for TP53 disruptions or the t(14;18) BCL-2 translocation. The t(14;18) translocations were confirmed by PCR analysis. These analyses were complemented by immunohistologic staining for BCL-2 and p53. By this technique we do detect cases of mutated TP53 that is usually associated with high p53 expression. Typical examples are depicted in Figure 6A. Tumors that depicted an intense p53 staining were further confirmed by TP53 sequencing, given that enough material was available (data not shown). According to this information the patients were allocated to different groups. The first group included all patients with tumors that showed neither abnormality for p53 nor elevated BCL-2 levels (n = 31). For most of these patients, pathologists diagnosed their condition as classic Burkitt lymphoma. The second group comprised persons that displayed tumors that either lacked p53 or showed signs of a mutated p53, indicated by a high p53 protein level (n = 14). The third group did not show any signs of a disturbed p53 signaling pathway but expressed high BCL-2 levels because of t(14;18) (n = 10). In groups 2 and 3 patients prevailed whose condition had been initially diagnosed as DLBCL by the pathologist. In 4 patients both alterations were detectable, a disrupted p53 signaling as well as elevated BCL-2 levels. All 4 patients' conditions had been diagnosed with lymphomas that had been classified as DLBCLs. Statistical analysis showed that patients with lymphoma with a disrupted p53 pathway had a significantly worse prognosis than did patients with tumors that showed neither abnormality for p53 nor elevated BCL-2 levels (P = .0104). All other comparisons did not reach the level of statistical significance, although a trend is evident. Figure 6B summarized the survival of these patients. A similar picture was obtained when we compared the survival of patients with DLBCL only (supplemental Figure 4). Interestingly, the 4 patients harboring both p53 loss and t(14;18) belong in this group. None of these patients died during the observation period of the study.
Patients with Burkitt lymphoma and DLBCL with deregulated MYC but high BCL-2 expression show increased disease latency. (A) IHC staining of tumors from patients with DLBCL with MYC translocations. One representative example of a TP53 mutation (patient #1; n = 11) and one example of a tumor with a BCL-2 overexpression because of t(14;18) translocation (patient #2; n = 10) is shown. (B) Kaplan-Meier plot; 4 different groups are shown: (1) patients showing wild-type p53 and regular BCL-2 expression [lack of t(14;18), n = 31; “no t(14;18); no p53 signaling disrupted”], (2) patients showing TP53 mutation or deletion alone (n = 14, “p53 signaling disrupted”), (3) patients showing high BCL-2 expression because of t(14;18) alone [n = 10 “t(14;18”)], and (4) patients showing both second hits [“t(14,18) and p53 signaling disrupted,” n = 4]. Patients with both second hits display an evident trend for an increase in survival time compared with patients with disrupted p53 signaling pathway alone. (P = .152).
Patients with Burkitt lymphoma and DLBCL with deregulated MYC but high BCL-2 expression show increased disease latency. (A) IHC staining of tumors from patients with DLBCL with MYC translocations. One representative example of a TP53 mutation (patient #1; n = 11) and one example of a tumor with a BCL-2 overexpression because of t(14;18) translocation (patient #2; n = 10) is shown. (B) Kaplan-Meier plot; 4 different groups are shown: (1) patients showing wild-type p53 and regular BCL-2 expression [lack of t(14;18), n = 31; “no t(14;18); no p53 signaling disrupted”], (2) patients showing TP53 mutation or deletion alone (n = 14, “p53 signaling disrupted”), (3) patients showing high BCL-2 expression because of t(14;18) alone [n = 10 “t(14;18”)], and (4) patients showing both second hits [“t(14,18) and p53 signaling disrupted,” n = 4]. Patients with both second hits display an evident trend for an increase in survival time compared with patients with disrupted p53 signaling pathway alone. (P = .152).
Discussion
In this study we investigated the effect of the nature of the second hit for c-MYC–driven tumorigenesis in the well-characterized Eμ-myc–induced Burkitt lymphoma model. To the best of our knowledge this is the first report that documents a link between the nature of the second hit and the immunologic visibility of Eμ-myc–driven lymphomas. We are aware that our study cannot accommodate the complex biology of MYC-driven lymphomas. Nevertheless, our data provide the first insights on the mechanisms underlying tumor surveillance in this disease entity. We found that Eμ-myc–induced lymphomas harboring a disrupted p53 signaling pathway progressed rapidly and were not subject to immune surveillance. In contrast, the fate of lymphomas endowed with excessive levels of BCL-2 was contingent on the host immune system. Several lines of evidence support this conclusion. (1) We did not observe any change in disease latency irrespective of whether we injected p53-disrupted lymphoma cells in wild-type or immunocompromised Tyk2−/− animals. (2) The depletion of CD8+ or NK cells with the use of mAbs did not affect tumor formation of p53-disrupted lymphomas. (3) In contrast, under the same experimental conditions, clear-cut and significant differences occurred when we studied BCL-2–overexpressing Eμ-myc–induced lymphomas. (4) The effect of high BCL-2 levels was phenotypically dominant: it prevailed in a p53-negative background. (5) The dominant effect of high BCL-2 levels depended on the presence of functional lymphoid cells because it disappeared on transplantation in Rag2−/− γc−/− animals. (6) BCL-2–overexpressing cells were efficiently lysed by NK and T cells in in vitro cytotoxicity assays.
There are, in principle, 2 possible explanations for the difference between loss of p53 and BCL-2 overexpression: (1) a cell-autonomous effect of these second hits and (2) an effect mediated by the immune system or the microenvironment of the tumor. The former explanation appeared intuitively more appealing. It was, for instance, conceivable that immature pre-B cells are more susceptible to mutations in the p53 signaling pathway because they have intrinsically low BCL-2 levels.40 Thus, transcriptional repression may preclude the emergence of BCL-2 overexpression at this early differentiation stage. However, there are several lines of evidence that argue against any major contribution of cell autonomous effects. First, in both Eμ-myc/vav-bcl-2 and Eμ-myc/p53+/− mice, the tumor cells emerged at the pre-B-cell stage. The tumor cells displayed a comparable profile of surface markers; that is, they only expressed CD19, CD43 but lacked IgM and IgD. Despite the similar differentiation state, the disease latency was consistently and significantly higher on transplantation of BCL-2–expressing lymphoma cells than in lymphomas with a disrupted p53 signaling network. The delayed emergence of Eμ-myc/vav-bcl-2 was consistently observed, irrespective of the experimental conditions used. The effect was repeatable also after secondary transplantation. Finally, we failed to detect any correlation between altered disease latency and a cell autonomous difference in growth, cell death, or directed migration. Individual cell lines differed modestly in their rate of proliferation and of apoptosis and in their homing, and these variations were not related to the nature of the second hit. Previous studies in Eμ-myc mice also did not observe that tumors of the pre-B lineage give rise to tumors earlier than IgM+ tumors.21,41 In contrast, manipulations in the level of immunosurveillance showed a clear-cut difference, and this interpretation is based on 3 lines of evidence. (1) Loss of TYK2 relaxes immunosurveillance as has been shown for several tumor models.28 We exploited this model to explore the contribution of the immune system to the different fate of p53-deficient and BCL-2–overexpressing tumor cells. (2) Consistent with the findings in TYK2-deficient animals we also observed that depletion of CD8+ T or NK cells accelerated the growth of transplanted tumors that overexpress BCL-2 but not of those lacking p53. (3) Last but not least, forced expression of BCL-2 in p53-deficient lymphoma cells restored susceptibility to immunosurveillance and thus delayed disease onset. The increase in disease latency critically depended on the presence of functional lymphoid cells, because it was not observed on injection into Rag2−/− γc−/− mice. (4) In vitro cytotoxicity assays confirmed that BCL-2–expressing cells are lysed at a higher rate by NK and T cells.
Our observations obtained in the Eμ-myc mouse model predicted that overexpression of BCL-2 and loss of p53 ought to result in different outcomes. The available literature on c-MYC–driven lymphoma in people only analyzes the effect of the absence or presence of either BCL-2 or p53 on disease progression: It is clear that patients whose tumor cells have neither incurred a loss of p53 nor acquired high levels of BCL-2 have a better prognosis and, unsurprisingly, respond better to chemotherapy.42-45 Unfortunately, these analyses do not provide any accessible information that allow for comparisons of loss of p53 versus BCL-2 overexpression. We therefore initiated a retrospective analysis of clinical data obtained from 59 patients who were admitted to the General Hospital of Vienna during the time period of 1999-2010 and whose condition was diagnosed as either Burkitt lymphoma or DLBCL with MYC translocation. If we grouped the patients according to their second hit, a more favorable outcome was seen in patients with BCL-2–expressing lymphomas than in patients with lymphomas with p53 mutations. Admittedly, because of low number of patients, the difference was not statistically significant. However, a trend is evident, and it is consistent with the animal experiments. We are aware that the interpretation of these data is not easy and that several confounders have to be considered. First of all, the Eμ-myc mouse model does not correspond to lymphoma evolution in people; it is neither a perfect model for studying Burkitt lymphoma nor DLBCL. In addition, all patients included into our analysis had been treated with standard chemotherapy. Any classic chemotherapy will per se interfere with the immune system. This limits the validity of any conclusions that can be drawn. In addition, some chemotherapeutics rely on the p53 pathway to induce apoptosis. Finally, we also cannot exclude additional genetic alterations occurring in individual tumors. Despite the limitations of the dataset, which force us to be precautious and not to overinterpret things, we consider this information important. This data provide the first evidence that a comparable scenario as defined in the mouse model may exist in humans. It is of particular interest that in 4 patients with DLBCL harboring the presence of both hits seems to have an improved survival compared with the patients harboring TP53 mutations only. This observation provides a first glimpse on a potentially powerful mechanism shaping tumor surveillance. The biology of c-MYC–driven lymphoid malignancies is complex; we are currently unable to predict in which disease entities the mechanisms that we described here become operative. Thus, our analysis calls for additional in-depth studies.
Furthermore, these findings in patients are in line with the conclusion obtained in mice that BCL-2 is dominant in certain settings. Although the introduction of a dnp53 variant did not interfere with disease progression and disease latency, BCL-2 expression was capable to extend it. At the very least, these data have 2 implications: (1) the expression of BCL-2 appears to define the effectiveness of tumor immunosurveillance, and (2) the genetic instability conferred to a cell by the loss of functional p53 does not per se promote escape from immune control. The forced expression of BCL-2 in tumor cells lacking functional p53 sufficed to restore an immunologic response. Vice versa, a dnp53 did not interfere with tumor surveillance because of high levels of BCL-2. One mechanism, how BCL-2 enhances tumor cell recognition, is via NKG2D ligand expression.46,47 Both, MULT1 and RAE1 were found to be up-regulated in cells that express high levels of BCL-2. The significance of NKG2D-mediated tumor surveillance is beyond doubt. However, it remains to be determined how BCL-2, which per se does not exert any activity as transcription factor, causes the up-regulation of these ligands.
Obviously, the findings have repercussions on the general concept of cancer immune therapy. If the nature of the second hit determines tumor immunogenicity, a prudent design of clinical trials ought to take this insight into account, because only those patients are likely to benefit who have the appropriate second hit. At the current stage, we only provide formal proof that the second hit is important for immunosurveillance of murine lymphoma. We have, however, good reasons to suspect that this is a more general phenomenon, because BCL-2 is overexpressed in many tumors and because immunosurveillance is required to clear minimal residual disease in cancers other than Burkitt lymphoma or their murine equivalent. Moreover, during the past years several small molecule inhibitors have been designed to target members of the BCL-2 family; these have undergone successful initial tests in clinical trials.48,49 It is currently unclear which features of BCL-2 are required to induce an immune response. Nevertheless, the possibility that these substances may impair the immunogenicity of tumors must be taken into consideration. This is in particular true, if these inhibitors are to be combined with immune-based therapy approaches.50
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank U. Losert and the staff of the Biomedical Research Institute (MUV) as well as G. Schöppl and the mouse facility of the Institute of Pharmacology (MUV) for taking excellent care of the mice. They thank M. Busslinger for providing mice. They also thank J. Adams R. Moriggl, R. Eferl, D. Kerjaschki, T. Decker, M. Karaghiosoff, and C. Schellack for helpful discussions. They thank M. Schlederer, I. Toeplitz, and B. Rüger for advice and help with histopathology.
This work was supported by the Austrian National Bank (grant 13342, C.S.); WWTF LS07-037 (V.S.); the Austrian Science Fund (FWF; SFB F28, V.S. and M.M.), grant 19534 (D.S.); START Y212-B13 (A.V.); the AICR (grant 06-440, A.V.); and the Austrian Federal Ministry of Science and Research (grant BM_WFa GZ200.112/1-VI/1/2004, GZ BMWF-200.191/1-II/1a/2008, M.M. and BM_WFa program GEN-AU II and III project Austromouse, M.M., DRAGON and Placebo GZ BMWF-70.081/0018-II/1a/2008, V.S.); Austrian Academy of Sciences DOC-FFORTE fellowship (E.M.P.); and FWF (P19346-B12, B.S.).
Authorship
Contribution: C.S., M.A.H., A.B., E.M.P., A.F., O.S., N.M., A.H., B.K., E.P., B.S., and A.-I.S. performed research; V.S., C.S., D.S., U.J., A.V., L.M., and M.M. designed research; C.S., A.H., U.J., M.F., A.V., and L.M. evaluated data and performed statistics; and V.S. and C.S. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for C.S. is Division of Signal Transduction and Growth Control, German Cancer Research Center, Heidelberg, Germany. The current affiliation for M.A.H. is Department of Biosciences and Nutrition, Karolinska Institutet, Huddinge, Sweden. The current affiliation for O.S. is Immunology Frontier Research Center, Osaka University, Osaka, Japan. The current affiliation for A.F. is Department of Mocrobiology, Tumor and Cell Biology, Karolinska Institutet, Huddinge, Sweden
Correspondence: Veronika Sexl, Institute of Pharmacology and Toxicology, Veterinary University of Vienna (VetmedUni), Veterinaerplatz 1, A-1210 Vienna, Austria; e-mail: veronika.sexl@vetmeduni.ac.at.
References
Author notes
C.S. and A.B. contributed equally to this study.
D.S. and V.S. contributed equally to this study.

![Figure 6. Patients with Burkitt lymphoma and DLBCL with deregulated MYC but high BCL-2 expression show increased disease latency. (A) IHC staining of tumors from patients with DLBCL with MYC translocations. One representative example of a TP53 mutation (patient #1; n = 11) and one example of a tumor with a BCL-2 overexpression because of t(14;18) translocation (patient #2; n = 10) is shown. (B) Kaplan-Meier plot; 4 different groups are shown: (1) patients showing wild-type p53 and regular BCL-2 expression [lack of t(14;18), n = 31; “no t(14;18); no p53 signaling disrupted”], (2) patients showing TP53 mutation or deletion alone (n = 14, “p53 signaling disrupted”), (3) patients showing high BCL-2 expression because of t(14;18) alone [n = 10 “t(14;18”)], and (4) patients showing both second hits [“t(14,18) and p53 signaling disrupted,” n = 4]. Patients with both second hits display an evident trend for an increase in survival time compared with patients with disrupted p53 signaling pathway alone. (P = .152).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/118/17/10.1182_blood-2010-10-313098/4/m_zh89991180210006.jpeg?Expires=1767708458&Signature=yNVaDRIieMztQyYTkkLxYOwC5xIHmSRPlUy1~vfVK8htfIHiy-NWh2YnQJVvWcTsItL3u83ISBkxfpozGHoGdxSezaX7x9T6o4OaFjxk9eC7Crc1ONcD65nwRjfDncOO2sCsZKU2N8OqjmpIRFaw6oz7hSVsPO5yjLX6cZd7TpHGtgLyd2BbEV6A8AW4PlnuQLN6cmfMnf-kEMkbyi655gUYl2nrMkRkfBhnllmnfSGG9A~5AYTqM2HS-dmvjrL6aPUsGzBAaSeB71rluyky9J1GD2tZrYuLzGu5lOnZLg6LQG1THkKpHgUE4UnpogmKpAEswPzigcrN4Bn8tnFW3w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal