Abstract
The mechanism of cytotoxicity of farnesyltransferase inhibitors is incompletely understood and seems to vary depending on the cell type. To identify potential determinants of sensitivity or resistance for study in the accompanying clinical trial (Witzig et al, page 4882), we examined the mechanism of cytotoxicity of tipifarnib in human lymphoid cell lines. Based on initial experiments showing that Jurkat variants lacking Fas-associated death domain or procaspase-8 undergo tipifarnib-induced apoptosis, whereas cells lacking caspase-9 or overexpressing Bcl-2 do not, we examined changes in Bcl-2 family members. Tipifarnib caused dose-dependent up-regulation of Bim in lymphoid cell lines (Jurkat, Molt3, H9, DoHH2, and RL) that undergo tipifarnib-induced apoptosis but not in lines (SKW6.4 and Hs445) that resist tipifarnib-induced apoptosis. Further analysis demonstrated that increased Bim levels reflect inhibition of signaling from c-Raf to MEK1/2 and ERK1/2. Additional experiments showed that down-regulation of the Ras guanine nucleotide exchange factor RasGRP1 diminished tipifarnib sensitivity, suggesting that H-Ras or N-Ras is a critical farnesylation target upstream of c-Raf in lymphoid cells. These results not only trace a pathway through c-Raf to Bim that contributes to tipifarnib cytotoxicity in human lymphoid cells but also identify potential determinants of sensitivity to this agent.
Introduction
Farnesyltransferase inhibitors (FTIs) are currently undergoing extensive clinical testing in various hematologic malignancies.1-3 These agents inhibit farnesyltransferase, an enzyme that transfers the 15-carbon farnesyl group from farnesyl pyrophosphate to a variety of polypeptide acceptors, including the chaperone heat shock protein 40/HDJ-2; the nuclear intermediate filament proteins prelamin A and lamin B; the centromere protein CENP E; and small GTP-binding proteins of the Ras, Rho, and Rheb families.4,5 Collectively, inhibition of farnesylation of these polypeptides leads to diminished cell proliferation. In addition, FTIs induce cell death in some model systems under certain conditions. These cytotoxic effects have been attributed to FTI-induced inhibition of prosurvival signaling by Akt,6,7 signal transducers and activators of transcription,8-10 mitogen-activated protein kinases (MAPKs),9,11-13 or the Rheb target mammalian target of rapamycin.14 Recent work has especially emphasized the role of Rheb inhibition as a mechanism of FTI-induced antilymphoma effects in murine lymphomas and leukemia.15 Alternatively, it has been suggested that FTIs induce apoptosis by causing up-regulation of the proapoptotic Bcl-2 family members Bax,16 Bak,17 or Puma.18
Although FTIs were initially developed based on the premise that inhibition of farnesylation would abrogate signaling by mutant Ras proteins,19 these agents have demonstrated little efficacy in solid tumors.20-22 In contrast, tantalizing activity was observed in several hematologic malignancies.1-3 In particular, the orally bioavailable nonpeptidimimetic FTI tipifarnib23 demonstrated activity in adults with acute leukemia. The initial phase 1 trial not only established a maximum tolerated dose in patients with relapsed and refractory acute leukemias but also determined that tipifarnib levels in bone marrow were 1.6-8 nmol/mg of tissue at this dose, demonstrated FT inhibition in leukemia cells in situ, and provided evidence of activity in relapsed AML.24 Subsequent phase 2 and phase 3 studies have demonstrated response rates of 11%-23% in elderly patients with previously untreated poor risk acute myeloid leukemia (AML).25,26 In an effort to select the subset of AML patients most likely to respond, Raponi et al empirically identified a 2-transcript signature, characterized by a high ratio of mRNA encoding the Ras guanine nucleotide exchange factor RasGRP127 relative to mRNA encoding the repair protein aprataxin, that had a 92% negative predictive value and a 28% positive predictive value in 2 single-agent phase 2 tipifarnib AML trials.28 Based on these results, gene signature-guided trials of tipifarnib in acute leukemia are being initiated.
Tipifarnib also has demonstrated activity in relapsed and refractory lymphoma. Although this agent exhibits little activity in mantle cell and follicular lymphomas,29,30 which universally exhibit high Bcl-2 expression, responses (including durable partial responses and complete responses) have been observed in 25%- 50% of patients with other types of relapsed lymphoma.30
Because prior work examining the mechanism of cytotoxicity of single-agent FTIs has largely been performed in rodent cell lines or human carcinoma cells, the realization that tipifarnib is active against certain subsets of human lymphomas prompted us to examine the mechanism of tipifarnib cytotoxicity specifically in malignant human lymphoid cells. Accordingly, the present studies were designed to (1) determine the mechanism by which tipifarnib induces apoptosis in lymphoid cell lines and (2) assess potential mechanisms of resistance that could be then be examined in lymphoma samples from patients enrolled in the phase 2 trial described in the accompanying paper.30 In contrast to results in murine lymphomas, results of the present study highlight the importance of a pathway involving RasGRP1, MAPKs, and Bim in tipifarnib-induced killing of human lymphoid cells.
Methods
Materials
Tipifarnib was provided by David End (Johnson & Johnson, New Brunswick, NJ). Antibodies that recognize the indicated antigens were obtained as follows: phospho-serine 473-Akt, phospho-threonine 308-Akt, Akt, phospho-ERK1/2, ERK1/2, phospho-MEK1/2, MEK1/2, phospho-S6-kinase, and S6 kinase (Cell Signaling Technology); H-Ras (EMD Chemicals); HDJ-2 (NeoMarkers/Thermo Fisher Scientific); heat shock protein 90β (Hsp90β; from David Toft, Mayo Clinic, Rochester, MN); and Bcl-2 family members as described previously.31 Reagents were purchased from the following suppliers: DNA oligonucleotides (Integrated DNA Technologies); 3-(4,5-dimethylthiazol-2-yl)-5(3-carboxymethonyphenol)-2-(4-sulfophenyl)-2H-tetrazolium (MTS; Promega); phenazine methosulfate (Sigma-Aldrich); the broad-spectrum caspase inhibitor Q-VD-OPh (SM Chemical); ABT-263 (ChemieTek); and U0126 (Promega). Short oligonucleotides targeting Bax (nucleotides 271-289, GenBank accession NM_138761), Bak (nucleotides 913-931, NM_001188), Bim (nucleotides 325-343, NM_138621), and RasGRP1 (nucleotides 1555-1573; AF081195) were from Ambion. All other reagents were obtained as described previously.31
Cell culture
Hs445 Hodgkin lymphoma cells were obtained from ATCC. All other cell lines were obtained as described previously.32 Cell lines were propagated at densities of < 1 × 106 cells/mL in RPMI 1640 medium containing 100 units/mL penicillin G, 100 μg/mL streptomycin, 2mM glutamine, and 15% (Hs445, H9, JB-6, I9.2, I2.1, and JMR) or 10% (other cell lines) heat-inactivated FBS (medium A). To generate resistant Jurkat cells, parental cells were diluted to a density of 1 × 105/mL and exposed to increasing concentrations of tipifarnib, starting with 50nM and increasing in 2-fold increments over a 6-month period as cells repeatedly grew back to a density of 4 to 8 × 105/mL. Thereafter, cells were cultured at twice-weekly intervals in medium A containing the final indicated tipifarnib concentration. Jurkat cells stably expressing MEK(DD), a constitutively active form of MEK1,33 were generated by electroporating 1.5 × 107 parental Jurkat cells with 40 μg of plasmid,31 selecting for stable integrants in 5 μg/mL puromycin after 48 hours, and cloning the resulting puromycin-resistant cells by limiting dilution.
Immunoblotting
Whole cell lysates were prepared from cell lines as described previously.34 In brief, cells were washed in serum-free RPMI 1640–HEPES and solubilized in 6M guanidine hydrochloride containing 250mM Tris-HCl (pH 8.5 at 21°C), 10mM EDTA, 1% (vol/vol) β-mercaptoethanol, and 1mM α-phenylmethylsulfonyl fluoride (freshly added from a 100mM stock in anhydrous isopropanol). After reaction with iodoacetamide, cell lysates were dialyzed sequentially into 4M urea followed by 0.1% (vol/vol) SDS.34 After lyophilization, aliquots were resuspended in SDS sample buffer at 5 mg protein/mL (assayed by the bicinchoninic acid method), separated by SDS-PAGE, transferred to nitrocellulose membranes, and probed with various antibodies.35
MTS assay
Tipifarnib-resistant cells were sedimented at 100g for 5 minutes, washed once in serum-free medium, and resuspended in medium A. Aliquots containing ∼ 2 × 104 cells in 120 μL of medium A were incubated at 37°C with varying concentrations of tipifarnib for 6 days. After reaction with MTS and phenazine methosulfate as instructed by the supplier, plates were incubated for 2-6 hours to obtain an absorbance of 0.5-1.0 at 490 nm in control samples.
Detection of apoptosis
DNA fragmentation, annexin V binding,36,37 and apoptotic nuclear morphologic changes were assayed as described previously.31,37,38 Briefly, cells were fixed in 3:1 (vol:vol) methanol:acetic acid and dropped onto glass slides. After staining with 1 μg/ml Hoechst 33258 in 50% (wt:vol) glycerol containing 100mM Tris-HC1 (pH 7.4 at 21°C), samples were examined on a Zeiss Axioplan microscope using a 63× (NA 1.4) lens, photographed with a Hamamatsu C4742-95 digital camera using QED Image Version 1.7.12 software (QED Imaging), and imported as PICT files into Canvas 8.0.
Immunoprecipitation
To assess changes in binding of BH3-only proteins to antiapoptotic Bcl-2 family members, Jurkat cells were treated with the indicated concentration of tipifarnib for 72 hours in the presence of the broad-spectrum caspase inhibitor 5μM Q-VD-OPh. At the completion of the incubation, cells were washed in ice-cold RPMI 1640–HEPES and solubilized at 4°C for 30 minutes in lysis buffer consisting of 1% (wt/vol) 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS, 20mM HEPES, pH 7.4, 150mM NaCl, 1% (vol/vol) glycerol, 1mM PMSF, 10 μg/mL leupeptin, 10 μg/mL pepstatin, 100mM NaF, 10mM sodium pyrophosphate, 1mM sodium vanadate, and 20nM microcystin. After sedimentation of insoluble material at 14 000g for 15 minutes, aliquots of supernatant containing 1000 μg of protein were incubated overnight at 4°C with anti–Bcl-2, Bcl-xL, or Mcl-1 that was precoupled to protein G-agarose beads using dimethyl pimelimidate. Beads were sedimented at 400g for 5 minutes, washed 5 times in lysis buffer, and heated for 20 minutes at 65°C in SDS sample buffer consisting of 4M urea, 2% (wt/vol) SDS, 62.5mM Tris-HCl, pH 6.8, 1mM EDTA, and 5% (vol/vol) 2-mercaptoethanol to release bound polypeptides.
Bak activation
After treatment with tipifarnib for 48 hours in the presence of 5μM Q-VD-OPh, parental Jurkat cells were washed with PBS and disrupted in the lysis buffer described under “Immunoprecipitation.” After lysates were precleared, immunoprecipitations were performed for 1 hour at 4°C using aliquots containing 500 μg of lysate protein and 5 μg of anti-active Bak Ab-1 (Calbiochem) that was precoupled to protein G-agarose beads using dimethyl pimelimidate. After 4 washes with isotonic wash buffer containing 1% CHAPS, bound polypeptides were solubilized in SDS sample buffer, subjected to SDS-PAGE, and probed with antibodies that recognize total Bak.
RT-PCR
After total RNA was isolated from control or tipifarnib-treated Jurkat cells, cDNA was synthesized using a SuperScript III First-Strand Synthesis kit (Invitrogen) following the supplier's instructions. PCR reactions (50 μL) were performed using 3 μg of cDNA product and Master Mix PCR reagents (Promega) according to the supplier's instructions. The following primers were used: Bim forward, 5′-ATGGCAAA-GCAACCTTCTGATG-3′ and Bim reverse, 5′-TCAATGCATTCTCCACACCAGG-3′; and GAPDH forward, 5′-GGCAAATTCCAT-GGCACCGTCAGG-3′ and GAPDH reverse, 5′-GGGAGGCATTGCTGATGATCTGAGG-3′. Amplification for Bim and GAPDH involved a 3-step program: 95°C for 60 seconds; 35 cycles of 95°C for 30 seconds, 55°C for 30 seconds, 72°C for 60 seconds; and 72°C for 7 minutes. After amplification, products were electrophoresed on a 2% (wt/vol) agarose gel containing 0.5 μg/mL ethidium bromide in 1× TAE buffer (30.7mM Tris, 20mM sodium acetate, and 1mM EDTA), visualized on a UV transilluminator, excised, and sequenced using automated dye terminator technology.
Cell fractionation
After treatment with diluent or 800nM tipifarnib for 72 hours, cells were sedimented at 200g for 10 minutes, washed twice with ice-cold PBS, and lysed by swelling for 20 minutes at 4°C in hypotonic buffer (25mM HEPES, pH 7.5 at 4°C, 5mM MgCl2, and 1mM EGTA supplemented immediately before use with 1mM α-phenylmethylsulfonyl fluoride, 10 μg/mL leupeptin, and 10 μg/mL pepstatin) followed by Dounce homogenization.37 After trypan blue staining confirmed that all cells were ruptured, samples were sedimented at 800g to remove nuclei. The postnuclear supernatant was sedimented at 4000g to isolate crude mitochondria, which were resuspended in hypotonic buffer with 300mM sucrose and sedimented at 4000g a second time. Fractions were prepared for SDS-PAGE as described under “Immunoblotting.”
Results
Antiproliferative effect of tipifarnib in lymphoid cell lines
To assess the potential activity of tipifarnib in lymphoid malignancies, a variety of cell lines derived from B-cell lymphomas, T-cell lymphomas, and AML were treated with therapeutically achievable tipifarnib concentrations.24 MTS dye reduction assays indicated that these cell lines exhibited a range of sensitivities, with complete suppression of growth at ≤ 100nM tipifarnib in several lines, including Molt3, SKW6.4, CEM, RPMI1666, DoHH2, and SeAx (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article; and data not shown). Flow cytometry demonstrated a G1 arrest by 72 hours after drug addition (supplemental Figure 1B). These results raised the possibility that tipifarnib might be active in lymphoid malignancies.
Tipifarnib induces apoptosis through the mitochondrial pathway
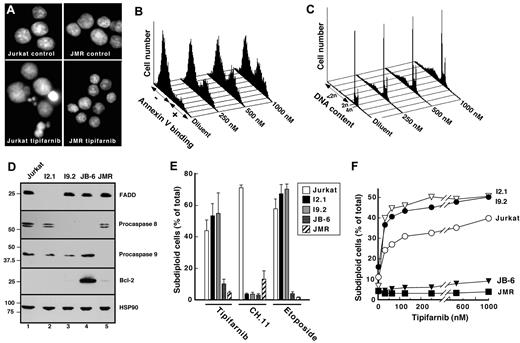
Because MTS assays do not distinguish between cell cycle arrest and induction of apoptosis, we next examined the ability of tipifarnib to induce apoptosis in these lines. Initial experiments focused on the T-cell ALL line Jurkat because of the availability of variants lacking various components of apoptotic pathways and the ease of transfecting this line. Previous studies demonstrated that tipifarnib elicits little cytotoxicity in Jurkat cells over 24 hours.38 In contrast, after a 48- to 72-hour exposure to tipifarnib, increasing numbers of cells displayed the typical morphologic characteristics of apoptosis (Figure 1A) as well as phosphatidylserine externalization (Figure 1B) and DNA fragmentation (Figure 1C).
Tipifarnib induces mitochondrial pathway-dependent apoptosis in Jurkat cells. (A-C) Aliquots containing 1 to 2 × 105 Jurkat cells/mL or Jurkat derivatives were incubated for 48 hours with diluent (0.1% DMSO), 1000nM tipifarnib (A; tipibarnib), or the concentration of tipifarnib indicated in panels B and C. At the completion of the incubation, cells were stained with Hoechst 33258 and examined by fluorescence microscopy (A), stained with APC-coupled annexin V and examined for phosphatidylserine externalization (B), or permeabilized with 0.1% Triton X-100 in 0.1% (wt/vol) sodium citrate containing 50 μg/mL propidium iodide and subjected to flow microfluorometry (C). (D) Whole cell lysates were subjected to immunoblotting for the indicated antigens. (E-F) Results obtained when the indicated Jurkat variants were treated for 72 hours with 1000nM tipifarnib, for 6 hours with 25 ng/mL CH.11 agonistic anti-Fas antibody, or for 24 hours with 2μM etoposide (E) or with varying tipifarnib concentrations (F) and analyzed as indicated in panel C. Errors bars in panel E indicate mean ± SD of 3 independent experiments.
Tipifarnib induces mitochondrial pathway-dependent apoptosis in Jurkat cells. (A-C) Aliquots containing 1 to 2 × 105 Jurkat cells/mL or Jurkat derivatives were incubated for 48 hours with diluent (0.1% DMSO), 1000nM tipifarnib (A; tipibarnib), or the concentration of tipifarnib indicated in panels B and C. At the completion of the incubation, cells were stained with Hoechst 33258 and examined by fluorescence microscopy (A), stained with APC-coupled annexin V and examined for phosphatidylserine externalization (B), or permeabilized with 0.1% Triton X-100 in 0.1% (wt/vol) sodium citrate containing 50 μg/mL propidium iodide and subjected to flow microfluorometry (C). (D) Whole cell lysates were subjected to immunoblotting for the indicated antigens. (E-F) Results obtained when the indicated Jurkat variants were treated for 72 hours with 1000nM tipifarnib, for 6 hours with 25 ng/mL CH.11 agonistic anti-Fas antibody, or for 24 hours with 2μM etoposide (E) or with varying tipifarnib concentrations (F) and analyzed as indicated in panel C. Errors bars in panel E indicate mean ± SD of 3 independent experiments.
In type II cells such as Jurkat cells, the mitochondrial pathway can be activated either downstream of death receptor ligation through the effects of cleaved Bid or by changes that affect other Bcl-2 family members (reviewed in Taylor et al39 ). Jurkat variants lacking procaspase-8 (I9.2; Figure 1D) or FADD (I2.1) were resistant to death ligands such as agonistic anti-Fas antibody CH.11 (Figure 1E)38 but were at least as sensitive as parental Jurkat cells to tipifarnib (Figure 1E-F). In contrast, Jurkat cells lacking caspase-9 (JMR; Figure 1D) or overexpressing Bcl-2 (JB-6; Figure 1D) were resistant to tipifarnib (Figure 1A,E-F). The overall pattern of sensitivity (Figure 1E) was similar to that of the established mitochondrial pathway activator etoposide40 and suggested that the mitochondrial pathway, but not the death receptor pathway, plays a critical role in tipifarnib-induced apoptosis.
Activation of Bax or Bak is thought to be an important event in apoptosis triggered through the mitochondrial pathway. To examine the potential contributions of these polypeptides, cells were treated with Bax or Bak siRNA before tipifarnib exposure. Down-regulation of Bax had relatively little impact on tipifarnib-induced apoptosis (supplemental Figure 2A-B). In contrast, Bak down-regulation diminished apoptosis by at least 80% (supplemental Figure 2A-B). Additional experiments demonstrated that Bak is activated (supplemental Figure 2C) and cytochrome c is released (see “Tipifarnib-induced killing depends on Bim up-regulation”) during tipifarnib-induced apoptosis.
Tipifarnib-induced killing depends on Bim up-regulation
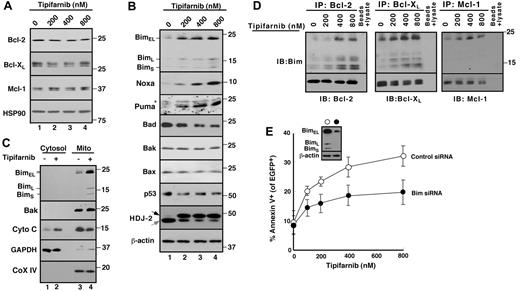
Because Bak activation results from changes in other pro- and antiapoptotic Bcl-2 family members,39 further experiments examined changes in levels and activation of these polypeptides. As indicated in Figure 2A, treatment with tipifarnib did not induce down-regulation of antiapoptotic Bcl-2 family members. Moreover, tipifarnib did not induce up-regulation of Bak, Bax, or the BH3-only family members Bid or Bad. In contrast, Bim increased markedly after tipifarnib treatment (Figure 2B). Cell fractionation demonstrated that a portion of the increased Bim was bound to the mitochondria, which released cytochrome c (Figure 2C). Consistent with the view that Bim has been activated, immunoprecipitation demonstrated increased binding of Bim to Bcl-2, Bcl-xL, and Mcl-1 (Figure 2D). In addition, siRNA experiments demonstrated that Bim down-regulation protected Jurkat cells (Figure 2E), consistent with a critical role for Bim in tipifarnib-induced apoptosis.
Role of Bim up-regulation in tipifarnib-induced apoptosis. (A-B) After Jurkat cells were treated for 72 hours with the indicated tipifarnib concentration in the presence of 5μM Q-VD-OPh, a broad-spectrum caspase inhibitor, whole cell lysates were subjected to SDS-PAGE followed by immunoblotting with antibodies that recognize the indicated antigens. Hsp90 and β-actin served as loading controls. The shift in mobility of the farnesyltransferase substrate HDJ-2 confirmed the inhibition of farnesylation. In this and subsequent figures, gray and black arrows indicate farnesylated and unfarnesylated species, respectively. Asterisk (*) represents nonspecific band present in all lanes. (C) After Jurkat cells were treated with diluent (0.1% DSMO, −) or 800nM tipifarnib (+) in the presence Q-VD-OPh for 72 hours, the indicated subcellular fractions were isolated and subjected to immunoblotting. GAPDH served as a marker for cytosol; and cytochrome c oxidase subunit IV (CoX IV) served as a marker for mitochondria. (D) After Jurkat cells were treated with the indicated concentration of tipifarnib for 72 hours in the presence 5μM Q-VD-OPh, cell lysates prepared in 1% CHAPS37 were subjected to immunoprecipitation with protein G-Sepharose cross-linked to antibodies that recognize Bcl-2, Bcl-xL, or Mcl-1. Immunoprecipitates were washed, resolved by SDS-PAGE, and subjected to immunoblotting as indicated. In each immunoprecipitation experiment, tubes lacking primary antibody served as controls to assess the specificity of protein recovery with the protein G-Sepharose beads. (E) Twenty-four hours after transfection of control oligonucleotide or Bim siRNA along with plasmid encoding EGFP-histone H2B (to mark successfully transfected cells), cells were treated for 48 hours with tipifarnib before staining with APC-conjugated annexin V and analysis by 2-color flow cytometry. Error bars indicate mean ± SD of 3 experiments. (E) Inset: Immunoblots of whole cell lysates prepared from siRNA-treated cells incubated in drug-free medium in parallel with samples harvested for flow cytometry.
Role of Bim up-regulation in tipifarnib-induced apoptosis. (A-B) After Jurkat cells were treated for 72 hours with the indicated tipifarnib concentration in the presence of 5μM Q-VD-OPh, a broad-spectrum caspase inhibitor, whole cell lysates were subjected to SDS-PAGE followed by immunoblotting with antibodies that recognize the indicated antigens. Hsp90 and β-actin served as loading controls. The shift in mobility of the farnesyltransferase substrate HDJ-2 confirmed the inhibition of farnesylation. In this and subsequent figures, gray and black arrows indicate farnesylated and unfarnesylated species, respectively. Asterisk (*) represents nonspecific band present in all lanes. (C) After Jurkat cells were treated with diluent (0.1% DSMO, −) or 800nM tipifarnib (+) in the presence Q-VD-OPh for 72 hours, the indicated subcellular fractions were isolated and subjected to immunoblotting. GAPDH served as a marker for cytosol; and cytochrome c oxidase subunit IV (CoX IV) served as a marker for mitochondria. (D) After Jurkat cells were treated with the indicated concentration of tipifarnib for 72 hours in the presence 5μM Q-VD-OPh, cell lysates prepared in 1% CHAPS37 were subjected to immunoprecipitation with protein G-Sepharose cross-linked to antibodies that recognize Bcl-2, Bcl-xL, or Mcl-1. Immunoprecipitates were washed, resolved by SDS-PAGE, and subjected to immunoblotting as indicated. In each immunoprecipitation experiment, tubes lacking primary antibody served as controls to assess the specificity of protein recovery with the protein G-Sepharose beads. (E) Twenty-four hours after transfection of control oligonucleotide or Bim siRNA along with plasmid encoding EGFP-histone H2B (to mark successfully transfected cells), cells were treated for 48 hours with tipifarnib before staining with APC-conjugated annexin V and analysis by 2-color flow cytometry. Error bars indicate mean ± SD of 3 experiments. (E) Inset: Immunoblots of whole cell lysates prepared from siRNA-treated cells incubated in drug-free medium in parallel with samples harvested for flow cytometry.
Although the BH3-only proteins Noxa and Puma also increased in tipifarnib-treated cells (Figure 2B), these proteins were unchanged or undetectable in Bcl-2, Bcl-xL, and Mcl-1 immunoprecipitates (data not shown). Accordingly, further experiments focused on tipifarnib-induced Bim up-regulation and activation.
Tipifarnib inhibits ERK phosphorylation
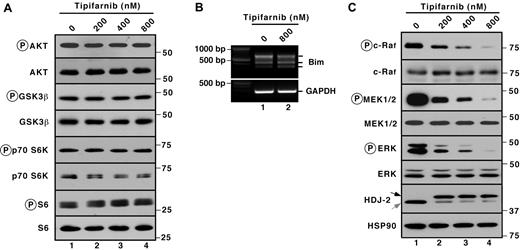
Several signal transduction pathways have been reported previously to regulate Bim levels. In particular, inhibition of the PI3K/Akt pathway reportedly enhances the activity of the transcription factor Foxo3a, leading to enhanced Bim transcription.41,42 Conversely, ERK- and p90 ribosomal S6 kinase-mediated Bim phosphorylation leads to increased βTrCP-mediated Bim ubiquitylation and proteasome-mediated degradation.43,44 In Jurkat cells, tipifarnib failed to inhibit phosphorylation of the Akt substrate glycogen synthase kinase 3β and downstream substrates p70S6 kinase and ribosomal protein S6 (Figure 3A). Additional experiments failed to demonstrate any change in mRNA encoding the various Bim isoforms (Figure 3B). Accordingly, we concluded that PI3K/Akt pathway inhibition was unlikely to contribute to tipifarnib-induced apoptosis in Jurkat cells. Instead, examination of components of the MAPK pathway demonstrated dose-dependent decreases in the autophosphorylation of c-Raf (on Ser338) as well as decreases of the activating phosphorylations of MEK and ERK (Figure 3C) after tipifarnib treatment.
Prominent tipifarnib-induced inhibition of MAPK signaling. (A,C) after Jurkat cells were treated for 72 hours with the indicated tipifarnib concentration in the presence of 5μM Q-VD-OPh, whole cell lysates were subjected to immunoblotting with antibodies that recognize the indicate polypeptides. Ribosomal protein S6 and Hsp90 served as loading controls. (B) After Jurkat cells were treated for 72 hours with the indicated tipifarnib concentration in the presence of 5μM Q-VD-OPh, cDNA was prepared and RT-PCR was performed using primers specific for Bim.
Prominent tipifarnib-induced inhibition of MAPK signaling. (A,C) after Jurkat cells were treated for 72 hours with the indicated tipifarnib concentration in the presence of 5μM Q-VD-OPh, whole cell lysates were subjected to immunoblotting with antibodies that recognize the indicate polypeptides. Ribosomal protein S6 and Hsp90 served as loading controls. (B) After Jurkat cells were treated for 72 hours with the indicated tipifarnib concentration in the presence of 5μM Q-VD-OPh, cDNA was prepared and RT-PCR was performed using primers specific for Bim.
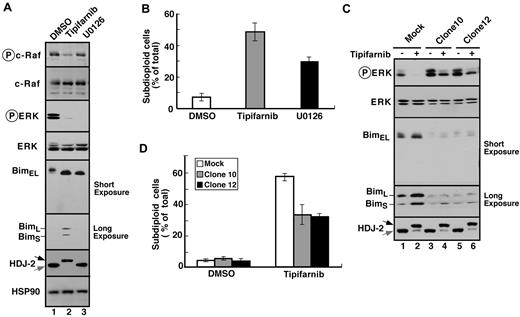
To further assess the importance of decreased signaling through the MAPK pathway, 2 types of experiments were performed. First, Jurkat cells were treated with the MEK1/2 inhibitor U0126. This agent inhibited ERK phosphorylation and, like tipifarnib, induced Bim up-regulation along with apoptosis (Figure 4A-B). Conversely, Jurkat cells were transfected with constitutively active MEK1. As indicated in Figure 4C and D for 2 stable clones, constitutive MEK1 activation blunted tipifarnib-induced Bim up-regulation and apoptosis.
Tipifarnib-induced Bim up-regulation reflects inhibition of MAPK signaling. (A) After Jurkat cells were treated for 72 hours with 800nM tipifarnib or 20μM U0126, in the presence of 5μM Q-VD-OPh, whole cell lysates were subjected to immunoblotting with antibodies that recognize the indicated polypeptides. (B) After Jurkat cells were treated for 72 hours with 800nM tipifarnib or 20μM U0126, samples were stained with propidium iodide and subjected to flow microfluorometry. (C) After Jurkat cells and constitutively active MEK1 clones 10 and 12 were treated for 72 hours with diluent (−) or 800nM tipifarnib (+) in the presence of 5μM Q-VD-OPh, whole cell lysates were subjected to immunoblotting with antibodies that recognize the indicated polypeptides. (D) After the indicated clones were treated for 72 hours with diluent or 800nM tipifarnib, samples were stained with propidium iodide and subjected to flow microfluorometry. Error bars in panels B and D indicate mean ± SD of at least 3 independent experiments.
Tipifarnib-induced Bim up-regulation reflects inhibition of MAPK signaling. (A) After Jurkat cells were treated for 72 hours with 800nM tipifarnib or 20μM U0126, in the presence of 5μM Q-VD-OPh, whole cell lysates were subjected to immunoblotting with antibodies that recognize the indicated polypeptides. (B) After Jurkat cells were treated for 72 hours with 800nM tipifarnib or 20μM U0126, samples were stained with propidium iodide and subjected to flow microfluorometry. (C) After Jurkat cells and constitutively active MEK1 clones 10 and 12 were treated for 72 hours with diluent (−) or 800nM tipifarnib (+) in the presence of 5μM Q-VD-OPh, whole cell lysates were subjected to immunoblotting with antibodies that recognize the indicated polypeptides. (D) After the indicated clones were treated for 72 hours with diluent or 800nM tipifarnib, samples were stained with propidium iodide and subjected to flow microfluorometry. Error bars in panels B and D indicate mean ± SD of at least 3 independent experiments.
Characterization of tipifarnib-resistant cells
To further evaluate the importance of the Raf/ERK/Bim pathway in tipifarnib-induced apoptosis, Jurkat cells were selected for the ability to grow during continuous tipifarnib exposure. Examination of these cells revealed that they were ∼ 50-fold resistant to tipifarnib and were cross-resistant to the farnesyltransferase inhibitor lonafarnib (supplemental Figure 3A-B). In contrast, they showed no change in sensitivity to the topoisomerase II poison etoposide (supplemental Figure 3C). Immunoblotting demonstrated that the resistant cells had restored phosphorylation of c-Raf, MEK1/2, and ERK, despite the presence of tipifarnib. Moreover, Bim levels were diminished in these cells relative to Jurkat cells treated acutely with the same tipifarnib concentration (supplemental Figure 3D). Collectively, results shown in Figure 4 and supplemental Figure 3 demonstrate that tipifarnib is selectively inhibiting signaling through the MAPK pathway, that this inhibition contributes to Bim up-regulation, and that changes resulting in restoration of signaling in this pathway down-regulate Bim and contribute to tipifarnib resistance.
Role of Ras GRP1 in tipifarnib sensitivity
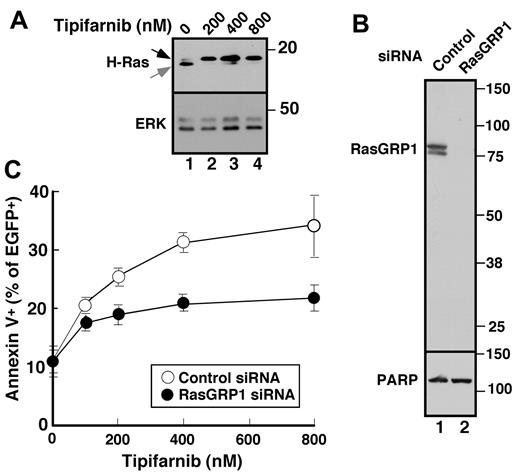
To assess the potential involvement of H-Ras upstream of c-Raf, we first examined the effect of tipifarnib on H-Ras prenylation and observed, as expected, that tipifarnib inhibited H-Ras farnesylation (Figure 5A). Further experiments examining the effects of tipifarnib in cells transfected with H-Ras-CVLL, a construct that undergoes geranylgeranylation rather than farnesylation,45 were hampered by profound toxicity of the H-Ras-CVLL construct itself (H. Ding, unpublished observations, May 28, 2009).
Role of RasGRP1 in tipifarnib sensitivity. (A) Twenty-four hours after transfection with cDNA encoding H-Ras, cells were treated with the indicated tipifarnib concentration for 72 hours in the presence of 5μM Q-VD-OPh and prepared for immunoblotting. (B) Forty-eight hours after transfection with control siRNA (lane 1) or RasGRP1 siRNA (lane 2), whole cell lysates were prepared and subjected to immunoblotting with antibody to RasGRP1 or, as a control, poly(ADP-ribose) polymerase (PARP). (C) Beginning 24 hours after transfection with control siRNA or RasGRP1 siRNA, cells were treated for 48 hours with tipifarnib, stained with propidium iodide, and subjected to flow cytometry. Error bars, mean ± SD of 3 independent experiments.
Role of RasGRP1 in tipifarnib sensitivity. (A) Twenty-four hours after transfection with cDNA encoding H-Ras, cells were treated with the indicated tipifarnib concentration for 72 hours in the presence of 5μM Q-VD-OPh and prepared for immunoblotting. (B) Forty-eight hours after transfection with control siRNA (lane 1) or RasGRP1 siRNA (lane 2), whole cell lysates were prepared and subjected to immunoblotting with antibody to RasGRP1 or, as a control, poly(ADP-ribose) polymerase (PARP). (C) Beginning 24 hours after transfection with control siRNA or RasGRP1 siRNA, cells were treated for 48 hours with tipifarnib, stained with propidium iodide, and subjected to flow cytometry. Error bars, mean ± SD of 3 independent experiments.
While the preceding experiments were in progress, Raponi et al28 reported that a 2-transcript signature, characterized by a high ratio of message encoding the Ras guanine nucleotide exchange factor RasGRP1 compared with aprataxin, was associated with a 28% complete response rate in two phase 2 AML trials of single-agent tipifarnib. To assess whether RasGRP1 contributes to tipifarnib sensitivity in lymphoid cells, we examined the effect of RasGRP1 down-regulation. Because we could not identify high-quality commercially available anti-Ras GRP1 antibodies, we generated a series of murine monoclonal antibodies against purified recombinant human RasGRP1 for these experiments. All 5 monoclonals recognized the native protein by ELISA; and 3 (typified by results in Figure 5B) recognized a doublet migrating at Mr ∼ 85 000 (the known molecular weight of RasGRP1) by immunoblotting. Using this antibody, we identified an siRNA oligonucleotide that down-regulates RasGRP1 by > 90% (Figure 5B lane 2). Importantly, Jurkat cells with diminished RasGRP1 were less sensitive to tipifarnib-induced apoptosis (Figure 5C), suggesting that Ras is a critical target of tipifarnib upstream of c-Raf in these cells and providing the first direct confirmation that RasGRP1 affects tipifarnib sensitivity.
Role of Bim up-regulation in other lymphoid lines
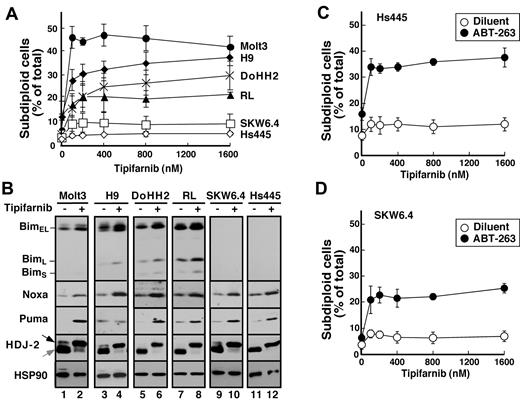
To extend these results to additional lymphoid cell lines, 6 additional lines were examined. Tipifarnib-induced apoptosis also was observed in Molt3, H9, DOHH2, and RL cells (Figure 6A), all of which exhibited Bim up-regulation (Figure 6B). In contrast, tipifarnib did not induce detectable apoptosis in SKW6.4 or Hs445 cells, which express undetectable Bim levels before and after treatment. Importantly, SKW6.4 and Hs445 cells failed to undergo apoptosis despite tipifarnib-induced up-regulation of Puma and Noxa (Figure 6B), again highlighting the predominant importance of Bim in tipifarnib sensitivity. In contrast, treatment of these latter cell lines with ABT-263 along with tipifarnib resulted in modest induction of apoptosis (Figure 6C-D), indicating that Puma and Noxa can contribute to tipifarnib-induced apoptosis when neutralization by Bcl-2 and Bcl-xL is diminished.
Correlation between tipifarnib-induced Bim up-regulation and apoptosis. (A) After cells were treated for 72 hours with the indicated tipifarnib concentration, samples were stained with propidium iodide and subjected to flow microfluorometry. (B) After cells were treated with diluent (odd lanes) or 800nM tipifarnib (even lanes) in the presence of 5μM Q-VD-OPh, whole cell lysates were subjected to SDS-PAGE followed by immunoblotting with antibodies to the indicated antigen. For each antigen, all lanes were derived from corresponding signals on a single piece of x-ray film but were rearranged for clarity. (C-D) Hs445 (C) or SKW6.4 cells (D) were treated for 72 hours with the indicated tipifarnib concentration in the absence (open circles) or presence (closed circles) of 125nM ABT-263, stained with propidium iodide, and subjected to flow microfluorometry. Error bars in panels A, C, and D indicate mean ± SD of 3 independent experiments.
Correlation between tipifarnib-induced Bim up-regulation and apoptosis. (A) After cells were treated for 72 hours with the indicated tipifarnib concentration, samples were stained with propidium iodide and subjected to flow microfluorometry. (B) After cells were treated with diluent (odd lanes) or 800nM tipifarnib (even lanes) in the presence of 5μM Q-VD-OPh, whole cell lysates were subjected to SDS-PAGE followed by immunoblotting with antibodies to the indicated antigen. For each antigen, all lanes were derived from corresponding signals on a single piece of x-ray film but were rearranged for clarity. (C-D) Hs445 (C) or SKW6.4 cells (D) were treated for 72 hours with the indicated tipifarnib concentration in the absence (open circles) or presence (closed circles) of 125nM ABT-263, stained with propidium iodide, and subjected to flow microfluorometry. Error bars in panels A, C, and D indicate mean ± SD of 3 independent experiments.
Discussion
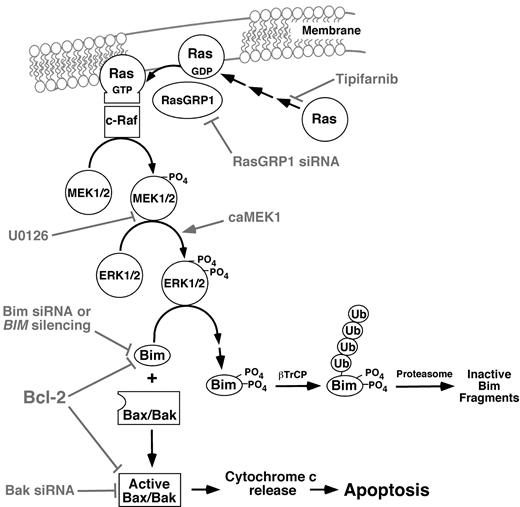
Results of the present manuscript demonstrate that tipifarnib, probably acting through H-Ras or N-Ras, decreases activation of the kinases c-Raf, MEK1/2, and ERK1/2, leading to up-regulation and activation of the BH3-only protein Bim (Figure 7). Several observations, including the ability of Bim or Bak down-regulation to protect cells and the ability of constitutively active MEK1 to blunt the effects of tipifarnib, point to the importance of these changes for tipifarnib-induced killing of lymphoid cells. Importantly, all of the tipifarnib-induced changes from c-Raf inhibition to Bim up-regulation are reversed in tipifarnib-resistant Jurkat cells. Collectively, these results provide new insight into the cytotoxic action of tipifarnib in human lymphoid cells.
Pathways potentially contributing to tipifarnib-induced apoptosis in lymphoid cells. Experimental manipulations examined in the present study are indicated in gray. As described in “Results,” the Raf/MEK/ERK pathway plays the predominant role in Bim up-regulation in the cell lines examined, and the PI3 kinase/Akt/Foxo3a pathway plays at most a limited role.
Pathways potentially contributing to tipifarnib-induced apoptosis in lymphoid cells. Experimental manipulations examined in the present study are indicated in gray. As described in “Results,” the Raf/MEK/ERK pathway plays the predominant role in Bim up-regulation in the cell lines examined, and the PI3 kinase/Akt/Foxo3a pathway plays at most a limited role.
Previous studies have identified several potential targets of FTIs, including members of the Ras, Rho, and Rheb families.4,5 Which of these are important for FTI-mediated killing in various cell types has not been fully resolved. Recent studies of murine lymphoid malignancies15 and human AML specimens38 have demonstrated that FTIs inhibit signaling downstream of mammalian target of rapamycin, probably reflecting inhibition of Rheb farnesylation. Although we cannot rule out the possibility that Rheb is inhibited in some human lymphoid cells, this did not seem to be a prominent feature in the cell lines examined in the present work (Figure 3A). Instead, based on the ability of the Ras guanine nucleotide exchange factor RasGRP1 to impact tipifarnib sensitivity (Figure 5C), along with changes from c-Raf through ERK (Figures 3–4; supplemental Figure 3), it seems that H-Ras or N-Ras is the pertinent tipifarnib target. These differences probably reflect context-dependent differences in signal transduction pathways between various cell types.
Previous studies using a variety of FTIs and cell line systems have identified a wide range of FTI-induced changes in apoptotic pathways. It has been suggested, for example, that FTIs up-regulate death receptors and enhance death ligand-induced apoptosis in colon cancer cells.46 Given the lack of activity of FTIs against colon cancer47,48 but the readily demonstrable activity in lymphoid neoplasms,30 we felt it important to reevaluate the mechanism of action of FTIs in lymphoid lines. Results of this analysis demonstrate that tipifarnib-induced killing is unaffected by loss of FADD or procaspase-8 (Figure 1E-F), both of which are required for death ligand-induced apoptosis (Figure 1E). These results point directly to the mitochondrial pathway as a primary target of tipifarnib cytotoxicity. Further analysis demonstrated an absolute reliance on the proapoptotic Bcl-2 family member Bak (supplemental Figure 2A-B), consistent with recent experiments showing that Jurkat cells express 10-fold more Bak than Bax.31 Upstream of Bak, we demonstrated that tipifarnib up-regulates Bim in a dose-dependent manner (Figure 2B). Additional experiments showed that Bim traffics to mitochondria (Figure 2C), where it bound other Bcl-2 family members (Figure 2D).
Several additional observations point to the importance of Bim in tipifarnib-induced apoptosis. First, Bim siRNA markedly diminished tipifarnib-induced cytotoxicity (Figure 2E). Second, signaling changes that rendered cells resistant to tipifarnib, including overexpression of constitutively active MEK1 (Figure 4C-D) or selection for tipifarnib resistance (supplemental Figure 3B,D), reversed the Bim up-regulation. Collectively, these results provide the first evidence that Bim plays a critical role in FTI-induced killing.
We also observed that Noxa and Puma were up-regulated in tipifarnib-treated Jurkat cells (Figure 2B). Because the same experiments failed to show changes in p53 levels in Jurkat cells (Figure 2B), the mechanism of tipifarnib-induced Puma and Noxa up-regulation is unknown. Although these polypeptides might contribute to apoptosis in Jurkat cells, as evidenced by further decreases in apoptosis after Bim plus Puma plus Noxa siRNA compared with Bim siRNA alone (H. Ding, unpublished observations, May 17, 2009), it is unlikely that they are by themselves major contributors in lymphoid cells. In particular, we observed that Hs445 and SKW6.4 cells up-regulate Puma and Noxa in response to tipifarnib yet fail to undergo tipifarnib-induced apoptosis (Figure 6A-B). Instead, tipifarnib-induced apoptosis was only observed in those lymphoid cell lines where Bim up-regulation also occurred.
At least 2 signal transduction pathways could have contributed to Bim up-regulation: the Akt pathway (through dephosphorylation of Foxo1 or Foxo3A)41,42 or the ERK pathway.49 Further experiments highlighted the importance of the ERK pathway, known previously to be sensitive to FTIs in some cell types9,11-13,45 but not others.38 In particular, we failed to observe the increase in Bim mRNA that would be expected if the Akt/Foxo pathway were involved (Figure 3B). Instead, we observed that the MEK1/2 inhibitor U0126, like tipifarnib, inhibited ERK phosphorylation and induced Bim up-regulation (Figure 4B).
The fact that Bim up-regulation plays a critical role in tipifarnib-induced apoptosis in lymphoid cells suggests several potential mechanisms of resistance. First, because Bcl-2 has a high affinity for Bim,50 massive Bcl-2 overexpression would be expected to render cells resistant to tipifarnib, a result confirmed in the JB-6 variant of Jurkat cells (Figure 1D-F). Consistent with these results, tipifarnib also failed to induce regressions in lymphomas with extremely high Bcl-2 levels, for example, follicular and mantle cell lymphomas, in the accompanying clinical trial.30 Second, cells that failed to up-regulate Bim would be expected to be resistant to tipifarnib. In the present study, we identified several lines that failed to express detectable levels of Bim either before or after tipifarnib treatment (Figure 6B). Interestingly, these lines remained sensitive to tipifarnib-induced inhibition of proliferation (supplemental Figure 1), stressing the difference between effects on proliferation as assessed by MTS assays and induction of apoptosis. Moreover, these cells could be sensitized tipifarnib-induced killing by ABT-263 (Figure 6C-D), indicating a possible role for other BH3-only proteins and suggesting that the tipifarnib/ABT263 combination might deserve further investigation.
In the accompanying paper, results of a phase 2 trial of tipifarnib in relapsed and refractory lymphoma are described.30 This study identifies certain subtypes of lymphoma that have a high probably of responding to tipifarnib as a single agent even after multiple previous therapies. Results of the present study identify the signaling pathway downstream of Ras that is triggered during tipifarnib-induced cell death in human lymphoid lines, thereby providing the background for the correlative studies performed in conjunction with the accompanying clinical trial.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors gratefully acknowledge discussions with J. Karp; gifts of reagents from D. Toft, I. Schmitz, T. Gomez, and D. Billadeau; assistance of personnel in the Mayo Clinic Flow Cytometry and Hybridoma Core facilities; and editorial assistance of D. Strauss.
This work was supported in part by grants from the Leukemia & Lymphoma Society (6047-08, S.H.K.) and the National Institutes of Health (R01 CA127433, T.E.W.).
National Institutes of Health
Authorship
Contribution: H. Ding, T.E.W., and S.H.K. designed the research; H. Ding, H. Dai, J.H., P.A.S., K.L.P., X.W.M., and S.H.K. performed the research; H. Ding and S.H.K. analyzed data and wrote the paper; and J.H. provided new reagents.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Scott Kaufmann, Division of Oncology Research, Gonda 19-212, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail: kaufmann.scott@mayo.edu.