Abstract
Little information exists regarding long-term psychological health of hematopoietic cell transplantation (HCT) survivors. Using resources offered by the Bone Marrow Transplant Survivor Study (BMTSS), we evaluated adverse psychological outcomes in 1065 long-term HCT survivors and a healthy comparison group composed of siblings. Psychological health status was evaluated using the Brief Symptom Inventory-18. Twenty-two percent of the HCT survivors reported adverse psychological outcomes, compared with 8% of the siblings. Exposure to prednisone was associated with psychological distress across all domains (anxiety, depression, and somatic distress). Fifteen percent of the HCT survivors reported somatic distress, representing an almost 3-fold higher risk comparing to siblings. Among survivors, in addition to low annual household income and self-reported poor health, having severe/life-threatening conditions and presence of active chronic GVHD were associated with a 2-fold increased risk for somatic distress. Seven percent of the HCT survivors expressed suicidal ideation; patients with higher scores on depression subscale were most vulnerable. This study demonstrates that somatic distress is the biggest challenge faced by survivors long after HCT. These results identify vulnerable subpopulations and provide patients, families, and healthcare providers with necessary information to plan for post-HCT needs many years after HCT.
Introduction
Hematopoietic cell transplantation (HCT) is an established curative option for hematologic malignancies. Advances in transplantation techniques and supportive care strategies have resulted in significant improvements in survival; > 70% of those who survive the first 2 years after HCT are expected to become long-term survivors.1-3 However, recovery after transplantation is prolonged, often because of complications such as chronic GVHD, cardiopulmonary compromise, endocrinopathies, musculoskeletal disorders, and subsequent malignancies.4-16 Recently, we have demonstrated that the cumulative incidence of severe or life-threatening chronic health conditions approaching 35% at 10 years after HCT.17 These complications contribute significantly to the late morbidity and mortality experienced by HCT survivors,2,3,17 with a potential impact on their psychological health.
Cross-sectional studies have described psychological health from 1 to 10 years after HCT, with varied and often contradictory results.18-26 This inconsistency in the reports is likely because of incomplete long-term follow-up of cohorts, resulting in participation bias of the relatively small cohorts of participants, and varied outcome measures used across studies. Longitudinal studies carry the advantage of a pre-HCT baseline assessment, and the ability to describe recovery after HCT, but are limited by relatively short follow-up (1-5 years), and small number of subjects when the follow-up extends beyond 3 years. Previous longitudinal studies indicate that 14% to 90% of HCT survivors report psychological distress,27-31 and that recovery after HCT occurs gradually over 1 to 5 years.32 However, it is difficult to extrapolate these short-term findings to long-term survivors. Furthermore, given the increasing burden of morbidity carried by long-term survivors of HCT, the impact of chronic health conditions on their psychological health remains to be determined.
The current study addressed these gaps by using the resources offered by the Bone Marrow Transplant Survivor Study (BMTSS). The aims of the current study were to: (1) describe the prevalence of adverse psychological outcomes in a large cohort of long-term HCT survivors and to understand whether this differed significantly from an unaffected population; (2) among HCT survivors, to identify demographic and clinical risk factors associated with adverse psychological outcomes; (3) examine the contribution of chronic health conditions to the psychological health of long-term HCT survivors; and (4) describe the prevalence of adverse outcomes with increasing time from HCT.
Methods
Subjects
BMTSS is a collaborative effort between City of Hope (COH) and University of Minnesota (UMN), describing long-term outcomes reported by HCT survivors. Eligible participants were individuals who had undergone autologous or allogeneic HCT at COH or UMN between 1974 and 1998 for a hematologic malignancy or severe aplastic anemia (SAA), had survived at least 2 years posttransplantation, and were 18 years of age or older and alive at study participation. Comparison with a noncancer population was made possible by asking participating survivors to invite their nearest-age siblings to the study. This methodology yielded a comparison group of 309 siblings. The human subjects committee at the participating institutions approved the protocol; informed consent was provided to each subject according to the Declaration of Helsinki.
Clinical characteristics
Information regarding clinical characteristics (primary cancer diagnosis, preparative regimens, source of stem cells [autologous, sibling, or unrelated donor], graft type [BM or peripheral blood stem cell], risk of relapse at HCT [standard or high risk], presence of chronic GVHD, and management of GVHD [prophylaxis and/or treatment]) was obtained from the institutional transplantation databases. Patients who received transplants in first or second complete remission after acute leukemia (acute myeloid [AML] or acute lymphoid [ALL]) leukemia) and lymphoma (Hodgkin [HL] or non-Hodgkin lymphoma [NHL]), first chronic phase of chronic myeloid leukemia [CML], and patients with SAA were considered being at standard risk for relapse; the remainder were considered at high risk.
Bone Marrow Transplant Survivor Study Questionnaire
HCT survivors and siblings completed a 255-item questionnaire, which covered the following general areas: sociodemographic characteristics (race, education, marital status, employment, household income, and insurance); diagnosis of specific physical health conditions; presence or absence of active chronic GVHD in the preceding 12 months; access to and use of medical care; self-reported health status (poor, fair, good, or excellent); and self-reported psychological health status (described in “Psychological outcomes”).
Chronic physical health conditions diagnosed after HCT were graded using the Common Terminology Criteria for Adverse Event, Version 3.0 (CTCAEv3.0).33 The CTCAEv3.0 is used to grade acute and chronic conditions in individuals with cancer, including cancer survivors, and distinguishes grades 1 through 5 with unique clinical descriptions of the severity for each event (grade 1, mild; grade 2, moderate; grade 3, severe; grade 4, life-threatening/disabling; grade 5, death because of chronic health conditions). The same scoring system was applied to responses from the sibling comparison group. A detailed description of the questions asked in the BMTSS questionnaire, the corresponding chronic health condition categories that were created from the responses, and the scoring of these conditions is presented by Sun et al.17
Psychological outcomes
Psychological health status was evaluated using the Brief Symptom Inventory-18 (BSI-18). BSI-18 measures psychological distress by using 18 five-point Likert scale items (from 0 = “not at all ” to 4 = “extremely”) related to symptoms experienced during the previous 7 days (Table 1).34 BSI-18 has been validated in healthy volunteers35 and in cancer patients.34,36 BSI-18 includes a summary scale, the Global Severity Index (GSI: all 18 items, with scores ranging from 0 to 72), and 3 subscales: depression, anxiety, and somatization (each with 6 items; scores ranging from 0 to 24). Higher scores indicate higher symptom levels. To facilitate interpretation, raw scores were converted to gender-specific T scores (mean = 50, SD = 10) based on a community sample of 1134 adults. Patients with elevations of T score for GSI ≥ 63 (90th percentile) were classified as having significant psychological distress; similarly, for each subscale, patients with T score ≥ 63 were classified as having distress in the corresponding subscale.37,38
Item content of the subscales constituting the Brief Symptom Inventory-18
| BSI-18 subscales/items . |
|---|
| Anxiety |
| 1. Nervousness or shaking inside |
| 2. Feeling tense or keyed up |
| 3. Suddenly scared for no reason |
| 4. Spells of terror or panic |
| 5. Feeling so restless you couldn't sit |
| 6. Feeling fearful |
| Depression |
| 1. Feeling no interest in things |
| 2. Feeling lonely |
| 3. Feeling blue |
| 4. Feeling of worthlessness |
| 5. Feeling hopeless about the future |
| 6. Thoughts of ending your life |
| Somatization |
| 1. Faintness or dizziness |
| 2. Pains in heart or chest |
| 3. Nausea or upset stomach |
| 4. Trouble getting your breath |
| 5. Numbness or tingling in parts of your body |
| 6. Feeling weak in parts of the body |
| BSI-18 subscales/items . |
|---|
| Anxiety |
| 1. Nervousness or shaking inside |
| 2. Feeling tense or keyed up |
| 3. Suddenly scared for no reason |
| 4. Spells of terror or panic |
| 5. Feeling so restless you couldn't sit |
| 6. Feeling fearful |
| Depression |
| 1. Feeling no interest in things |
| 2. Feeling lonely |
| 3. Feeling blue |
| 4. Feeling of worthlessness |
| 5. Feeling hopeless about the future |
| 6. Thoughts of ending your life |
| Somatization |
| 1. Faintness or dizziness |
| 2. Pains in heart or chest |
| 3. Nausea or upset stomach |
| 4. Trouble getting your breath |
| 5. Numbness or tingling in parts of your body |
| 6. Feeling weak in parts of the body |
BSI-18 indicates Brief Symptom Inventory-18.
Suicidal ideation was elicited as part of the BSI-18 depression subscale. Participants who endorsed the question “thoughts of ending your life” were considered to have suicidal ideation. To evaluate the association between depression and suicidal ideation, an alternative method was used to calculate the depression scale by treating suicidal ideation as a missing item, and then scoring the depression scale using published missing data rules.34
Statistical analysis
Standard descriptive statistics were used to summarize the demographic and clinical variables for survivors and siblings. Logistic regression techniques were used to identify demographic and clinical variables associated with adverse psychological outcomes (global distress and each subscale [anxiety, depression, and somatization]).
Comparison between HCT survivors and siblings was adjusted for sex, age at study participation (treated as a continuous variable), race/ethnicity (non-Hispanic whites vs others), education (less than high school/completed high school vs some college or higher education), annual household income (< $20 000 vs $20 000-$60 000 vs > $60 000), marital status, health insurance status (yes vs no), grade of chronic health conditions (none vs grades 1 or 2 vs grades 3 or 4) and self-reported health status (poor or fair vs good or excellent).
In the analysis restricted to HCT survivors, a fixed set of explanatory variables were selected a priori and were retained in the model to assess their simultaneous impact on adverse psychological outcomes. These variables included sex, age at HCT (per 10 years), time since HCT (per 10 years), race (non-Hispanic whites vs others), marital status, education (less than high school/completed high school vs some college or higher education), annual household income (< $20 000 vs $20 000-$60 000 vs > $60 000), health insurance coverage (yes vs no), risk of relapse at HCT (standard vs high risk) and grade of chronic health conditions (none vs grades 1 or 2 vs grades 3 or 4). After taking these variables into consideration, the following variables were included in the multivariate model one at time to avoid collinearity: primary cancer diagnosis, stem cell donor type (autologous vs related vs unrelated donor), exposure to total body irradiation (TBI), presence of chronic GVHD (for allogeneic HCT survivors only), exposure to immunosuppressants (for allogeneic HCT survivors only). The chronic GVHD variable was categorized into 3 groups; “active chronic GVHD,” “resolved chronic GVHD,” and “no chronic GVHD.” Active chronic GVHD was defined as presence of chronic GVHD requiring active intervention within the 12 months before study participation. Resolved chronic GVHD was defined as history of chronic GVHD that had not required active intervention in the past 12 months. Exposure to immunosuppressants referred to exposure to any agents that were used to prevent or treat acute or chronic GVHD. Three agents were primarily used during this time period when these transplants were performed—methotrexate, cyclosporin, and prednisone. Exposure to these agents was collected as a “yes/no” variable, and included current as well as past exposures. The prevalence of adverse psychological outcomes by time since HCT was examined using the Cochran-Armitage test for trend. Data were analyzed using SAS 9.2 (SAS Institute).
Results
Characteristics of the study population
Of the 1717 eligible survivors, 1539 (90%) were successfully contacted; of these, 1065 (69%) participated in the study. Participation rate did not differ by survivors' disease status at HCT or by transplanting institution. However, non-Hispanic whites (P = .001) and females (P = .01) were more likely to participate; participants were older at HCT (mean age: 35 vs 29 years, P < .001) and had been followed for a shorter period of time after HCT (mean length of follow-up: 8.7 vs 10.4 years, P = .01). Patients who had undergone HCT for SAA were less likely to participate compared with those with other diagnoses (P = .05).
Table 2 summarizes the characteristics of the study participants. Of the 1065 HCT survivors, 585 (54.9%) were male, and 825 (77.5%) were whites. Median age at study participation was 44 years (range, 18-73), and median time from HCT was 7.4 years (range, 2.0-28). Primary diagnoses included AML (n = 255), CML (n = 249), NHL (n = 208), ALL (n = 106), HL (n = 92), SAA (n = 56), and other miscellaneous diagnoses (n = 99). Four hundred and sixty-nine (44%) patients had received an autologous HCT, 497 (46.7%) a related donor HCT, and 99 (9.3%) an unrelated donor HCT. TBI was used in the preparative regimen for 815 (76.7%) patients, and 35.1% were at high risk for relapse at HCT.
Characteristics of HCT survivors and siblings
| Characteristic . | Survivors, no. (%), N = 1065 . | Siblings, no. (%), N = 309 . | P . |
|---|---|---|---|
| Male sex, % | 585 (54.9) | 113 (36.6) | < .001 |
| Race: non-Hispanic white* | 825 (77.5) | 270 (88.0) | < .001 |
| Did not complete HS/ HS graduate* | 222 (20.9) | 38 (12.3) | < .001 |
| Household income, $ | < .001 | ||
| > 60 000/y | 438 (44.2) | 189 (64.3) | |
| 20 000-60 000/y | 397 (40.1) | 93 (31.6) | |
| < 20 000/y | 155 (15.7) | 12 (4.1) | |
| No health insurance* | 88 (8.4) | 14 (4.6) | 0.03 |
| Chronic health condition | < .001 | ||
| None | 386 (36.2) | 189 (61.2) | |
| Grade1/2 | 492 (46.2) | 96 (31.0) | |
| Grade3/4 | 187 (17.6) | 24 (7.8) | |
| Major categories of chronic health conditions† | |||
| Cardiovascular | 6.5 | 2.6 | .01 |
| Coronary artery disease | 1.7 | 1.6 | .93 |
| Congestive heart failure | 0.5 | 0 | .59 |
| Stroke | 1.3 | 0.3 | .21 |
| Auditory or visual | 2.5 | 1.0 | .12 |
| Legally blind or loss of an eye | 1.4 | 0 | .03 |
| Hearing loss not corrected by an aid | 1.1 | 1.0 | 1.00 |
| Gastrointestinal | 2.8 | 0.7 | .03 |
| Surgery (intestinal obstruction/ colostomy) | 1.3 | 0 | .05 |
| Rectal or anal fissure | 0.9 | 0.7 | 1.00 |
| Cirrhosis | 0.8 | 0 | .21 |
| Endocrine | 2.3 | 1.0 | .24 |
| Diabetes | 1.8 | 0.7 | .19 |
| Musculoskeletal problems | 3.3 | 0.7 | .01 |
| Joint replacement | 3.3 | 0.7 | .01 |
| Renal | 0.4 | 0 | .58 |
| Dialysis support | 0.4 | 0 | .58 |
| New malignancies | 2.4 | 2.9 | .54 |
| Median age at study participation, y (range) | 44 (18-73)‡ | 45.2 (19-79)‡ | .05 |
| Interval between HCT and study, y | |||
| Median (range) | 7.4 (2-28)‡ | NA | |
| 2-5 y | 328 (30.8) | NA | |
| 6-10 y | 397 (37.3) | NA | |
| 11+ y | 340 (31.9) | NA | |
| Primary cancer diagnosis | |||
| Aplastic anemia | 56 (5.3) | NA | |
| Chronic myeloid leukemia | 249 (23.4) | NA | |
| Acute myeloid leukemia | 255 (23.9) | NA | |
| Hodgkin lymphoma | 92 (8.9) | NA | |
| Non-Hodgkin lymphoma | 208 (19.5) | NA | |
| Acute lymphoblastic leukemia | 106 (10.0) | NA | |
| Multiple myeloma | 47 (4.4) | NA | |
| Others | 52 (4.9) | NA | |
| Stem cell donor | |||
| Autologous HCT | 469 (44.0) | NA | |
| Allogeneic, sibling donor | 497 (46.7) | NA | |
| Allogeneic, unrelated donor | 99 (9.3) | NA | |
| High risk of relapse at HCT* | 373 (35.1) | NA | |
| Total body irradiation–based transplant regimens | 815 (76.7) | NA | |
| Among allogeneic HCT survivors only, cGVHD | |||
| No | 276 (46.4) | NA | |
| Active cGVHD | 134 (22.5) | NA | |
| Resolved cGVHD | 185 (31.1) | NA | |
| Immune suppression | |||
| Any immunosuppression | 576 (96.8) | NA | |
| Methotrexate | 475 (80.0) | NA | |
| Cyclosporine | 403 (67.7) | NA | |
| Prednisone | 336 (56.6) | NA |
| Characteristic . | Survivors, no. (%), N = 1065 . | Siblings, no. (%), N = 309 . | P . |
|---|---|---|---|
| Male sex, % | 585 (54.9) | 113 (36.6) | < .001 |
| Race: non-Hispanic white* | 825 (77.5) | 270 (88.0) | < .001 |
| Did not complete HS/ HS graduate* | 222 (20.9) | 38 (12.3) | < .001 |
| Household income, $ | < .001 | ||
| > 60 000/y | 438 (44.2) | 189 (64.3) | |
| 20 000-60 000/y | 397 (40.1) | 93 (31.6) | |
| < 20 000/y | 155 (15.7) | 12 (4.1) | |
| No health insurance* | 88 (8.4) | 14 (4.6) | 0.03 |
| Chronic health condition | < .001 | ||
| None | 386 (36.2) | 189 (61.2) | |
| Grade1/2 | 492 (46.2) | 96 (31.0) | |
| Grade3/4 | 187 (17.6) | 24 (7.8) | |
| Major categories of chronic health conditions† | |||
| Cardiovascular | 6.5 | 2.6 | .01 |
| Coronary artery disease | 1.7 | 1.6 | .93 |
| Congestive heart failure | 0.5 | 0 | .59 |
| Stroke | 1.3 | 0.3 | .21 |
| Auditory or visual | 2.5 | 1.0 | .12 |
| Legally blind or loss of an eye | 1.4 | 0 | .03 |
| Hearing loss not corrected by an aid | 1.1 | 1.0 | 1.00 |
| Gastrointestinal | 2.8 | 0.7 | .03 |
| Surgery (intestinal obstruction/ colostomy) | 1.3 | 0 | .05 |
| Rectal or anal fissure | 0.9 | 0.7 | 1.00 |
| Cirrhosis | 0.8 | 0 | .21 |
| Endocrine | 2.3 | 1.0 | .24 |
| Diabetes | 1.8 | 0.7 | .19 |
| Musculoskeletal problems | 3.3 | 0.7 | .01 |
| Joint replacement | 3.3 | 0.7 | .01 |
| Renal | 0.4 | 0 | .58 |
| Dialysis support | 0.4 | 0 | .58 |
| New malignancies | 2.4 | 2.9 | .54 |
| Median age at study participation, y (range) | 44 (18-73)‡ | 45.2 (19-79)‡ | .05 |
| Interval between HCT and study, y | |||
| Median (range) | 7.4 (2-28)‡ | NA | |
| 2-5 y | 328 (30.8) | NA | |
| 6-10 y | 397 (37.3) | NA | |
| 11+ y | 340 (31.9) | NA | |
| Primary cancer diagnosis | |||
| Aplastic anemia | 56 (5.3) | NA | |
| Chronic myeloid leukemia | 249 (23.4) | NA | |
| Acute myeloid leukemia | 255 (23.9) | NA | |
| Hodgkin lymphoma | 92 (8.9) | NA | |
| Non-Hodgkin lymphoma | 208 (19.5) | NA | |
| Acute lymphoblastic leukemia | 106 (10.0) | NA | |
| Multiple myeloma | 47 (4.4) | NA | |
| Others | 52 (4.9) | NA | |
| Stem cell donor | |||
| Autologous HCT | 469 (44.0) | NA | |
| Allogeneic, sibling donor | 497 (46.7) | NA | |
| Allogeneic, unrelated donor | 99 (9.3) | NA | |
| High risk of relapse at HCT* | 373 (35.1) | NA | |
| Total body irradiation–based transplant regimens | 815 (76.7) | NA | |
| Among allogeneic HCT survivors only, cGVHD | |||
| No | 276 (46.4) | NA | |
| Active cGVHD | 134 (22.5) | NA | |
| Resolved cGVHD | 185 (31.1) | NA | |
| Immune suppression | |||
| Any immunosuppression | 576 (96.8) | NA | |
| Methotrexate | 475 (80.0) | NA | |
| Cyclosporine | 403 (67.7) | NA | |
| Prednisone | 336 (56.6) | NA |
HCT indicates hematopoietic cell transplantation; HS, high school; NA, not applicable; and cGVHD, chronic GVHD.
Two subjects with unknown race; 6 subjects with unknown education; 90 subjects with unknown household income; 18 subjects with unknown insurance status; and 3 patients with unknown risk of relapse status at HCT.
Key chronic health conditions within each category shown here. Some participants reported > 1 condition, and therefore the total number within each category does not total the sum of conditions show here. Percentages are based on the total number of participants who provided data for each variable, rather than on the total number of subjects in the cohort; percentages may not total 100 because of rounding.
Median (range), not no. (%).
Siblings were slightly older than the survivors (median age, 45.2 vs 44 years) at study participation. In addition, there was an overrepresentation of females (63.4% vs 45.1%), non-Hispanic whites (88.0% vs 77.5%), individuals with higher education (some college or higher: 87.7% vs 79.1%) and individuals with higher household income (> $60 000 64.3% vs 44.2%) among the siblings. All these variables were adjusted for in the statistical analyses comparing survivors with siblings.
Adverse psychological outcomes: a comparison of HCT survivors with siblings
Of the 1065 survivors, 232 (21.8%) reported psychological distress on at least one subscale; 52 (4.9%) on 2 subscales and 30 (2.8%) on all 3 subscales. In comparison, a significantly smaller proportion of siblings reported psychological distress: 25 (8.1%) on at least 1 subscale; 5 (1.6%) on 2 subscales, and 2 (0.6%) on all 3 subscales (P < .001). Compared with siblings, survivors were more likely to report anxiety (5.6% vs 2.6%, P = .03), depression (11% vs 4.5%, P < .001), somatization (15.7% vs 3.9%, P < .001), and global distress (9.8% vs 3.2%, P < .001; Figure 1). After adjustment for age at study participation, sex, race, education, household income, health insurance status, self-reported health status, and presence and severity of chronic health conditions, survivors were 2.9 times more likely to report somatic distress (odds ratio [OR] = 2.88, 95% confidence interval [CI] = 1.4-5.8), but not anxiety, depression, or global distress (Figure 1, Table 3).
Magnitude of risk of poor psychological outcomes. Survivors were compared with siblings, adjusted for sex, age at study participation (continuous), race/ethnicity (non-Hispanic white, others), marital status (married, not married), income (< $20 000/y, $20 000-$60 000/y, > $60 000/y), education (did not complete high school (HS)/HS graduate, some college or higher), insurance status (yes, no), health status (poor/fair, good/excellent), and grade of chronic health conditions (none, grade 1 or 2, grade 3 or 4).
Magnitude of risk of poor psychological outcomes. Survivors were compared with siblings, adjusted for sex, age at study participation (continuous), race/ethnicity (non-Hispanic white, others), marital status (married, not married), income (< $20 000/y, $20 000-$60 000/y, > $60 000/y), education (did not complete high school (HS)/HS graduate, some college or higher), insurance status (yes, no), health status (poor/fair, good/excellent), and grade of chronic health conditions (none, grade 1 or 2, grade 3 or 4).
Multivariate analysis of poor psychological outcomes: survivor and survivor subgroups compared with siblings
| . | Anxiety . | Depression . | Somatic distress . | Global distress . | ||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) . | P . | OR (95% CI) . | P . | OR (95% CI) . | P . | OR (95% CI) . | P . | |
| Siblings | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Survivors | 1.12 (0.47-2.67) | .80 | 1.15 (0.60-2.18) | .67 | 2.88 (1.44-5.76) | .003 | 1.79 (0.81-3.93) | .15 |
| By primary cancer diagnosis | ||||||||
| Aplastic anemia | 0.72 (0.13-3.97) | .70 | 0.72 (0.21-2.52) | .61 | 2.06 (0.62-6.80) | .23 | 1.34 (0.35-5.16) | .67 |
| Chronic myeloid leukemia | 1.01 (0.36-2.79) | .99 | 1.34 (0.64-2.81) | .43 | 3.53 (1.65-7.55) | .001 | 2.19 (0.92-5.24) | .08 |
| Acute myeloid leukemia | 1.14 (0.41-3.16) | .81 | 1.47 (0.70-3.06) | .31 | 3.25 (1.50-7.01) | .003 | 1.88 (0.77-4.57) | .16 |
| Hodgkin lymphoma | 0.20 (0.02-1.75) | .14 | 0.21 (0.04-1.00) | .05 | 2.18 (0.82-5.81) | .12 | 0.53 (0.13-2.23) | .39 |
| Non-Hodgkin lymphoma | 1.64 (0.59-4.55) | .34 | 1.14 (0.51-2.58) | .75 | 2.77 (1.24-6.21) | .01 | 2.43 (0.97-6.08) | .06 |
| Acute lymphoblastic leukemia | 1.32 (0.40-4.37) | .65 | 1.52 (0.64-3.59) | .34 | 2.35 (0.91-6.08) | .08 | 1.53 (0.53-4.43) | .43 |
| Multiple myeloma | 1.06 (0.22-5.09) | .95 | 0.60 (0.14-2.53) | .49 | 4.45 (1.53-12.92) | .01 | 1.26 (0.31-5.19) | .75 |
| Others | 1.45 (0.28-7.53) | .66 | 0.96 (0.25-3.76) | .96 | 0.47 (0.06-3.90) | .49 | 1.16 (0.22-6.01) | .86 |
| By stem cell donor | ||||||||
| Autologous HCT | 1.01 (0.39-2.58) | .99 | 1.02 (0.51-2.06) | .95 | 2.60 (1.25-5.38) | .01 | 1.53 (0.66-3.55) | .32 |
| Allogeneic, sibling donor | 1.15 (0.45-2.92) | .78 | 1.34 (0.68-2.65) | .40 | 2.88 (1.38-6.02) | .00 | 1.96 (0.85-4.49) | .11 |
| Allogeneic, unrelated donor | 1.54 (0.48-4.90) | .47 | 0.91 (0.35-2.36) | .85 | 4.32 (1.84-10.18) | .001 | 2.28 (0.83-6.21) | .11 |
| By risk of relapse at HCT | ||||||||
| Standard risk | 0.89 (0.32-2.44) | .82 | 1.15 (0.55-2.39) | .71 | 2.67 (1.22-5.84) | .01 | 1.75 (0.73-4.18) | .21 |
| High risk | 1.84 (0.64-5.29) | .26 | 1.17 (0.51-2.68) | .72 | 4.18 (1.79-9.74) | .001 | 1.99 (0.76-5.24) | .16 |
| By transplant regimens | ||||||||
| Chemotherapy alone | 0.70 (0.16-3.11) | .64 | 0.75 (0.26-2.20) | .60 | 2.42 (0.87-6.79) | .09 | 0.90 (0.25-3.22) | .87 |
| Total body irradiation–based | 1.24 (0.47-3.24) | .66 | 1.23 (0.60-2.51) | .57 | 3.15 (1.46-6.76) | .003 | 2.00 (0.85-4.70) | .11 |
| By cGVHD status (among allogeneic HCT survivors only) | ||||||||
| No | 1.17 (0.41-3.33) | .77 | 0.89 (0.40-1.99) | .78 | 2.32 (1.01-5.33) | .05 | 1.32 (0.51-3.41) | .56 |
| Active cGVHD | 1.96 (0.66-5.79) | .22 | 1.40 (0.60-3.25) | .44 | 5.14 (2.21-11.89) | < .001 | 3.04 (1.17-7.88) | .02 |
| Resolved cGVHD | 0.56 (0.16-1.99) | .37 | 1.34 (0.60-3.00) | .47 | 2.49 (1.04-5.97) | .04 | 1.61 (0.61-4.28) | .34 |
| By exposure to immunosuppressants (among allogeneic HCT survivors only) | ||||||||
| Use of any immunosuppression | 1.23 (0.47-3.19) | .68 | 1.20 (0.59-2.44) | .61 | 3.00 (1.40-6.43) | .005 | 1.90 (0.81-4.44) | .14 |
| Methotrexate | 1.22 (0.46-0.00) | .69 | 1.06 (0.51-2.21) | .87 | 3.15 (1.46-6.79) | .08 | 1.75 (0.73-4.19) | .21 |
| Cyclosporine | 1.22 (0.46-3.26) | .69 | 1.46 (0.71-2.99) | .31 | 3.32 (1.54-7.15) | .003 | 2.04 (0.86-4.85) | .11 |
| Prednisone | 1.63 (0.62-4.33) | .33 | 1.53 (0.74-3.18) | .25 | 3.76 (1.72-8.21) | .106 | 2.46 (1.02-5.91) | .04 |
| . | Anxiety . | Depression . | Somatic distress . | Global distress . | ||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) . | P . | OR (95% CI) . | P . | OR (95% CI) . | P . | OR (95% CI) . | P . | |
| Siblings | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Survivors | 1.12 (0.47-2.67) | .80 | 1.15 (0.60-2.18) | .67 | 2.88 (1.44-5.76) | .003 | 1.79 (0.81-3.93) | .15 |
| By primary cancer diagnosis | ||||||||
| Aplastic anemia | 0.72 (0.13-3.97) | .70 | 0.72 (0.21-2.52) | .61 | 2.06 (0.62-6.80) | .23 | 1.34 (0.35-5.16) | .67 |
| Chronic myeloid leukemia | 1.01 (0.36-2.79) | .99 | 1.34 (0.64-2.81) | .43 | 3.53 (1.65-7.55) | .001 | 2.19 (0.92-5.24) | .08 |
| Acute myeloid leukemia | 1.14 (0.41-3.16) | .81 | 1.47 (0.70-3.06) | .31 | 3.25 (1.50-7.01) | .003 | 1.88 (0.77-4.57) | .16 |
| Hodgkin lymphoma | 0.20 (0.02-1.75) | .14 | 0.21 (0.04-1.00) | .05 | 2.18 (0.82-5.81) | .12 | 0.53 (0.13-2.23) | .39 |
| Non-Hodgkin lymphoma | 1.64 (0.59-4.55) | .34 | 1.14 (0.51-2.58) | .75 | 2.77 (1.24-6.21) | .01 | 2.43 (0.97-6.08) | .06 |
| Acute lymphoblastic leukemia | 1.32 (0.40-4.37) | .65 | 1.52 (0.64-3.59) | .34 | 2.35 (0.91-6.08) | .08 | 1.53 (0.53-4.43) | .43 |
| Multiple myeloma | 1.06 (0.22-5.09) | .95 | 0.60 (0.14-2.53) | .49 | 4.45 (1.53-12.92) | .01 | 1.26 (0.31-5.19) | .75 |
| Others | 1.45 (0.28-7.53) | .66 | 0.96 (0.25-3.76) | .96 | 0.47 (0.06-3.90) | .49 | 1.16 (0.22-6.01) | .86 |
| By stem cell donor | ||||||||
| Autologous HCT | 1.01 (0.39-2.58) | .99 | 1.02 (0.51-2.06) | .95 | 2.60 (1.25-5.38) | .01 | 1.53 (0.66-3.55) | .32 |
| Allogeneic, sibling donor | 1.15 (0.45-2.92) | .78 | 1.34 (0.68-2.65) | .40 | 2.88 (1.38-6.02) | .00 | 1.96 (0.85-4.49) | .11 |
| Allogeneic, unrelated donor | 1.54 (0.48-4.90) | .47 | 0.91 (0.35-2.36) | .85 | 4.32 (1.84-10.18) | .001 | 2.28 (0.83-6.21) | .11 |
| By risk of relapse at HCT | ||||||||
| Standard risk | 0.89 (0.32-2.44) | .82 | 1.15 (0.55-2.39) | .71 | 2.67 (1.22-5.84) | .01 | 1.75 (0.73-4.18) | .21 |
| High risk | 1.84 (0.64-5.29) | .26 | 1.17 (0.51-2.68) | .72 | 4.18 (1.79-9.74) | .001 | 1.99 (0.76-5.24) | .16 |
| By transplant regimens | ||||||||
| Chemotherapy alone | 0.70 (0.16-3.11) | .64 | 0.75 (0.26-2.20) | .60 | 2.42 (0.87-6.79) | .09 | 0.90 (0.25-3.22) | .87 |
| Total body irradiation–based | 1.24 (0.47-3.24) | .66 | 1.23 (0.60-2.51) | .57 | 3.15 (1.46-6.76) | .003 | 2.00 (0.85-4.70) | .11 |
| By cGVHD status (among allogeneic HCT survivors only) | ||||||||
| No | 1.17 (0.41-3.33) | .77 | 0.89 (0.40-1.99) | .78 | 2.32 (1.01-5.33) | .05 | 1.32 (0.51-3.41) | .56 |
| Active cGVHD | 1.96 (0.66-5.79) | .22 | 1.40 (0.60-3.25) | .44 | 5.14 (2.21-11.89) | < .001 | 3.04 (1.17-7.88) | .02 |
| Resolved cGVHD | 0.56 (0.16-1.99) | .37 | 1.34 (0.60-3.00) | .47 | 2.49 (1.04-5.97) | .04 | 1.61 (0.61-4.28) | .34 |
| By exposure to immunosuppressants (among allogeneic HCT survivors only) | ||||||||
| Use of any immunosuppression | 1.23 (0.47-3.19) | .68 | 1.20 (0.59-2.44) | .61 | 3.00 (1.40-6.43) | .005 | 1.90 (0.81-4.44) | .14 |
| Methotrexate | 1.22 (0.46-0.00) | .69 | 1.06 (0.51-2.21) | .87 | 3.15 (1.46-6.79) | .08 | 1.75 (0.73-4.19) | .21 |
| Cyclosporine | 1.22 (0.46-3.26) | .69 | 1.46 (0.71-2.99) | .31 | 3.32 (1.54-7.15) | .003 | 2.04 (0.86-4.85) | .11 |
| Prednisone | 1.63 (0.62-4.33) | .33 | 1.53 (0.74-3.18) | .25 | 3.76 (1.72-8.21) | .106 | 2.46 (1.02-5.91) | .04 |
OR indicates odds ratio; CI, confidence interval; HCT, hematopoietic cell transplantation; and cGVHD, chronic GVHD.
Adverse psychological outcomes among HCT survivors
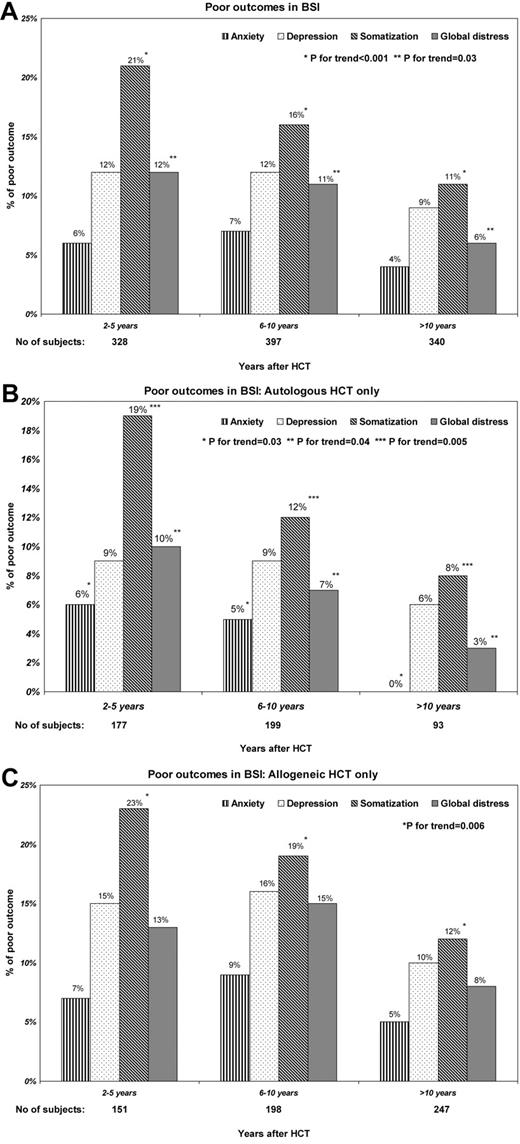
The proportion of survivors reporting somatic distress declined significantly over time (2-5 years from HCT: 21%; 6-10 years: 16%; and 11+ years: 11%, P for trend < .001). On the other hand, the proportion of survivors with anxiety or depression remained unchanged over this time period (Figure 2A). These trends differed by type of HCT. Among autologous HCT recipients, the proportion of survivors reporting anxiety (P for trend = .03), somatic distress (P for trend = .005), and global distress (P for trend = .04) declined over time (Figure 2B). On the other hand, among allogeneic HCT recipients, while the proportion of survivors with somatic distress (P for trend = .006) declined over time (Figure 2C), the proportion of survivors with anxiety, depression, and global distress appeared to increase for the first 10 years, with declines thereafter.
Prevalence of HCT survivors with adverse psychosocial outcomes as a function of time from HCT. (A) Among all survivors by years after HCT. (B) Among autologous survivors only. (C) Among allogeneic survivors only.
Prevalence of HCT survivors with adverse psychosocial outcomes as a function of time from HCT. (A) Among all survivors by years after HCT. (B) Among autologous survivors only. (C) Among allogeneic survivors only.
Examination of demographic and clinical variables associated with adverse psychological outcomes using multivariate logistic regression techniques are detailed in Table 4. The likelihood of reporting adverse psychological outcomes did not differ by primary cancer diagnosis and type of HCT. Relevant sociodemographic and clinical variables identified to be associated with global distress, anxiety, depression, and somatization are summarized here.
Multivariate analysis of sociodemographic and clinical factors in relation to poor psychological outcomes among HCT survivors
| . | Anxiety . | Depression . | Somatization . | Global distress . | ||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) . | P . | OR (95% CI) . | P . | OR (95% CI) . | P . | OR (95% CI) . | P . | |
| Sex | ||||||||
| Female | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Male | 1.02 (0.56-1.86) | .95 | 1.80 (1.14-2.85) | .01 | 0.89 (0.60-1.31) | .55 | 1.09 (0.68-1.76) | .71 |
| Race/ethnicity | ||||||||
| Non-Hispanic white | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Others | 1.94 (0.98-3.87) | .06 | 1.28 (0.75-2.18) | .36 | 1.60 (0.99-2.60) | .05 | 2.04 (1.17-3.55) | .01 |
| Education | ||||||||
| Did not complete HS/HS graduate | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Some college or above | 0.62 (0.31-1.21) | .16 | 0.64 (0.38-1.09) | .10 | 1.26 (0.75-2.11) | .38 | 0.81 (0.46-1.43) | .47 |
| Household income, $ | ||||||||
| > 60 000/y | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| 20 000-60 000/y | 3.28 (1.41-7.59) | .01 | 2.12 (1.21-3.71) | .01 | 1.92 (1.21-3.05) | .01 | 2.04 (1.11-3.74) | .02 |
| < 20 000/y | 3.22 (1.08-9.64) | .04 | 3.10 (1.56-6.19) | < .001 | 2.28 (1.23-4.24) | .01 | 2.54 (1.21-5.35) | .01 |
| Health insurance | ||||||||
| Yes | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| No | 0.87 (0.31-2.43) | .80 | 1.16 (0.59-2.32) | .66 | 1.20 (0.61-2.37) | .60 | 1.35 (0.66-2.78) | .42 |
| Marital status | ||||||||
| Married | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| No | 0.82 (0.42-1.59) | .55 | 1.45 (0.88-2.37) | .14 | 1.02 (0.65-1.57) | .95 | 1.28 (0.76-2.17) | .35 |
| Health status | ||||||||
| Excellent/good | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Fair/poor | 3.80 (2.03-7.12) | < .0001 | 3.56 (2.20-5.74) | < .0001 | 5.58 (3.71-8.39) | < .0001 | 5.98 (3.65-9.81) | < .0001 |
| Chronic health condition | ||||||||
| None | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Grade1/2 | 1.36 (0.68-2.73) | .39 | 1.48 (0.88-2.48) | .14 | 1.44 (0.91-2.28) | .12 | 1.21 (0.70-2.10) | .49 |
| Grade3/4 | 1.39 (0.57-3.38) | .47 | 1.49 (0.76-2.90) | .24 | 1.91 (1.09-3.36) | .02 | 1.37 (0.68-2.74) | .38 |
| Risk of relapse at HCT | ||||||||
| Standard risk | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| High risk | 1.18 (0.64-2.20) | .59 | 0.75 (0.46-1.21) | .23 | 0.95 (0.63-1.43) | .81 | 0.77 (0.46-1.28) | .31 |
| Age at HCT, year (per 10 y) | 0.91 (0.70-1.20) | .52 | 0.80 (0.66-0.98) | .03 | 0.98 (0.82-1.17) | .86 | 0.88 (0.71-1.09) | .25 |
| Time since HCT (per 10 y) | 0.62 (0.29-1.29) | .20 | 0.55 (0.32-0.93) | .03 | 0.56 (0.35-0.92) | .02 | 0.56 (0.31-0.99) | .05 |
| Primary cancer diagnosis | ||||||||
| CML | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| AML/ALL | 1.32 (0.60-2.92) | .49 | 1.19 (0.69-2.08) | .53 | 0.94 (0.57-1.54) | .79 | 0.92 (0.50-1.67) | .78 |
| HL/NHL | 0.99 (0.41-2.38) | .99 | 0.61 (0.31-1.19) | .14 | 0.68 (0.39-1.17) | .16 | 0.82 (0.42-1.60) | .56 |
| SAA/MM/others | 0.95 (0.33-2.69) | .92 | 0.59 (0.26-1.35) | .21 | 0.65 (0.33-1.29) | .22 | 0.65 (0.28-1.52) | .32 |
| Stem cell donor | ||||||||
| Autologous HCT | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Allogeneic, sibling donor | 1.35 (0.67-2.71) | .40 | 1.41 (0.85-2.33) | .19 | 1.28 (0.82-2.01) | .28 | 1.41 (0.81-2.46) | .22 |
| Allogeneic, unrelated donor | 1.61 (0.61-4.26) | .34 | 0.82 (0.36-1.87) | .64 | 1.60 (0.86-2.96) | .14 | 1.42 (0.65-3.08) | .38 |
| Transplant regimens | ||||||||
| Chemotherapy alone | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Total body irradiation–based | 1.63 (0.75-3.53) | .22 | 1.96 (1.06-3.63) | .03 | 1.63 (0.98-2.71) | .06 | 1.98 (1.03-3.80) | .04 |
| cGVHD (among allogeneic HCT survivors only) | ||||||||
| No | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Active cGVHD | 1.69 (0.66-4.33) | .27 | 1.47 (0.69-3.14) | .32 | 2.00 (1.03-3.89) | .04 | 2.20 (1.01-4.79) | .05 |
| Resolved cGVHD | 0.44 (0.15-1.33) | .15 | 1.49 (0.76-2.93) | .25 | 0.99 (0.51-1.93) | .98 | 1.13 (0.53-2.41) | .75 |
| Any immunosuppression (among allogeneic HCT survivors only) | ||||||||
| Methotrexate | 1.50 (0.54-4.19) | .44 | 0.66 (0.33-1.31) | .23 | 1.31 (0.66-2.58) | .44 | 0.79 (0.38-1.64) | .53 |
| Cyclosporine | 0.97 (0.32-2.91) | .95 | 2.93 (1.15-7.49) | .02 | 1.00 (0.46-2.20) | 1.00 | 1.31 (0.53-3.27) | .56 |
| Prednisone | 2.62 (1.11-6.19) | .03 | 2.27 (1.24-4.18) | .01 | 1.80 (1.04-3.11) | .04 | 2.28 (1.19-4.36) | .01 |
| . | Anxiety . | Depression . | Somatization . | Global distress . | ||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) . | P . | OR (95% CI) . | P . | OR (95% CI) . | P . | OR (95% CI) . | P . | |
| Sex | ||||||||
| Female | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Male | 1.02 (0.56-1.86) | .95 | 1.80 (1.14-2.85) | .01 | 0.89 (0.60-1.31) | .55 | 1.09 (0.68-1.76) | .71 |
| Race/ethnicity | ||||||||
| Non-Hispanic white | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Others | 1.94 (0.98-3.87) | .06 | 1.28 (0.75-2.18) | .36 | 1.60 (0.99-2.60) | .05 | 2.04 (1.17-3.55) | .01 |
| Education | ||||||||
| Did not complete HS/HS graduate | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Some college or above | 0.62 (0.31-1.21) | .16 | 0.64 (0.38-1.09) | .10 | 1.26 (0.75-2.11) | .38 | 0.81 (0.46-1.43) | .47 |
| Household income, $ | ||||||||
| > 60 000/y | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| 20 000-60 000/y | 3.28 (1.41-7.59) | .01 | 2.12 (1.21-3.71) | .01 | 1.92 (1.21-3.05) | .01 | 2.04 (1.11-3.74) | .02 |
| < 20 000/y | 3.22 (1.08-9.64) | .04 | 3.10 (1.56-6.19) | < .001 | 2.28 (1.23-4.24) | .01 | 2.54 (1.21-5.35) | .01 |
| Health insurance | ||||||||
| Yes | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| No | 0.87 (0.31-2.43) | .80 | 1.16 (0.59-2.32) | .66 | 1.20 (0.61-2.37) | .60 | 1.35 (0.66-2.78) | .42 |
| Marital status | ||||||||
| Married | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| No | 0.82 (0.42-1.59) | .55 | 1.45 (0.88-2.37) | .14 | 1.02 (0.65-1.57) | .95 | 1.28 (0.76-2.17) | .35 |
| Health status | ||||||||
| Excellent/good | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Fair/poor | 3.80 (2.03-7.12) | < .0001 | 3.56 (2.20-5.74) | < .0001 | 5.58 (3.71-8.39) | < .0001 | 5.98 (3.65-9.81) | < .0001 |
| Chronic health condition | ||||||||
| None | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Grade1/2 | 1.36 (0.68-2.73) | .39 | 1.48 (0.88-2.48) | .14 | 1.44 (0.91-2.28) | .12 | 1.21 (0.70-2.10) | .49 |
| Grade3/4 | 1.39 (0.57-3.38) | .47 | 1.49 (0.76-2.90) | .24 | 1.91 (1.09-3.36) | .02 | 1.37 (0.68-2.74) | .38 |
| Risk of relapse at HCT | ||||||||
| Standard risk | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| High risk | 1.18 (0.64-2.20) | .59 | 0.75 (0.46-1.21) | .23 | 0.95 (0.63-1.43) | .81 | 0.77 (0.46-1.28) | .31 |
| Age at HCT, year (per 10 y) | 0.91 (0.70-1.20) | .52 | 0.80 (0.66-0.98) | .03 | 0.98 (0.82-1.17) | .86 | 0.88 (0.71-1.09) | .25 |
| Time since HCT (per 10 y) | 0.62 (0.29-1.29) | .20 | 0.55 (0.32-0.93) | .03 | 0.56 (0.35-0.92) | .02 | 0.56 (0.31-0.99) | .05 |
| Primary cancer diagnosis | ||||||||
| CML | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| AML/ALL | 1.32 (0.60-2.92) | .49 | 1.19 (0.69-2.08) | .53 | 0.94 (0.57-1.54) | .79 | 0.92 (0.50-1.67) | .78 |
| HL/NHL | 0.99 (0.41-2.38) | .99 | 0.61 (0.31-1.19) | .14 | 0.68 (0.39-1.17) | .16 | 0.82 (0.42-1.60) | .56 |
| SAA/MM/others | 0.95 (0.33-2.69) | .92 | 0.59 (0.26-1.35) | .21 | 0.65 (0.33-1.29) | .22 | 0.65 (0.28-1.52) | .32 |
| Stem cell donor | ||||||||
| Autologous HCT | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Allogeneic, sibling donor | 1.35 (0.67-2.71) | .40 | 1.41 (0.85-2.33) | .19 | 1.28 (0.82-2.01) | .28 | 1.41 (0.81-2.46) | .22 |
| Allogeneic, unrelated donor | 1.61 (0.61-4.26) | .34 | 0.82 (0.36-1.87) | .64 | 1.60 (0.86-2.96) | .14 | 1.42 (0.65-3.08) | .38 |
| Transplant regimens | ||||||||
| Chemotherapy alone | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Total body irradiation–based | 1.63 (0.75-3.53) | .22 | 1.96 (1.06-3.63) | .03 | 1.63 (0.98-2.71) | .06 | 1.98 (1.03-3.80) | .04 |
| cGVHD (among allogeneic HCT survivors only) | ||||||||
| No | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Active cGVHD | 1.69 (0.66-4.33) | .27 | 1.47 (0.69-3.14) | .32 | 2.00 (1.03-3.89) | .04 | 2.20 (1.01-4.79) | .05 |
| Resolved cGVHD | 0.44 (0.15-1.33) | .15 | 1.49 (0.76-2.93) | .25 | 0.99 (0.51-1.93) | .98 | 1.13 (0.53-2.41) | .75 |
| Any immunosuppression (among allogeneic HCT survivors only) | ||||||||
| Methotrexate | 1.50 (0.54-4.19) | .44 | 0.66 (0.33-1.31) | .23 | 1.31 (0.66-2.58) | .44 | 0.79 (0.38-1.64) | .53 |
| Cyclosporine | 0.97 (0.32-2.91) | .95 | 2.93 (1.15-7.49) | .02 | 1.00 (0.46-2.20) | 1.00 | 1.31 (0.53-3.27) | .56 |
| Prednisone | 2.62 (1.11-6.19) | .03 | 2.27 (1.24-4.18) | .01 | 1.80 (1.04-3.11) | .04 | 2.28 (1.19-4.36) | .01 |
Primary diagnosis, stem cell donor, transplant regimens, chronic GVHD, and use of immunosuppressants were included in the model one at a time. Adjusted for sex, age at study participation (continuous), race/ethnicity (non-Hispanic white, others), marital status (married, not married), income (< $20 000/y, 20 000-60 000/y, > 60 000/y), education (did not complete high school/high-school graduate, some college or above), insurance status (yes, no) and health status (poor/fair, good/excellent), grade of chronic health conditions (none, grade 1 or 2, grade 3 or 4).
HCT indicates hematopoietic cell transplantation; OR, odds ratio; CI, confidence interval; HS, high school; CML, chronic myeloid leukemia; AML, acute myeloid leukemia; ALL, acute lymphoid leukemia; HL, Hodgkin lymphoma; NHL, non-Hodgkin lymphoma; SAA, severe aplastic anemia; MM, multiple myeloma; and cGVHD, chronic GVHD.
Global distress.
Lower household income, poor self-reported health status, shorter time since HCT, and TBI-based conditioning were associated with global distress. Among allogeneic HCT recipients, exposure to prednisone and presence of active chronic GVHD were associated with global distress in separate models. To explore the impact of prednisone further, prednisone and chronic GVHD were introduced in the same model; results demonstrated that the association between prednisone and global distress remained unchanged (without chronic GVHD in model: OR = 2.28, P = .01; with chronic GVHD in the same model: OR = 2.16, P = .02).
Anxiety.
Lower household income and poor self-reported health status were associated with anxiety. Among allogeneic HCT recipients, exposure to prednisone was also associated with anxiety. This association persisted after adjusting for chronic GVHD (without chronic GVHD in the model: OR = 2.62, P = .03; with chronic GVHD in the model: OR = 2.66, P = .03).
Depression.
Male sex, younger age at study participation, lower household income, poor self-reported health status, TBI-based conditioning, and shorter time since HCT were associated with depression. Among allogeneic HCT recipients, exposure to prednisone was also associated with a higher likelihood of reporting depression. Again, the association persisted after adjusting for chronic GVHD in the model (without chronic GVHD in model: OR = 2.27, P = .01; with chronic GVHD in the same model: OR = 2.21, P = .01).
Somatization.
Lower household income, poor self-reported health status, shorter time since HCT, and TBI-based conditioning were associated with somatization. Severe/life-threatening conditions (grades 3 or 4) were associated with a 2.3-fold increased risk of reporting somatization. Among allogeneic HCT recipients, exposure to prednisone and presence of active chronic GVHD were associated with an increased risk of reporting somatic distress in separate models. However, inclusion of chronic GVHD in the model with prednisone somewhat attenuated the association between prednisone and somatization (without chronic GVHD in model: OR = 1.80, P = .04; with chronic GVHD in the same model: OR = 1.68, P = .07).
Suicidal ideation
Although a higher proportion of HCT survivors reported suicidal ideation (6.7%) compared with siblings (2.3%), adjustment for household income eliminated the difference between survivors and siblings (P = .18).
Among HCT survivors, low household income (OR = 2.23, 95% CI, 1.1-4.4 for income $20 000-$60 000; OR = 3.06, 95% CI, 1.3-7.1 for income < $20 000; referent group > $60 000) and poor self-reported health status (OR = 3.15, 95% CI, 1.7-5.7) were associated with a higher likelihood of reporting suicidal ideation. Of note, presence and severity of chronic health conditions did not increase the likelihood of reporting suicidal ideation. In general, survivors with poor psychological outcomes were more likely to report suicidal ideation (OR for global distress = 7.98, 95% CI, 4.3-14.7). Each of the subscales (anxiety, depression, and somatic distress) was associated with suicidal ideation when examined individually. However, inclusion of these subscales in the same model resulted in depression as the only subscale associated with suicidal ideation (OR = 11.59, 95% CI, 6.0-22.5). To further explore the association between suicidal ideation and depression, we classified patients with depression into categories according to the increasing T score (63-65 and ≥ 66) indicating higher severity of depression. As anticipated, while the risk of suicidal ideation was elevated for both levels, patients with T score ≥ 66 were at the highest risk of suicidal ideation (OR = 15.20, 95% CI, 7.1-32.4), while those with T score between 63 and 65 were at a lower risk (OR = 8.24, 95% CI, 3.6-19.0: referent group T score < 63).
Discussion
The overall goal of this study was to describe the prevalence of adverse psychological outcomes experienced by long-term HCT survivors; to understand whether the prevalence differed significantly from a healthy population consisting of siblings; and among those treated with HCT, to identify clinical and demographic variables associated with an increased risk of adverse psychological outcomes. One in 5 HCT survivors reported poor psychological outcomes, in comparison to < 1 in 10 siblings. While adjustment for relevant sociodemographic variables abrogated the difference in global distress, depression, and anxiety between HCT survivors and siblings, somatic distress remained a significant challenge faced by survivors. HCT survivors were at an almost 3-fold higher risk of reporting somatic distress compared with siblings, even after accounting for chronic health conditions.
Most of the previous studies have focused on anxiety and depression in the first few years after HCT31,39-42 ; anxiety and/or depression have been reported in up to 40% of patients before HCT,39,40 gradually declining during the first year.31,42 However, Hjermstad et al did not find any differences in the rates of anxiety or depression in a cohort of patients followed prospectively for 3 to 5 years after HCT, compared with population norms.42 The latter findings are similar to the current report, where, with the exception of somatic distress, the prevalence of survivors with poor psychological outcomes is not different from siblings. Furthermore, the current study demonstrates that the risk of psychological distress declines with time from HCT, indicating that adverse psychological outcomes are less pronounced once the initial intense period surrounding the HCT procedure is over. However, certain subgroups continue to be at increased risk, as detailed here.
Low annual household income was associated with global distress, anxiety, depression, and somatic distress in this study. These findings are similar to those observed in the general population, where the National Health and Nutrition Examination survey data demonstrate that depression rates are higher among socioeconomically disadvantaged individuals.43 Given the high morbidity after HCT and difficulty in resuming gainful employment,44 survivors with low income are especially vulnerable for poor psychological outcomes. Adequate social support should be provided to these survivors to alleviate the risk of developing psychological problems. Self-reported impaired health status was associated with global distress, as well as poor outcome in all 3 subscales. The fact that this association is independent of the presence of chronic health conditions calls for close attention to the reasons for survivors' self-rated health status. Active chronic GVHD was associated with somatic distress and global distress, while resolved chronic GVHD was not. Finally, exposure to prednisone for management of chronic GVHD was associated with psychological distress across all domains. This finding is consistent with existing literature suggesting that corticosteroid exposure is associated with an elevated level of psychological distress. Patten reported that in the general population, persons taking corticosteroids have a higher prevalence of major depression than those not taking the drug.45 Gift et al reported that depression rate was higher among COPD patients receiving steroids than those not receiving steroids.46 Holsboer suggested that impaired corticosteroid receptor signaling might be a key mechanism in the pathogenesis of corticosteroid-related depression.47 To examine whether the association with prednisone was explained by active chronic GVHD in the current study, we examined this association adjusting for active chronic GVHD in the model. The fact that the association remained intact after adjusting for active chronic GVHD suggests that prednisone plays an independent role in the development of adverse psychological outcome. The only exception was somatization, where the association with prednisone was somewhat attenuated, indicating that the association between prednisone and somatic distress was explained to some extent by the presence of active chronic GVHD.
The current study demonstrates that after a median follow-up of 7 years, somatic distress persists as a significant problem faced by survivors. HCT survivors with lower household income, poor self-reported health status, shorter time since HCT, severe/life-threatening conditions (grades 3 or 4), and among allogeneic HCT recipients, exposure to prednisone and presence of active chronic GVHD, were associated with an increased risk of reporting somatic distress. To reduce the burden of somatic distress in this population, specialized multidisciplinary management involving both physician and psychologist is needed.
Although 11% of the HCT survivors reported high scores on the depression subscale, this rate did not differ significantly from the siblings after adjustment for relevant sociodemographic factors. Of note, individuals with a low annual household income were more likely to report higher scores on the depression subscale, consistent with the reports on childhood cancer survivors.48 In addition, among HCT survivors, those who reported poor health status were more likely to report higher scores on the depression subscale. Furthermore, younger individuals, males, and those with shorter follow-up from HCT were more likely to report depression.
Survivors were more likely to report suicidal ideation compared with siblings—a difference that was accounted for by low income. Among HCT survivors, depression was the single most important factor associated with suicidal ideation, consistent with reports from studies focusing on childhood cancer survivors.49 Furthermore, increasing severity of depression was associated with a higher risk of suicidal ideation. However, unlike childhood cancer survivors, presence of chronic health conditions was not associated with suicidal ideation in HCT survivors.
Only 5% of the survivors reported anxiety—a proportion that did not differ significantly from the siblings, after adjustment for sociodemographic variables. Low income and poor self-reported health status were again associated with a higher level of anxiety. The comparable level of anxiety between survivors and siblings is expected because of the long follow-up time after HCT. Previous longitudinal studies indicate that recovery after HCT occurs gradually over 1 to 5 years.32 Hjermstad et al also did not find any differences in the rates of anxiety or depression in a cohort of patients followed prospectively for 3 to 5 years after HCT, compared with population norms.42
Certain limitations should be considered when interpreting these findings. In the current, we did not control for variables such as coping and social support that are known to impact psychological well-being.31 To clearly identify the impact of HCT on the risk of adverse psychological outcomes, a control population, consisting of patients with similar diseases but treated with conventional therapy without transplantation would be ideal. However, there are very few clinical scenarios where otherwise identical patients are randomized to conventional treatment versus HCT. In essence, patients undergoing HCT are likely to be more heavily pretreated, and at a higher risk of relapse. Thus, a direct comparison between patients treated with conventional therapy and those treated with transplantation is impractical. Siblings were used as a control group, rather than relying on normative data for comparison. The proportion of siblings whose GSI scores approximated clinical distress levels fell well within the range found in the general population, making them more like the general population than different. While there are some areas where siblings might indicate more problems than the general population, the survivor-sibling comparisons would make significant differences clinically meaningful and conservative. The participation rate was 69%, and could have resulted in self-selection and reporting biases, contributing to an underestimation of deficits by excluding survivors who are having more difficulty adjusting or are unable or unwilling to participate. The study relies on self-reported psychological symptoms experienced within the 7 days before participation. Such a cross-sectional evaluation does not capture other relevant psychosocial adjustment issues and may not be sensitive to other temporal patterns of psychological distress or the possibility that symptoms may have originated before HCT. Finally, the cohort included patients who received transplants between 1974 and 1998. Although this allowed for a long follow-up time, the outcomes reported in this study might not necessarily represent the expected (psychological) outcomes of patients receiving transplants today because of changes in transplantation technology and supportive care. However, by the time the patients receiving transplants today become long-term survivors, the transplantation strategies of today will have evolved, making the findings less applicable again. It is for this reason that the current study focused on examining certain disease and treatment variables that have remained relatively stable over the years, such as exposure to TBI (acknowledging that dose, fractionation, and schedule may have evolved), development of chronic GVHD, and exposure to steroids. These issues are as pertinent and broadly applicable today as they were in the 1980s and 1990s.
These limitations notwithstanding, the major strengths of this study are the large patient population followed long-term; evaluation of psychological outcomes using a well-validated instrument; and utilization of a relevant comparison group. Most of the previous studies of HCT survivors have been limited because of small, homogeneous clinical samples, and do not include appropriate comparison groups. This study recruited a diverse patient population, representing a full spectrum of HCT populations thus allowing much greater generalizability of the findings. Unlike some of the previous studies, the large sample permitted analyses that identified vulnerable subgroups and specific factors that are associated with poor psychological outcome. Finally, the current study is the first to examine the contribution of chronic health conditions in the overall magnitude and severity of psychological distress reported by HCT survivors.
Although the prevalence of adverse psychological outcomes reported by HCT survivors is similar to that reported by an unaffected sibling comparison group (with the exception of the nearly 3-fold higher risk of somatic distress reported by the survivors), there are well-characterized subpopulations that are at a significantly higher risk for psychological distress. HCT survivors with low annual household income, those with impaired self-reported health status, those with active chronic GVHD, and those managed with prednisone for their chronic GVHD are at significantly increased risk for psychological distress. Secondary and tertiary prevention of the psychosocial consequences in the HCT survivors may be realized by interventions that help healthcare providers optimize the medical care for conditions such as chronic GVHD, as well as interventions aimed at helping them cope more effectively with the burden of illness. Finally, understanding that poverty and self-perceived poor health play a large role in psychological distress could help institute targeted interventional strategies to provide aggressive support to those at risk.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by grants from the National Institutes of Health (R01 CA078938, S.B.; P01 CA 30 206, S.J.F.; K23 CA85503-01, K.S.B.) and the Leukemia & Lymphoma Society (2192, S.B.).
National Institutes of Health
Authorship
Contribution: C.-L.S. analyzed the data and wrote the manuscript; L.F. performed the research and interpreted the data; K.S.B. D.J.W., and S.J.F. provided critical insight into the interpretation of the data; and S.B. designed the study, supervised and performed the research, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Smita Bhatia, City of Hope, 1500 E Duarte Rd, Duarte, CA 91010-3000; e-mail: sbhatia@coh.org.