Abstract
The precise molecular mechanism of action and targets through which thalidomide and related immunomodulatory drugs (IMiDs) exert their antitumor effects remains unclear. We investigated the role of cereblon (CRBN), a primary teratogenic target of thalidomide, in the antimyeloma activity of IMiDs. CRBN depletion is initially cytotoxic to human myeloma cells, but surviving cells with stable CRBN depletion become highly resistant to both lenalidomide and pomalidomide, but not to the unrelated drugs bortezomib, dexamethasone, and melphalan. Acquired deletion of CRBN was found to be the primary genetic event differentiating isogenic MM1.S cell lines cultured to be sensitive or resistant to lenalidomide and pomalidomide. Gene expression changes induced by lenalidomide were dramatically suppressed in the presence of CRBN depletion, further demonstrating that CRBN is required for lenalidomide activity. Downstream targets of CRBN include interferon regulatory factor 4 (IRF4) previously reported to also be a target of lenalidomide. Patients exposed to, and putatively resistant to, lenalidomide had lower CRBN levels in paired samples before and after therapy. In summary, CRBN is an essential requirement for IMiD activity and a possible biomarker for the clinical assessment of antimyeloma efficacy.
Introduction
Thalidomide, initially developed for use as a sedative and for the treatment of morning sickness, is a compound with teratogenic effects.1 However, recent findings also demonstrate the efficacy of thalidomide and related immunomodulatory drugs (IMiDs) for the treatment of several hematologic malignancies, including multiple myeloma.2-6 IMiDs, including lenalidomide (CC-5013) and pomalidomide (CC-4047), thus represent a novel class of small-molecule antitumor drugs.7 Nevertheless, only 30% of patients respond to these drugs when used as single agents, and many patients will develop resistance.
Although several mechanisms of action have been proposed to explain the direct and indirect antimyeloma effect of thalidomide and related IMiDs, such as antiangiogenic,8 proapoptotic, antiproliferative, and immunomodulatory effects, the precise molecular mechanisms and targets through which thalidomide and IMiDs exert their effects remain unclear.9 A landmark paper has recently identified cereblon (CRBN) as a primary target of thalidomide teratogenicity.10 The authors show, in a zebrafish model, how thalidomide binds to CRBN at specific sites and demonstrate that this interaction disrupts the function of the E3 ubiquitin ligase complex (composed by proteins CRBN, DDB1, and Cul4), ultimately leading to the down-regulation of fibroblast growth factor genes and the teratogenic effects associated with thalidomide. However, whether the mechanism of action of thalidomide and IMiDs teratogenicity is linked to their efficacy as antitumor agents remains unclear.
In this study, we tested whether the absence of CRBN in human multiple myeloma cells modified the cellular cytotoxicity generated by IMiDs. Our data showed that CRBN down-regulation resulted in the development of marked IMiD resistance in human MM cells, thus confirming that the presence of CRBN is an absolute requirement for the IMiD activity.
Methods
CRBN shRNA lentiviral experiments
The lentiviral constructs expressing nontargeting (NT) and CRBN shRNAs (Sigma-Aldrich) were modified by replacing the puromycin resistance gene with a GFP-expressing cassette. Lentiviruses were prepared and used to infect various human multiple myeloma cell lines (HMCLs) as previously described.11 HMCLs were grown in RPMI 1640 media supplemented with 10% FCS and antibiotics. Infection efficiency was measured by FACScan analysis of GFP expression at 48 hours after infection. Cell viability was measured by 3-(4,5-dimethylthiazol)-2,5-diphenyl tetrazolium (MTT) dye absorbance at 6 days after infection, according to the manufacturer's instructions (Boehringer Mannheim). Apoptosis, using annexin V (BD Biosciences), and cell cycle assays were performed as previously described.2 To confirm CRBN knockdown, real-time quantitative PCR analysis of CRBN level was performed in control and CRBN shRNA-expressing cells harvested at 72 hours after infection. GFP-positive cells were sorted and expanded at 3 weeks after infection, and quantitative PCR was performed in sorted cells to confirm the low CRBN expression level.
HMCLs were incubated with serial doses of lenalidomide (ChemPacific), pomalidomide (Selleck Chemicals), dexamethasone, melphalan, and bortezomib for 3 to 6 days. Cell viability was determined using MTT assay. Each experimental condition was performed in triplicate and repeated at least once.
Quantitative PCR
Total RNA was isolated using RNeasy Plus Mini kit (QIAGEN) and reverse-transcribed using QuantiTect Reverse Transcription kit (QIAGEN). Quantitative PCR was performed using TaqMan Universal PCR Master Mix with predesigned probe (Applied Biosystems), and the comparative CT method was used for relative quantification on an ABI 7900HT Fast Real-Time PCR system (Applied Biosystems). Patients provided written informed consent for correlative studies according to the Declaration of Helsinki on a Mayo Clinic Institutional Review Board–approved protocol for the collection and use of samples for research purposes from both participating institution.
Immunoblotting
Western blot was performed using the manufacturer's protocol. Briefly, equal amounts of protein were subjected to SDS-PAGE gels followed by transfer to polyvinylidene difluoride membranes. Membranes were probed with primary antibodies, such as anti-Flag (Sigma-Aldrich), anti-CRBN (kindly provided by Dr Hiroshi Handa), and anti-IRF4 (Cell Signaling Technology) overnight, and then washed and incubated with HRP-conjugated secondary antibodies (Santa Cruz Biotechnology). Detection was performed by the enhanced chemical luminescence method. The membranes were stripped and reprobed with β-actin antibody (Cell Signaling Technology) to confirm protein loading.
Array-based comparative genomic hybridization
Genomic DNA of the original, lenalidomide sensitive, MM1.S cell line, and the derivative, lenalidomide-resistant, MM1.Sres cell line was obtained using Puregene blood kit (QIAGEN) according to the manufacturer's recommendations. High-resolution array-based comparative genomic hybridization was performed with the Human Genome 244A microarray (Agilent Technologies). DNA samples from a pool of 9 human female, lymphoblastoid cell lines (from Coriell repository) were used as the normal reference in the hybridization experiments. The digestion, labeling, and hybridization steps were done as previously described with minor modifications.12 Briefly, 1.2 μg of tumor and reference DNAs was separately digested with bovine DNaseI (Ambion) for 12 minutes at room temperature. Next, random primers and exo-Klenow fragment (Invitrogen) were used to differentially label tumor (Cy5) and reference (Cy3) genomic DNA samples (GE Healthcare). Labeled genomic reactions were cleaned up with purification columns (Invitrogen) and hybridized at 65°C for 40 hours. Microarrays were scanned in a DNA Microarray Scanner (Agilent Technologies). Feature extraction was performed with Feature Version 9.5 extraction software (Agilent Technologies). Log2 ratio data were imported and analyzed using DNA Analytics Version 4.0.85 software (Agilent Technologies). Copy number abnormalities were calculated using aberration detection module-1 algorithm13 with a threshold of 7.5. A 2 probe, 0.25-log2 filters were used in the aberration detection, obtaining an average genomic resolution of 17 kb. The complete dataset is accessible through GEO series accession number GSE31451.
FISH
Interphase FISH was used to analyze the ploidy status and copy number of CRBN in MM1.S and MM1.Sres. A total of 100 cells were counted in each case. Cut-off for scoring distinctive copy number abnormality was determined using normal controls and varied depending on probe set. The commercial and custom FISH probes used are listed in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
GEP analysis
OPM2 cells infected with NT and CRBN shRNA-expressing lentivirus were harvested and total RNA was prepared using RNeasy Plus Mini Kit (QIAGEN). Gene expression profiling (GEP) was generated from total RNA labeled with the Affymetrix OneStep IVT labeling kit and hybridized to the Affymetrix U133Plus Version 2.0 array. All labeling, hybridization, washing, and scanning steps were performed by the MicroArray facility at the Mayo Clinic Advanced Genomic Technology Center following the manufacturer's protocol. CEL files were processed and normalized by MAPP application with the following default settings: BG correction, gcrma; normalization, fastlo; PM correction, affinities_only; summarization, medianpolish; and computeCalls, TRUE. A 2-fold expression threshold was used to find differentially expressed genes between the treated and control samples. The complete dataset is accessible through GEO series accession number GSE31421.
DNA sequencing
Genome sequencing was performed on the CRBN coding exons and adjacent intron-exon junctions on MM1.Sres. All the coding regions were amplified using 10 ng of genomic DNA in 25-μL reactions. The specific primers used in this study were previously published.14 Capillary electrophoresis was performed on an ABI3730 sequencer (Applied Biosystems). DNA sequences were analyzed using Sequencher Version 4.5.
Pathway analysis
Pathway analysis was performed using MetaCore database software (GeneGo). The subset of probes that shared similar changes from GEP analysis in lenalidomide-treated OPM2 cells and CRBN shRNA-transduced OPM2 were uploaded as the input list for generation of biologic networks. Enrichment analysis consisted of matching gene IDs of possible targets for the “common,” “similar,” and “unique” sets with gene IDs in functional ontologies in MetaCore. The probability of a random intersection between a set of IDs and the size of target list with ontology entities was estimated in a P value of hypergeometric intersection. The lower the P value, the higher the relevance of the entity to the dataset, which shows in higher rating for the entity. The network of key transcription factors and targets was generated using Transcription Regulation algorithm or Transcription Factor Targets Modeling algorithm with default settings.
Results
In vitro responsiveness of HMCLs to IMiDs
The effect of lenalidomide, pomalidomide, and thalidomide on cell growth was first established on a panel of 12 HMCLs by MTT assay. Myeloma cell lines exhibit variable degrees of sensitivity to both lenalidomide and pomalidomide; and, in general, high concentrations and prolonged exposure to these drugs are required to produce cell death. The growth of 3 HMCLs (ie, KMS11, MM1.S, and OPM2) was substantially inhibited when treated either with lenalidomide or pomalidomide under these conditions. Conversely, 4 HMCLs (OCIMY5, OPM1, SKMM2, and KMS12 PE) were very resistant to treatment with both drugs. Thalidomide had no effects on MM cell growth using similar drug doses (data not shown). This absence of in vitro activity of thalidomide has been previously reported, suggesting a requirement for in vivo metabolism for the proper function of thalidomide.
Suppression of CRBN is cytotoxic
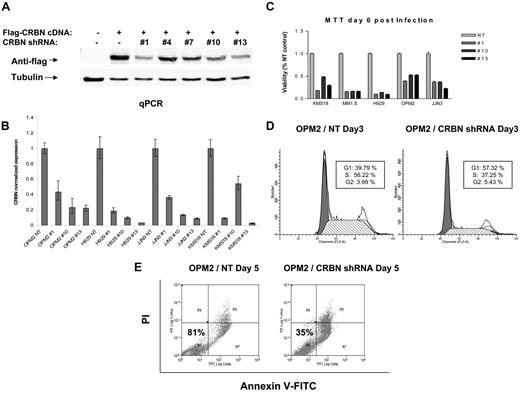
Five predesigned CRBN shRNA lentiviral expression constructs were obtained, and 3 of the 5 shRNA were confirmed to efficiently knockdown CRBN using a Flag-tagged CRBN in 293 cells (Figure 1A). Lentiviruses expressing these 3 CRBN shRNAs and the corresponding NT shRNA controls were then used to infect 5 HMCLs (KMS18, MM1.S, H929, OPM2, and JJN3). The infection efficiency of each lentivirus was measured at 48 hours after infection by FACScan analysis of GFP-positive cells (supplemental Figure 1). CRBN expression and cell viability were measured at days 3 and 6 after infection by quantitative PCR and MTT, respectively. HMCLs infected with CRBN shRNAs have a significant reduction of CRBN expression and also cell viability (reduced 65%-78%) compared with cells infected with control virus (Figure 1B-C). Furthermore, CRBN knockdown inhibited proliferation (Figure 1D) and induced apoptosis (Figure 1E).
CRBN knockdown induces myeloma cell cytotoxicity. (A) Five lentiviral CRBN shRNA constructs were cotransfected with Flag-tagged CRBN cDNA expression constructs into 293 cells, and Western blot was performed at day 3 after transfection to measure flag-tagged CRBN level. Lentiviruses were prepared from 3 lentiviral CRBN shRNA constructs (1, 10, and 13) and the corresponding NT constructs and subsequently were used to infect 4 different myeloma cell lines. CRBN knockdown was detected by quantitative PCR at day 3 after infection (B), and the cell viability was measured by MTT assay at day 6 after infection (C). Cell cycle (D) and apoptosis assays (E) were performed on OPM2 cells at day 3 and day 5 after lentiviral infection, respectively.
CRBN knockdown induces myeloma cell cytotoxicity. (A) Five lentiviral CRBN shRNA constructs were cotransfected with Flag-tagged CRBN cDNA expression constructs into 293 cells, and Western blot was performed at day 3 after transfection to measure flag-tagged CRBN level. Lentiviruses were prepared from 3 lentiviral CRBN shRNA constructs (1, 10, and 13) and the corresponding NT constructs and subsequently were used to infect 4 different myeloma cell lines. CRBN knockdown was detected by quantitative PCR at day 3 after infection (B), and the cell viability was measured by MTT assay at day 6 after infection (C). Cell cycle (D) and apoptosis assays (E) were performed on OPM2 cells at day 3 and day 5 after lentiviral infection, respectively.
Suppression of CRBN confers resistance to lenalidomide and pomalidomide
Depletion of CRBN significantly reduced the growth and viability of HMCLs. However, ∼ 30% of infected cells survived at 6 days after infection across different HMCLs analyzed. To confirm that CRBN remained depleted, surviving cells were sorted by GFP expression (Figure 2A), and CRBN expression level was analyzed by quantitative PCR. Overall, a 5- to 10-fold reduction of CRBN expression level was observed across different HMCLs (Figure 2B). Next, we tested the proliferation of CRBN-depleted HMCLs in the presence of different antimyeloma drugs. Three HMCLs with > 98% GFP-positive cells and CRBN knockdown (OPM2, H929, and KMS18) demonstrated an acquired (essentially complete) resistance to lenalidomide compared with their NT controls (Figure 2C). OPM2 was subsequently tested with other antimyeloma drugs, showing resistance to lenalidomide and pomalidomide (Figure 3A-B), but retained sensitivity to melphalan, dexamethasone, and bortezomib (Figure 3C-E). In addition, no cytotoxic effect was found in any HMCL tested in vitro with phthalimide (data not shown), an analog of thalidomide that was shown to be unable to bind to CRBN.10 These data confirmed that the suppression of CRBN specifically affects the response of HMCLs to IMiDs but not to other drugs.
Suppression of CRBN confers lenalidomide resistance. Three myeloma cell lines were infected with NT and CRBN shRNA-expressing lentivirus. Most CRBN shRNA-expressing myeloma cells died within the first week after infection. An average of 30% of myeloma cells survived, and those cells plus NT control expressing cells were sorted for GFP expression at 3 weeks after infection (A). CRBN expression level in each pair of sorted NT and CRBN shRNA-expressing cells was measured by quantitative PCR (B). (C) Activity of lenalidomide on sorted myeloma cell lines was measured by MTT assay.
Suppression of CRBN confers lenalidomide resistance. Three myeloma cell lines were infected with NT and CRBN shRNA-expressing lentivirus. Most CRBN shRNA-expressing myeloma cells died within the first week after infection. An average of 30% of myeloma cells survived, and those cells plus NT control expressing cells were sorted for GFP expression at 3 weeks after infection (A). CRBN expression level in each pair of sorted NT and CRBN shRNA-expressing cells was measured by quantitative PCR (B). (C) Activity of lenalidomide on sorted myeloma cell lines was measured by MTT assay.
Drug resistance mediated by CRBN depletion is specific for IMiDs, not for other unrelated antimyeloma compounds. OPM2 cells stably expressing either NT or CRBN shRNA were seeded and incubated with lenalidomide (A), pomalidomide (B), and other antimyeloma drugs, including bortezomib (C), dexamethasone (D), and melphalan (E) at the indicated concentration, followed by MTT assay at day 3 after adding drugs. Each experimental condition was performed in triplicate and repeated at least once.
Drug resistance mediated by CRBN depletion is specific for IMiDs, not for other unrelated antimyeloma compounds. OPM2 cells stably expressing either NT or CRBN shRNA were seeded and incubated with lenalidomide (A), pomalidomide (B), and other antimyeloma drugs, including bortezomib (C), dexamethasone (D), and melphalan (E) at the indicated concentration, followed by MTT assay at day 3 after adding drugs. Each experimental condition was performed in triplicate and repeated at least once.
Genomic abnormalities affecting CRBN confer MM1.S cell line resistance to IMiDs
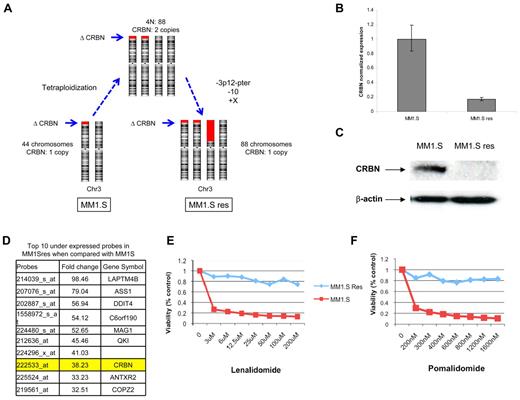
MM1.Sres was previously generated by culturing MM1.S in gradually increasing concentrations of lenalidomide.15 Thus, we conducted a genomic analysis searching for genomic abnormalities and gene expression differences between MM1.S (lenalidomide-sensitive) and isogenic MM1.Sres (lenalidomide-resistant) HMCLs. Based on array-based comparative genomic hybridization and FISH analyses, the cellular karyotype evolved from near-diploid harboring one copy of CRBN in MM1.S (44 chromosomes) to tetraploid with 2 copies of CRBN in MM1.Sres (88 chromosomes). Subsequent to the development of tetraploidy, the MM1.Sres karyotype was further characterized by the loss of one additional copy of 3p (containing the locus CRBN) as well as one copy of chromosome 10 and a gain of an extra copy of chromosome X (Figure 4A). Although MM1.Sres still has one copy (3 copies absent) of CRBN, GEP comparison between cell lines shows a 40-fold reduction of CRBN expression on MM1.Sres compared with the original MM1.S cell line. The CRBN RNA and protein are also confirmed as absent using quantitative PCR and Western blot (Figure 4B-C). Furthermore, CRBN was in the top 10 underexpressed genes in the MM1.Sres compared with the original MM1.S cell line (Figure 4D). The lack of CRBN expression was associated with complete resistance to lenalidomide and pomalidomide in MM1.Sres but not in MM1.S (Figure 4E-F).
Loss of CRBN expression in MM1.S cell line is associated with lenalidomide resistance. (A) Diagram of the karyotype evolution observed from MM1.S (lenalidomide-sensitive) to MM1.Sres cell line (lenalidomide-resistant). Red blocks represent the deletions on chromosome 3p, including CRBN (arrows indicate its location). Although 1 CRBN allele is present in the MM1.Sres cell line, there is a significant reduction of CRBN transcript (B) and lack of protein expression (C). (D) GEP comparison showed CRBN as one of the top 10 underexpressed genes in MM1.Sres compared with MM1.S. The lack of or very low CRBN expression was associated with lenalidomide (E) and pomalidomide (F) resistance.
Loss of CRBN expression in MM1.S cell line is associated with lenalidomide resistance. (A) Diagram of the karyotype evolution observed from MM1.S (lenalidomide-sensitive) to MM1.Sres cell line (lenalidomide-resistant). Red blocks represent the deletions on chromosome 3p, including CRBN (arrows indicate its location). Although 1 CRBN allele is present in the MM1.Sres cell line, there is a significant reduction of CRBN transcript (B) and lack of protein expression (C). (D) GEP comparison showed CRBN as one of the top 10 underexpressed genes in MM1.Sres compared with MM1.S. The lack of or very low CRBN expression was associated with lenalidomide (E) and pomalidomide (F) resistance.
Myeloma patients with lenalidomide resistance demonstrated reduction on CRBN expression levels
Because CRBN is required for lenalidomide and pomalidomide response, we further investigated the CRBN expression level in 9 MM patients with physician-reported lenalidomide resistance. In 8 of 9 MM patients, with pre- and post-lenalidomide therapy samples analyzed by quantitative PCR, the CRBN expression level showed a significant reduction (20%-90% reduction) at the time of drug resistance (Figure 5A).
Low CRBN expression is common in lenalidomide refractory patients. (A) Nine MM patients, whose pretreatment and post-treatment samples were available, were analyzed by real-time PCR for the expression of CRBN in MM cells collected at both diagnosis (D) and relapse (R) stages. The data for each sample represent the mean values of 4 independent experiments (mean ± SD). (B) An analysis of CRBN expression across different differentiation stages of normal B cells and a variety of B-cell tumors, including marginal zone lymphoma (MZL, n = 86), lymphoplasmacytic lymphoma/Waldenström macroglobulinemia (LPL/WM, n = 21), follicular lymphoma (FL, n = 38), diffuse large B-cell lymphoma (DLBCL, n = 73), MM (n = 238), and HMCLs (n = 60), shows a similar expression level across entities without low expression outliers. Arrows indicate the CRBN expression level in 4 HMCLs that show resistance to lenalidomide (OCIMY5, OPM1, SKMM2, and KMS12 PE). (C) The low CRBN gene expression level found in OCIMY5 and OPM1 was correlated with low protein expression level.
Low CRBN expression is common in lenalidomide refractory patients. (A) Nine MM patients, whose pretreatment and post-treatment samples were available, were analyzed by real-time PCR for the expression of CRBN in MM cells collected at both diagnosis (D) and relapse (R) stages. The data for each sample represent the mean values of 4 independent experiments (mean ± SD). (B) An analysis of CRBN expression across different differentiation stages of normal B cells and a variety of B-cell tumors, including marginal zone lymphoma (MZL, n = 86), lymphoplasmacytic lymphoma/Waldenström macroglobulinemia (LPL/WM, n = 21), follicular lymphoma (FL, n = 38), diffuse large B-cell lymphoma (DLBCL, n = 73), MM (n = 238), and HMCLs (n = 60), shows a similar expression level across entities without low expression outliers. Arrows indicate the CRBN expression level in 4 HMCLs that show resistance to lenalidomide (OCIMY5, OPM1, SKMM2, and KMS12 PE). (C) The low CRBN gene expression level found in OCIMY5 and OPM1 was correlated with low protein expression level.
Further examination of CRBN status on additional HMCLs and MM patients irrespective of therapy and stage suggested that copy number abnormalities affecting this gene are rare events in MM, with only 12% of HMCLs (7 of 60) and 1.2% of MM patients (3 of 238) showing CRBN monoallelic deletion (data not shown). In addition, GEP analysis did not identify additional HMCLs or MM cases with complete lack of CRBN expression (Figure 5B). From the original 4 HMCLs showing high resistance to treatment with lenalidomide and pomalidomide (OCIMY5, OPM1, SKMM2, and KMS12 PE), OCIMY5 and OPM1 showed CRBN gene expression levels at the bottom 10% across HMCLs (Figure 5B). The low mRNA expression observed in both HMCLs was correlated with low protein levels (Figure 5C). The remaining 2 resistant HMCLs (SKMM2 and KMS12 PE) showed normal levels of CRBN protein. Thus, although the data are generally consistent with our hypothesis that absence of CRBN confers resistance, numerous other factors may contribute to acquire drug resistance in patient populations, including pharmacokinetics, inability to tolerate full-dose drug without side effects, and likely other molecular acquired resistance mechanisms. Thus, the frequency with which whole or partial CRBN depletion is the responsible mechanism of resistance will require more detailed analysis on carefully selected patient populations and will be a subject for future intense scrutiny.
The molecular basis of IMiD resistance after CRBN knockdown
Next, we wanted to know whether the effect of depleting CRBN induces similar gene expression changes to in vitro therapy with lenalidomide. The GEP data obtained from OPM2 cells treated with lenalidomide for 0, 24, 48, and 72 hours were compared with the GEP data obtained from OPM2 cells with CRBN knockdown by shRNA. Overall, 123 genes were identified to have shared expression changes between cells treated with lenalidomide and with CRBN shRNA (Table 1). Pathway analysis performed on that set of genes indicated enrichment on cell survival and immune response cell signaling pathways as many affected genes were found to be targets of critical transcription factors, such as MYC, SP1, and TP53 (Figure 6A-B). We noticed that CRBN knockdown induced down-regulation of IRF4 (> 2 fold expression decrease). The effect of CRBN knockdown and lenalidomide treatment on IRF4 protein expression was subsequently analyzed in HMCLs. Both CRBN knockdown and lenalidomide treatment reduced IRF4 protein expression in HMCLs (Figure 6C). Interestingly, we demonstrated that after the initial CRBN knockdown and induced IRF4 reduction myeloma cytotoxicity are marked, however, in the surviving CRBN-depleted cells (now lenalidomide-resistant) IRF4 protein levels return to normal, suggesting alternate means of up-regulating IRF4. As further partial evidence of the CRBN-IRF4 axis, IRF4 expression levels are reduced in response to lenalidomide in lenalidomide-sensitive, but not in established lenalidomide-resistant, HMCLs (supplemental Figure 2).
Gene alterations shared in OPM2 cells after either CRBN knockdown or lenalidomide treatment
| Down-regulated . | Up-regulated . | |||
|---|---|---|---|---|
| AGPS | HAUS7///TREX2 | ROR1 | ADAT3 | HLA-DRB1///HLA-DRB4 |
| AJAP1 | HINT3 | RRP1B | ATXN1 | HLA-DRB1///HLA-DRB4 |
| APTX | HS3ST3B1 | SEC24A | AUTS2 | IFIH1 |
| BCL11B | ITPR1 | SEL1L | BTBD7 | IGF2BP3 |
| BEST3 | LOC730631 | SETD7 | C10orf10 | LIMA1 |
| BID | LOC84740 | SETMAR | C2orf68 | LRRC4C |
| C12orf66 | LPL | SLC7A2 | CARD16 | MAML2 |
| C13orf1 | LRRC8B | SMCR7L | CASP1 | 2-Mar |
| C18orf54 | MMACHC | SMEK2 | CTTNBP2 | MB |
| C7orf68 | MRS2 | SNHG3 | DCC | NFIB |
| CEP72 | MTAP | SNRPN | DHTKD1 | NMI |
| CNKSR2 | MYB | STAMBPL1 | DLEU2 | PCBP2 |
| COX11 | NEK6 | SUV39H2 | DNAJC5B | PLOD2 |
| DFFA | NETO2 | TET1 | ELF3 | PTEN |
| DNAJC25 | NUDCD1 | TMEM33 | EXTL2 | PTPRM |
| DTNA | NUP43 | TMEM64 | FCHO2 | RASGRP3 |
| EIF3M | ORC5L | TNFRSF21 | GIMAP2 | RCAN3 |
| FAM76B | PDK1 | TRIM4 | HIPK2 | RTP4 |
| FANCI | PGM2 | USP9X | HIST1H2AD///HIST1H3D | SUMF1 |
| FKBP11 | PPIF | WDR4 | HIST1H2BG | TMEM185A |
| FLJ25006 | PPP2R1B | WDR72 | HIST2H4A///HIST2H4B | UTRN |
| GABRB2 | RAD18 | ZNF148 | HLA-DPB1 | WDFY3 |
| GALNTL4 | RFC3 | ZNF561 | HLA-DQA1 | |
| GINS4 | RGS1 | ZNF706 | HLA-DQA1/// HLA-DRB1///HLA-DRB3 | |
| GNPNAT1 | RGS16 | ZWILCH | ||
| Down-regulated . | Up-regulated . | |||
|---|---|---|---|---|
| AGPS | HAUS7///TREX2 | ROR1 | ADAT3 | HLA-DRB1///HLA-DRB4 |
| AJAP1 | HINT3 | RRP1B | ATXN1 | HLA-DRB1///HLA-DRB4 |
| APTX | HS3ST3B1 | SEC24A | AUTS2 | IFIH1 |
| BCL11B | ITPR1 | SEL1L | BTBD7 | IGF2BP3 |
| BEST3 | LOC730631 | SETD7 | C10orf10 | LIMA1 |
| BID | LOC84740 | SETMAR | C2orf68 | LRRC4C |
| C12orf66 | LPL | SLC7A2 | CARD16 | MAML2 |
| C13orf1 | LRRC8B | SMCR7L | CASP1 | 2-Mar |
| C18orf54 | MMACHC | SMEK2 | CTTNBP2 | MB |
| C7orf68 | MRS2 | SNHG3 | DCC | NFIB |
| CEP72 | MTAP | SNRPN | DHTKD1 | NMI |
| CNKSR2 | MYB | STAMBPL1 | DLEU2 | PCBP2 |
| COX11 | NEK6 | SUV39H2 | DNAJC5B | PLOD2 |
| DFFA | NETO2 | TET1 | ELF3 | PTEN |
| DNAJC25 | NUDCD1 | TMEM33 | EXTL2 | PTPRM |
| DTNA | NUP43 | TMEM64 | FCHO2 | RASGRP3 |
| EIF3M | ORC5L | TNFRSF21 | GIMAP2 | RCAN3 |
| FAM76B | PDK1 | TRIM4 | HIPK2 | RTP4 |
| FANCI | PGM2 | USP9X | HIST1H2AD///HIST1H3D | SUMF1 |
| FKBP11 | PPIF | WDR4 | HIST1H2BG | TMEM185A |
| FLJ25006 | PPP2R1B | WDR72 | HIST2H4A///HIST2H4B | UTRN |
| GABRB2 | RAD18 | ZNF148 | HLA-DPB1 | WDFY3 |
| GALNTL4 | RFC3 | ZNF561 | HLA-DQA1 | |
| GINS4 | RGS1 | ZNF706 | HLA-DQA1/// HLA-DRB1///HLA-DRB3 | |
| GNPNAT1 | RGS16 | ZWILCH | ||
GEP identified common changes in OPM2 cells treated either with lenalidomide or with CRBN shRNA. GEP analysis was performed on OPM2 cells either treated with lenalidomide for 48 and 72 hours or transduced with CRBN shRNA (for 48 and 72 hours). (A) The numbers of genes that have expression changed at least 2-fold in both treatments (lenalidomide vs vehicle and CRBN shRNA vs NT shRNA) are summarized at the top. Overall, 123 gene changes were shared between lenalidomide treatment and CRBN knockdown. Those 123 genes were uploaded as the input list for generation of biologic networks using MetaCore pathway analysis. (B) Illustration of one of the top scored networks from active experiments. Green lines indicate activation; red lines, inhibition; and gray lines, unspecified. Red circles represent up-regulated genes; and blue circles, down-regulated genes. (C) Western blot analysis was performed to detect IRF4 expression in HMCLs treated either with lenalidomide or transfected with CRBN shRNA.
GEP identified common changes in OPM2 cells treated either with lenalidomide or with CRBN shRNA. GEP analysis was performed on OPM2 cells either treated with lenalidomide for 48 and 72 hours or transduced with CRBN shRNA (for 48 and 72 hours). (A) The numbers of genes that have expression changed at least 2-fold in both treatments (lenalidomide vs vehicle and CRBN shRNA vs NT shRNA) are summarized at the top. Overall, 123 gene changes were shared between lenalidomide treatment and CRBN knockdown. Those 123 genes were uploaded as the input list for generation of biologic networks using MetaCore pathway analysis. (B) Illustration of one of the top scored networks from active experiments. Green lines indicate activation; red lines, inhibition; and gray lines, unspecified. Red circles represent up-regulated genes; and blue circles, down-regulated genes. (C) Western blot analysis was performed to detect IRF4 expression in HMCLs treated either with lenalidomide or transfected with CRBN shRNA.
To further examine the molecular basis of lenalidomide resistance after CRBN depletion, gene expression from OPM2 cells stably expressing CRBN shRNA (resistant to lenalidomide) and NT control shRNA (sensitive to lenalidomide) was compared. The experiment was performed in cells treated with lenalidomide for 48 hours. The NT control, lenalidomide-sensitive, cells showed 2-fold expression changes in approximately 1200 genes (600 up-regulated and 600 down-regulated), whereas CRBN-depleted, lenalidomide-resistant, OPM2 cells only showed 30 down-regulated (3% of control) and 150 up-regulated genes (24% of control) after treatment (supplemental Table 2). The marked depression of gene expression changes in CRBN-depleted OPM2 cells after treatment with lenalidomide reinforces the evidence that the presence of CRBN is critical for the activity of lenalidomide in HMCLs.
Discussion
In recent years, preclinical and clinical studies demonstrating the efficacy of thalidomide and IMiDs for the treatment of MM and other hematologic malignancies have generated substantial interest in the development and use of these compounds.4-6 Nevertheless, although thalidomide has been studied for < 40 years, key questions remain unanswered regarding its mechanism of action and whether its teratogenicity and antitumor activity are related. A recent seminal study has identified CRBN as a primary target of thalidomide teratogenicity.10 Thalidomide was demonstrated to bind to CRBN and inhibit the function of the E3 protein ligase complex, which is required for limb outgrowth and expression of the fibroblast growth factor FGF8. However, whether CRBN is involved in the antimyeloma effects of thalidomide and IMiDs is unclear.
In the present study, we demonstrated that CRBN is an essential requirement for IMiD antimyeloma activity. Our study clearly shows that CRBN is involved in IMiD antimyeloma activity based on the following evidence: (1) CRBN depletion is directly cytotoxic to myeloma cells; (2) IMiD-sensitive HMCLs become profoundly resistant after CRBN is knocked down; (3) acquired loss of CRBN expression in the MM1.S cell line was associated with IMiD resistance; (4) phthalimide, an analog of thalidomide that does not bind to CRBN,10 did not show activity on any HMCL tested; (5) loss of CRBN expression did not affect the HMCL response to other unrelated drugs, such as bortezomib, dexamethasone, and melphalan; and (6) gene expression changes induced by lenalidomide exposure are, to a large extent, vanished when CRBN is depleted. In addition, preliminary data suggest that a reduction on CRBN expression was observed in > 85% of lenalidomide-resistant MM patients, although larger and carefully selected patient cohorts (clearly resistant and unresponsive to full dose of IMiD) need to be evaluated to confirm this finding.
We also found that significant gene expression changes were shared between cells with CRBN depletion compared with cells with normal CRBN and treated with lenalidomide.
Therefore, the presence of CRBN is an absolute requirement for activity of these compounds; and although its consequent suppression mediates cell death, its complete loss renders the drug class ineffective. Thus, CRBN is apparently the essential central orchestrator of thalidomide, lenalidomide, and pomalidomide action. It is, however, important to note that these drugs may well have other off-target effects as their clinical profiles are not identical and thus mechanisms of action and resistance that do not implicate CRBN as a primary mediator are likely.
When seeking a common pathway for activity, we found that both CRBN knockdown and lenalidomide treatment initially resulted in IRF4 down-regulation, a finding that may explain cytotoxicity because IRF4 is a critical transcription factor for myeloma cell survival. Specifically, it has been shown that knockdown of IRF4 mRNA and protein is sufficient to kill myeloma cell lines.16 It is therefore intriguing to propose that direct antimyeloma activity of lenalidomide could be associated with CRBN-mediated down-regulation of IRF4. Indeed, our data show that, although the initial CRBN knockdown leads to IRF4 reduction, in surviving cells with stable CRBN knockdown the expression level of IRF4 is subsequently restored, presumably as the survival mechanism in a non–CRBN-dependent process. Together, this suggests that lenalidomide-resistant HMCLs might use an alternative pathway to overcome the CRBN-mediated down-regulation of IRF4. Further studies are needed to better understand the mechanisms underlying how IRF4 expression is regulated by CRBN-mediated signaling and how resistant cells with CRBN depletion restore IRF4 expression.
Our data confirm that CRBN is a major target of lenalidomide and related IMiDs and the presence of CRBN is essential for the activity of these drugs. Our studies do not, however, exclude the possibility that IMiDs target other molecules or that resistance evolves from multiple distinct reasons. Indeed, the fact that a significant percent of MM patients shows resistance to IMiDs as single agents and yet few unselected myeloma cases show genomic abnormalities affecting CRBN suggested that other molecules downstream of CRBN and/or completely different pathways may also be targeted by IMiDs. Recent studies demonstrated that high IRF4 levels correlated with increased lenalidomide sensitivity.17,18 Another study demonstrated that activation of the Wnt/β-catenin pathway mediates lenalidomide resistance in MM.15 In a study performed on Del(5q) myelodysplastic syndrome, the secondary resistance to lenalidomide was found to be associated with CDC25C and PP2A overexpression.19 Future studies may then focus on investigating whether inactivation of CRBN-mediated signaling is linked/connected with downstream up-regulation of Wnt signaling pathway or other immune or signaling pathways previously related with lenalidomide resistance.
In conclusion, we demonstrate here that CRBN is essential for IMiD activity and low levels of CRBN correlate with poor drug response. In addition, our data suggested that CRBN is a critical molecule but not the unique source of IMiD resistance. The confirmation that CRBN down-regulation could lead to lack of response to IMiDs in the clinic identifies CRBN as a potentially useful biomarker for the clinical assessment of antimyeloma efficacy. Thus, the future analysis of CRBN expression in MM cells may help to distinguish MM patients that will or will not benefit from thalidomide and/or IMiD-based therapy. Finally, a better understanding of the mechanism of action of IMiDs via binding to CRBN will open the door to screening of drugs that target not only CRBN itself but also its downstream effectors, perhaps allowing the identification of novel agents that distinguish the antimyeloma from teratogenic activity associated with these compounds.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: Y.X.Z., R.F., P.L.B., and A.K.S. designed the study; Y.X.Z., E.B., C.-X.S., L.A.B., J.E.S., S.V.W., and X.-B.C. performed the research; C.C.B. and R.Z.O. contributed vital new cell lines; Y.X.Z., E.B., and A.K.S. wrote the paper; and all authors reviewed and gave final approval of the manuscript.
Conflict-of-interest disclosure: R.F. is a consultant for Genzyme, Medtronic, BMS, Amgen, Otsuka, Celgene, Intellikine, and Lilly (all < $10 000) and receives research support from Cylene, Onyx, and Celgene. A.K.S. is a consultant for Onyx, Millenium-Takeda, has received honoraria from Celgene, and has received research support from Millenium-Takeda. The remaining authors declare no competing financial interests.
Correspondence: A. Keith Stewart, Mayo Clinic, 13400 E. Shea Blvd, MCCRB3-008, Scottsdale, AZ 85259; e-mail: stewart.keith@mayo.edu.
References
National Institutes of Health