Abstract
Rapid blood perfusion is critical for postimplantation survival of thick, prevascularized bioartificial tissues. Yet the mechanism by which implanted vascular networks inosculate, or anastomose, with the host vasculature has been unknown, making it difficult to develop optimized strategies for facilitating perfusion. Here we show that implanted vascular networks anastomose with host vessels through a previously unidentified process of “wrapping and tapping” between the engrafted endothelial cells (ECs) and the host vasculature. At the host-implant interface, implanted ECs first wrap around nearby host vessels and then cause basement membrane and pericyte reorganization and localized displacement of the underlying host endothelium. In this way, the implanted ECs replace segments of host vessels to divert blood flow to the developing implanted vascular network. The process is facilitated by high levels of matrix metalloproteinase-14 and matrix metalloproteinase-9 expressed by the wrapping ECs. These findings open the door to new strategies for improving perfusion of tissue grafts and may have implications for other physiologic and pathologic processes involving postnatal vasculogenesis.
Introduction
A major obstacle in tissue engineering is poor postimplantation graft survival because of insufficient blood perfusion. A potential solution is to populate the engineered tissue with endothelial cells (ECs) or endothelial progenitor cells (EPCs), which can quickly organize into interconnected networks, undergo lumenogenesis, and anastomose with the host vasculature to redirect blood flow into the graft.1 Human umbilical vein endothelial cells (HUVECs),2-4 ECs derived from human embryonic stem cells,5 and human adult and cord blood EPCs6,7 are all capable of generating such patent vascular networks in vivo. This approach has proven effective in improving the quality of engineered skeletal muscle8 and bone9 tissues.
Limited studies suggest that, during embryonic vasculogenesis and sprouting angiogenesis, anastomosis is accomplished via connection of extended cellular processes followed by lumen propagation through intracellular and intercellular vacuole fusion,10,11 with macrophages playing an accessory role.12 However, it is not known whether this is the only mechanism for connecting vessels. Without a basic understanding of the cellular mechanisms of anastomosis, it is difficult to develop strategies for accelerating this critical step for perfusing engrafted tissues.
To investigate the process of anastomosis, we used a previously established model in which HUVECs and mouse mesenchymal precursor cells are embedded in collagen-fibronectin gels and placed in cranial window preparations of severe combined immunodeficient mice2 (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). In this system, anastomosis between host vessels and implanted EC networks occurs as early as 2 weeks after implantation, and the engineered vessels remain stable and functional for one year in vivo.2 Similar results can be achieved when the mouse mesenchymal precursor cells are replaced with human mesenchymal stem cells13 or human lung fibroblasts.14,15 Tracking fluorescently labeled implanted ECs and host ECs simultaneously in live animals, we found that tip cell connections and vacuole fusion were not involved in host-implant vascular anastomosis; surprisingly, the engrafted endothelial networks wrapped around host vessels at the host-implant interface and then replaced sections of the underlying vessel wall to tap into the host blood supply.
Methods
Cell culture
HUVECs were obtained from the Center for Excellence in Vascular Biology, Brigham & Women's Hospital, Boston and maintained in endothelial growth medium (Lonza Switzerland). The 10T1/2 cells were purchased from ATCC and maintained in Dulbecco modified Eagle medium (Invitrogen) supplemented with 10% FBS (HyClone). Primary mouse brain endothelial cells (PMBECs) were separated from mouse brain tissues and maintained in MCDB131 (Invitrogen) supplemented with 5% bovine platelet-poor derived serum (Biomedical Technologies), 1% penicillin/streptomycin (Invitrogen), 60 μg/mL of endothelial cell growth supplement (Sigma-Aldrich), 85 μg/mL heparin, 280 μg/mL glutathione, 1 μL/mL 2-Mercaptoethanol, and insulin/transferring/selenium (Invitrogen).
Retrovirus packaging and transduction with green or red fluorescent protein
The retrovirus vector for transducing enhanced green fluorescent protein (GFP) or dsRed fluorescent protein (dsRed) into HUVECs or 10T1/2 was kindly provided by Dr Gary Nolan at Stanford University (Stanford, CA). For retrovirus packaging, the plasmids of enhanced GFP or dsRed protein, gag/pol, and VSVG (15 μg, 7 μg, and 5 μg, respectively) were mixed and cotransfected into 293ET cells with Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol. After overnight incubation, the 293ET cells were washed with PBS and replaced with fresh media. The next day, the supernatant containing retrovirus was collected and fresh medium was added; this step was repeated 3 more times. After the supernatant was collected, it was passed through a 0.45-μm filter (Whatman) and was either used immediately or kept at −80°C.
For the transduction, the supernatant was first diluted 1:1 with fresh cell culture medium and supplemented with polybrene (8 μg/mL). The diluted supernatant was then added to a subconfluent monolayer of cells and allowed to incubate for 4 hours. Fresh cell culture medium was exchanged at the end of the incubation period, and this step was repeated 2 to 3 times on consecutive days. By comparing fluorescent images of GFP-transduced HUVECs with human CD31 staining, we confirmed that > 90% of cells were effectively labeled for intravital imaging (supplemental Figure 2B).
Isolation of PMBECs
Because PMBECs were difficult to label ex vivo, we used endogenous expression of GFP driven by the Tie2 promoter to distinguish implanted from host PMBECs. Implantation of PMBECs was performed between Tie2-GFP/Rag1−/− and Rag1−/− mice (both 8 weeks old). The Tie2-GFP/Rag1−/− mice were created by crossing Tie2-GFP mice with Rag1−/− mice (both from The Jackson Laboratory).
For PMBEC isolation, animal cerebrocortices were first cut into small pieces, homogenized, and diluted with dextran/PBS to a final concentration of 15% (vol/vol). Homogenate was centrifuged at 10 000g and 4°C for 15 minutes. The resulting pellet was digested with collagenase/dispase at 1 mg/mL in MCDB131 with 2% FBS for 3 hours at 37°C with constant shaking. The digested tissues were subsequently resuspended with Percoll/PBS to a final concentration of 45% (volume/volume). Tissues were then centrifuged at 20 000g and 4°C for 10 minutes. Supernatant containing vessel fragments was saved, washed once with PBS, resuspended with PMBEC medium, and plated on fibronectin-coated plates. All PMBECs were implanted at passage zero.
Mouse window models and implantation of collagen gels embedded with cells
A total of 8 × 105 HUVECs or PMBECs and 2 × 105 10T1/2 cells were suspended in 1 mL of a solution of rat-tail type 1 collagen (1.5 mg/mL; BD Biosciences) and mixed with human plasma fibronectin (90 μg/mL; Sigma-Aldrich) in endothelial growth medium or PMBEC medium at 4°C. pH was adjusted to 7.6 using 1N NaOH. The cell suspension was pipetted into 12-well plates (BD Biosciences) and warmed to 37°C for 30 minutes to allow collagen polymerization. Each solidified gel construct was covered by 2 mL of warmed endothelial growth medium or PMBEC medium. After 18 to 24 hours of culture at 37°C and 5% CO2, a 4-mm biopsy punch was used to cut disk-shaped pieces of the gel construct; these were implanted into mouse cranial windows16 as described previously.2 Dorsal skinfold chambers were created as previously described.17 For gel construct implantation, the cover glass on the chamber was removed and the disk-shaped gel construct was placed directly on top of the tissue. The chamber was then sealed again with the cover glass and a metal tension ring. Care was taken, and saline solution was applied, to prevent the formation of air bubbles inside the chamber during this procedure.
Intravital confocal microscopy
A Fluoview 500 confocal laser-scanning microscope (Olympus America) was used for intravital microscopy. During the imaging, the animal's head was stabilized with a custom-made cranial window holder, its body was placed on a heating pad with circulating 37°C water (Paragon Medical), and anesthesia was maintained with a table-top isofluorane anesthesia system (VetEquip). Imaging medium was air, water, or oil per objective type. Images were acquired with the software provided with the Fluoview 500 system. Maltab (MathWorks) was used to apply pseudo-color to capture images for presentation.
Visualization of the host vasculature in live mice with mCD31-X647 antibody
To visualize the host vasculature in mice implanted with HUVECs, azide-free and low-endotoxin mouse-specific CD31 antibody (BD Biosciences PharMingen) was labeled using an Alexa647 (referred to here as X647) antibody labeling kit (Invitrogen) and then injected intravenously. The antibody, mCD31-X647, was allowed ∼ 5 minutes to circulate before imaging started. This treatment was very well tolerated by the animal, and the fluorescence from vessel-bound mCD31-X647antibody persisted for ∼ 3 days.
Fluorescent labeling of mouse red blood cells
To visualize blood flow during intravital microscopy, mouse RBCs were collected, labeled with highly lipophilic carbocyanine dyes DiI or DiD (Invitrogen), and injected into the animal intravenously at 50% hematocrit. Fluorescence from the labeled RBCs was sufficiently bright for imaging for ∼ 30 days.
Whole-mount immunohistochemistry
For preparations requiring lectin staining, anesthetized animals were first perfused with biotinylated lectin (Vector Laboratories) for 10 minutes.18 Otherwise, animals were perfused with 4% paraformaldehyde in PBS through cardiac puncture. The cover glass forming the cranial window was then removed, and the implanted collagen gel (∼ 300 μm in thickness) was harvested and put into 4% paraformaldehyde in PBS to be fixed for 1.5 hours on ice. For immunohistochemical (IHC) staining, the fixed collagen gels were washed with 1× PBS 3 times, 10 minutes each, blocked with 3% BSA + 0.1% Triton X-100 for 1 hour, incubated with primary antibodies at 4°C overnight, and washed with 1× PBS + 0.1% Triton X-100 for 10 minutes 3 times. The sample was then incubated with secondary antibodies at room temperature for 4 hours or at 4°C overnight and washed again with 1× PBS + 0.1% Triton X-100 for 10 minutes 3 times. The collagen gels were then mounted directly on glass slides and covered with mounting media (Vector Laboratories) and a cover glass for imaging. The primary antibodies used were rat anti–mouse CD11b (BioLegend), rat anti–mouse CD31 (BD Biosciences PharMingen), rabbit anti–mouse collagen IV (Millipore), rat anti–mouse F4/80 (AbD Serotec), rat anti–mouse MECA32 (BD Biosciences PharMingen), mouse anti–mouse matrix metalloproteinase-14 (MMP-14; Millipore), rabbit anti–mouse MMP-9 (Abcam), rabbit anti–mouse NG2 (Millipore), mouse anti–human Desmin (Dako North America), and mouse anti–human α smooth muscle actin (Sigma-Aldrich). The MOM kit (Vector Laboratories) was used for mouse anti–mouse primary antibodies.
Correlation of pericyte disruption and wrapping
To determine whether pericyte disruption occurred specifically in regions of HUVEC wrapping, 3 researchers familiar with pericyte morphology classified the pericytes as “normal” or “disrupted” based only on CD31 and NG2 staining patterns (blinded to the wrapping status and HUVEC staining). A total of 80.0% ± 6.9% of the wrapped vessels were classified as having abnormal morphology; none of the unwrapped vessels was classified as having abnormal pericyte morphology. According to the Pearson χ2 test, there was a correlation between wrapping and pericyte disruption (P < .01).
Quantification of MMP-14/MMP-9 expression
Images were analyzed with code developed in-house using Matlab (MathWorks). Operations were performed on confocal images of MMP-14/MMP-9 staining and HUVECs. Multiple rectangular regions of interest (ROI) were placed at random positions in the HUVEC network, each locally orthogonal to, and overlapping with, the vessel segment; ROI were 40 μm in length and extended 50 μm past the vessel wall on both sides. For each ROI, segmentation was performed to identify the HUVEC-positive pixels; and within this area, the average intensity of the corresponding MMP-9 or MMP-14 signal was recorded. The local vessel diameter was also calculated within each ROI, and the wrapping status recorded (ie, “wrapped” or “not wrapped”). In each field, all intensity data were normalized to the average level associated with wrapped HUVECs, which served as the internal standard. Thus, each data point in Figure 4E-F represents the intensity within a given ROI divided by the field-averaged, wrapped vessel intensity. Analysis of expression by other cell types followed a similar procedure.
Quantification of the perfused fraction of engrafted vessels
Fluorescently conjugated dextran was injected intravenously into the animal, and confocal image stacks were acquired of both the implanted vessels and the dextran. Image projections were then processed by Matlab (MathWorks), and the perfused fraction was calculated as the ratio of the projected area with dextran over the projected area of all the implanted vessels.
GM6001 treatment
Nine days after implantation, locations were identified where HUVECs were wrapping around host vessels but had not yet caused host vessel dissolution. The mice then received daily intraperitoneal injection of either 100 mg/kg GM6001 (Ryss Lab) in 4% carboxymethylcellulose (treated group) or the same volume of carboxymethylcellulose (control group) for 2 weeks. The same wrapped segments were revisited 23 days after implantation, and the status of the wrapped host segment was recorded as either intact or degraded.
MMP-14 and/or MMP-9 antibody treatment
Mice with implants in cranial windows (see “Mouse window models and implantation of collagen gels embedded with cells”) were treated with control IgG, DX-240019 (a highly selective monoclonal MMP-14 antibody from Dyax), DX-2802 (a monoclonal MMP-9 antibody from Dyax), or DX-2400 + DX-2802 at 10 mg/kg every other day starting from one day before gel implantation. At 2 weeks, 4 weeks, and 6 weeks after implantation, the perfused fraction of the implant EC network was quantified (see “Quantification of the perfused fraction of engrafted vessels”).
Statistics
Values are reported as arithmetic mean with SD. In general, the Student unpaired t test was used to determine significance, with P < .05. For testing the correlation between vessel wrapping and pericyte disruption, the Pearson χ2 test was used, with P < .05 considered significant.
Results
Implanted vascular networks connect to host vasculature through WAT anastomosis in skin and brain tissues
Performing intravital confocal microscopy on gels implanted in cranial windows, we found that, within 2 weeks, HUVECs in the bulk of the gel self-organized into networks by tip-cell extension and homotypic (HUVEC-HUVEC) anastomosis (supplemental Video 1) and formed contiguous lumens via vacuole fusion (supplemental Video 2). However, the interactions between engrafted ECs and the host vessels were quite different. As early as one week after implantation, HUVECs at the brain-implant interface began wrapping around nearby host vessels (Figure 1A day 7). High-resolution 3D image rendering confirmed that the wrapping occurred around angiogenic host vessels that had invaded the gel (supplemental Figure 2A; see supplemental Videos 3 and 4 for 3D renderings). Over the next 2 weeks, host endothelium in the wrapped areas disappeared, and the overlying HUVEC segments were in contact with the blood flow that had previously been carried by the host vessels (Figure 1A day 16 and day 23). During the tapping process, stable, long-lasting junctions were formed between the HUVECs and the remaining host ECs. As this wrapping-and-tapping (WAT) anastomosis occurred at the host-implant interface, blood perfusion gradually propagated through the HUVEC networks in the bulk of the implant, which matured over time (supplemental Figure 3). This WAT process appeared to be the exclusive mechanism for host-implant vascular anastomosis in this model, as we detected no evidence for heterotypic (HUVEC-host EC) tip-cell anastomosis.
WAT anastomosis between implanted HUVECs and host (mouse) vasculature in cranial windows and dorsal skinfold chambers. (A) In cranial windows, HUVECs (green, GFP transduction) first wrap around host vessels (red, Alexa647-conjugated mouse-specific CD31, which is denoted mCD31-X647, injected intravenously), which have invaded the implant through angiogenesis, and eventually replace them to tap into the blood flow. Perfused vessels were visualized by intravenous injection of cascade blue-conjugated dextran (denoted CB-dextran, blue) on day 23. White dashed curves indicate the edge of the engrafted gel. Empty arrowheads in the central panels indicate a host vessel within the implant. Filled arrowheads in the central and right-hand panels indicate a host segment wrapped and then completely replaced by HUVECs. The arrow indicates a HUVEC-host junction. Intravital confocal images; 4×/0.13 NA air objective (left column) and 20×/0.4 NA air objective at 2.0× digital zoom (middle and right columns); scale bar represents 500 μm. (B) Overview of host-implant vascular anastomosis in dorsal skinfold chambers. HUVECs are green (GFP transduction), host vessels are red (mCD31-X647 injected intravenously), and blood perfusion is shown by intravenous injection of rhodamine-conjugated dextran (denoted Rho-dextran, light blue). Empty arrowheads track a typical example of WAT anastomosis: significant host vessel angiogenesis and extensive HUVEC wrapping are evident at day 14; more host vessels grow into the implant and wrapping continues at day 19; at day 23, there is obvious host vessel degradation and extensive HUVEC network perfusion; at day 28, host vessel degradation continues and perfusion propagates deeper into the bulk of the implant. Details of the areas within the white boxes in “Day 14” and “Day 23” images are shown in supplemental Figure 2C and Figure 1C, respectively. Intravital confocal images; 10×/0.3 NA air objective (each presented image is a montage of 16 captured images); scale bar represents 500 μm. (C) Detail of the area within the white box in “Day 23” image of panel B. Wrapping HUVECs (green, GFP transduction) “tap” a host vessel (red, mCD31-X647 injected intravenously) for blood flow (light blue, Rho-dextran injected intravenously). Arrows indicate where “tapping” is taking place. The right-hand panel shows the detail within the white box in the left-hand panel. Empty arrowhead points to where HUVECs have opened the host vessel wall to divert the blood flow. Intravital confocal images; 20×/0.4 NA air objective at 2.5× (left and middle columns) and 9.0× digital zoom (right column); scale bar represents 40 μm. (D) Regressing host vessel segments (red, mCD31-X647 injected intravenously; empty arrowheads) inside developing HUVEC vessels (green, GFP transduction) in a 30-day-old implant. Perfused vessels (blue) are visualized by intravenous injection of CB-dextran. Arrow indicates a HUVEC-host junction. Intravital confocal images; 10×/0.3 NA air objective at 1.5× digital zoom; scale bar represents 100 μm. (E) Detail of a HUVEC-host junction at day 48 showing that the HUVEC segment encapsulates the end of the remaining host vessel. Vessel perfusion is marked by biotin staining (blue) of lectin injected intravenously before tissue fixation. “Box” shows the detail of the area within the white box in the “Merged” panel. “Illustration” shows the microanatomy of the junction. Whole-mount IHC stain; 20×/0.95 NA water objective (row 1) and 60×/1.1 NA objective at 2.0× digital zoom (row 2); scale bar represents 100 μm.
WAT anastomosis between implanted HUVECs and host (mouse) vasculature in cranial windows and dorsal skinfold chambers. (A) In cranial windows, HUVECs (green, GFP transduction) first wrap around host vessels (red, Alexa647-conjugated mouse-specific CD31, which is denoted mCD31-X647, injected intravenously), which have invaded the implant through angiogenesis, and eventually replace them to tap into the blood flow. Perfused vessels were visualized by intravenous injection of cascade blue-conjugated dextran (denoted CB-dextran, blue) on day 23. White dashed curves indicate the edge of the engrafted gel. Empty arrowheads in the central panels indicate a host vessel within the implant. Filled arrowheads in the central and right-hand panels indicate a host segment wrapped and then completely replaced by HUVECs. The arrow indicates a HUVEC-host junction. Intravital confocal images; 4×/0.13 NA air objective (left column) and 20×/0.4 NA air objective at 2.0× digital zoom (middle and right columns); scale bar represents 500 μm. (B) Overview of host-implant vascular anastomosis in dorsal skinfold chambers. HUVECs are green (GFP transduction), host vessels are red (mCD31-X647 injected intravenously), and blood perfusion is shown by intravenous injection of rhodamine-conjugated dextran (denoted Rho-dextran, light blue). Empty arrowheads track a typical example of WAT anastomosis: significant host vessel angiogenesis and extensive HUVEC wrapping are evident at day 14; more host vessels grow into the implant and wrapping continues at day 19; at day 23, there is obvious host vessel degradation and extensive HUVEC network perfusion; at day 28, host vessel degradation continues and perfusion propagates deeper into the bulk of the implant. Details of the areas within the white boxes in “Day 14” and “Day 23” images are shown in supplemental Figure 2C and Figure 1C, respectively. Intravital confocal images; 10×/0.3 NA air objective (each presented image is a montage of 16 captured images); scale bar represents 500 μm. (C) Detail of the area within the white box in “Day 23” image of panel B. Wrapping HUVECs (green, GFP transduction) “tap” a host vessel (red, mCD31-X647 injected intravenously) for blood flow (light blue, Rho-dextran injected intravenously). Arrows indicate where “tapping” is taking place. The right-hand panel shows the detail within the white box in the left-hand panel. Empty arrowhead points to where HUVECs have opened the host vessel wall to divert the blood flow. Intravital confocal images; 20×/0.4 NA air objective at 2.5× (left and middle columns) and 9.0× digital zoom (right column); scale bar represents 40 μm. (D) Regressing host vessel segments (red, mCD31-X647 injected intravenously; empty arrowheads) inside developing HUVEC vessels (green, GFP transduction) in a 30-day-old implant. Perfused vessels (blue) are visualized by intravenous injection of CB-dextran. Arrow indicates a HUVEC-host junction. Intravital confocal images; 10×/0.3 NA air objective at 1.5× digital zoom; scale bar represents 100 μm. (E) Detail of a HUVEC-host junction at day 48 showing that the HUVEC segment encapsulates the end of the remaining host vessel. Vessel perfusion is marked by biotin staining (blue) of lectin injected intravenously before tissue fixation. “Box” shows the detail of the area within the white box in the “Merged” panel. “Illustration” shows the microanatomy of the junction. Whole-mount IHC stain; 20×/0.95 NA water objective (row 1) and 60×/1.1 NA objective at 2.0× digital zoom (row 2); scale bar represents 100 μm.
Because the brain has a unique vasculature20 that may influence anastomosis, we also investigated this process in dorsal skinfold chambers of severe combined immunodeficient mice (supplemental Figure 1). To induce levels of host angiogenesis similar to those in the brain microenvironment, we needed to increase the seeding density of HUVECs and 10T1/2 cells 3-fold (maintaining the same ratio, see “Mouse window models and implantation of collagen gels embedded with cells”). Two weeks after implantation, many host vessels had grown into the implant and were extensively wrapped by HUVECs (Figure 1B day 14). In some locations, HUVECs had tapped into the blood flow of host vessels (supplemental Figure 2C; supplemental Video 5), using the same WAT process seen in cranial windows. After 5 more days, host vessel networks had expanded farther into the implant and more HUVEC vessels were perfused at the interface (Figure 1B day 19). By day 23, there was evidence of host vessel regression inside the implant and more extensive perfusion of the HUVEC networks (Figure 1B day 23). WAT by HUVECs was evident in many locations (Figure 1C). At the final time point (dorsal skinfold chambers can be maintained for no more than 4 weeks), the disappearance of host vessels within the implant continued and blood perfusion propagated farther into the HUVEC networks (Figure 1B day 28). Thus, WAT anastomosis occurs in skin as well as brain tissue.
Because of the limited useable life span of the dorsal skinfold chambers, we further characterized WAT in the cranial window, which can be maintained for more than a year. Thirty days after implantation, isolated patches of host vessel were frequently observed inside continuous and perfused HUVEC vessels near the host-implant interface (Figure 1D), suggesting that previously patent and continuous parts of the host vasculature were being degraded and replaced by the overlying HUVECs. This arrangement was also observed in dorsal skinfold chambers (supplemental Figure 2D). Higher magnification images revealed more structural detail of the HUVEC-host junctions (Figure 1E; see supplemental Video 6 for 3D rendering of a similar junction). In this late stage of the process (48 days after implantation), the HUVECs still formed “cuffs” around the host vessel, consistent with regression or dissolution of the inner host EC layer. During WAT anastomosis, there were no significant changes in the length of the wrapped segments over time, suggesting that the HUVEC and host EC bilayers did not simply slide relative to one another, as in an expanding telescope, but that host ECs were lost.
Wrapping results in pericyte reorganization
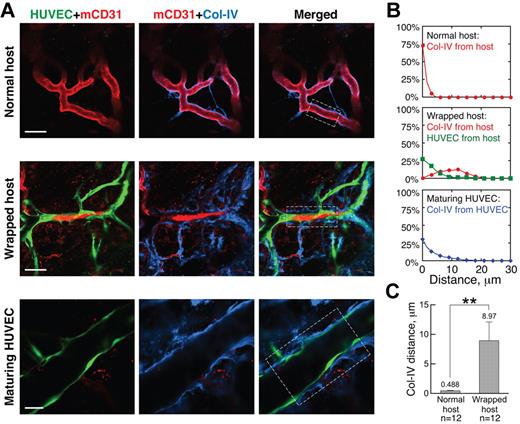
We next examined the structure of the wrapped vessel segments, focusing first on pericytes, which are known to provide structural integrity and survival factors to blood vessels, making them refractory to remodeling.21-23 Because the expression of pericyte markers, including α-smooth muscle actin, desmin, and NG2, is known to change during vessel maturation and destabilization,24,25 we assessed the association of α-smooth muscle actin-, desmin- and NG2-positive pericytes with the vessels in our gels and host tissue. Almost all nascent host vessels that had grown into the implanted gels through angiogenesis lacked coverage by α-smooth muscle actin- or desmin-positive pericytes at the inception of WAT anastomosis; in contrast, NG2 was expressed by all host vessels in both the host tissue and the implanted gels (supplemental Figure 4). Intact, unwrapped host vessels were fortified by a layer of regularly distributed NG2-positive pericytes (Figure 2A “Normal host”); in contrast, host vessels wrapped by engrafted HUVECs had a highly disorganized NG2 pattern (Figure 2A, “Wrapped host”). Even along contiguous stretches of host vessels, wrapped and unwrapped regions had very different pericyte morphology (Figure 2A “Wrapped host” and “Box 2”). Quantification revealed a significant correlation between disruption of pericyte morphology and the presence of wrapping HUVECs: 80.0% ± 6.9% of the wrapped vessels had abnormal morphology, whereas none of the unwrapped vessels had abnormal NG2-positive pericyte morphology (significant according to the Pearson χ2 test, P < .01). Once perfused, HUVEC vessels matured, as evidenced by increasingly normal pericyte coverage (Figure 2A “Maturing HUVEC”). Individual confocal slices revealed that the wrapping HUVECs were distinct from the host vessel pericytes (Figure 2B), with only 5.69% ± 2.98% overlap between HUVEC and NG2 antibody signals. The NG2-positive cells were frequently located between the HUVECs and the host vessel (Figure 2B).
Wrapping HUVECs reorganize pericytes on host vessels. (A) Pericytes (blue, NG2 staining) appear normal around unwrapped, intact host vessels (row 1). Wrapping by HUVECs dramatically changes pericyte morphology (rows 2-4), but pericytes around maturing HUVEC vessels appear more normal (row 5). “Box 1” and “Box 2” show details of the areas within the white boxes in the “Wrapped host” panel. Empty and filled arrowheads highlight unwrapped and wrapped regions, respectively. Note that the disruption of host pericytes requires complete and tight wrapping by the HUVECs, not casual alignment as shown on the host vessel with empty arrowheads in “Box 1.” Whole-mount IHC stain; 60×/1.1 NA oil objective (rows 1, 3, 4 and 5) and 20×/0.95 NA water objective (row 2); scale bar represents 120 μm for row 2 and 40 μm for all the other rows. (B) When wrapping around host vessels (blue, mCD31 staining), HUVECs (green, GFP transduction) often lay on top of pericytes (red, NG2 staining) with little colocalization between the 2 (ie, very few pixels are yellow in the “Merged” panel). Filled arrowheads indicate where wrapping is taking place. Note that pericyte morphology on the wrapped host vessel is more abnormal in “Spot 2.” Whole-mount IHC stain; 20×/0.95 NA water objectie at 4.0× digital zoom; scale bar represents 30 μm.
Wrapping HUVECs reorganize pericytes on host vessels. (A) Pericytes (blue, NG2 staining) appear normal around unwrapped, intact host vessels (row 1). Wrapping by HUVECs dramatically changes pericyte morphology (rows 2-4), but pericytes around maturing HUVEC vessels appear more normal (row 5). “Box 1” and “Box 2” show details of the areas within the white boxes in the “Wrapped host” panel. Empty and filled arrowheads highlight unwrapped and wrapped regions, respectively. Note that the disruption of host pericytes requires complete and tight wrapping by the HUVECs, not casual alignment as shown on the host vessel with empty arrowheads in “Box 1.” Whole-mount IHC stain; 60×/1.1 NA oil objective (rows 1, 3, 4 and 5) and 20×/0.95 NA water objective (row 2); scale bar represents 120 μm for row 2 and 40 μm for all the other rows. (B) When wrapping around host vessels (blue, mCD31 staining), HUVECs (green, GFP transduction) often lay on top of pericytes (red, NG2 staining) with little colocalization between the 2 (ie, very few pixels are yellow in the “Merged” panel). Filled arrowheads indicate where wrapping is taking place. Note that pericyte morphology on the wrapped host vessel is more abnormal in “Spot 2.” Whole-mount IHC stain; 20×/0.95 NA water objectie at 4.0× digital zoom; scale bar represents 30 μm.
Wrapping ECs disrupt host vessel BM
Suspecting that the tapping process also requires the degradation of host vessel basement membrane (BM), we next analyzed the distribution of collagen IV, a major component of the BM, around wrapped vessels. Normal, unwrapped host vessels were ensheathed in a thin, compact layer of BM (Figure 3A “Normal host”). This structure was dramatically disrupted by the wrapping HUVECs, which in many regions were directly adjacent to the underlying host ECs, separating them from the BM (Figure 3A “Wrapped host”). This observation is further confirmed by quantification of the spatial distribution of collagen IV around normal and wrapped host vessels (Figure 3B-C).
Wrapping HUVECs reorganize BM of host vessels. (A) BM (blue, collagen IV staining) morphology is normal around unwrapped host vessels (row 1). On wrapping by HUVECs, the collagen IV is more distant from the host ECs, lying outside the HUVEC layer (row 2). In maturing HUVEC segments, the collagen IV pattern appears more normal (row 3). Whole-mount IHC stain; 20×/0.95NA water objective at 3.0× (row 1), 2.0× (row 2), and 4.0× (row 3) digital zoom; scale bar represents 40 μm, 60 μm, and 25 μm for rows 1, 2, and 3, respectively. (B) Typical spatial distributions of collagen IV relative to the host vessels (red line), HUVECs relative to host vessels (green line), and collagen IV relative to HUVEC vessels (blue line) for the areas within the white boxes in the “Merged” panels in panel A. (C) Quantification showing that the collagen IV is closer to normal host vessels than those that are wrapped. **P < .0001.
Wrapping HUVECs reorganize BM of host vessels. (A) BM (blue, collagen IV staining) morphology is normal around unwrapped host vessels (row 1). On wrapping by HUVECs, the collagen IV is more distant from the host ECs, lying outside the HUVEC layer (row 2). In maturing HUVEC segments, the collagen IV pattern appears more normal (row 3). Whole-mount IHC stain; 20×/0.95NA water objective at 3.0× (row 1), 2.0× (row 2), and 4.0× (row 3) digital zoom; scale bar represents 40 μm, 60 μm, and 25 μm for rows 1, 2, and 3, respectively. (B) Typical spatial distributions of collagen IV relative to the host vessels (red line), HUVECs relative to host vessels (green line), and collagen IV relative to HUVEC vessels (blue line) for the areas within the white boxes in the “Merged” panels in panel A. (C) Quantification showing that the collagen IV is closer to normal host vessels than those that are wrapped. **P < .0001.
Wrapping ECs express high levels of MMP-14 and MMP-9
Because rearrangement of the BM generally requires MMPs, we assessed the expression of collagen IV-specific MMPs during WAT by the various cell populations. MMP-14 (also known as membrane type 1 MMP or MT1-MMP) causes the degradation of collagen IV through activation of MMP-226 and was expressed strongly by HUVECs wrapping around host vessels. Staining was significantly weaker on host ECs and pericytes (Figure 4A,D; see explanation of analysis in “Quantification of MMP-14/MMP-9 expression”). MMP-9, another MMP with collagen IV activity,27,28 showed similar expression patterns with the strongest staining associated with wrapping HUVECs (Figure 4D; supplemental Figure 5A). We also checked MMP expression by the coimplanted 10T1/2 cells (supplemental Figure 5B) as well as F4/80-positive host macrophages (supplemental Figure 5C). Both cell populations had even less MMP expression than nonwrapping HUVECs (supplemental Figure 5D). In addition, these cells did not associate closely with regressing host vessels, suggesting that they are not physically involved in WAT anastomosis.
Wrapping HUVECs express high levels of MMP-14 and MMP-9, and inhibition of MMPs interferes with WAT anastomosis. (A) Strong MMP-14 expression (blue) colocalizes precisely with HUVECs (green, GFP transduction), but not with unwrapped parts of host vessels (red, mouse-specific MECA32, which is denoted mMECA32, staining) or pericytes (gray, NG2 staining). Filled arrowheads indicate unwrapped host ECs with weak MMP-14 staining; empty arrowheads indicate strong pericyte staining with weak MMP-14 signal. Whole-mount IHC stain; 20×/0.95 NA water objective at 3.0× digital zoom; scale bar represents 40 μm. (B) MMP-14 expression (blue) by wrapping HUVECs (filled arrowheads) is stronger than that by nonwrapping HUVECs (empty arrowheads) or maturing HUVEC vessels (arrows). Whole-mount IHC stain; 20×/0.95 NA water objective; scale bar represents 120 μm. (C) MMP-9 (blue) expression is strongest on wrapping HUVECs (filled arrowheads) compared with nonwrapping HUVECs (empty arrowheads) and maturing HUVEC vessels (arrows). “Box” shows the detail of the area within the white box. The dashed line divides the main HUVEC vessel in this panel into 2 parts: the one on the right is wrapping around some host vessel segments (empty arrows) and has very strong MMP9 staining, whereas the one on the left has no host vessel segments inside, is apparently maturing, and has much weaker MMP-9 staining. Whole-mount stain; 20×/0.95NA water objective; scale bar represents 120 μm. (D) MMP-14 expression (white bars) on unwrapped host ECs and pericytes is 46% ± 18% (n = 12) and 49% ± 16% (n = 12), respectively, that of wrapping HUVECs. MMP-9 expression (gray bar) by unwrapped host ECs is 59% ± 9% (n = 13) that of wrapping HUVECs. (E) MMP-14 expression by HUVECs changes with segment diameter and wrapping status. The normalized value is 0.67 ± 0.23 (n = 94) for “Individual,” nonwrapping HUVECs, 1.00 ± 0.18 (n = 25) for “Wrapping” HUVECs, and 0.54 ± 0.16 (n = 38) for “Maturing” HUVEC vessels. (F) MMP-9 expression by HUVECs also changes with segment diameter and wrapping status. The normalized value is 0.67 ± 0.20 (n = 153) for “Individual,” nonwrapping HUVECs, 1.000 ± 0.176 (n = 37) for “Wrapping” HUVECs, and 0.569 ± 0.126 (n = 47) for “Maturing” HUVEC vessels. (G) Typical perfusion (visualized by intravenous injection of Rho-dextran, which is shown in red) of implanted HUVEC networks (green, GFP transduction) 2 weeks after implantation when the animal is treated with control IgG, monoclonal antibody against MMP-14 (DX-2400), monoclonal antibody against MMP-9 (DX-2802), or DX-2400 + DX-2802. Intravital confocal images; 10×/0.3 NA air objective; scale bar represents 200 μm. (H) Treatment with DX-2400 and/or DX-2802 delays the onset of WAT anastomosis. Data were generated from the analysis of images, such as those shown in panel G. ***P < .05 between “Control IgG” and each of the other 3 groups. *P < .05 only between “Control IgG” and “DX-2400 + DX-2802.”
Wrapping HUVECs express high levels of MMP-14 and MMP-9, and inhibition of MMPs interferes with WAT anastomosis. (A) Strong MMP-14 expression (blue) colocalizes precisely with HUVECs (green, GFP transduction), but not with unwrapped parts of host vessels (red, mouse-specific MECA32, which is denoted mMECA32, staining) or pericytes (gray, NG2 staining). Filled arrowheads indicate unwrapped host ECs with weak MMP-14 staining; empty arrowheads indicate strong pericyte staining with weak MMP-14 signal. Whole-mount IHC stain; 20×/0.95 NA water objective at 3.0× digital zoom; scale bar represents 40 μm. (B) MMP-14 expression (blue) by wrapping HUVECs (filled arrowheads) is stronger than that by nonwrapping HUVECs (empty arrowheads) or maturing HUVEC vessels (arrows). Whole-mount IHC stain; 20×/0.95 NA water objective; scale bar represents 120 μm. (C) MMP-9 (blue) expression is strongest on wrapping HUVECs (filled arrowheads) compared with nonwrapping HUVECs (empty arrowheads) and maturing HUVEC vessels (arrows). “Box” shows the detail of the area within the white box. The dashed line divides the main HUVEC vessel in this panel into 2 parts: the one on the right is wrapping around some host vessel segments (empty arrows) and has very strong MMP9 staining, whereas the one on the left has no host vessel segments inside, is apparently maturing, and has much weaker MMP-9 staining. Whole-mount stain; 20×/0.95NA water objective; scale bar represents 120 μm. (D) MMP-14 expression (white bars) on unwrapped host ECs and pericytes is 46% ± 18% (n = 12) and 49% ± 16% (n = 12), respectively, that of wrapping HUVECs. MMP-9 expression (gray bar) by unwrapped host ECs is 59% ± 9% (n = 13) that of wrapping HUVECs. (E) MMP-14 expression by HUVECs changes with segment diameter and wrapping status. The normalized value is 0.67 ± 0.23 (n = 94) for “Individual,” nonwrapping HUVECs, 1.00 ± 0.18 (n = 25) for “Wrapping” HUVECs, and 0.54 ± 0.16 (n = 38) for “Maturing” HUVEC vessels. (F) MMP-9 expression by HUVECs also changes with segment diameter and wrapping status. The normalized value is 0.67 ± 0.20 (n = 153) for “Individual,” nonwrapping HUVECs, 1.000 ± 0.176 (n = 37) for “Wrapping” HUVECs, and 0.569 ± 0.126 (n = 47) for “Maturing” HUVEC vessels. (G) Typical perfusion (visualized by intravenous injection of Rho-dextran, which is shown in red) of implanted HUVEC networks (green, GFP transduction) 2 weeks after implantation when the animal is treated with control IgG, monoclonal antibody against MMP-14 (DX-2400), monoclonal antibody against MMP-9 (DX-2802), or DX-2400 + DX-2802. Intravital confocal images; 10×/0.3 NA air objective; scale bar represents 200 μm. (H) Treatment with DX-2400 and/or DX-2802 delays the onset of WAT anastomosis. Data were generated from the analysis of images, such as those shown in panel G. ***P < .05 between “Control IgG” and each of the other 3 groups. *P < .05 only between “Control IgG” and “DX-2400 + DX-2802.”
Interestingly, MMP expression correlated with the size and wrapping status of HUVEC structures: MMP-14 (Figure 4B,E) and MMP-9 (Figure 4C,F) levels were low on individual, nonwrapping HUVEC structures, higher on HUVECs wrapping around host vessels, and low again on larger, maturing HUVEC segments. Other studies have shown that MMP-14 and MMP-9 expression is necessary for autologous endothelial lumen formation and angiogenesis in vitro29 and in vivo.30,31 Our results support this finding: these MMPs are expressed by practically all HUVECs, including those involved in lumen formation. However, our results further suggest a previously unknown function of MMP-14 and MMP-9 during WAT anastomosis: their activities are up-regulated in the wrapping ECs, apparently to destabilize the existing host vessel wall structure. After disappearance of the underlying host BM and ECs, HUVEC MMP levels returned to baseline.
WAT anastomosis is delayed when MMP-9 and/or MMP-14 are pharmacologically inhibited
To determine whether MMP activity is necessary for host vessel degradation during WAT anastomosis, we treated animals with GM6001, a broad-spectrum MMP inhibitor. After waiting for the engrafted HUVECs to wrap around many of the host vessels (9 days), we started treatment and then tracked 8 or 9 prewrapped host vessel segments in the cranial window for 2 weeks. This procedure allowed us to focus specifically on MMP involvement in host vessel degradation by wrapping HUVECs, rather than other processes, such as EC migration or lumenogenesis. GM6001 treatment significantly reduced the degradation of the underlying host segments, allowing many of the wrapped, bilayer structures to persist. The percentage of wrapped host segments that had degraded was 68.1% ± 6.3% in control animals and 38.9% ± 9.6% in those treated with GM6001 (P < .05; see also supplemental Figure 5E). To further investigate the roles of MMP-14 and MMP-9 in WAT anastomosis, we treated animals with monoclonal antibodies against MMP-14 (DX-2400) and/or MMP-9 (DX-2802) every other day for 6 weeks and quantified the fraction of HUVEC network that was perfused during this period (see “Quantification of the perfused fraction of engrated vessels”). Blocking either MMP-14 or MMP-9 significantly suppressed perfusion of the HUVEC network during the first 4 weeks after implantation, and there was an additional reduction with simultaneous inhibition of both MMPs (Figure 4G-H). However, the effect of MMP inhibition gradually diminished, and the difference was only significant between the control group and the group treated with both antibodies at 6 weeks after implantation. Therefore, it is possible that activation of other gelatinases can compensate for the loss of MMP-14 and MMP-9 activity.
Considering the complex cellular interactions required by WAT anastomosis, it would be surprising if the host endothelial barrier were maintained throughout the process. Although, for the most part, this seemed to be achieved, we did observe transient increases in vessel leakiness in wrapped host vessels (supplemental Figure 5F; see also supplemental Video 7). Such transient plasma leaks were not observed in normal host vessels under similar imaging conditions.
WAT anastomosis also occurs during engraftment of syngeneic vascular networks
To verify that WAT anastomosis is not an artifact of xenografting human ECs into mice, we repeated the experiment with syngeneic PMBECs. We collected GFP-expressing PMBECs from Tie2-GFP/Rag1−/− mice and implanted them into cranial windows of Rag1−/− mice, allowing fluorescence visualization of the engrafted PMBECs. The PMBECs formed networks and anastomosed with the host vasculature even more vigorously than the HUVECs: at the same seeding density, PMBECs formed vascular networks in which 53.2% ± 6.3% of all segments were perfused 2 weeks after implantation, compared with 5.90% ± 4.7% for HUVECs (Figure 5A; supplemental Figure 6). Consistent with WAT anastomosis, segments of host vessels near the gel-tissue interface were replaced by vessels that had formed from the implanted PMBECs (Figure 5B). This was often accompanied by significant remodeling of the affected local host vascular network and alteration of blood flow (Figure 5B). Furthermore, by performing the inverse experiment (implanting PMBECs from Rag1−/− mice into cranial windows of Tie2-GFP/Rag1−/− mice) to visualize the host ECs specifically, we found isolated host vessel remnants within perfused, implanted vessels (Figure 5C; compare with Figure 1D), consistent with WAT anastomosis.
WAT anastomosis with syngeneic EC networks. (A) The perfused fraction of engrafted vessels was assessed at days 3, 6, 9, and 13 after implantation. PMBECs anastomosed with the host vasculature much more rapidly and efficiently than HUVECs. Three to 5 images (1280 μm × 1280 μm) from each of 3 animals were analyzed for each group at each time point. (B) Dynamics at the interface of engrafted and host networks. PMBECs from Tie2-GFP/Rag1−/− mice (green) migrate to and wrap around host vessels (red, mCD31-X647 injected intravenously) of a Rag1−/− mouse. Note that because perfused host vessels, perfused PMBEC vessels, and wrapping PMBECs are all labeled with the injected mCD31-X647 antibody, the replacement of host vessels by implanted PMBECs can only be identified when a vessel that was previously red (ie, unwrapped and perfused host vessel) has become yellow (PMBECs with or without regressing host vessel segments inside). The illustration in the right-hand column shows the major segments involved and indicates the locations of attachment to nearby networks consisting only of implanted PMBECs (green arrows) or host ECs (red arrows). This column also shows the fate of various segments in the tracked host vascular network. Once the orange section has been wrapped by the engrafted PMBECs, the adjacent purple segment regresses and the blue segment dilates. By day 11, there is extensive fluid communication between the implanted and host networks because of WAT anastomosis in this region. Intravital confocal images; 20×/0.4 NA air objective at 2.0× digital zoom; scale bar represents 50 μm. (C) Ten-day-old implant showing remnants of host vessels from the Tie2-GFP/Rag1−/− mouse (green, filled arrowheads) within vessels formed from implanted PMBECs collected from Rag1−/− mice (red, mCD31 staining). Note that mCD31 stains both the host and the implanted ECs. “Box” shows the area within the white box, but with fewer z-slices included in the projection to eliminate some of the overlying and underlying vessels. Whole-mount IHC stain; 60×/1.1 NA oil objective; scale bar represents 30 μm.
WAT anastomosis with syngeneic EC networks. (A) The perfused fraction of engrafted vessels was assessed at days 3, 6, 9, and 13 after implantation. PMBECs anastomosed with the host vasculature much more rapidly and efficiently than HUVECs. Three to 5 images (1280 μm × 1280 μm) from each of 3 animals were analyzed for each group at each time point. (B) Dynamics at the interface of engrafted and host networks. PMBECs from Tie2-GFP/Rag1−/− mice (green) migrate to and wrap around host vessels (red, mCD31-X647 injected intravenously) of a Rag1−/− mouse. Note that because perfused host vessels, perfused PMBEC vessels, and wrapping PMBECs are all labeled with the injected mCD31-X647 antibody, the replacement of host vessels by implanted PMBECs can only be identified when a vessel that was previously red (ie, unwrapped and perfused host vessel) has become yellow (PMBECs with or without regressing host vessel segments inside). The illustration in the right-hand column shows the major segments involved and indicates the locations of attachment to nearby networks consisting only of implanted PMBECs (green arrows) or host ECs (red arrows). This column also shows the fate of various segments in the tracked host vascular network. Once the orange section has been wrapped by the engrafted PMBECs, the adjacent purple segment regresses and the blue segment dilates. By day 11, there is extensive fluid communication between the implanted and host networks because of WAT anastomosis in this region. Intravital confocal images; 20×/0.4 NA air objective at 2.0× digital zoom; scale bar represents 50 μm. (C) Ten-day-old implant showing remnants of host vessels from the Tie2-GFP/Rag1−/− mouse (green, filled arrowheads) within vessels formed from implanted PMBECs collected from Rag1−/− mice (red, mCD31 staining). Note that mCD31 stains both the host and the implanted ECs. “Box” shows the area within the white box, but with fewer z-slices included in the projection to eliminate some of the overlying and underlying vessels. Whole-mount IHC stain; 60×/1.1 NA oil objective; scale bar represents 30 μm.
Discussion
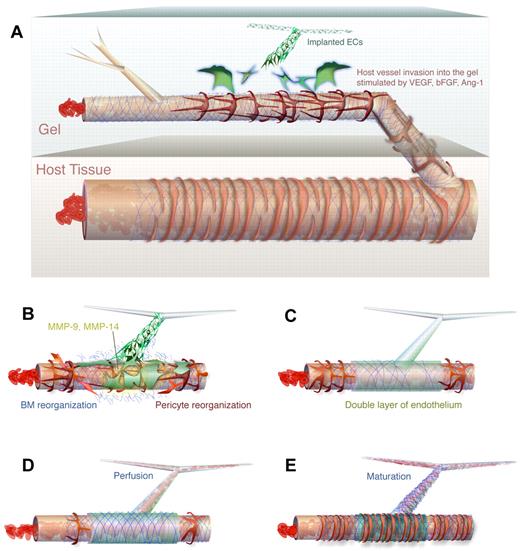
In this study, we have identified the cellular interactions that result in anastomosis of implanted vascular networks with the host vasculature. Instead of the expected mechanism involving connections between endothelial tip cells and vacuole fusion, engrafted vascular networks wrap around existing host vessels and express high levels of MMP-14 and MMP-9. The underlying host vessel is disrupted, thereby diverting blood flow into the nascent, implanted network (Figure 6). There is little other information in the literature concerning processes similar to WAT anastomosis. One study in zebrafish suggests that ECs from one vessel can replace those in another during anastomosis; however, further analysis is needed to determine whether this process indeed occurs by WAT anastomosis.32 For tissue engineering applications or transplants, this study points to possible strategies for promoting WAT anastomosis. Appropriate manipulation of local MMP-14 and/or MMP-9 levels, for example, may produce faster perfusion of engrafted vascular networks.
Mechanism of WAT anastomosis. (A) Nonincorporated ECs (green) organize in the implanted gel to form an unperfused network in the vicinity of a perfused host vessel that has grown into the gel. The host vessel consists of host ECs (brown), BM (blue), and pericytes (red) and contains flowing blood. (B) Engrafted ECs associated with the nascent network wrap around the existing host vessel segment and produce MMPs (yellow), which cause reorganization of the existing BM and pericytes. (C) The engrafted ECs lie on top of the host segment to form a bilayer. At this stage, there is not yet perfusion of the nascent vascular network. Note that the wrapping coverage is generally not as complete as depicted. (D) On degradation of a portion of the underlying host endothelium, blood is allowed to flow into the nascent vascular network. (E) The engrafted ECs are fully incorporated, forming junctions with the host ECs. The segment has matured, achieving normal BM and pericyte fortification.
Mechanism of WAT anastomosis. (A) Nonincorporated ECs (green) organize in the implanted gel to form an unperfused network in the vicinity of a perfused host vessel that has grown into the gel. The host vessel consists of host ECs (brown), BM (blue), and pericytes (red) and contains flowing blood. (B) Engrafted ECs associated with the nascent network wrap around the existing host vessel segment and produce MMPs (yellow), which cause reorganization of the existing BM and pericytes. (C) The engrafted ECs lie on top of the host segment to form a bilayer. At this stage, there is not yet perfusion of the nascent vascular network. Note that the wrapping coverage is generally not as complete as depicted. (D) On degradation of a portion of the underlying host endothelium, blood is allowed to flow into the nascent vascular network. (E) The engrafted ECs are fully incorporated, forming junctions with the host ECs. The segment has matured, achieving normal BM and pericyte fortification.
An interesting question raised by this study is: in what natural physiologic or pathologic conditions would WAT anastomosis operate? We suspect that this mechanism is not prevalent in systems in which the tissue and vasculature grow in concert, as in developmental vasculogenesis or angiogenesis. Instead, it is probably more frequent during revascularization of tissues with relatively large regions of ischemia, caused by vascular damage or occlusion. Rather than relying only on sprouting angiogenesis from existing vessels, resident ECs and EPCs could form networks independent of the existing vasculature and then tap into the existing blood flow at the periphery of the ischemic region. Similarly, it may play a role in vascularization of tumors, where rapid proliferation of cancer cells can produce large regions of hypoxia. This concept is supported by studies showing that vasculogenesis by bone marrow-derived cells is the major mode of vascularization of irradiated glioblastomas33 and naive Ewing sarcomas.34 These cells might infiltrate the hypoxic regions, form networks independent of the existing vasculature, and then connect to the blood supply via WAT anastomosis. High-resolution, longitudinal, intravital imaging of specifically labeled cell populations would be necessary to verify this mechanism in tumors.
Our findings underscore the importance of microenvironmental context in determining cell behavior. The sequence of events resulting in formation and perfusion of the engineered vascular networks included (1) individual, isolated ECs embedded in the 3D gel extending to connect with neighbors and then forming lumens via vacuole fusion, (2) engrafted ECs at the host-implant interface contacting an existing vessel wall and spreading on it, (3) host ECs with improper BM disappearing, and (4) maturation of newly perfused, engrafted vessels. At each step, the ECs were integrating signals from the environment to determine behavior, including microanatomic information (eg, the nature and distribution of adjacent extracellular matrix, other cells, and blood flow) as well as soluble signals, such as angiogenic growth factors and cytokines. A better understanding of such microenvironmental cues, and how they are integrated by the ECs, may allow development of more effective, and more precise, strategies for modulating vasculogenesis and angiogenesis in the clinic.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Peigen Huang for his support with the animal care, Lei Xu for her advice on the molecular dissection and Sylvie Roberge and Julia Kahn for preparation of the window models.
This work was supported by the National Institutes of Health (grant HL64240, L.L.M.; grant PO1 CA80124, R.K.J., L.L.M., and D.F.; and grant R01-CA126642, L.L.M.) and Federal Share/National Cancer Institute (Proton Beam Program Income Grants, R.K.J., L.L.M., and D.F.; and grant R01-CA096915, D.F.).
National Institutes of Health
Authorship
Contribution: G.C. performed experiments, was involved in project direction, analyzed data, and wrote the manuscript; S.L. performed experiments and analyzed data; D.A.L. and E.d.T. provided IHC expertise and helped with the NG2 staining analysis; H.K.W. helped with the isolation of PMBECs; P.A., D.F., and R.K.J. provided expertise on the engineered vessel model and MMP blockade studies; D.F. developed the Tie2-GFP/Rag1−/− mice; and L.L.M. conceived the study, provided overall direction, and cowrote the manuscript.
Conflict-of-interest disclosure: R.K.J. is a consultant for and grantee of Dyax Corp; Dyax supplied antibodies for the studies. The remaining authors declare no competing financial interests.
The current affiliation of G.C. is BeiGene Co Ltd, Beijing, China. The current affiliation of E.d.T. is Novartis Institute for Biomedical Research Inc, Cambridge, MA. The current affiliation of P.A. is Food and Drug Administration, Office of Cellular, Tissue and Gene Therapies, Rockville, MD.
Correspondence: Lance L. Munn, Department of Radiation Oncology, Massachusetts General Hospital and Harvard Medical School, 100 Blossom Street, Cox-7, Boston, MA 02114; e-mail: munn@steele.mgh.harvard.edu.
References
Author notes
G.C. and S.L. contributed equally to this study.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal