Abstract
Studies of haploidentical hematopoietic stem cell transplantation (HSCT) have identified threshold doses of T cells below which severe GVHD is usually absent. However, little is known regarding optimal T-cell dosing as it relates to engraftment, immune reconstitution, and relapse. To begin to address this question, we developed a 2-step myeloablative approach to haploidentical HSCT in which 27 patients conditioned with total body irradiation (TBI) were given a fixed dose of donor T cells (HSCT step 1), followed by cyclophosphamide (CY) for T-cell tolerization. A CD34-selected HSC product (HSCT step 2) was infused after CY. A dose of 2 × 108/kg of T cells resulted in consistent engraftment, immune reconstitution, and acceptable rates of GVHD. Cumulative incidences of grade III-IV GVHD, nonrelapse mortality (NRM), and relapse-related mortality were 7.4%, 22.2%, and 29.6%, respectively. With a follow-up of 28-56 months, the 3-year probability of overall survival for the whole cohort is 48% and 75% in patients without disease at HSCT. In the context of CY tolerization, a high, fixed dose of haploidentical T cells was associated with encouraging outcomes, especially in good-risk patients, and can serve as the basis for further exploration and optimization of this 2-step approach. This study is registered at www.clinicaltrials.gov as NCT00429143.
Introduction
Until recently, HLA haploidentical hematopoietic stem cell transplantation (HSCT) has often been associated with disappointing clinical outcomes, limiting the widespread application of this approach. Higher rates of infection and relapse, 2 consequences of the T-cell depletion required to prevent severe GVHD in recipients of HLA mismatched grafts, adversely affect long-term survival, particularly in patients receiving HSCT late in their disease course. Because only a subset of appropriate transplantation candidates has an HLA-identical sibling or unrelated donor, the development of safer, more efficacious transplantation approaches using haploidentical donors would provide potential transplantation options for patients who lack well-matched donors.
Based on murine models, clinical approaches to haploidentical transplantation initially relied on aggressive T-cell depletion techniques. Ex vivo T-cell depletion with soybean agglutinin and E rosetting or the use of mAbs such as T10B9 resulted in BM products containing T-cell doses in the range of 104-105 T cells/kg of recipient body weight. This degree of T-cell depletion was associated with the attenuation of severe GVHD and provided consistent engraftment, particularly when antithymocyte globulin was also administered.1,2 The correlate of a high degree of T-cell depletion to avoid GVHD is delayed posttransplantation immune recovery,3-9 mortality from infection and relapse,1,10-16 and higher rates of graft rejection compared with T cell–containing regimens.17 Ruggeri et al18 demonstrated that the higher relapse rates associated with T-cell depletion can be moderated in part by maximizing natural killer cell alloreactivity. However, infectious mortality still remains an obstacle to long-term survival even with this approach.
Much of the data regarding the permissible content of T cells in the donor inocula were necessarily based on the correlation between T-cell doses and the development of GVHD as opposed to immune reconstitution. Severe GVHD was infrequent to absent with T-cell doses of < 1 × 105/kg in adults undergoing matched-sibling HSCT 19 and < 5 × 104/kg in children undergoing haploidentical HSCT.20 Huang et al21 reported encouraging rates of disease-free survival but a cumulative incidence of chronic GVHD that exceeded 50% when a median T-cell dose of 2.4 × 108/kg was administered with antithymocyte globulin to recipients of haploidentical HSCT using combined blood and BM products from recombinant human G-CSF–primed donors.
The use of T-cell add-back,22 selective lymphocyte depletion of donor grafts,23,24 and preemptive donor lymphocyte infusion (DLI)25,26 are strategies used after T cell–depleted haploidentical HSCT to preserve or restore the beneficial immune reconstituting effects of T cells. Another approach involves the use of posttransplantation cyclophosphamide (CY) after nonmyeloablative BM grafts from haploidentical donors to preferentially delete activated lymphocytes as opposed to a nonselective depletion of all CD3+ T cells.27-29 In one study,28 there were low infectious rates and little significant GVHD associated with the infusion of donor products containing a mean number of 4.2 × 107/kg of T cells. Despite the use of these relatively high T cell–containing products, the rejection rate of 13% was higher and the disease free survival rate was lower than that reported by Huang et al,21 possibly because of the administration of comparatively fewer T cells, the use of a nonmyeloablative conditioning regimen, or a combination of the two.
These trials demonstrate that outcomes after haploidentical HSCT can be influenced by the dosing, timing, and treatment of donor T cells. Potential barriers to further progress include the lack of consistency in T-cell dosing from which to compare and optimize outcomes and methodologies to deliver consistent T-cell doses at the time of HSCT. An ideal approach would maximize the number of “safe,” non-alloreactive T cells, avoiding the problems of GVHD while preserving the beneficial effects of T cells with regard to engraftment, infectious complications, and relapse. Moreover, whereas murine models of transplantation virtually always administer a fixed dose of T cells to produce more consistent immunologic outcomes, human HSCT grafts contain a more highly variable number of passenger lymphocytes.
We developed a 2-step myeloablative approach to haploidentical HSCT for which the primary goal was to provide a fixed—and ideally maximized—dose of T cells in the context of CY tolerization. Myeloablative rather than reduced intensity conditioning was used to provide more treatment intensity to high-risk patients. In the setting of myeloablative conditioning, we sought to maximize the number of T cells that could be safely administered and to use the higher number of stem cells that can be obtained from peripheral blood rather than BM to avoid rejection. Because there was no data regarding T-cell dosing with CY tolerization in a myeloablative setting, the trial was designed as a phase 1/2 study in which an optimal dose of T cells would be initially determined based on the incidences of graft rejection and of GVHD. The goal of the phase 2 part of the study was to assess whether this optimized dose of CY-tolerized T cells would result in rapid immune constitution and low rates of severe infection, rejection, relapse, and significant GVHD, thus resulting in improved overall survival.
Methods
The 2-step transplantation regimen
Patients received 12 Gy of total body irradiation (TBI) administered in 8 fractions over 4 days on days −9 to −6. After the last fraction of TBI, a DLI product was administered to deliver a specific dose of donor CD3+ T cells (see next paragraph), representing step 1 (the lymphoid portion) of the transplantation. Days −5 and −4 were rest days. CY 60 mg/kg/d was given on days −3 and −2. Tacrolimus and mycophenolate mofetil were initiated on day −1 for GVHD prophylaxis. A CD34-selected donor stem cell product was infused on day 0, representing step 2 (the stem cell portion) of the transplantation. GM-CSF 250 μg/m2 was begun on day +1. No steroids were permissible until after the second dose of CY. In the absence of GVHD, mycophenolate mofetil was discontinued on day 28 after HSCT and a tacrolimus taper was initiated by day +60.
Study design and end points
The primary end point of the phase 1 part of the study was to determine the optimal (or maximum feasible) dose of CD3+ T cells that could be given with CY tolerization that would result in reliable engraftment without significant GVHD. After review of T-cell numbers in allogeneic peripheral stem cell products at our institution, we hypothesized that 2 × 108 CD3+ cells/kg would produce consistent engraftment, and therefore started the trial at that dose. The study design was such that the dose of T cells would be escalated if excessive graft failure was observed or decreased if excessive GVHD was observed. If excessive graft failure and GVHD were both observed at the same T-cell dose, the study would close. The study was also designed to close if after 4 dose adjustments (up or down), an appropriate T-cell dose could not be identified. Once a dose was identified at which 6 patients achieved successful engraftment without significant (grade III/IV) GVHD, the phase 1 part of this protocol would close, and subsequent patients would be treated at this dose in the phase 2 portion of the trial.
Because our goal was to develop a regimen that allowed haploidentical HSCT to be performed with low treatment-related mortality (TRM) and because we anticipated a high relapse rate in the high-risk patients likely to enter such a trial, the primary end point of the phase 2 part of the trial was to demonstrate an overall survival of ≥ of 30% at 6 months. Secondary end points included the assessment of engraftment rates, immune reconstitution and infection, and incidence and severity of GVHD.
Recipient consent, eligibility, and donor selection
Written informed consent was obtained for all of the patients in accordance with the Declaration of Helsinki. The study was approved by the institutional review board of Thomas Jefferson University. Patients were eligible for inclusion if they had received front-line therapy for their disease, were without an available genotypically identical related donor, had an available related donor that was mismatched for > 2 HLA antigens (HLA-A, B, C, DRB1) in the GVH direction, adequate organ function as defined by a serum creatinine of ≤ 2.0 mg/dL or creatinine clearance of > 40 mL/min, pulmonary diffusion capacity > 45% (corrected for hemoglobin), cardiac ejection fraction > 45%, a Karnofsky Performance Status > 70%, were HIV negative, were not pregnant, and had no other active malignancies. Donors were selected to try to maximize anti-host alloreactivity based on factors such as a higher degree of HLA mismatch or the presence of KIR mismatches.
Collection of cells, graft characteristics, and processing
Donors underwent apheresis on days −7 and −6 to collect the DLI product. The desired dose of CD3+ cells was infused without manipulation after the last fraction of TBI on day −6. After collection of the DLI product, donors received subcutaneous injections of G-CSF, 5 μg/kg twice a day on days −5 through −1 and underwent apheresis for HSCs on days −2 and −1. The HSC product underwent CD34 selection using the Isolex 300i magnetic cell selection system (Baxter), followed by treatment with muromonab-CD3 (OKT-3; Ortho-Biotech) to decrease residual T-cell numbers after selection. The product was washed after OKT-3 incubation before infusion to ensure that any infused OKT-3 was cell bound and that free OKT-3 was not administered. Processing and infusion of the HSC product occurred on day 0.
Definitions
White cell engraftment was defined as an absolute neutrophil count of > 0.5 × 109/L for at least 3 consecutive days after transplantation. Platelet engraftment was defined as a platelet count of > 20 000/μL without transfusion for the 7 preceding days. Toxicities were graded using National Cancer Institute (NCI) Common Toxicity Criteria. Acute GVHD was scored based on the Glucksberg system.30 Grades III-IV GVHD were termed “severe” GVHD. Chronic GVHD was based on the National Institutes of Health Consensus Criteria.31
Posttransplantation supportive care
Patients were monitored weekly with a quantitative CMV PCR assay performed on blood samples. If the test became positive, patients were preemptively treated with foscarnet or valganciclovir. Patients were given IVIG therapy every 3-4 weeks until the IgG levels returned to the normal range.
Statistical analysis
The phase 1 portion of the trial was based on a continual reassessment method and the final T-cell dose was determined based on the observed clinical outcomes as described in “Study design and end points.” The 6-month survival was estimated using the Kaplan-Meier method (SPSS Version 12 software). Cumulative incidence of grades II-IV GVHD, grades III-IV GVHD, engraftment, and relapse were all calculated with death as a competing risk using R Version 2.11.1.
Results
Patients
A total of 27 patients with a median age of 52 years (range, 19-67) with high-risk hematologic malignancies were treated between the years of 2006 and 2009. Patient, donor, and disease characteristics are listed in Table 1. Patients and donors were mismatched for 2 (n = 2), 3 (n = 11), or 4 (n = 13) HLA-A, B, C, or DR antigens in the GVHD direction. A single patient with 0 mismatches in the GVH direction and 4 mismatches in the host-versus-graft (HVG) direction because of HLA homozygosity was treated on the trial.
Patient characteristics
| Subjects, n | 27 |
| Median age, y (range) | |
| Recipient | 52 (19-67) |
| Donor | 39 (24-65) |
| Sex | |
| Male | 11 |
| Female | 16 |
| Race | |
| White | 19 |
| Black | 6 |
| Asian | 2 |
| Disease and disease status at HSCT | |
| AML CR1 with high-risk features* | 5 |
| AML CR2 | 2 |
| AML primary induction failure | 2 |
| AML in resistant relapse | 7 |
| Biphenotypic leukemia with disease at HSCT | 1 |
| ALL CR2 (Ph−) | 3 |
| ALL (Ph+) morphologic remission | 1 |
| MDS | 2 |
| NHL chemotherapy resistant | 3 |
| Aplastic anemia | 1 |
| Previous transplant | 2 |
| Secondary malignancy | 2 |
| Recipient/donor transplantation combinations | |
| Sibling-to-sibling | 7 |
| Parent-to-child | 4 |
| Child-to-parent | 16 |
| CMV serostatus recipient (R) and donor (D) | |
| R+/D+ | 12 |
| R+/D− | 6 |
| R−/D− | 9 |
| HLA antigen mismatches (GVH direction) (A, B, Cw, DRB1), n | |
| 4 | 13 |
| 3 | 11 |
| 2 | 2 |
| 0† | 1 |
| KIR mismatches‡ | |
| HLA-C group 1 | 5 |
| HLA-C group 2 | 4 |
| HLA-Bw4 | 1 |
| HLA-C and HLA-Bw4 | 2 |
| No KIR mismatch | 15 |
| Subjects, n | 27 |
| Median age, y (range) | |
| Recipient | 52 (19-67) |
| Donor | 39 (24-65) |
| Sex | |
| Male | 11 |
| Female | 16 |
| Race | |
| White | 19 |
| Black | 6 |
| Asian | 2 |
| Disease and disease status at HSCT | |
| AML CR1 with high-risk features* | 5 |
| AML CR2 | 2 |
| AML primary induction failure | 2 |
| AML in resistant relapse | 7 |
| Biphenotypic leukemia with disease at HSCT | 1 |
| ALL CR2 (Ph−) | 3 |
| ALL (Ph+) morphologic remission | 1 |
| MDS | 2 |
| NHL chemotherapy resistant | 3 |
| Aplastic anemia | 1 |
| Previous transplant | 2 |
| Secondary malignancy | 2 |
| Recipient/donor transplantation combinations | |
| Sibling-to-sibling | 7 |
| Parent-to-child | 4 |
| Child-to-parent | 16 |
| CMV serostatus recipient (R) and donor (D) | |
| R+/D+ | 12 |
| R+/D− | 6 |
| R−/D− | 9 |
| HLA antigen mismatches (GVH direction) (A, B, Cw, DRB1), n | |
| 4 | 13 |
| 3 | 11 |
| 2 | 2 |
| 0† | 1 |
| KIR mismatches‡ | |
| HLA-C group 1 | 5 |
| HLA-C group 2 | 4 |
| HLA-Bw4 | 1 |
| HLA-C and HLA-Bw4 | 2 |
| No KIR mismatch | 15 |
Based on cytogenetics, secondary disease, CNS/tissue involvement, or arising from MDS.
Patient had 4 mismatches in HVG direction only and was counted for toxicity only
KIR ligand missing in recipient but present in donor. Missing self as defined by Ruggeri et al.18
AML indicates acute myeloid leukemia; CR, complete remission; Ph, Philadelphia chromosome; ALL, acute lymphoblastic leukemia; MDS, myelodysplastic syndrome; and NHL, nonHodgkin lymphoma.
T-cell dose (transplantation step 1) and subsequent in vivo alloreaction
The initial T-cell dose in this trial was 2 × 108 CD3+ cells/kg. This dose resulted in consistent engraftment and acceptable rates of severe GVHD. Consequently, no dose escalation or de-escalation was performed and all study patients received this dose—except for 1 patient whose donor product contained only 1.7 × 108 T cells despite 2 days of apheresis. This patient engrafted and did not develop significant GVHD.
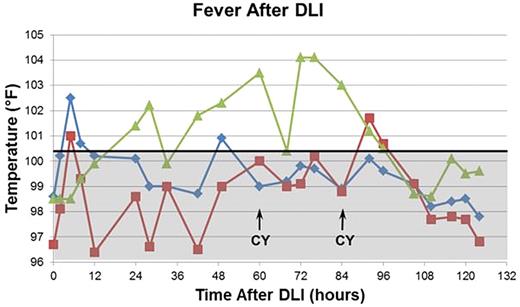
Unexpectedly, patients developed fever (median temperature, 103.8°F) within 24 hours of the DLI. Virtually all patients developed diarrhea coincident with or shortly after onset of the fever. Approximately 25% of patients developed rash in the same time frame. Two of the patients who developed a rash and diarrhea in this time frame underwent biopsies, which showed histopathologic evidence of GVHD. Fever and diarrhea were resistant to antipyretics and other supportive measures; however, in all cases, these symptoms abated after the second dose of CY. A typical fever curve is shown in Figure 1.
Fever curves after DLI are correlated with clinical events. A typical temperature curve from an engrafting patient is shown in green. Curves from the 2 patients with anti-donor antibodies who rejected their grafts are shown in red and blue. The boundary between the febrile and afebrile ranges (100.4°F) is shown by the horizontal solid black line. The afebrile range is shaded gray. Engrafting patients generally developed fever within 24 hours after DLI. The fever spikes persisted despite the use of acetaminophen and other comfort measures until after the second dose of CY. In the setting of anti-donor antibodies, patients developed fever within a few hours of the DLI, rapidly defervesced, and remained afebrile thereafter.
Fever curves after DLI are correlated with clinical events. A typical temperature curve from an engrafting patient is shown in green. Curves from the 2 patients with anti-donor antibodies who rejected their grafts are shown in red and blue. The boundary between the febrile and afebrile ranges (100.4°F) is shown by the horizontal solid black line. The afebrile range is shaded gray. Engrafting patients generally developed fever within 24 hours after DLI. The fever spikes persisted despite the use of acetaminophen and other comfort measures until after the second dose of CY. In the setting of anti-donor antibodies, patients developed fever within a few hours of the DLI, rapidly defervesced, and remained afebrile thereafter.
CD34 dose (transplantation step 2) and engraftment
CD34 and residual CD3 content of the second step of the graft is summarized in Table 2. Two patients died before engraftment could be evaluated. One patient with a flare of Crohn disease the week before HSCT (GVH direction mismatches = 0) developed hypotension and adult respiratory distress syndrome shortly after transplantation. He died on day +9 from a presumed bowel event related to his Crohn disease. A second patient died of respiratory syncytial virus pneumonitis (GVH direction mismatches = 3) on day +1. These 2 patients were evaluated for toxicity only.
Graft characteristics
| Step 1 . | Step 2 . | |
|---|---|---|
| CD3+/kg × 108 median (range) . | CD34+/kg × 106 median (range) . | Residual CD3+ cells/kg in CD34+ product × 104 median (range) . |
| 2.0 (1.7 in 1 patient, 2.0 in 26 patients) | 3.6 (1.3-7.4) | 0.51 (0.13-6.9) |
| Step 1 . | Step 2 . | |
|---|---|---|
| CD3+/kg × 108 median (range) . | CD34+/kg × 106 median (range) . | Residual CD3+ cells/kg in CD34+ product × 104 median (range) . |
| 2.0 (1.7 in 1 patient, 2.0 in 26 patients) | 3.6 (1.3-7.4) | 0.51 (0.13-6.9) |
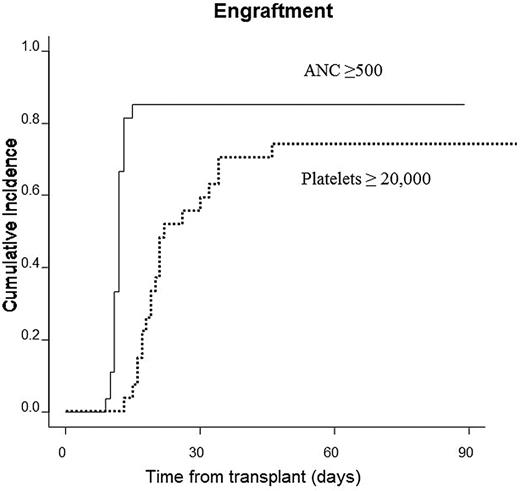
Twenty-three of the remaining 25 patients had full donor engraftment. Neutrophil recovery occurred at a median of 12 days (range = 9-15) and platelet recovery occurred at a median of 20.5 days (range = 15-46). Cumulative incidence of engraftment for neutrophils and platelets was 85.2% and 74.1%, respectively (Figure 2). Two multiparous females with multiple HLA antibodies rejected grafts from their daughters. The first patient had an HLA class I anti-donor antibody and was successfully engrafted using a reduced intensity conditioning regimen and an alternate haploidentical donor. The second patient demonstrated an HLA class II anti-donor antibody. This patient, who had no suitable alternate donor, was given 4 doses of rituximab and apheresis, followed by a reduced intensity conditioning regimen, and was successfully engrafted using the original donor. Both patients who rejected their transplantations had markedly different temperature curves compared with those who successfully engrafted, with an earlier appearance of the initial temperature rise but a far milder fever later in the course. Their fever curves were more typical during their second transplantations. The fever curves of these patients are shown in Figure 1.
Neutrophil and platelet engraftment. Cumulative incidences of neutrophil and platelet engraftment were 85.2% and 74.1%, respectively.
Neutrophil and platelet engraftment. Cumulative incidences of neutrophil and platelet engraftment were 85.2% and 74.1%, respectively.
TRM
There were 3 deaths because of multiorgan failure. These included the patient described above who had a flare of Crohn disease. The other 2 deaths occurred in the patients who experienced primary graft failure. Although these patients were subsequently successfully engrafted, both died of multiorgan failure presumably because of the combined toxicities of the conditioning regimens used for 2 transplantations in rapid succession.
Infection and immune reconstitution
Three of 27 patients (11%) died of infection (1 due to bacterial sepsis with subsequent brain abscess, 1 due to progression of preexisting fungal pneumonia, and 1 due to respiratory syncytial virus pneumonia) during the transplantation admission.
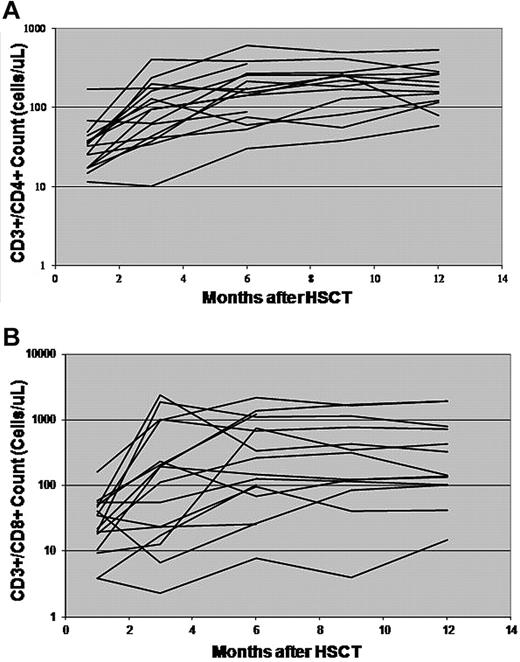
Sixteen patients alive and without evidence of recurrent disease at 6 months after HSCT were analyzed with regard to their immune reconstitution. Their median CD3+/CD4+ cell count at day +28 was 33.6 cells/μL (range, 11.5-171.8), increasing to a median of 104.6 cells/μL (range, 10-403.27) at day +100. The median CD3+/CD8+ cell count at day +28 was 28.7 cells/μL (range, 3.83-160.09), increasing to 151.3 cells/μL (range, 2.31-2379.8) at day +100. Lower CD3+/CD4+ and CD3+/CD8+ counts were associated with the use of corticosteroids for GVHD. The CD3+/CD4+ and CD3+/CD8+ counts for these patients are shown in Figure 3. Patients treated earlier in the trial who developed GVHD were treated more aggressively and appeared to have slower CD4 count recovery. There was no consistent pattern in the CD4/CD8 ratios, although several patients developed very elevated CD3+/CD8+ counts at the time of infections.
Immune recovery after haploidentical transplantation. CD3+/CD4+ cell counts (A) and CD3+/CD8+ cell counts (B) of 16 patients alive and disease-free at least 6 months after HSCT are shown.
Immune recovery after haploidentical transplantation. CD3+/CD4+ cell counts (A) and CD3+/CD8+ cell counts (B) of 16 patients alive and disease-free at least 6 months after HSCT are shown.
No patient died of complications related to CMV reactivation, although the majority of patients who were CMV seropositive developed evidence of reactivation (15 of 18) regardless of whether their donor was CMV seropositive or not. There were no cases of CMV tissue infection.
GVHD
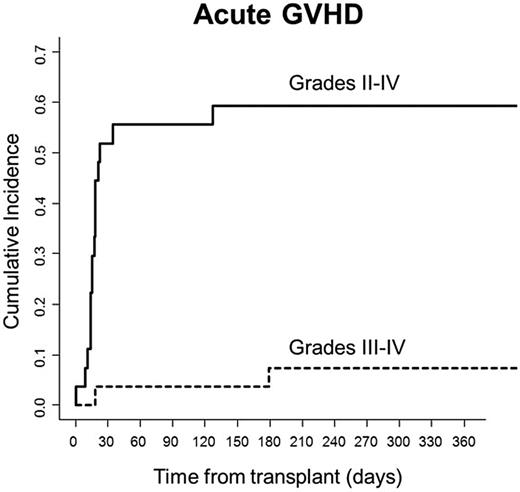
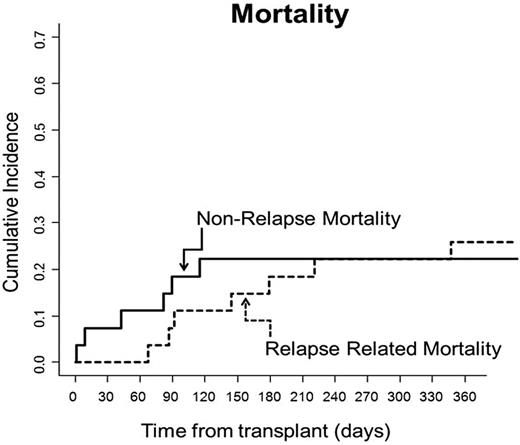
None of the 25 evaluable patients died from GVHD. Only 2 of 25 (8%) patients developed severe acute GVHD. One patient developed steroid-responsive grade 3 intestinal GVHD, and one patient, having previously developed stage III skin GVHD that resolved with steroids and photopheresis, later developed grade 4 liver GVHD that responded to treatment with OKT-3. Fourteen others developed grade 2 skin GVHD, with the majority of these patients (11) having stage III skin disease only without evidence of GVHD in the liver or gastrointestinal tract. Their skin GVHD responded to steroids (n = 8) or steroids plus photopheresis (n = 3). Three patients had grade 1 GVHD responding primarily to topical steroids. Four of 25 evaluable patients (16%) developed chronic GVHD with a score of 1. Cumulative incidences of grades II-IV and grades III-IV GVHD were 59.2% and 7.4%, respectively (Figure 4). Cumulative incidence of nonrelapse mortality (NRM) at the time of the most recent follow-up was 22.2% (Figure 5).
Acute GVHD. Cumulative incidences of grades II-IV and III-IV GVHD were 59.2% and 7.4%, respectively.
Acute GVHD. Cumulative incidences of grades II-IV and III-IV GVHD were 59.2% and 7.4%, respectively.
Relapse-related mortality and NRM. Cumulative incidences of relapse-related mortality and NRM were 29.6% and 22.2%, respectively.
Relapse-related mortality and NRM. Cumulative incidences of relapse-related mortality and NRM were 29.6% and 22.2%, respectively.
Relapse and overall survival
Relapsed disease was the primary cause of death in patients treated on this trial. Eight of 25 patients (32%) experienced a relapse of their malignancies 49-327 days after HSCT. Cumulative incidence of death due to relapse was 29.6% (Figure 5). Six of 13 patients with active malignancy at the time of HSCT subsequently relapsed, whereas only 2 of 12 patients who underwent transplantation while in remission relapsed afterward. All patients who relapsed ultimately died from their malignancy, and all patients who are alive are at least 28 months after HSCT without evidence of their disease.
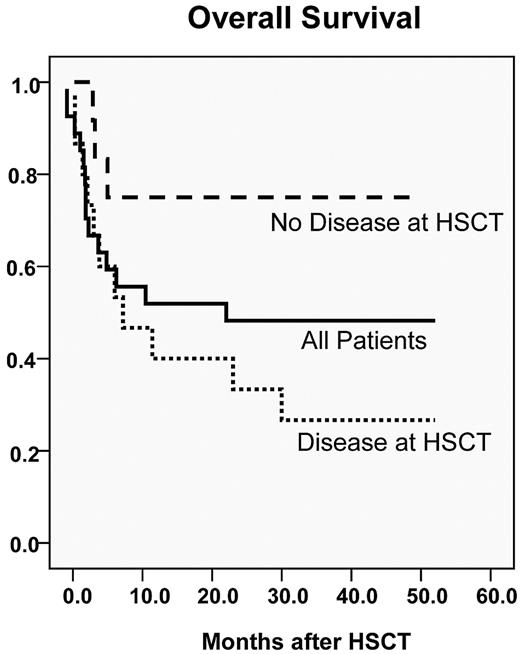
Seventeen of 27 patients (63%) were alive 6 months after their transplantation, satisfying the primary end point of the trial. Kaplan-Meier estimate of overall survival was 54% at 1 year and 48% at 3 years (median follow-up, 40 months; range, 28-56 months). Patients without disease at the time of HSCT fared better, with a projected overall survival of 75% at 3 years. Survival curves are shown in Figure 6. All surviving patients remain in complete remission and have been followed for a minimum of 28 months after transplantation.
Probability of overall survival. Survival for all patients in the trial is shown as the solid black line (48% 3-year overall survival). Patients without BM morphologic or radiographic evidence of disease at the time of transplantation are shown with the dashed line (75% 3-year overall survival). Patients with BM or radiographic evidence of disease are shown in the dotted line (27% 3-year overall survival).
Probability of overall survival. Survival for all patients in the trial is shown as the solid black line (48% 3-year overall survival). Patients without BM morphologic or radiographic evidence of disease at the time of transplantation are shown with the dashed line (75% 3-year overall survival). Patients with BM or radiographic evidence of disease are shown in the dotted line (27% 3-year overall survival).
Discussion
Our goals in developing this 2-step regimen were: (1) to develop a myeloablative regimen for haploidentical HSCT that could be administered to patients with refractory or relapsed hematologic malignancies initially, but would subsequently be appropriate for high-risk patients earlier in their disease course who lacked other donor options; (2) to avoid exposure of newly transplanted HSCs to high-dose CY; (3) to use peripheral blood rather than BM as the stem cell source; and (4) to fix and maximize the number of CD3 cells patients would receive in an effort to produce consistent outcomes after transplantation. Of these 4 goals, we considered the standardization of the CD3 dose to be the most essential. Every animal transplantation model carefully controls and fixes CD3 content, yet this is rarely done in clinical transplantation (with the exception of trials of T-cell depletion, in which the focus is often on a T-cell threshold rather than a dose). Essentially we believed that controlling the T-cell dose was equally important to the outcome of transplantation as prescribing specific doses of radiation and chemotherapy.
The initially tested T-cell dose of 2 × 108/kg was associated with prompt engraftment, little significant GVHD, and prompt immunologic recovery, and therefore met our phase 1 criteria for an optimal dose of T cells within the context of these immunologic outcome measures. In contrast to our own experience with T cell–depleted haploidentical HSCT, deaths from infection at this T-cell dose were very low despite a high rate of CMV reactivation. In many patients, the reactivation was accompanied by an increase in CD3+/CD4+ and CD3+/CD8+ T cells, although the circulating T cells were not tested for CMV specificity. In our prior experience with global T-cell depletion, patients remained severely T-cell lymphopenic throughout the course of viral infections. With CY tolerization, CMV reactivation was typically rapidly controlled once immune suppression was tapered. Twenty-one of 27 patients (78%) survived until discharge and all subsequent deaths were related solely to relapsed disease and not to TRM.
We did not anticipate the 2 rejections in patients with donor specific antibodies (DSAs), especially in the context of a large T-cell dose and the successful engraftment of a patient treated earlier in the trial with very broadly reactive anti-HLA antibodies who was without DSAs. It is possible that the atypical early fevers in these 2 patients were a reflection of an antibody-mediated lysis of the donor lymphocytes shortly after infusion, which allowed residual host humoral and cellular immunity to reject the graft. Rejection resulting from DSAs was not well described at the time this trial was launched, and a retrospective review of the literature revealed only one trial that described this phenomenon.32 Few additional reports have been published since this time, and the cumulative experience—including our own—suggests that the presence of DSAs is a significant risk factor for rejection of haploidentical grafts.33,34 In the absence of DSAs, > 60 patients treated with this 2-step approach either within this particular trial or subsequent to it have engrafted, demonstrating that without anti-donor antibodies, engraftment is consistent.
The incidence of GVHD was higher in this trial compared with that reported in other haploidentical trials in which CY tolerization was used as GVHD prophylaxis.28 This likely reflects the 5-fold higher T-cell dose that was administered, the aggressive taper of immune suppression, efforts to select the donor predicted to be most alloreactive among the available family members, and the more intensive conditioning regimen. Nonetheless, GVHD was primarily limited to the skin and was easily controlled with steroids or steroids plus early photopheresis. Photopheresis is thought to ameliorate GVHD in part by increasing CD4+CD25+/FoxP3+ Tregs.35,36 The role of Tregs in controlling haploidentical GVHD after using this 2-step HSCT approach is an area of current inquiry.
Chronic GVHD was infrequent and was not severe. This positive outcome likely contributed to the absence of late NRM because most patients did not require chronic immune suppression.
The febrile reaction, rash, and diarrhea that resulted from the T-cell infusion are reminiscent of the “haplo immunostorm” (HIS) described by Colvin et al37 when they infused similar doses of T cells after 2 Gy of radiation without other cytoreduction. In that setting, HIS was thought to be mediated by a cytokine storm and not to be associated with GVHD or engraftment syndrome. In our 2-step approach, the reaction resolved completely after 2 doses of CY and morphological analysis of skin and gut biopsies were consistent with GVHD. In the Colvin et al study, this phenomenon was observed when the CD3 dose reached 1 × 108/kg and was most pronounced at 2 × 108/kg, the dose used in this trial.37 Similar febrile reactions were not reported by O'Donnell et al,29 probably because their median T-cell dose was 5-fold lower than that used in our trial.
We believe that during this in vivo alloreaction, donor lymphocytes contribute to the immunologic elimination of residual malignancy. This is supported by the observation by Colvin et al that patients receiving haploidentical cellular therapy for resistant malignancy had clinical responses at the doses of T cells associated with HIS despite the minimal conditioning administered.
One of the rationales for the 2-step approach described here was that donor lymphocytes would be exposed to CY, whereas HSCs and progenitor cells would not. Whereas stem cells may be protected from mutagenic effects of CY because of their expression of aldehyde dehydrogenase, levels of this enzyme decrease as cells diverge from the stem cell phenotype. Some current models of leukemogenesis posit that mutations occur in a poststem-precursor cell that, as a consequence, reacquires stem properties. Whether secondary myelodysplastic syndrome or acute myeloid leukemia will emerge as problems in regimens in which HSCs are exposed to CY remains to be determined. The 2-step approach described herein eliminates that issue, however big or small it may be. It does require 4 days of apheresis for most donors (2 for DLI and 2 for HSC), but this has been well tolerated to date.
The 52% and 48% 1- and 3-year overall survival rates, respectively, observed in patients treated on this 2-step regimen met the protocol criteria for an effective regimen. Based on the current literature, the results appear to be comparable to outcomes in matched-sibling and unrelated-donor HSCT in similar high-risk groups of patients. All 6 patients treated for lymphoid diseases, 4 patients with high-risk acute lymphoblastic leukemia, and 2 patients with chemotherapy-resistant nonHodgkin lymphoma are disease free at 28-52 months after transplantation. Whether these favorable outcomes were because of the use of TBI in the conditioning regimen or to the large number of haploidentical T cells administered with this approach is unknown. If the latter is true, it may alter the spectrum of diseases in which strong graft-versus-tumor effects are observed after haploidentical transplantation.
Administration of the graft in 2 steps prevented the infusion of donor T cells that were skewed toward a TH2 phenotype, because T cells were collected before administering G-CSF to the donors. Recognizing the high-risk nature of the patients treated, we also administered GM-CSF rather than G-CSF after transplantation in a further attempt to avoid G-CSF–induced polarization to a TH2 phenotype.38 Despite this strategy, relapsed disease in patients with secondary myelodysplastic syndrome or acute myeloid leukemia was the primary cause of mortality. Interestingly, 6 of 8 (75%) recipients who relapsed, including the only 2 patients who relapsed after transplantation while in remission, were mothers receiving grafts from their children. Recent data support a mechanism of long-lasting Treg-mediated tolerance of maternal cells by offspring.39 Whereas child-to-mother transplantations have been associated with superior outcomes in other transplantation settings,40 in our limited population, they were associated with higher rates of relapse. It is possible that the outcomes associated with this donor-recipient combination are dependent on the particular disease state and transplantation regimen, which together will determine whether an increase or decrease in alloreactivity is likely to be beneficial or detrimental.
Building on the platform of a large and consistent T-cell dose, additional strategies can be explored with the potential to improve relapse rates in patients with advanced malignancies. Mayumi and Good demonstrated in murine models that the administration of CY 1-3 days after antigenic stimulation is the optimal time to establish allogeneic tolerance.41 The optimal timing in humans has not yet been studied directly. It may be worthwhile to assess whether the interval between DLI and CY can be safely increased modestly in future trials as a means of allowing further immunologically mediated cytoreduction by the DLI before abrogation by CY. Other drugs preferentially cytoxic to activated cells, such as melphalan,42,43 could potentially be introduced in place of CY using this 2-step approach without concerns about cytotoxicity toward HSCs. This platform also allows the use of 2 haploidentical donors to further increase the graft-versus-tumor effect and eliminate the potential for leukemic escape due to uniparental disomy.44
The results from this trial have been encouraging among patients undergoing transplantation in remission with a follow-up of in excess of 2 years for all patients. The low regimen-related mortality and high overall survival rates suggest that this approach can be extended to better-risk patients earlier in their disease course and should be further studied in larger trials.
Presented in part in poster form at the 51st Annual Meeting of the American Society of Hematology, New Orleans, LA, December 6, 2009.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are grateful to all of the clinicians at the Jefferson Kimmel Cancer Center for their excellent care of our transplantation patients.
Authorship
Contribution: D.G. and N.F. designed and performed the research, analyzed and interpreted the results, and drafted the manuscript; D.G. and T.H. performed the statistical analyses; M.C., J.F.-O, M.K., J.L.W, P.F., W.O., M.W.-W., B.M., and J.B., performed the research and contributed to the writing of the manuscript; P.C.-F. and B.C. performed and analyzed the research; and M.W. analyzed the research and contributed to the drafting of the manuscript.
Conflict-of-interest disclosure: B.M. has stock options with Incyte Corporation and AstraZeneca and is currently employed by Incyte Corporation. The remaining authors declare no competing financial interests.
Correspondence: Dolores Grosso, DNP, Blood and Marrow Transplant Program, Thomas Jefferson Kimmel Cancer Center, Suite 320 Ben Franklin Bldg, Philadelphia, PA 19107; e-mail: dolores.grosso@jefferson.edu.