Abstract
Randomized control trials show beneficial effects of heparin in high-risk pregnancies to prevent preeclampsia and intrauterine growth restriction. However, the lack of placental pathology data in these trials challenges the assumption that heparin is a placental anticoagulant. Recent data show that placental infarction is probably associated with abnormalities in development of the placenta, characterized by poor maternal perfusion and an abnormal villous trophoblast compartment in contact with maternal blood, than with maternal thrombophilia. At-risk pregnancies may therefore be predicted by noninvasive prenatal testing of placental function in mid-pregnancy. Heparin has diverse cellular functions that include direct actions on the trophoblast. Dissecting the non–anticoagulant actions of heparin may indicate novel and safer therapeutic targets to prevent the major placental complications of pregnancy.
Introduction
A small subset of reproductive-aged women require anticoagulation in the context of a mechanical heart valve1 or to prevent recurrent venous thromboembolism as in women with antiphospholipid syndrome.2 When planning pregnancy, they mostly convert from an oral anticoagulant such as Coumadin to subcutaneous injections of heparin to avoid the potential embryopathy induced by transplacental passage of Coumadin.3 By doing so, they expose themselves to relatively minor side effects such as skin bruising and, because heparin promotes bone loss, to the rare possibility of vertebral bone compression fractures and neurologic complications.4 In this context heparin is a success story for women whose predecessors were counseled to avoid attempts at motherhood. More than 35 years ago the concept of placental anticoagulation was proposed for women in subsequent pregnancies after recurrent placental infarction.5 Since then we have witnessed an exponential increase in the prescription of heparin to pregnant women for a wide variety of indications, based on a common theme that superior placental function can be attained via its anticoagulant properties. The fashion has spread widely according to claims that heparin promotes successful implantation after in vitro fertilization,6,7 prevents recurrent miscarriages,8 promotes better outcomes in the perinatal period,9 and, finally, may be used vaginally to induce labor in full-term pregnancies.10 A common aspect of these studies is the use of safer low molecular weight heparins (LMWHs) in prophylactic regimes that have a low risk of serious side effects when used over a relatively short duration of time in pregnancy. However, the current cost per pregnancy to prescribe prophylactic LMWH is ∼ $3000, a significant burden to uninsured women in countries, such as Canada, with variable employer-based drug plans. Ongoing use of such drugs for these indications in pregnancy therefore deserves more rigorous evidence. Recent impressive but negative trials are making progress by limiting the scope of use in women with recurrent miscarriage. Heparins are complex macromolecules with diverse actions extending beyond the classic anticoagulant role.11 Likewise diseases, such as severe preeclampsia (PE), that may be prevented by LMWH are equally complex in their pathogenesis. It is important to fully appreciate the mechanisms by which LMWH may favorably interact with the human placenta.
Evidence from randomized control trials
In 2009-2010, 3 well-designed trials reported a lack of efficacy of subcutaneous LMWH in the prevention of recurrent miscarriages, across both thrombophilia-positive and -negative women.12-14 A subsequent review of the management of recurrent miscarriage concluded that LMWH is not indicated in thrombophilia-negative women but that the evidence remains insufficient to make the same conclusion in thrombophilia-positive women pending the results of ongoing registered randomized control trials (listed in the review).15 From a pathologic standpoint these data are not surprising, because placental bed biopsies from a large series of euploid first-trimester miscarriages show no histologic evidence of disease (abnormal spiral artery transformation or lumen thrombosis) that might theoretically be prevented by heparin.16
The evidence that LMWH may promote improved perinatal outcomes is more promising but is confined to smaller underpowered trials in thrombophilia screen–negative women.17 The recent Canadian pilot trial18 randomly assigned 114 perceived high-risk women without thrombophilia to LMWH or no drug, mostly on the basis of either previous severe PE or placental infarction. LMWH was commenced in the first trimester. All 3 perinatal deaths (at 21-24 weeks) were potentially because of placental complications, but no placental pathology was reported. A composite outcome was significantly different that favored LMWH, mostly comprising a reduction (1 vs 8) in severe PE. A subsequent similarly designed pilot trial, not yet included in the Cochrane Review,17 randomly assigned 160 women with previous pathologically proven placental abruption and no antiphospholipid antibodies equally to receive LMWH or no drug.19 The study design apparently excluded pregnancies with placental pathologies despite the stated inclusion criteria. LMWH was commenced as soon as pregnancy was chemically diagnosed in the first trimester. As in the Rey trial, a composite outcome was significantly different favoring LMWH and interestingly had a similar (2 vs 12) reduction in severe PE. Neither trial reported placental pathology, yet both trials required placental pathology as an entry criterion. More recently, an Israeli group published 2 retrospective case-control studies of LMWH in women with previous severe perinatal complications of pregnancy with20 and without21 thrombophilia. Because of trial design no meaningful clinical conclusions can be drawn, although data in women without thrombophilia21 merits closer scrutiny because the publication included detailed placental pathology. Of 32 LMWH-treated women, 50% had placental infarcts and 25% had multiple placental infarcts, strikingly similar to their control arm. These high rates of infarction rates suggest that LMWH in the doses used are incapable of preventing placental infarction in clinically high-risk women.
Placental development and hemostasis in the intervillous space
The human placenta is termed hemochorial because the maternal vascular integrity is disrupted by the invasive extravillous cytotrophoblast cells (EVTs) to bring maternal blood directly in contact with placental villi (Figure 1). Although this anatomical arrangement dominates at the end of the first trimester, it is attained cautiously during embryogenesis, so as to avoid oxygen toxicity.22 The growth requirements of the postimplantation blastocyst are provided by the endometrial glands secreting their contents into the primitive intervillous space until 10 weeks of gestation.23 Cytotrophoblasts form an outer shell with the intention of occluding capillaries breached within the decidualized gland stroma.24 These interact with decidual cells that express tissue factor (TF) the potent initiator of the rapid external pathway of hemostasis via thrombin generation (reviewed in Lockwood et al25 ). Deletion of TF in mice is lethal with abnormal vascular development and hemorrhage.26 TF is also expressed in placental trophoblast, but is normal effectively counterbalanced by protein C activation via the endothelial cell protein receptor.27 Human embryogenesis is therefore characterized by low oxygen tension because of effective but controlled local hemostasis.
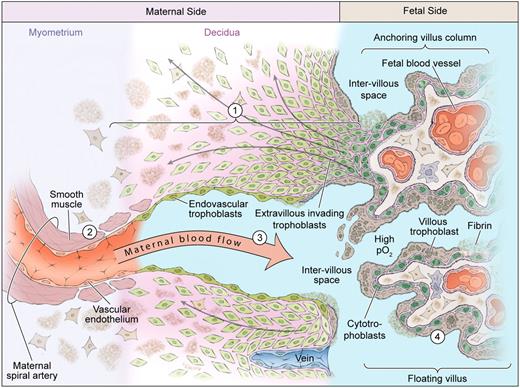
Normal placental development. Extravillous cytotrophoblasts proliferate in anchoring columns to successful invade through the decidua (1) and transform the distal spiral arteries (2). These changes mediate high volume flow at low pressure into the intervillous space (3). The placental villi are covered by the villous trophoblast compartment (4), comprising cytotrophoblasts that proliferate to generate the outer syncytiotrophoblast in direct contact with maternal blood.
Normal placental development. Extravillous cytotrophoblasts proliferate in anchoring columns to successful invade through the decidua (1) and transform the distal spiral arteries (2). These changes mediate high volume flow at low pressure into the intervillous space (3). The placental villi are covered by the villous trophoblast compartment (4), comprising cytotrophoblasts that proliferate to generate the outer syncytiotrophoblast in direct contact with maternal blood.
EVTs subsequently dilate the distal spiral arterioles by removing the muscular walls,28 and, where exposed to maternal blood, they switch to a pseudoendothelial phenotype.29 Under normal circumstances, EVTs are able to suppress proinflammatory responses (complement C3 activation and IL-6 expression).30 Uteroplacental blood flow then increases exponentially in the second trimester, to a much greater extent that is required for transplacental transfer of oxygen and nutrients; this spraying the villi evenly at low pressure31 may be an important contributor to hemostasis from the principles of Virchov triad.32
Pregnancy is associated with significant changes in circulating proteins that participate in hemostasis (increased procoagulant proteins,33 decreased anticoagulant proteins34 presumably designed to limit blood loss at birth; reviewed in Thornton and Douglas35 and Sarig and Brenner36 ). This shift to a procoagulant state is counterbalanced, in the systemic circulation, by reduced hematocrit37 and increased cardiac output,38 whereas rises in tissue factor pathway inhibitors (TFPI1 and TFPI2) counteract the elevated circulating and placental TF activity.39
From the second trimester onward, maternal blood is in contact with the outer syncytiotrophoblast surface of the developing placental villi, analogous to the labyrinth trophoblast in mice.40 In both species, this is a terminally differentiated postmitotic structure. In contrast with mice the human placenta retains its population of proliferating villous cytotrophoblasts until term.41 Although a limited degree of transcription can be detected in the outer syncytiotrophoblast,42 it is mostly confined to the underlying cytotrophoblasts; continuous syncytial fusion thus maintains the transcriptional machinery of functionality of the outer syncytiotrophoblast. Syncytial fusion also donates antiapoptotic proteins to focally restrict syncytiotrophoblast apoptosis under normal conditions (reviewed in Huppertz et al43 ). The dual requirements of continuous syncytial fusion and the need to retain a population of proliferating progenitor cells require that cytotrophoblast division is asymmetric; this is achieved via expression of the transcription factor glial cell missing-1 (GCM1) in the daughter cell destined for syncytial fusion.44 GCM1 is similarly required for the formation of extravillous trophoblast such that defective GCM1 expression is a common molecular explanation for the dual defect in both extravillous and villous trophoblast lineages observed in placentas from severe preeclamptic pregnancies.44 GCM1 is promoted by peroxisome proliferator-activated receptor-γ45 and in turn promotes expression of the fusogenic protein Syncytin1 required to effect fusion.46
This differentiated outer syncytiotrophoblast layer expresses a number of regulators of hemostasis normally found in the systemic endothelium (summarized in Sood et al47 ). These include the procoagulant proteins TF, VWF, factor VIII,48 and tissue plasminogen activator inhibitor type 1. Inhibitory proteins of the cascade include TFPI1 and TFPI2,49,50 thrombomodulin,51,52 annexin V,53 and tissue plasminogen activators such as urokinase. The placenta is theoretically vulnerable to thrombosis because TF is constitutively expressed, although as trophoblast cells differentiate, they adopt a thromboresistant phenotype.47 As an example, differentiating cytotrophoblast cells in the first-trimester placenta strongly express endothelial cell protein receptor that, after syncytial fusion, will participate in hemostasis within the intervillous space.47
Pathogenesis of severe PE
PE is a potentially life-threatening hypertensive disorder affecting ∼ 2%-7% of all pregnancies.54 Approximately 1% of cases are severe, causing stillbirth or the need for extreme preterm delivery.55 The pathogenesis of the disease is a variable blend of host risk factors (metabolic syndrome, maternal age, medical comorbidities)55,56 and severe defects in placental development (M. Walker, B. Fitzgerald, S. Keating, J. Ray, and J.C.P.K., manuscript submitted, 2011) and pathology, most commonly villous infarction.57-59 Host susceptibility alone more commonly induces near-term disease that is safely managed by delivery,60 whereas the early severe forms of the disease are more challenging to manage because of the early gestational age and coexistent IUGR.61 Although host factors contribute to the susceptibility of late-gestation PE,55 early/severe disease is strikingly predicted by abnormal uterine artery Doppler (area under the receiver operator curve, 0.922) with no significant added value by incorporating maternal characteristics.62 This ultrasound-based test is easily integrated into the standard 20-week fetal anatomical ultrasound scan and can be combined with an assessment of placental dimensions and texture.63
The maternal disease is characterized by hypertension because of vascular dysfunction. The consequent high systemic vascular resistance and depressed cardiac output can be observed during the second trimester64 and is associated with the release, from the placental villi into maternal venous blood, of splice-variant decoy receptor proteins, such as soluble fms-like tyrosine kinase (sFLT1), that competitively antagonizes the actions of proangiogenic growth factors (vascular endothelial growth factor [VEGF] and placenta-like growth factor) that contribute to the physiologic angiogenesis and systemic vasodilation characteristic of normal pregnancy65 (for review see Powe et al66 ). Animal models in both rats67 and mice68 support a pathogenic role for sFlt1 in the pathogenesis of severe PE. Despite this evidence for a pathogenic role for sFLT1, other investigators have found conflicting evidence. First, in a nested case-control study of ∼ 2000 women, high levels of sFLT1 at 10-14 weeks predicted lower risks for a range of adverse perinatal outcomes and did not associate with PE.69 Second, when LMWH is administered to pregnant women, it elevates circulating sFlt1 levels ∼ 3-fold.70 sFLT1 has physiologic actions, for example to arrest de novo blood vessel formation in the cornea.71 sFlt1 is locally retained on the surface of placental villi, where it can be released via the heparanase action of LMWH.70,72 Interestingly, LMWH induces transcription and translation of sFlt1 in floating first-trimester villous explants, further contributing to the LMWH-induced secretion in a manner that antagonizes VEGF receptor phosphorylation.73 This disparity in clinical and in vitro observations suggests more complex interactions between the placenta and LMWH to mediate a preventive role of heparin in women at high risk of severe PE.
Placental pathology of severe PE
The placental disease is characterized by maternal underperfusion. As such, bilateral abnormal waveforms strongly predict early placental disease and are associated with pathology of the maternal-fetal interface and the placental villi shown in Figure 2. Physiologic transformation of the spiral arteries is impaired, resulting in decidual vasculopathy and an unstable perfusion of the placenta by maternal blood (for detailed review see Burton et al31 ). Note this is not primarily a thrombotic vasculopathy; rather, it is characterized mostly by lack of transformation of the distal spiral arterioles because of maternal macrophage-mediated cell death of the invading extravillous cytotrophoblasts,74 recruited by local complement activation and generation of IL-6, resulting in acute atherosis.30 The lesion of focus in the context of heparin and anticoagulation is infarction of placental villi. Large or central placental infarction is the dominant lesion, in one cohort affecting > 50% of pregnancies delivering before 32 weeks.57 Placental infarction associates with abnormal uterine artery Doppler, suggesting that reduced uteroplacental perfusion as a contributory factor.75 We recently explored the relative importance of maternal thrombophilia and structural disorders of the placenta in a single center 10-year cohort study of 180 singleton pregnancies with histologic-confirmed placental infarction.59 High rates of mostly severe PE (61%), HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome (39%), small for gestational age (70%) and stillbirth (39%) among the 108 screened for thrombophilia show the serious implications of placental infarction. Thrombophilia (defined as > 1 factor V Leiden or prothrombin gene mutations or antiphospholipid antibodies) was found in only 14 of 108 (13%), whereas a range of underlying structural abnormalities of the placenta (small placenta, decidual vasculopathy, abnormal development of the placental villi), collectively classified as abnormal placental development, were found in ∼ 70% of cases, > 7-fold more common than thrombophilia. Of the 108 cases with thrombophilia testing, only 4 placentas had infarcts in an otherwise structurally normal placenta. These findings suggest that, from early pregnancy, there are defects in the development of placental structure that prevents normal hemostasis and somehow confers a risk of infarction. Heparin is unlikely to be capable of reversing these early developmental changes in placental structure, and the high rates of infarction (50%) observed recently by Kupfermic et al21 in high-risk women treated with LMWH supports this conclusion.
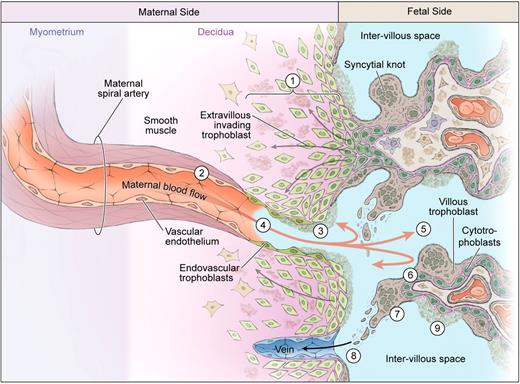
Uteroplacental vascular insufficiency. Extravillous cytotrophoblasts are less successful in invading the maternal decidua and may be removed by the maternal immune system (1). Consequently the distal spiral arteries are narrower (2) and diseased, accompanied by atherosis or local fibrin deposition (3) and reduced endovascular invasion (4). Hypoxia or hypoxia-reoxygenation injury (5) has direct effects on the villous trophoblast compartment, reducing syncytial fusion (6) that may trigger the formation of syncytial knots (7). These accumulate but may fragment and shed into maternal blood (8), whereas areas deficient in syncytial fusion may exhibit focal necrosis (9).
Uteroplacental vascular insufficiency. Extravillous cytotrophoblasts are less successful in invading the maternal decidua and may be removed by the maternal immune system (1). Consequently the distal spiral arteries are narrower (2) and diseased, accompanied by atherosis or local fibrin deposition (3) and reduced endovascular invasion (4). Hypoxia or hypoxia-reoxygenation injury (5) has direct effects on the villous trophoblast compartment, reducing syncytial fusion (6) that may trigger the formation of syncytial knots (7). These accumulate but may fragment and shed into maternal blood (8), whereas areas deficient in syncytial fusion may exhibit focal necrosis (9).
Placental infarcts alone do not cause the maternal vascular injury of severe PE; rather they mediate the coexistent IUGR because of defective global transplacental transfer. How, therefore, does the placenta induce the maternal syndrome and at the same time become prone to thrombosis? The key is abnormal differentiation of the placental villi, summarized in Figure 3. The underlying decidual vasculopathy, established in early pregnancy, renders the placental villi poorly perfused, inducing static local hypoxia76 or more likely a dynamic process of ischemia-reperfusion injury.77 The GCM1 axis, which controls differentiation of both types of trophoblasts, is disrupted in severely preeclamptic placental villi to favor a reduction in syncytial fusion78-81 and is accentuated by hypoxia-mediated degradation of GCM1.82 Patchy areas of apoptosis, excess syncytial knot formation, and areas of necrosis are found in severe preeclamptic placental villi83 and can be reproduced in vitro, respectively, by hypoxic culture84 or hypoxia-reoxygenation77 in cultured first-trimester placental villous explants. Large syncytial knots secrete sFlt1 into maternal blood in severely preeclamptic women.85
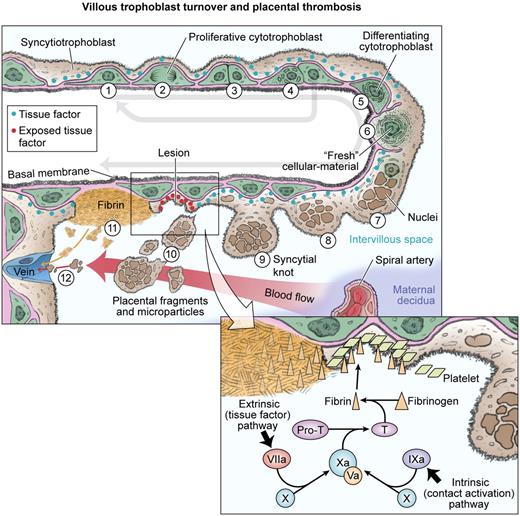
Abnormal villus trophoblast differentiation and placental thrombosis. Progenitor cytotrophoblasts proliferate (1) and divide asymmetrically (2 and 3) so that the progenitor is conserved (4) for subsequent rounds of syncytial fusion. The daughter cytotrophoblast prepares for syncytial fusion (5), focally donating transcriptional machinery and antiapoptotic proteins into the outer syncytiotrophoblast (6). Syncytial nuclei gradually progress toward apoptosis and aggregate in syncytial knots (7 and 8). Syncytial fusion is restricted in severe PE, increasing the number and size of syncytial knots (9 and 10). Some knots may fragment into the intervillous space, whereas other parts of the abnormal villi have exposed cytotrophoblasts and basal lamina (11) that trigger local thrombosis (see inset). Syncytiotrophoblast fragments are filtered in the maternal lungs, whereas microparticles pass through and may exert systemic effects in the maternal vasculature.
Abnormal villus trophoblast differentiation and placental thrombosis. Progenitor cytotrophoblasts proliferate (1) and divide asymmetrically (2 and 3) so that the progenitor is conserved (4) for subsequent rounds of syncytial fusion. The daughter cytotrophoblast prepares for syncytial fusion (5), focally donating transcriptional machinery and antiapoptotic proteins into the outer syncytiotrophoblast (6). Syncytial nuclei gradually progress toward apoptosis and aggregate in syncytial knots (7 and 8). Syncytial fusion is restricted in severe PE, increasing the number and size of syncytial knots (9 and 10). Some knots may fragment into the intervillous space, whereas other parts of the abnormal villi have exposed cytotrophoblasts and basal lamina (11) that trigger local thrombosis (see inset). Syncytiotrophoblast fragments are filtered in the maternal lungs, whereas microparticles pass through and may exert systemic effects in the maternal vasculature.
These alterations in trophoblast cell biology are directly relevant to hemostasis because normal trophoblast differentiation, in both mice and humans, is accompanied by the adoption of an endothelial-like anticoagulant phenotype.47 The extensive literature supporting an arrest of syncytial fusion in severe PE disrupts surface regulation of hemostasis to favor local thrombosis; as an example, isolated first trimester villous cytotrophoblasts subjected to either hypoxia and hypoxia-reoxygenation were recently shown to double TF gene expression.86 TF is also released by the placenta into the maternal circulation in microparticles87 that are shed excessively in severe PE.88
Non-anticoagulant actions of heparin in the placenta
In the nonpregnant state, LMWH prolongs survival in metastatic cancer.89 These effects are supported by in vitro data90 and are retained in truncated forms of heparin that lack anti–thrombin-3 binding sites.11 These clinical effects seem paradoxical, because heparin promotes dimerization of basic growth factors such as fibroblast growth factor (FGF) and VEGF and to enhance their cell signaling. The explanation may be that smaller LMWH, especially < 6 kDa,91 are unable to facilitate growth factor-receptor interactions that promote angiogenesis. In the context of the trophoblast layer (Figure 4), heparin is required along with FGF4 to maintain murine trophoblast stem cells.92 Withdrawal of FGF4/heparin allows spontaneous differentiation to extravillous trophoblast.93 Some of these murine effects can be observed in human placental villi. Denuded of the outer syncytiotrophoblast to expose villous cytotrophoblasts in first-trimester placental villi to FGF4 and heparin, they proliferate to form lumps of cells expressing the receptor FGFR2.94 Lower doses of LMWH alone (0.25 IU/mL) in such experiments promote villous cytotrophoblast mitosis and syncytiotrophoblast formation in first-trimester villi.94 Heparin also induces cytotrophoblast proliferation in trophoblast-derived cell lines.94,95 These observations are consistent with previous data, suggesting that heparin reverses the proapoptotic effects in villous explant syncytiotrophoblast induced by serum from antiphospholipid antibody syndrome,96 presumably by promoting mitosis in the trophoblast progenitors within the explants that express FGFR2. We recently demonstrated a more direct and beneficial effect of heparin on placental villi in the context of preventing severe PE. We demonstrated that both unfractionated heparin (UFH) and LMWH, in comparable doses used clinically in pregnancy, are capable of reversing the natural antiangiogenic tendency of first-trimester placental villi.97 These heparins exert this proangiogenic effect despite increasing secretion of sFLT170,72 that at least in vitro impairs VEGF receptor signaling.75 This paradox between in vitro and in vivo data in the context of sFLT1 may be because of the restoration additional functions of the outer syncytiotrophoblast that override the endothelial-specific effects of excess sFLT1. First, in the context of hemostasis regulation, restoration of syncytial fusion in severe preeclamptic placental villi is predicted to promote improved expression of anticoagulant proteins on the syncytiotrophoblast surface. Second, this restoration may promote other important signaling systems, such as hemoxygenase-1, to reverse disease progression toward the vasculopathy and end-organ damage that characterizes severe PE.98
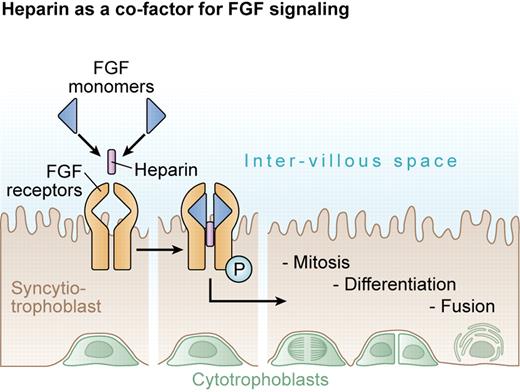
Heparin promotes cytotrophoblast proliferation. Heparin facilitates dimerization of FGFs to enhance mitotic signaling. This action of heparin may reduce the risk of severe PE by promoting the production of cytotrophoblasts for syncytial fusion and maintenance of a healthy outer syncytiotrophoblast in contact with maternal blood.
Heparin promotes cytotrophoblast proliferation. Heparin facilitates dimerization of FGFs to enhance mitotic signaling. This action of heparin may reduce the risk of severe PE by promoting the production of cytotrophoblasts for syncytial fusion and maintenance of a healthy outer syncytiotrophoblast in contact with maternal blood.
Complement and inflammation of the preeclamptic placenta
Heparin may also play a substantial non-anticoagulant role in the prevention of severe PE via suppression of complement pathway activation. Women destined to develop PE have elevated circulating levels of complement-activation factor Bb, but not C3a or sC5b-9.99 Because Bb levels are associated with maternal obesity, complement activation may be a dominant pathway in the maternal phenotype of PE. Interestingly, a mutation (MCP/A304V) in the complement regulatory protein MCP was recently shown in 4 of 59 maternal DNA samples of women who had developed severe PE/HELLP syndrome.100 Although antiphospholipid syndrome is deliberately excluded from this review, there is good experimental evidence to suggest that heparin inhibits complement activation in this context.101 Noninfectious leukocyte infiltration of both the maternal-fetal interface (deciduitis) and villi (villitis) is a feature in both severe PE and IUGR that, interestingly, is more common with male than female fetuses. Heparin may limit the adverse effects of this type of pathology via suppression of T-cell adhesion and migration.102
Implications for the design of future heparin trials
The consistency of the data in 2 recent trials of LMWH to prevent severe PE, together with an awareness of their limitations, should be influential for future definitive trial designs.18,19 There has been a substantial interest in developing algorithms to screen pregnancies for severe PE, variously combining maternal characteristics,55 uterine artery Doppler,62 and a variety of maternal blood tests,103 a group of which are used primarily to screen at 12-16 weeks of pregnancy for Down syndrome104 and are therefore available “free” for real-time prediction of PE and IUGR. Because > 70% of infarcted placentas are small-for-dates59 and small dysmorphic placentas on ultrasound scanning (< 10 cm long at 19-22 weeks' gestation),58 increase the risk of severe PE and IUGR,105 we have combined these tests in high-risk clinical practice routinely since 2007, when we demonstrated that normal tests substantially reduce the risk extreme preterm birth from severe PE/HELLP syndrome (odds ratio, 0.2; 95% CI, 0.1-0.4) and severe early-onset IUGR (odds ratio, 0).106 With the use of this concept we subsequently screened our high-risk population for multiparameter placental dysfunction to recruit to our pilot HEPRIN (HEparin for the PRevention of placental INsufficiency) trial that was recently reported.107 Of ∼ 250 women screened, we randomly assigned 32 of 41 eligible women to either 7500 IU of UFH twice daily or no drug. Despite the much smaller sample size, we had more perinatal deaths (3; 9.3%) and deliveries < 32 weeks (9 of 32; 28.1%) than either recent trial of LMWH to prevent adverse perinatal outcomes18,19 that recruited on clinical risk factors alone. Only 5 of 31 examined placentas (16.1%) were normal. UFH did not reduce the risk of placental infarction (UFH, 3 of 16, 18.8% vs standard care, 4 of 15, 26.7%), consistent with recent Israeli observational data.21
Future directions
Given the exciting potential of prophylactic LMWH to prevent recurrent severe PE, it is important to step back from the assumption that it acts as a direct placental anticoagulant. Consequently, investigators will focus on the more diverse biologic actions of heparins and the potential that non-anticoagulant forms of heparin, without their attendant obstetric/delivery risks, might conserve beneficial actions that may operate more directly at the level of the syncytiotrophoblast layer covering the placental villi. Future trial designs, focusing on a smaller subset of women at most risk of severe PE, is now possible by embracing tools that permit a prenatal diagnosis of “placental insufficiency” in the early second trimester; this strategy is logical because at-risk women can be identified before the maternal vasculopathy becomes established. Given the diversity of potential actions of heparin together with the heterogeneity of cell-specific pathologies in the pathologic placenta, it is imperative that all future LMWH trials capture the placenta. A good start is the determination of gross and microscopic pathologies, but the subtle molecular actions of heparin will only become understood in a clinical context if immediate sampling is done that preserves the snapshot of cellular functions and protein transcription/translation. Our understanding of the molecular control of villous syncytiotrophoblast formation already offers several additional therapeutic possibilities that might restore placental self-anticoagulation via restoration of syncytial fusion. The future for the prevention of severe PE therefore has a new focus.
Acknowledgments
This work was supported by the PSI (11-02, J.C.P.K.) and Rose Torno, Chair, Mount Sinai Hospital (J.C.P.K.), and a Molly Towell Perinatal Research Foundation Fellowship (S.D.).
Authorship
Contribution: J.C.P.K. and S.D. designed and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: John Kingdom, Department of Obstetrics & Gynecology, Rm 3265, Mount Sinai Hospital, 600 University Ave, Toronto, ON, Canada M5G 1X5; e-mail: jkingdom@mtsinai.on.ca.