Abstract
Previous studies from our group have demonstrated that bone morphogenetic protein receptor-II (BMPR-II), expressed on pulmonary artery endothelial cells, imparts profound anti-inflammatory effects by regulating the release of proinflammatory cytokines and promoting barrier function by suppressing the transmigration of leukocytes into the pulmonary vessel wall. Here we demonstrate that, in mice with endothelial-specific loss of BMPR-II expression (L1Cre(+);Bmpr2f/f), reduction in barrier function and the resultant pulmonary hypertension observed in vivo are the result of increased leukocyte recruitment through increased CXCR1/2 signaling. Loss of endothelial expressed BMPR-II leads to elevated plasma levels of a wide range of soluble mediators important in regulating leukocyte migration and extravasation, including the CXCR1/2 ligand, KC. Treatment of L1Cre(+);Bmpr2f/f mice with the CXCR1/2 antagonist SCH527123 inhibits leukocyte transmigration into lung and subsequently reverses the pulmonary hypertension. Our data have uncovered a previously unrecognized regulatory function of BMPR-II, which acts to regulate the expression of CXCR2 on endothelial cells, suggesting that increased CXCR2 signaling may also be a feature of the human pathology and that CXCR1/2 pathway antagonists may represent a novel therapeutic approach for treating pulmonary hypertension because of defects in BMPR-II expression.
Introduction
Intensive research efforts have helped explain how germline mutations in the bone morphogenetic protein receptor-2 (BMPR-II) gene can lead to the development of pulmonary arterial hypertension (PAH). These studies have shown that BMPR-II exerts cell-specific effects in the pulmonary vasculature and that loss of expression and function can directly lead to pulmonary artery vasoconstriction and remodeling, luminal occlusion, and right ventricular hypertrophy and failure.1 It is now widely appreciated that inflammation and immune mechanisms play a key role in the initiation and progression of the disease by actively contributing to chronic vasoconstriction, remodeling of the pulmonary vessel wall, and thrombosis.1 However, our knowledge of how these inflammatory processes may contribute to the pathogenesis and progression of heritable PAH is still limited.
Inflammatory cells, such as lymphocytes and monocytes, are increased in and around plexiform lesions of hypertensive pulmonary vessels.2 In addition, elevated circulating levels of proinflammatory cytokines, such as IL-8 (CXCL8), monocyte chemoattractant protein-1 (MCP-1; CCL2), macrophage inflammatory protein-1α (MIP-1α; CCL3), IL-1β, and IL-6 are observed in PAH patients3-7 and in experimental models of PAH.8,9 However, the contribution of inflammatory cells and inflammatory mediators to the vascular remodeling and development of PAH in patients is still unclear. The role of inflammatory cytokines in the initiation and progression of PAH has been considered in animal models of PAH, whereby blockade of the MCP-1 signaling pathway using anti-MCP-1 gene therapy was shown to attenuate the progression of PAH induced by monocrotoline.9 Similarly, Voelkel et al showed that treatment of monocrotoline-treated rats with repeated injection of an IL-1 receptor antagonist resulted in reduced PH and right heart hypertrophy.8 These studies support a role for inflammatory cytokines in the development of PAH.
Genetic studies have linked germline mutations in the gene encoding the bone morphogenetic protein receptor II (BMPR-II) to underlie the development of heritable forms of PAH.10 BMPR-II protein expression is also significantly reduced in the lungs of iPAH patients, in whom no mutation in the BMPR-II gene was identified, suggesting that a reduction of BMPR-II function may be important in the pathogenesis of PAH, irrespective of whether there is a mutation in the gene.11
In PAH, it has been suggested that loss of endothelial integrity may result in the abnormal exposure of underlying smooth muscle cells to proliferative mediators resulting in their uncontrolled proliferation. Many authors have suggested that abnormal BMP signaling could lead to an alteration in the permeability of the endothelial cells within the vascular wall,12-14 which could in turn be responsible for the inappropriate leukocyte infiltration observed in PAH patients. The CXCL8 receptor CXCR2 has a well-described role in mediating leukocyte degranulation, phagocytosis, and transmigration.15 CXCR2 on endothelial cells has been shown to regulate pulmonary vascular permeability whereby leukocyte recruitment and vascular permeability were reduced in CXCR2-deficient mice in a model of acute lung injury.16 Since this report, a number of studies have supported a role for CXCR2 in pulmonary diseases, including asthma, chronic obstructive pulmonary disease, and cystic fibrosis17 ; however, no studies to date have considered a possible role for CXCR2 in PAH.
Using a murine model lacking Bmpr-II expression in endothelial cells, we have previously shown a role for endothelial Bmpr-II in maintaining pulmonary vascular integrity and dampening inflammatory signals.13 In addition, a reduction of BMPR-II in human pulmonary artery endothelial cells (HPAECs) facilitated leukocyte transmigration in a CXCR1/2-dependent manner. Here we demonstrate that pharmacologic inhibition of CXCR1/2 inhibits vascular leakage and leukocyte infiltration in mice with endothelial-specific loss of Bmpr-II, which spontaneously develop PAH. Importantly, we demonstrate that inhibition of leukocyte recruitment by inhibition of CXCR1/2 attenuates the progression of PAH and reverses established pathology. Taken together, our results suggest a key role for CXCR1/2 in the progression of experimental PAH and that pharmacologic intervention of CXCR1/2 function may provide therapeutic benefit for the treatment of BMPR-II–associated PAH.
Methods
Endothelial cell culture
HPAECs were obtained from Promocell and maintained as previously described.13 Each experiment used HPAECs isolated from a different donor.
Transduction of HPAECs with gene silencing BMPR-II shRNA
For BMPR-II silencing, HPAECs were transduced with lentiviral particles encoding 3 different shRNAs targeting BMPR-II (BMPR-IIsh; SMARTvector 2.0 lentiviral particles, Dharmacon, Thermo Fisher Scientific), scrambled nontargeting control shRNA (NTCsh), or empty vector (EV) particles. Optimal cell density and multiplicity of infection for transduction were determined in-house according to the manufacturer's suggestions. The day before transduction, HPAECs were seeded into 6-well plates at 4 × 105 cells per well in complete growth medium. Lentiviral shRNA particles were diluted in complete growth medium to give a multiplicity of infection of 20 and added to each well. Twenty-four hours after transduction, puromycin (3 ng/mL) was added to the complete growth medium for enrichment of transduced cells. Efficiency of gene silencing was determined 72 hours after transduction by quantitative RT-PCR and Western blot (see “Western blotting”).
Quantitative RT-PCR
See supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Western blotting
CXCR2 expression in HPAECs transduced with NTCsh, BMPR-IIsh, or an EV was assessed by Western blot as previously described.13 Blots were probed with murine anti-CXCR2 antibody (Abcam) overnight at 4°C and then incubated with an HRP-conjugated goat anti–mouse secondary antibody (Zymed) for 2 hours at room temperature. Bands were visualized using chemiluminescence (GE Healthcare Life Sciences). All blots were reprobed with anti–human GAPDH (Sigma-Aldrich) to confirm equal loading.
Measurement of TEER
Endothelial integrity was measured using the electric cell-substrate impedance sensing system (Applied Biophysics). Wells containing 40 gold electrodes (8W10E+, Wolf Laboratories) were coated for 1 hour with fibronectin after which HPAECs (16 000 cells/well), previously transduced with shRNA targeting BMPR-II, NTCsh, or an EV, were dissociated from flasks using trypsin/EDTA (Invitrogen), then seeded into each well and cultured for 24 hours in complete endothelial growth medium. Capacitance as a measure of cell confluence and transendothelial electrical resistance (TEER) were measured over a 2-hour period; then vehicle (1:1 solution of polyethylene glycol [PEG] 200/saline) or the well-characterized CXCR1/2 antagonist SCH527123 (10nM)18,19 was added to the wells and capacitance and TEER measured for a further 2 hours.
Animals
All animal procedures were conducted in accordance with the British Home Office regulations (Scientific Procedures) Act 1986, United Kingdom.
L1Cre(+);Bmpr2f/f and L1Cre(−);Bmpr2f/f mice, which spontaneously develop PAH at 5 months, were obtained as a gift from Dr S. Paul Oh (University of Florida, Gainesville, FL) and generated as previously described.13,20
L1Cre(−);Bmpr2f/f and L1Cre(+);Bmpr2f/f mice were dosed orally, twice daily with 10 mg/kg (20 mg/kg per day) SCH527123, and suspended in 1:1 solution of polyethylene glycol (PEG) 200/saline or with PEG200/saline (vehicle) alone for 21 days. On day 22, mice were injected intravenously with Evans blue (30 mg/kg, Sigma-Aldrich) for 1 hour and then anesthetized; echocardiographic and surgical assessment of hemodynamics was carried out (see “Echocardiographic assessment”), after which blood was collected into EDTA-coated tubes for future cytokine analysis (see “Echocardiographic assessment”). Lungs were perfused free of blood via the right ventricle (RV) and then excised from the animals, and the right lobe was snap frozen for estimation of Evans blue leakage and myeloperoxidase (MPO) determination (see “Echocardiographic assessment”). The left lobe was inflated with 10% (volume/volume) neutral buffered formalin for immunohistologic examination.
Echocardiographic assessment
Echocardiographic assessments were performed by ultrasound on anesthetized L1Cre(−);Bmpr2f/f and L1Cre(+);Bmpr2f/f mice. The pediatric probe was adjusted to ∼ 400 images per second and placed in a parasternal short axis position to visualize the aorta. The flow through the aorta was determined and the diameter of the aorta measured in 3 areas to calculate cardiac output. Cardiac output was calculated by multiplying the stroke volume by heart rate and dividing by 1000. Analysis was performed using EchoPAC dimension software (GE Healthcare Version 6).
Surgical assessment of PAH pathology
The jugular vein was surgically exposed, and blood flow was isolated with a distal ligature. A small hole was made in the vessel, and a 2-F Millar pressure/volume catheter introduced and progressed into the RV, where an average RV pressure was measured during systole.
Measurement of Evans blue leakage and MPO
Accumulation of Evans blue dye as a measure of vascular leakage and concentration of MPO in the right lung of L1Cre(−)Bmpr2f/f or L1Cre(+)Bmpr2f/f mice was assessed as previously described.13
Immunohistochemistry
See supplemental Methods.
Image analysis
See supplemental Methods.
Measurement of proinflammatory cytokines
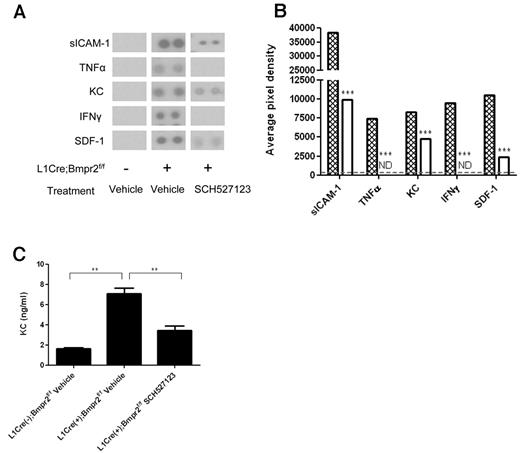
Blood from L1Cre(−);Bmpr2f/f and L1Cre(+);Bmpr2f/f mice was collected into EDTA coated tubes. Plasma was collected by centrifugation of whole blood at 3000g for 3 minutes. Plasma from each group of mice was pooled, and levels of soluble intercellular adhesion moelcule-1 (sICAM-1), TNFα, CXCL1 (KC), IFNγ, and stromal-derived growth factor (SDF-1;CXCL12) were profiled using commercially available sandwich ELISA according to the manufacturer's instructions (Proteome Profiler Array ARY006; R&D Systems). The average pixel density from each pair of duplicate spots representing each cytokine was determined by subtracting the background pixel density from the total pixel density using Image-Pro Plus Version 6.3 (Datacell). KC levels in pooled plasma from each group of mice were quantified by commercial ELISA as described by the manufacturer (R&D Systems).
Statistical analysis
Data analysis was performed using the statistical software GraphPad Prism Version 5 (GraphPad Software). Data are the mean ± SEM for a minimum 8 mice per treatment group or 3 independent experiments using a different HPAEC donor for in vitro studies. For in vitro studies, statistical significance was assessed by comparison of treatment groups by 1-way ANOVA with Tukey test. For in vivo studies, comparison of each group was assessed by 1-way ANOVA with Dunnett test. P < .05 was considered significant.
Results
Pharmacologic intervention of CXCR1/2 reduces pulmonary vascular leakage and leukocyte infiltration in vivo
We previously found that loss of endothelial BMPR-II in vitro and in vivo resulted in loss of barrier function as evidenced by increased pulmonary vascular leakage and increased leukocyte transmigration. In vitro this increased leukocyte transmigration could be ablated by pharmacologic inhibition of CXCR1/2. Here we sought to investigate whether vascular leakage and leukocyte infiltration in vivo were also CXCR1/2-dependent.
Using the CXCR1/2 antagonist SCH527123, we investigated the effect of CXCR1/2 blockade on pulmonary vascular leakage by measuring the accumulation of Evans blue in the right lung of L1Cre(+)Bmpr2f/f mice treated with 20 mg/kg per day SCH527123 or vehicle. Leakage of Evans blue was significantly increased in L1Cre(+)Bmpr2f/f mice compared with L1Cre(−)Bmpr2f/f mice (Figure 1A); however, this was dramatically reduced after treatment with the CXCR1/2 antagonist SCH527123. To investigate the impact of SCH527123 on leukocyte infiltration into the lungs of L1Cre(+)Bmpr2f/f mice, we measured MPO activity in lung homogenates from these animals.20 Here we found that leukocyte infiltration into the lungs of L1Cre(+)Bmpr2f/f mice was significantly increased compared with L1Cre(−)Bmpr2f/f mice (Figure 1B), which could be greatly reduced on treatment with SCH527123, suggesting that CXCR1/2 may play a role in mediating vascular permeability and leukocyte infiltration in this model.
Assessment of vascular leakage and leukocyte infiltration in endothelial restricted Bmpr-II–deficient mice with PAH. To assess vascular leakage (A), L1Cre(−)Bmpr2f/f mice or L1Cre(+)Bmpr2f/f mice were treated with SCH527123 or vehicle orally twice daily for 21 days. Evans blue dye was then administered intravenously via the tail vein for 1 hour, after which the lungs were flushed with PBS and the heart/lung block excised. Evans blue was extracted from the right lung, quantified, and expressed as micrograms per milliliter Evans blue per right lung. To assess leukocyte infiltration (B), the concentration of MPO in the right lung of L1Cre(−)Bmpr2f/f and L1Cre(+)Bmpr2f/f mice treated with vehicle or SCH527123 was determined by ELISA. Leukocyte infiltration was further assessed by observing leukocyte infiltration (arrows) (Ci) into the small pulmonary vessels by histologic sections stained with H&E and scoring the number and location of infiltrated leukocytes (Cii). Identification of specific leukocyte subsets was assessed in lung sections stained with specific antibodies targeting monocyte/macrophages, T cells, B cells, and neutrophils (Ciii). Data are the mean ± SEM for minimum 8 animals per group. (A) ANOVA showed a significant effect of treatment with SCH527123 and genotype (P < .001). ***P < .001, compared with L1Cre(+)Bmpr2f/f animals treated with vehicle by Dunnett test. (B) ANOVA showed a significant effect of treatment with SCH527123 and genotype (P < .001). ***P < .001, compared with L1Cre(+)Bmpr2f/f animals treated with vehicle by Dunnett test. Slides were mounted using VECTORSHIELD (Vector Laboratories) and viewed using a 20× objective lens. Images were captured using a DMLB microscope and digital camera. Digital image acquisition was performed using IM50 Version 4.0 imaging sofware (Leica Microsystems) and digital image slide viewing using Image Scope software (Aperio Technologies).
Assessment of vascular leakage and leukocyte infiltration in endothelial restricted Bmpr-II–deficient mice with PAH. To assess vascular leakage (A), L1Cre(−)Bmpr2f/f mice or L1Cre(+)Bmpr2f/f mice were treated with SCH527123 or vehicle orally twice daily for 21 days. Evans blue dye was then administered intravenously via the tail vein for 1 hour, after which the lungs were flushed with PBS and the heart/lung block excised. Evans blue was extracted from the right lung, quantified, and expressed as micrograms per milliliter Evans blue per right lung. To assess leukocyte infiltration (B), the concentration of MPO in the right lung of L1Cre(−)Bmpr2f/f and L1Cre(+)Bmpr2f/f mice treated with vehicle or SCH527123 was determined by ELISA. Leukocyte infiltration was further assessed by observing leukocyte infiltration (arrows) (Ci) into the small pulmonary vessels by histologic sections stained with H&E and scoring the number and location of infiltrated leukocytes (Cii). Identification of specific leukocyte subsets was assessed in lung sections stained with specific antibodies targeting monocyte/macrophages, T cells, B cells, and neutrophils (Ciii). Data are the mean ± SEM for minimum 8 animals per group. (A) ANOVA showed a significant effect of treatment with SCH527123 and genotype (P < .001). ***P < .001, compared with L1Cre(+)Bmpr2f/f animals treated with vehicle by Dunnett test. (B) ANOVA showed a significant effect of treatment with SCH527123 and genotype (P < .001). ***P < .001, compared with L1Cre(+)Bmpr2f/f animals treated with vehicle by Dunnett test. Slides were mounted using VECTORSHIELD (Vector Laboratories) and viewed using a 20× objective lens. Images were captured using a DMLB microscope and digital camera. Digital image acquisition was performed using IM50 Version 4.0 imaging sofware (Leica Microsystems) and digital image slide viewing using Image Scope software (Aperio Technologies).
To further investigate the effect of CXCR1/2 inhibition on leukocyte infiltration, we performed a histologic examination to determine the number and location of leukocytes in the lungs of these mice. Consistent with the elevated MPO levels, a higher incidence of leukocytic infiltrates surrounding the pulmonary vessels was observed in the L1Cre(+)Bmpr2f/f mice treated with vehicle compared with L1Cre(−)Bmpr2f/f animals (Figure 1Ci). L1Cre(+)Bmpr2f/f mice treated with SCH527123 exhibited substantially fewer leukocytes in the pulmonary vessel wall. It was interesting to note that the few leukocytes that were present in the lung of these animals appeared to be adhered to the luminal wall (Figure 1Cii). This is in contrast to what we observed in the L1Cre(+)Bmpr2f/f vehicle-treated mice where leukocytes were found adhered to within the vessel wall, supporting the idea that CXCR1/2 mediates the later stages of leukocyte recruitment to the tissue (ie, transmigration rather than the earlier stages, such as adhesion to the vessel wall). To determine whether CXCR1/2 inhibition had any effect on the infiltration of specific leukocyte subsets, we carried out immunostaining for T cells, neutrophils, monocyte/macrophages, and B cells (Figure 1Ciii). Numbers of T cells, neutrophils, monocyte/macrophages, and B cells were increased in L1Cre(+)Bmpr2f/f mice compared with L1Cre(−)Bmpr2f/f control mice. Treatment with SCH527123 decreased the number of T cells, neutrophils, and monocyte/macrophages; however, the number of B cells was unchanged.
Effect of the CXCR1/2 antagonist SCH527123 on circulating cytokine levels in vivo
We previously found that loss of BMPR-II in HPAECs resulted in an increase in the release of the proinflammatory cytokine IL-8. To investigate the impact of loss of endothelial BMPR-II on the levels of circulating proinflammatory cytokines in vivo, we assessed proinflammatory cytokine levels in the plasma of L1Cre(−)Bmpr2f/f, L1Cre(+)Bmpr2f/f mice, and L1Cre(+)Bmpr2f/f mice treated with SCH527123 using an array-based approach.
Levels of the proinflammatory mediators sICAM-1, TNF-α, KC, IFN-γ, and SDF-1 were significantly increased in L1Cre(+)Bmpr2f/f mice compared with L1Cre(−)Bmpr2f/f control mice (Figure 2A-B). Treatment with SCH527123 significantly reduced levels of sICAM-1, KC, and SDF-1 and reduced levels of TNFα and IFNγ to nondetectable levels (Figure 2A-B). Because the detection of cytokine levels using the array approach was semiquantitative, we compared levels of KC using a quantitative ELISA method. Levels of KC were 4 times higher in L1Cre(+)Bmpr2f/f mice (7 ng/mL) compared with control mice (1.6 ng/mL; Figure 2C). These levels were dramatically reduced on treatment with SCH527123 (3 ng/mL; Figure 2C).
Assessment of plasma cytokine levels in L1Cre(−)Bmpr2f/f and L1Cre(−)Bmpr2f/f mice treated with SCH527123 or vehicle. Plasma from L1Cre(−)Bmpr2f/f mice or L1Cre(+)Bmpr2f/f mice treated with SCH527123 or vehicle was assessed for levels of multiple proinflammatory cytokines using a commercial proteome profile array (A-B). The levels of each cytokine could be observed as a pair of duplicate spots (A) and semiquantified by subtracting the background pixel density from the total pixel density of each duplicate spot and represented as the average of both pixel densities for each pair of spots (B). (B) Hatched bars represent L1Cre(+)Bmpr2f/f mice treated with vehicle; and open bars, L1Cre(+)Bmpr2f/f mice treated with SCH527123. KC levels in pooled plasma from each group of mice were then quantified by commercial ELISA (C). Data are the mean ± SEM for a minimum of 8 animals per group. ***P < .001, compared with L1Cre(−)Bmpr2f/f mice by Dunnett test. **P < .01, compared with vehicle-treated L1Cre(+)Bmpr2f/f mice by Dunnett test.
Assessment of plasma cytokine levels in L1Cre(−)Bmpr2f/f and L1Cre(−)Bmpr2f/f mice treated with SCH527123 or vehicle. Plasma from L1Cre(−)Bmpr2f/f mice or L1Cre(+)Bmpr2f/f mice treated with SCH527123 or vehicle was assessed for levels of multiple proinflammatory cytokines using a commercial proteome profile array (A-B). The levels of each cytokine could be observed as a pair of duplicate spots (A) and semiquantified by subtracting the background pixel density from the total pixel density of each duplicate spot and represented as the average of both pixel densities for each pair of spots (B). (B) Hatched bars represent L1Cre(+)Bmpr2f/f mice treated with vehicle; and open bars, L1Cre(+)Bmpr2f/f mice treated with SCH527123. KC levels in pooled plasma from each group of mice were then quantified by commercial ELISA (C). Data are the mean ± SEM for a minimum of 8 animals per group. ***P < .001, compared with L1Cre(−)Bmpr2f/f mice by Dunnett test. **P < .01, compared with vehicle-treated L1Cre(+)Bmpr2f/f mice by Dunnett test.
Effect of the CXCR1/2 antagonist SCH527123 on hemodynamic assessment of PAH
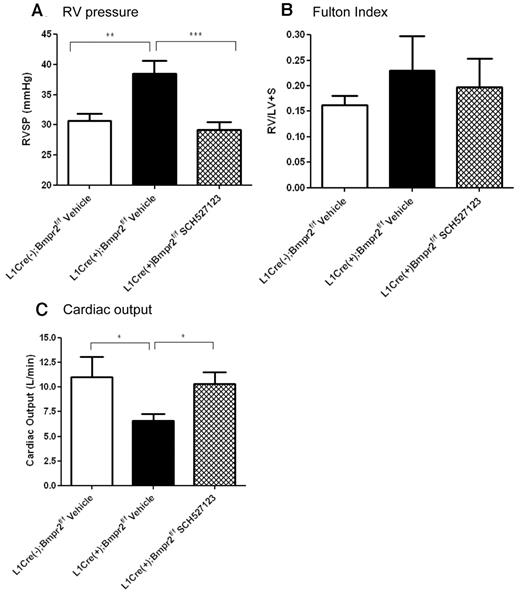
Because we found that inhibition of CXCR1/2 reduced the increased pulmonary vascular leakage and leukocyte infiltration observed in L1Cre(+)Bmpr2f/f mice, we investigated whether CXCR1/2 played a role in the progression of PAH in these animals. We examined right ventricular systolic pressure (RVSP), Fulton index (RV/LV + S), and cardiac output in L1Cre(−)Bmpr2f/f and L1Cre(+)Bmpr2f/f mice after a 21-day dosing period of 20 mg/kg per day SCH527123 or vehicle. After 21 days of dosing with vehicle or SCH527123, L1Cre(+)Bmpr2f/f mice treated with vehicle exhibited significantly elevated RVSP (40.00 ± 2.10 mmHg) compared with L1Cre(−)Bmpr2f/f mice (30.00 ± 1.13 mmHg; Figure 3A). However, treatment with SCH527123 reduced the RVSP in these animals to similar pressures observed in the L1Cre(−)Bmpr2f/f mice (29.00 ± 1.20 mmHg).
RVSP pressure, Fulton index, and cardiac output in L1Cre(−)BMPR2f/f and L1Cre(+)Bmpr2f/f mice treated with vehicle or SCH527123. L1Cre(−)Bmpr2f/f mice or L1Cre(+)Bmpr2f/f mice were treated with vehicle or the CXCR1/2 antagonist SCH527123 for 21 days after which RVSP levels (A), Fulton indexes (B), and cardiac output (C) were assessed. Data are the mean ± SEM for minimum of 8 animals per group. *P < .05, **P < .01, ***P < .001 compared with L1Cre(+)Bmpr2f/f mice treated with vehicle by Dunnett test.
RVSP pressure, Fulton index, and cardiac output in L1Cre(−)BMPR2f/f and L1Cre(+)Bmpr2f/f mice treated with vehicle or SCH527123. L1Cre(−)Bmpr2f/f mice or L1Cre(+)Bmpr2f/f mice were treated with vehicle or the CXCR1/2 antagonist SCH527123 for 21 days after which RVSP levels (A), Fulton indexes (B), and cardiac output (C) were assessed. Data are the mean ± SEM for minimum of 8 animals per group. *P < .05, **P < .01, ***P < .001 compared with L1Cre(+)Bmpr2f/f mice treated with vehicle by Dunnett test.
The progression of RV hypertrophy was measured by the Fulton index. The Fulton index appeared slightly more pronounced in L1Cre(+)Bmpr2f/f mice compared with L1Cre(−)Bmpr2f/f mice and appeared reduced on treatment with SCH527123 (Figure 3B), although none of these changes achieved statistical significance. In contrast, the cardiac output of L1Cre(+)Bmpr2f/f animals was significantly reduced compared with L1Cre(−)Bmpr2f/f control mice (Figure 3C), and this was substantially increased on treatment with SCH527123 and returned to a similar level to that observed in L1Cre(−)Bmpr2f/f mice.
Effect of the CXCR1/2 antagonist SCH527123 on histopathologic features of PAH
To examine whether treatment with SCH527123 had any effect on the histopathologic features of PAH with regards to pulmonary vascular remodeling, we investigated the extent of α-SMA staining in the small pulmonary arteries as a measure of pulmonary artery muscularization.
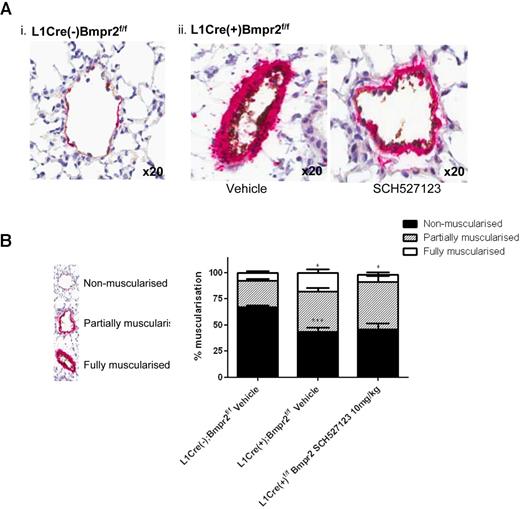
The majority of small vessels in the L1Cre(−)Bmpr2f/f mice were nonmuscularized (68%), with the remaining vessels showing partial (7%) or full muscularization (25%; Figure 4Ai,B). L1Cre(+)Bmpr2f/f mice displayed a significant decrease in the number of nonmuscularized vessels (43%) with an increased proportion of partial (38%) and fully muscularized (18%) vessels (Figure 4Aii,B) with some vessels almost occluded (Figure 4Aii). Treatment with SCH527123 significantly reduced the proportion of fully muscularized vessels in L1Cre(+)Bmpr2f/f mice to 6% and increased the number of partially muscularized vessels (45%), suggesting that CXCR1/2 may play a role in vascular remodeling in this model.
Inhibition of CXCR1/2 reduces muscularization and leukocyte infiltration into lungs of L1Cre(+)Bmpr2f/f mice. The left lung from L1Cre(−)Bmpr2f/f (Ai) and L1Cre(+)Bmpr2f/f mice (Aii) treated with vehicle or SCH527123 was inflated with 1% (volume/volume) formalin, processed for histologic assessment, and then stained with anti-VWF and anti–α-SMA. The extent of α-SMA staining as a measure of pulmonary artery muscularization was assessed in 100 small vessels (30-70 μm) per animal and assigned as nonmuscularized (no α-SMA staining), partially muscularized, or fully muscularized (thick unbroken wall of smooth muscle), and the percentage distribution of each calculated per group (B). Data are the mean ± SEM for a minimum of 8 animals per group. *P < .05, ***P < .001 compared with L1Cre(−)Bmpr2f/f mice by Dunnett test. Slides were mounted using VECTORSHIELD (Vector Laboratories) and viewed using a 20× objective lens. Images were captured using a DMLB microscope and digital camera. Digital image acquisition was performed using IM50 Version 4.0 imaging software (Leica Microsystems) and digital image slide viewing using ImageScope software (Aperio Technologies).
Inhibition of CXCR1/2 reduces muscularization and leukocyte infiltration into lungs of L1Cre(+)Bmpr2f/f mice. The left lung from L1Cre(−)Bmpr2f/f (Ai) and L1Cre(+)Bmpr2f/f mice (Aii) treated with vehicle or SCH527123 was inflated with 1% (volume/volume) formalin, processed for histologic assessment, and then stained with anti-VWF and anti–α-SMA. The extent of α-SMA staining as a measure of pulmonary artery muscularization was assessed in 100 small vessels (30-70 μm) per animal and assigned as nonmuscularized (no α-SMA staining), partially muscularized, or fully muscularized (thick unbroken wall of smooth muscle), and the percentage distribution of each calculated per group (B). Data are the mean ± SEM for a minimum of 8 animals per group. *P < .05, ***P < .001 compared with L1Cre(−)Bmpr2f/f mice by Dunnett test. Slides were mounted using VECTORSHIELD (Vector Laboratories) and viewed using a 20× objective lens. Images were captured using a DMLB microscope and digital camera. Digital image acquisition was performed using IM50 Version 4.0 imaging software (Leica Microsystems) and digital image slide viewing using ImageScope software (Aperio Technologies).
Effect of the CXCR1/2 antagonist SCH527123 on pulmonary endothelial cell barrier function
Because we observed a reduction in the increased vascular leakage observed in L1Cre(+);Bmpr2f/f mice on inhibition of CXCR1/2, we hypothesized that this effect was the result of either inhibition of CXCR1/2 on endothelial cells or reducing leukocyte emigration into the pulmonary artery vessel wall. To investigate the direct effects of endothelial CXCR1/2 inhibition on barrier function, we used an electric cell-substrate impedance sensing system to assess the transcellular resistance of HPAEC monolayers transduced with NTCsh or BMPR-II shRNA or an EV treated with vehicle or SCH527123 over time. The capacitance, as a measure of cell confluence, remained similar over the 2-hour time period for all HPAEC monolayers regardless of shRNA transduction (Figure 5B). However, the resistance of those HPAECs transduced with shRNA targeting BMPR-II was significantly lower than those transduced with NTC shRNA or the EV (Figure 5C). This decreased resistance remained constant over 2 hours. Treatment of the endothelial monolayer with SCH527123 had no effect on the resistance of endothelial monolayers regardless of shRNA treatment (Figure 5D). This would suggest that inhibition of endothelial CXCR1/2 does not directly affect the integrity of the endothelial monolayer over the time studied in this experiment.
Effect of the CXCR1/2 antagonist SCH527123 on TEER of HPAEC monolayers transduced with BMPR-IIsh, NTCsh, or an EV. Confirmation of efficient knockdown of BMPR-II using lentivirus transduction of HPAECs transduced with BMPR-II shRNA, NTC shRNA, or an EV was confirmed at the RNA and protein level (A). HPAECs were then seeded into wells containing 40 gold electrodes and cultured for 24 hours. Capacitance (B,E) and TEER (C-D,F) were measured over a 2-hour period. Then vehicle (C,F) or the CXCR1/2 antagonist SCH527123 (10nM) (D,F) was added to each well and TEER measured for a further 2 hours. (B-D) Representative traces of capacitance and TEER before the addition of vehicle (B-C) and after the addition of SCH527123 (D). (E-F) Data are the mean ± SEM for a minimum 6 experiments. (A) One-way ANOVA showed a significant effect of transduction with shRNA targeting BMPR-II. ***P < .001, compared with EV and NTC shRNA-transduced cells by Tukey test. (B) One-way ANOVA showed a significant effect of transduction with shRNA targeting BMPR-II. *P < .05, **P < .01, and ***P < .001, compared with EV and NTC shRNA-transduced cells by Tukey test.
Effect of the CXCR1/2 antagonist SCH527123 on TEER of HPAEC monolayers transduced with BMPR-IIsh, NTCsh, or an EV. Confirmation of efficient knockdown of BMPR-II using lentivirus transduction of HPAECs transduced with BMPR-II shRNA, NTC shRNA, or an EV was confirmed at the RNA and protein level (A). HPAECs were then seeded into wells containing 40 gold electrodes and cultured for 24 hours. Capacitance (B,E) and TEER (C-D,F) were measured over a 2-hour period. Then vehicle (C,F) or the CXCR1/2 antagonist SCH527123 (10nM) (D,F) was added to each well and TEER measured for a further 2 hours. (B-D) Representative traces of capacitance and TEER before the addition of vehicle (B-C) and after the addition of SCH527123 (D). (E-F) Data are the mean ± SEM for a minimum 6 experiments. (A) One-way ANOVA showed a significant effect of transduction with shRNA targeting BMPR-II. ***P < .001, compared with EV and NTC shRNA-transduced cells by Tukey test. (B) One-way ANOVA showed a significant effect of transduction with shRNA targeting BMPR-II. *P < .05, **P < .01, and ***P < .001, compared with EV and NTC shRNA-transduced cells by Tukey test.
Effect of reduced BMPR-II expression on endothelial CXCR2 expression
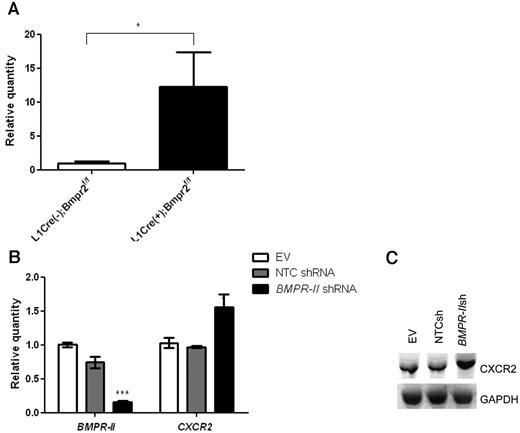
Because we observed such dramatic effects of CXCR1/2 inhibition on vascular integrity, leukocyte infiltration, and PAH pathology, we investigated whether the expression of CXCR2 was also altered in L1Cre(+)Bmpr2f/f mice. CXCR2 mRNA expression from L1Cre(+)Bmpr2f/f mice was significantly higher compared with L1Cre(−)Bmpr2f/f mice (Figure 6A). Because CXCR2 is widely expressed on a number of cell types, we could not determine in which cell type CXCR2 expression was increased in vivo; therefore, we assessed the effect of reduced BMPR-II expression on mRNA and protein expression of CXCR2 in HPAECs after knockdown of BMPR-II by shRNA treatment. CXCR2 expression on HPAECs transduced with shRNA targeting BMPR-II resulted in a significant increase in the expression of CXCR2 at the mRNA and protein levels compared with HPAECs transduced with NTC shRNA (Figure 6B). This suggests that reduced BMPR-II expression may lead to a concomitant increase in CXCR2 expression and uncovers a previously unrecognized role for BMPR-II in controlling the expression of proinflammatory cytokine receptors.
Effect of reduced BMPR-II expression on the expression of CXCR2 in vitro and in vivo. RNA was harvested from the lungs of L1Cre(−)Bmpr2f/f and L1Cre(+)Bmpr2f/f mice (A) or HPAECs transduced with shRNA targeting Bmpr-II, NTC shRNA, or an EV (B). Expression of BMPR-II and CXCR2 was determined with quantitative PCR (TaqMan) using primers specific for BMPR-II and CXCR2 or Western blotting (C). For TaqMan, data are the mean ± SEM for a minimum 3 independent experiments.
Effect of reduced BMPR-II expression on the expression of CXCR2 in vitro and in vivo. RNA was harvested from the lungs of L1Cre(−)Bmpr2f/f and L1Cre(+)Bmpr2f/f mice (A) or HPAECs transduced with shRNA targeting Bmpr-II, NTC shRNA, or an EV (B). Expression of BMPR-II and CXCR2 was determined with quantitative PCR (TaqMan) using primers specific for BMPR-II and CXCR2 or Western blotting (C). For TaqMan, data are the mean ± SEM for a minimum 3 independent experiments.
Discussion
We previously found that BMPR-II played a key role in vivo in dampening inflammatory signals in the pulmonary endothelium and that reduced BMPR-II in the endothelial layer resulted in the loss of vascular integrity, evidenced by increased vascular leakage and immune cell recruitment. In vitro, leukocyte recruitment could be ablated by inhibition of CXCR1/2. Here we show that increased leukocyte infiltration and pulmonary vascular leakage observed in mice with endothelial specific deletion of Bmpr-II can be inhibited by pharmacologic inhibition of the CXCL8 receptors CXCR1/2 with the CXCR1/2 antagonist SCH527123. In addition, we found that the reduction in leukocyte infiltration and inflammatory mediators as a result of treatment with SCH527123 reversed the hemodynamics associated with PAH and vascular remodeling observed in these animals.
Elevated circulating levels of proinflammatory cytokines have been frequently reported in PAH patients and animal models of PAH.3-9 Consistent with these studies, we also found that levels of a wide range of proinflammatory cytokines and soluble adhesion molecules, including sICAM-1, TNFα, KC, IFNγ, and SDF-1, were elevated in the plasma of mice with PAH arising from the deletion of endothelial-specific Bmpr2, suggesting that inflammation may play a role in the development of heritable forms of PAH. Treatment of mice with the CXCR1/2 antagonist SCH527123 unexpectedly resulted in a marked reduction in the plasma levels of all of these mediators. We speculate that the increase in some of these proinflammatory mediators may be mediated through the secondary recruitment of cell types, such as platelets to the pulmonary vasculature of L1Cre(+)Bmpr2f/f mice, and that antagonism of the CXCR1/2 receptor with SCH527123 inhibits recruitment of these cells and the subsequent increase in plasma levels of proinflammatory cytokines. In support of this hypothesis, evidence of increased platelets associated with the pulmonary vasculature has been described in L1Cre(+)Bmpr2f/f mice.20
How inflammatory cells and mediators contribute to vascular remodeling and the development of PAH is still unclear. The role of inflammatory cytokines in the initiation and progression of PAH has been considered in animal models whereby blockade of the IL-1β receptor8 or the MCP-1 signaling pathway9 appears to attenuate the progression of PAH induced by monocrotoline. Although these studies support a role for the contribution of these mediators to the development of PAH, to our knowledge no studies have described any beneficial effect of inhibiting inflammatory chemokines or their receptors once experimental PAH is established. Here we show, for the first time, that therapeutic inhibition of the IL-8 receptors, CXCR1/2, results in a reduction in the increased pulmonary artery pressure and restoration of cardiac output observed in L1Cre(+)Bmpr2f/f mice. In addition, we observed a decrease in the extent of muscularization in these animals when treated with SCH527123, suggesting that inflammatory cells and mediators contribute to the vascular remodeling observed in this model and by reducing the infiltration of these cells through inhibition of CXCR1/2 vascular remodeling can be reversed.
A major role of the endothelium is to shield the underlying stromal cells from angiogenic factors present in serum. We previously showed that loss of BMPR-II in the endothelial layer resulted in reduced integrity of the endothelium resulting in the development of a “leaky” vasculature.13 Here we found that treatment with the CXCR1/2 antagonist SCH527123 reduced the increased vascular permeability observed in L1Cre(+)Bmpr2f/f mice, suggesting that CXCR1/2 plays a role in mediating vascular leakage in this model. Pulmonary vascular leakage has been shown to be CXCR2-dependent in a number of models of lung injury,16,21 which appears to be almost completely regulated by CXCR2 on endothelial cells.16 Whether CXCR1/2 on endothelial cells was directly responsible for mediating the vascular leakage observed in our L1Cre(+)Bmpr2f/f mice is not known; however, TEER in HPAECs treated with shRNA targeting BMPR-II was unchanged when CXCR1/2 was inhibited, thus suggesting that CXCR1/2 on endothelial cells may not be responsible for the increased permeability in our studies. It is probable, therefore, that the effects of SCH527123 on vascular leakage result from its effects on reducing leukocyte infiltration into the pulmonary artery wall.
Because CXCR1/2 blockade inhibits both vascular leakage and leukocyte infiltration in vivo, we cannot distinguish between these 2 factors in establishing PAH pathology; however, the inhibition of PAH observed after CXCR1/2 blockade in this model is probably the result of the effect of preventing the recruitment of leukocytes into the lung. Our data suggest that loss of endothelial BMPR-II leads to reduced barrier function and elevated levels of proinflammatory mediators, such as IL-8, which in turn promote leukocyte recruitment and facilitate transmigration.
We found that mice with endothelial-specific deletion of Bmpr-II showed higher CXCR2 mRNA expression in the lung compared with mice with normal Bmpr-II expression. Because these animals also exhibited an increased number of leukocytes in the lungs, we cannot determine the origin of these CXCR2-expressing cells. However, in vitro, reduced BMPR-II expression on HPAECs resulted in increased CXCR2 expression at the mRNA and protein levels, suggesting that it is probable that the increased CXCR2 expression observed in vivo was in part the result of elevated endothelial cell-specific expression of CXCR2. To our knowledge, this is the first time that a reduction in BMPR-II has been shown to increase CXCR2 expression. The pathophysiologic role of increased CXCR2 expression in the pulmonary endothelium resulting from loss of BMPR-II is unclear, particularly as we could not demonstrate a role for endothelial-expressed CXCR2 in mediating an increase in endothelial monolayer permeability in vitro. In addition, how BMPR-II may regulate CXCR2 expression is currently unknown, and further investigations are in progress to address this question.
In conclusion, our studies show that increased vascular leakage and leukocyte infiltration resulting from reduced BMPR-II in the endothelial layer can be ablated by pharmacologic inhibition of CXCR1/2. Furthermore, our data reveal a pivotal role for inflammatory cells and their mediators in driving the development and progression of PAH that results from loss of endothelial-specific BMPR-II, suggesting that pharmacologic inhibition of CXCR1/2 may provide a promising and novel therapeutic approach for PAH patients with BMPR-II mutations.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: V.J.B. designed and performed the in vitro and in vivo experiments, analyzed the data, and contributed to the writing of the manuscript; A.M.H. performed in vitro experiments and provided intellectual input; L.I.C. provided help performing the in vivo experiments; A.R. performed the CXCR2 Western blot; J.S.R. performed the immunohistochemistry; G.J. provided intellectual input; A.C.P. and D.C.B. provided project supervision; and D.C.B. contributed to the writing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for D. C. B. is Inflammation, Hoffmann-La Roche Inc, Nutley, NJ.
Correspondence: Victoria J. Burton, Respiratory Disease Area, Novartis Institutes for BioMedical Research, Wimblehurst Road, Horsham, West Sussex RH12 5AB, United Kingdom; e-mail: victoria.burton@novartis.com.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal