Abstract
Non-Hodgkin lymphoma (NHL) presents as both localized and disseminated disease with spread to secondary sites carrying a worse prognosis. Although pathways driving NHL dissemination have been identified, there are few therapies capable of inhibiting them. Here, we report a novel role for the immunomodulatory protein CD47 in NHL dissemination, and we demonstrate that therapeutic targeting of CD47 can prevent such spread. We developed 2 in vivo lymphoma metastasis models using Raji cells, a human NHL cell line, and primary cells from a lymphoma patient. CD47 expression was required for Raji cell dissemination to the liver in mouse xenotransplants. Targeting of CD47 with a blocking antibody inhibited Raji cell dissemination to major organs, including the central nervous system, and inhibited hematogenous dissemination of primary lymphoma cells. We hypothesized that anti-CD47 antibody-mediated elimination of circulating tumor cells occurred through phagocytosis, a previously described mechanism for blocking anti-CD47 antibodies. As predicted, inhibition of dissemination by anti-CD47 antibodies was dependent on blockade of phagocyte SIRPα and required macrophage effector cells. These results demonstrate that CD47 is required for NHL dissemination, which can be therapeutically targeted with a blocking anti-CD47 antibody. Ultimately, these findings are potentially applicable to the dissemination and metastasis of other solid tumors.
Introduction
Lymphocyte trafficking is essential for the regulation of systemic immune processes, as well as lymphocyte differentiation and development. Most mature lymphocytes recirculate continuously from blood to tissue and back to the blood again.1 This recirculation is not random but rather is guided by lymphocyte-endothelial interactions mediated by adhesion molecules (L-selectin, CD44, integrin α4β7, VLA-4, and LFA-1) and select chemokines.2,3
Malignant transformation of normal lymphocytes results in lymphoma, many subtypes of which migrate and disseminate. Unlike the metastasis of other cancers, lymphoma dissemination probably reflects conserved physiologic behavior, rather than acquisition of metastatic potential. Indeed, mechanisms of normal lymphocyte homing and recirculation have been implicated in lymphoma dissemination and invasion. For example, adhesion molecules involved in normal lymphocyte trafficking have been shown to play a role in lymphoma dissemination, including LFA-1, α3β3, and other homing-associated integrins.4-6 Furthermore, several of these adhesion molecules have been therapeutically exploited, as antibodies targeting the adhesion receptors LFA-1, integrin αvβ3, and CD44 can inhibit dissemination of lymphoma in experimental models.5,7-9
CD47, also known as integrin-associated protein, has been implicated in the migration and mobilization of normal leukocytes.10-14 In cancer, we recently demonstrated that CD47 regulates lymphoma pathogenesis by enabling evasion of phagocytosis through binding of the inhibitory receptor SIRPα on phagocytes.15 Furthermore, a blocking monoclonal antibody targeting CD47 eliminated human lymphoma in xenotransplant models through phagocytosis of tumor cells, and synergized with rituximab, a therapeutic antibody commonly used in non-Hodgkin lymphoma (NHL) therapy.15 Given the roles of CD47 in normal cell migration and lymphoma pathogenesis, we investigated the function of CD47 in NHL dissemination and whether therapeutic targeting of CD47 could inhibit such spread.
Methods
Human samples and antibodies
NHL samples were obtained as previously described15 from patients at the Stanford University Medical Center with informed consent according to an Institutional Review Board–approved protocol (Stanford IRB #13500) or with informed consent from the Norwegian Radium Hospital (Oslo, Norway) according to a Regional Ethic Committee–approved protocol (REK #2.2007.2949) following the Declaration of Helsinki. For all in vivo experiments, anti–human CD47 (clone B6H12.2) was used and obtained as previously described.15 The nonblocking anti–human CD47 antibody clone 2D3, mouse IgG1 isotype control, and anti–human CD45 antibodies were obtained from eBioscience.
Flow cytometric analysis
For analysis of primary and xenografted NHL cells, human CD19, human CD45, mouse Terr19, mouse CD45, and mouse F4/80 were used (Invitrogen and BD Biosciences). Analysis of human CD47 expression was performed with an anti–human CD47 FITC antibody (clone B6H12.2, BD Biosciences).
Generation of luciferase-positive Raji cells and luciferase imaging analysis
A luciferase-positive Raji cell line was generated and analyzed by luciferase imaging as previously described.15
In vivo anti-CD47 antibody treatment in a localized and disseminated lymphoma xenograft model
For the localized model, 3 × 106 luciferase-labeled Raji cells were injected subcutaneously into the right flank of 6- to 10-week-old NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NSG) mice. After 1 week, mice were treated with daily 200 μg intraperitoneal injections of either mouse control IgG or anti-CD47 antibody. These mice were then followed weekly by luciferase imaging for the presence of dissemination of luciferase-positive disease. Tumor volume was measured weekly with calipers and determined by (length × width)/2. Appearance of metastatic luciferase-positive disease was present at ∼ 2-3 weeks for control IgG-treated mice. For the disseminated model, 1.5 × 106 luciferase-labeled Raji cells were injected intravenously into the retro-orbital sinus of adult NSG mice. Antibody treatment consisted of coating Raji cells ex vivo with 30 μg/mL of IgG1 isotype control antibody, anti-CD45 antibody, anti-CD47 antibody clone B6H12.2, or anti-CD47 antibody clone 2D3 before intravenous transplantation. Tumor engraftment was analyzed 5 days after transplantation by luciferase imaging.
In vivo anti-CD47 antibody treatment in a primary localized DLBCL xenograft model
A total of 2-3 × 106 bulk diffuse large B-cell lymphoma (DLBCL) cells were transplanted into sublethally irradiated (200 cGy) NSG mice. Three weeks later, mice were treated with daily 400 μg intraperitoneal injections of either control mouse IgG or anti-CD47 antibody. The presence of DLBCL disease was analyzed by flow cytometry for the presence of human CD45+CD19+ cells in the peripheral blood or bone marrow.
Lentiviral-encoded shRNA knockdown of Raji cells
shRNA constructs targeting knockdown of human CD47 or a GFP control were used and transduced into Raji cells as previously described.15 Knockdown of CD47 protein levels was determined by staining with CD47 antibody (clone B6H12.2) with fold knockdown calculated by reduction of mean fluorescence intensity normalized over isotype control.
Histopathologic analysis of lymphoma-engrafted mice
NSG mice engrafted subcutaneously with luciferase-labeled Raji cells were killed after control IgG or anti-CD47 antibody treatment, and organs harvested and analyzed for human lymphoma dissemination by H&E staining according to standard protocols. Hematoxylin and eosin sections were analyzed in a double-blinded fashion by a pathologist who determined whether dissemination of human lymphoma was present in each mouse organ. Micrographs were obtained using an Olympus BX41 microscope with a 40× objective, and images were acquired with a Canon G9 digital camera and processed using Axiovision 4.7 software.
In vivo macrophage depletion
Depletion of macrophages in NSG mice was performed using liposomal clodronate or liposomal control as previously described.15 Clodronate or liposomal control was administered to human DLBCL-engrafted mice for the duration of anti-CD47 antibody or control IgG therapy. Macrophage depletion was assessed by flow cytometric analysis of the percentage of F4/80+ macrophages in the bone marrow.
MRI of NHL-engrafted mice
For in vivo MRI acquisition, mice were anesthetized with isoflurane (IsoFlo; Abbott Laboratories; 1%-1.75%). Gadolinium-based contrast, gadopentate dimeglumine (Berlex Laboratories), was injected intraperitoneally 20-30 minutes before image acquisition (2.5 mL/kg in PBS, 100 μL). Temperature and respiration were monitored continuously. Body temperature was maintained at 37 ± 1°C using a warm-air circulation system. The mice lay supine with the liver region positioned at the coil center. Anatomic images were obtained through the upper abdomen. MRI experiments were performed on Magnex/Varian self-shielded 30-cm bore 7-Tesla magnet, a Research Resonance Instruments 9-cm bore gradient insert, and the GEHC “Micro”-Signa software environment. Acquisition parameters are as follows: T1-weighted fast spin echo, TE 5.7 ms, TR 800, thickness 0.5 mm, FOV 6 × 6, NEX 1.0. For image analysis, an off-line workstation was used (Osirix).
Chemotaxis assay
The chemotaxis assay across polycarbonate transwell inserts were performed as previously described.16 Raji cells were incubated with IgG1 isotype control, anti-CD45, anti-CD47 (B6H12), or anti-CD47 (2D3) antibody for 20 minutes at 4°C and washed before chemotaxis. A total of 1 × 106 Raji cells were then suspended in 100 μL of serum-free IMDM and added to the top chamber of a 24 well transwell plate with 5 μm pore-size filters (Corning/Costar). In the bottom chamber, there was 600 μL of media with either chemokine 10nM SDF-1α or 1 μg/mL CXCL13 (PeproTech). SDF-1α and CXCL13 were chosen as chemokines previously shown to enable migration of lymphoma cells.17,18 Chemokine dosing was determined by dose titration for maximal migration of Raji cells (data not shown). Cells were incubated for 3-3.5 hours at 37°C. In chemotaxis assays involving antibodies, Raji cells were preincubated with 10 μg/mL of IgG1 isotype control or anti-CD47 antibodies for 20 minutes at 4°C before subjecting them to the transwell assay. Upper chamber inserts were removed, and migrated cells in the lower chamber were standardized using 100-μL microbeads per sample and counted on a flow cytometer (LSRII, BD Biosciences). Percentage migration was calculated using the number of starting and migrated cells.
Static adhesion assay
To evaluate static adhesion, Raji cells were washed and resuspended at 0.5 to 1 × 106 cells/mL in adhesion buffer (DMEM, 1% BSA, 2mM Ca2+/Mg2+). For antibody blocking experiments, cells were incubated for 20 minutes at 4°C with antibodies at indicated concentrations before their use. The 96-well plates were coated with recombinant human VCAM (R&D Systems) at 1 μg/mL overnight at 4°C. Plates were then blocked for 1-3 hours with PBS + 0.1% BSA at room temperature; 100-μL cells were plated per well in triplicate. Cells were incubated at 37°C for 1-2 hours and washed gently more than 4 times with PBS + Ca2+/Mg2+. To quantify cells, 20 μL CellTiter 96 (Invitrogen) was added to each well containing adherent cells in 100 μL PBS and incubated at 37°C for 1-3 hours. Absorbance at 490 nm was read on a plate reader and calculated relative to Raji cells incubated with BSA-coated plates.
Results
Dissemination of NHL is dependent on CD47
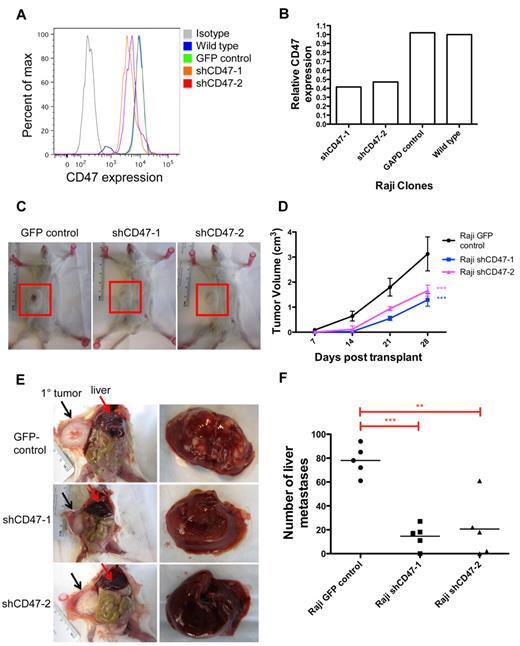
To investigate the role of CD47 in NHL dissemination, we first determined the effect of knocking down CD47 on NHL spread in vivo. We previously developed a human xenotransplant model of NHL whereby Raji cells, a human Burkitt lymphoma cell line, transplanted subcutaneously into the flanks of NOD/SCID/IL2R-γ null (NSG) immunodeficient mice developed nodular disease.15 In this model, engrafted lymphoma disseminated to extranodal secondary sites, including the liver (Figure 1E). Lentiviral shRNA vectors were used to knock down CD47 expression in Raji cells (Figure 1A-B) as previously described.15 Raji cells with > 50% reduction in CD47 expression (shCD47-1 and shCD47-2) exhibited no difference in proliferation rate compared with control-transduced cells in vitro (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). However, CD47 knockdown Raji cells (shCD47-1,2) grew at a slower rate in vivo (Figure 1C-D). Transplanted mice were killed 28 days later and analyzed for secondary liver lesions. Although mice transplanted with control cells developed numerous liver masses, the number of lesions was dramatically reduced with CD47 knockdown cells (Figure 1E-F). Liver involvement did not occur by direct extension as the primary subcutaneous tumor did not directly invade into the vasculature or adjacent organs (Figure 1E). CD47 knockdown Raji cells developed an average 4-fold fewer liver lesions, compared with control, with several mice showing complete absence of secondary lesions (Figure 1F). Thus, dissemination of Raji cells in vivo is dependent on the level of CD47 expression.
Dissemination of NHL is dependent on CD47. (A) Raji cells were transduced with lentiviruses encoding shRNA CD47 knockdown constructs (shCD47) or a GFP control, and CD47 protein expression was determined by flow cytometry. (B) Relative CD47 expression levels were quantified by comparing mean fluorescence intensity with wild-type Raji cells. (C) Raji cells from panels A and B were transplanted subcutaneously into the right flank of adult NSG mice and monitored weekly for tumor growth. Representative mice are shown 28 days after transplantation with flank tumors demarcated (red box). (D) Tumor volume was quantified for all mice (n = 5 per cohort), demonstrating reduced growth from shCD47-1,2–transduced cells compared with GFP control. Statistical comparison was performed using a 2-way ANOVA. Data are mean ± SD. (E) Posttransplantation day 28, these mice were killed and analyzed for the number of gross liver metastases with representative mice shown (livers are shown in right panels). Direct invasion from the primary tumor (black arrows) to adjacent organs was not observed. (F) Number of liver metastases was quantified for all mice, with each data point representing an individual mouse. P values were calculated by Student t test: **P < .005. ***P < .0005.
Dissemination of NHL is dependent on CD47. (A) Raji cells were transduced with lentiviruses encoding shRNA CD47 knockdown constructs (shCD47) or a GFP control, and CD47 protein expression was determined by flow cytometry. (B) Relative CD47 expression levels were quantified by comparing mean fluorescence intensity with wild-type Raji cells. (C) Raji cells from panels A and B were transplanted subcutaneously into the right flank of adult NSG mice and monitored weekly for tumor growth. Representative mice are shown 28 days after transplantation with flank tumors demarcated (red box). (D) Tumor volume was quantified for all mice (n = 5 per cohort), demonstrating reduced growth from shCD47-1,2–transduced cells compared with GFP control. Statistical comparison was performed using a 2-way ANOVA. Data are mean ± SD. (E) Posttransplantation day 28, these mice were killed and analyzed for the number of gross liver metastases with representative mice shown (livers are shown in right panels). Direct invasion from the primary tumor (black arrows) to adjacent organs was not observed. (F) Number of liver metastases was quantified for all mice, with each data point representing an individual mouse. P values were calculated by Student t test: **P < .005. ***P < .0005.
A blocking anti-CD47 antibody inhibits formation of extranodal disease in Raji-engrafted mice
Next, we investigated whether extranodal NHL dissemination could be prevented by targeting CD47 with a blocking antibody that was previously shown to induce phagocytosis and elimination of several human tumors.15,19-22 Luciferase-labeled Raji cells were transplanted subcutaneously into NSG mice. One week later, mice were administered daily treatment with 200 μg of either anti-CD47 antibody or control mouse IgG. Compared with control, anti-CD47 antibody therapy decreased the rate of tumor growth (Figure 2A) but did not eliminate the primary disease as previously reported.15 However, anti-CD47 antibody prevented lymphoma dissemination as assessed by 3 independent modalities. First, serial luciferase imaging demonstrated dissemination to secondary sites 3 weeks after transplantation in controls, whereas no spread was observed in the anti-CD47 antibody-treated mice (Figure 2B). Second, MRI with gadolinium-based contrast at 3 weeks after transplantation showed that mice treated with control IgG developed multiple liver tumor nodules, whereas anti-CD47 antibody inhibited liver dissemination (Figure 2C). Lastly, mice were killed and analyzed for extranodal disease by gross tissue analysis. Lastly, multiple liver tumor nodules were grossly observed at necropsy in controls, whereas no tumor nodules were detected in anti-CD47 antibody-treated mice (Figure 2D).
A blocking anti-CD47 antibody inhibits formation of extranodal disease in Raji-engrafted mice. (A) Luciferase-labeled Raji cells were transplanted subcutaneously into the right flank of NSG mice. One week later, mice were administered daily therapy with 200 μg anti-CD47 antibody or mouse IgG control. Tumor volume was measured until end of treatment (posttransplantation day 28). Anti-CD47 antibody treatment reduced tumor volume compared with IgG control. ***P < .0001 (2-way ANOVA). (B) These mice were analyzed in parallel for the presence of luciferase-positive disease to assess tumor dissemination. Spread of disease to secondary sites (red arrows) was detected in IgG control-treated mice 3 weeks after transplantation, whereas no dissemination was observed in anti-CD47 antibody-treated mice. Representative mice are shown (n = 6 per treatment group). (C) Analysis of tumor lesions was performed by MRI at 3 weeks after transplantation, demonstrating tumor nodules (red circles) in controls, but not in mice treated with anti-CD47 antibody. The table reports the number of tumor nodules observed for each mouse, as assessed by a blinded-clinically trained radiation oncologist. (D) Mice were then killed and assessed for gross lesions, which demonstrated diffuse liver involvement in representative mice treated with control IgG, but not with anti-CD47 antibody.
A blocking anti-CD47 antibody inhibits formation of extranodal disease in Raji-engrafted mice. (A) Luciferase-labeled Raji cells were transplanted subcutaneously into the right flank of NSG mice. One week later, mice were administered daily therapy with 200 μg anti-CD47 antibody or mouse IgG control. Tumor volume was measured until end of treatment (posttransplantation day 28). Anti-CD47 antibody treatment reduced tumor volume compared with IgG control. ***P < .0001 (2-way ANOVA). (B) These mice were analyzed in parallel for the presence of luciferase-positive disease to assess tumor dissemination. Spread of disease to secondary sites (red arrows) was detected in IgG control-treated mice 3 weeks after transplantation, whereas no dissemination was observed in anti-CD47 antibody-treated mice. Representative mice are shown (n = 6 per treatment group). (C) Analysis of tumor lesions was performed by MRI at 3 weeks after transplantation, demonstrating tumor nodules (red circles) in controls, but not in mice treated with anti-CD47 antibody. The table reports the number of tumor nodules observed for each mouse, as assessed by a blinded-clinically trained radiation oncologist. (D) Mice were then killed and assessed for gross lesions, which demonstrated diffuse liver involvement in representative mice treated with control IgG, but not with anti-CD47 antibody.
Although liver dissemination is observed in patients with NHL, extranodal spread has been observed in almost every organ, particularly the gastrointestinal tract and CNS. Therefore, control and anti-CD47 antibody-treated mice were analyzed for lymphoma invasion in major organs by tissue histopathology. IgG control-treated mice displayed evidence of diffuse micro-dissemination, including invasion into liver, pancreas, kidney, nasal cavity, and bone (Figure 3A,C). Hematogenous dissemination and invasion into the brain were also observed as evidenced by disease in the bone marrow and cerebral cortex (and pituitary gland), respectively (Figure 3A,C). In contrast, mice treated with anti-CD47 antibody exhibited no evidence of disease in any major organ (Figure 3B-C).
A blocking anti-CD47 antibody inhibits dissemination of Raji cells to multiple major organs. Mice engrafted subcutaneously with Raji cells and treated with either IgG control (A) or anti-CD47 antibody (B; from Figure 2) were killed after 1 month, and major organs were analyzed for dissemination of human lymphoma by H&E staining. Lymphoma dissemination was observed in several major organs of IgG control-treated mice as demonstrated by invasion of monomorphic lymphoma cells and disruption of normal cellular architecture. (A) Red asterisks indicate areas of lymphoma invasion. (B) No evidence of human lymphoma was observed in anti-CD47 antibody-treated mice. (C) Human lymphoma organ invasion was assessed by a blinded pathologist. The number of mice with disseminated lymphoma in each organ is indicated.
A blocking anti-CD47 antibody inhibits dissemination of Raji cells to multiple major organs. Mice engrafted subcutaneously with Raji cells and treated with either IgG control (A) or anti-CD47 antibody (B; from Figure 2) were killed after 1 month, and major organs were analyzed for dissemination of human lymphoma by H&E staining. Lymphoma dissemination was observed in several major organs of IgG control-treated mice as demonstrated by invasion of monomorphic lymphoma cells and disruption of normal cellular architecture. (A) Red asterisks indicate areas of lymphoma invasion. (B) No evidence of human lymphoma was observed in anti-CD47 antibody-treated mice. (C) Human lymphoma organ invasion was assessed by a blinded pathologist. The number of mice with disseminated lymphoma in each organ is indicated.
Anti-CD47 antibody inhibits hematogenous dissemination of primary human NHL
We extended these studies with Raji cells to next investigate the effect of anti-CD47 antibody on tumor dissemination of primary human lymphoma cells. We developed a novel localized DLBCL xenotransplant model by subcutaneous transplantation of cells from a patient with DLBCL (previously described as sample NHL1715 ) into sublethally irradiated NSG mice. Three weeks after transplantation, mice developed a palpable mass and were treated daily with 400 μg of either control mouse IgG or anti-CD47 antibody. Similar to Raji-engrafted mice, anti-CD47 antibody decreased the rate of tumor growth compared with control; however, this effect was not statistically significant (Figure 4A). Antibody treatment was administered for only 11 days, at which point mice were killed because of large tumor masses. Tumor dissemination to secondary sites was first investigated by MRI and gross pathology, which showed no disease outside of the primary subcutaneous transplant site (data not shown). However, analysis of the bone marrow and peripheral blood demonstrated hematogenous spread of human CD45+CD19+ DLBCL cells in controls, which was completely inhibited in anti-CD47 antibody-treated mice (Figure 4B-D).
Anti-CD47 antibody inhibits hematogenous dissemination of primary human NHL. (A) NSG mice were transplanted subcutaneously with bulk cells from a primary human DLBCL patient (NHL7, supplemental Table 1). Mice with a palpable mass (3 weeks after transplantation) were treated with daily injections of control IgG or anti-CD47 antibody for 11 days. Anti-CD47 antibody treatment had no effect on decreasing tumor volume compared with control IgG (P = .49, 2-way ANOVA). Data are mean ± SD. The presence of human CD45+CD19+ lymphoma cells in the bone marrow (B-C) and peripheral blood (D) of these mice after treatment (treatment day 11, posttransplantation day 32) was analyzed by flow cytometry. (C-D) Statistical analysis was calculated by Fisher exact test measuring engraftment versus no engraftment. ***P < .0005.
Anti-CD47 antibody inhibits hematogenous dissemination of primary human NHL. (A) NSG mice were transplanted subcutaneously with bulk cells from a primary human DLBCL patient (NHL7, supplemental Table 1). Mice with a palpable mass (3 weeks after transplantation) were treated with daily injections of control IgG or anti-CD47 antibody for 11 days. Anti-CD47 antibody treatment had no effect on decreasing tumor volume compared with control IgG (P = .49, 2-way ANOVA). Data are mean ± SD. The presence of human CD45+CD19+ lymphoma cells in the bone marrow (B-C) and peripheral blood (D) of these mice after treatment (treatment day 11, posttransplantation day 32) was analyzed by flow cytometry. (C-D) Statistical analysis was calculated by Fisher exact test measuring engraftment versus no engraftment. ***P < .0005.
CD47 is increased on disseminated lymphoma cells compared with primary lesions
CD47 binds several ligands, including integrins, thrombospondin-1, and SIRPα, which regulates functions of cell adhesion, migration, immune modulation, and phagocytic evasion.14 We previously showed that blockade of the CD47-SIRPα interaction eliminates tumors through induction of phagocytosis.19,20 From this, we postulated that anti-CD47 antibody eliminated circulating lymphoma cells by inducing their phagocytosis, leading to the testable hypothesis that higher levels of CD47 are required for lymphoma cells to evade perivascular macrophages to disseminate and invade secondary sites.
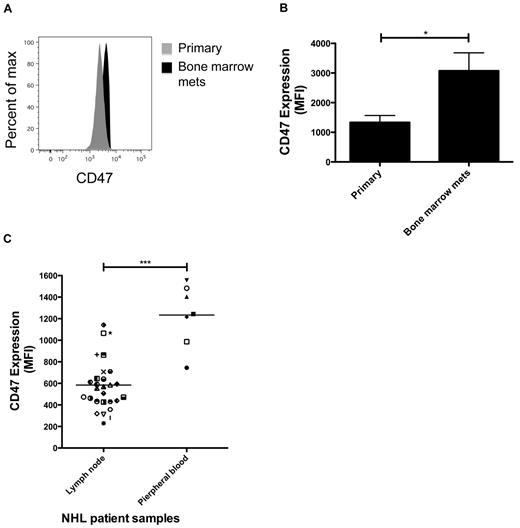
To test this hypothesis, we analyzed CD47 expression on lymphoma cells from both the primary subcutaneous and secondary bone marrow sites in NSG mice engrafted subcutaneously with primary DLBCL. Average CD47 expression was increased on disseminated cells in the bone marrow ∼ 2-fold compared with cells from the primary site (Figure 5A-B). To determine whether this finding was recapitulated in human lymphoma patients, CD47 expression was examined on a panel of patient samples from various NHL subtypes (supplemental Table 1), with either primary nodal disease or hematogenous dissemination to the peripheral blood. Peripheral blood lymphoma cells exhibited > 2-fold increased expression of CD47 protein compared with cells isolated from lymph nodes (Figure 5C), suggesting that lymphoma dissemination is associated with increased CD47 expression. Although higher CD47 expression was observed on peripheral blood compared with lymph node-resident lymphoma cells, this difference may not only be the result of dissemination but the result of intrinsic differences between NHL disease subtypes, as lymph node and peripheral blood lymphoma cells isolated from the same patient were not available for analysis.
CD47 expression is increased on disseminated lymphoma cells compared with primary lesions. (A) DLBCL cells from patient sample NHL7 were engrafted subcutaneously into NSG mice where they eventually disseminated to the bone marrow. Three weeks after transplantation, engrafted human lymphoma cells from the primary subcutaneous injection site and from secondary bone marrow disease were profiled for CD47 expression by flow cytometry (representative plot shown). CD47 expression was analyzed on human CD45+CD19+ cells. (B) CD47 expression was increased on lymphoma cells from the primary engraftment site compared with bone marrow metastases (P = .026, t test) when a panel of engrafted mice was profiled (n = 3 in each group). Data are mean ± SD. (C) When analyzing a panel of NHL patient samples, CD47 protein expression was increased on lymphoma cells from peripheral blood compared with lymph node. Normalized mean expression (and range) was determined for lymph node 584.0 (229.5-1142.0) and peripheral blood 1234.0 (745.0-1556). Each symbol represents a distinct NHL sample (supplemental Table 1). *P < .05. ***P < .0005.
CD47 expression is increased on disseminated lymphoma cells compared with primary lesions. (A) DLBCL cells from patient sample NHL7 were engrafted subcutaneously into NSG mice where they eventually disseminated to the bone marrow. Three weeks after transplantation, engrafted human lymphoma cells from the primary subcutaneous injection site and from secondary bone marrow disease were profiled for CD47 expression by flow cytometry (representative plot shown). CD47 expression was analyzed on human CD45+CD19+ cells. (B) CD47 expression was increased on lymphoma cells from the primary engraftment site compared with bone marrow metastases (P = .026, t test) when a panel of engrafted mice was profiled (n = 3 in each group). Data are mean ± SD. (C) When analyzing a panel of NHL patient samples, CD47 protein expression was increased on lymphoma cells from peripheral blood compared with lymph node. Normalized mean expression (and range) was determined for lymph node 584.0 (229.5-1142.0) and peripheral blood 1234.0 (745.0-1556). Each symbol represents a distinct NHL sample (supplemental Table 1). *P < .05. ***P < .0005.
Anti-CD47 antibody inhibits chemokine-mediated migration of lymphoma cells but does not affect integrin-mediated adhesion
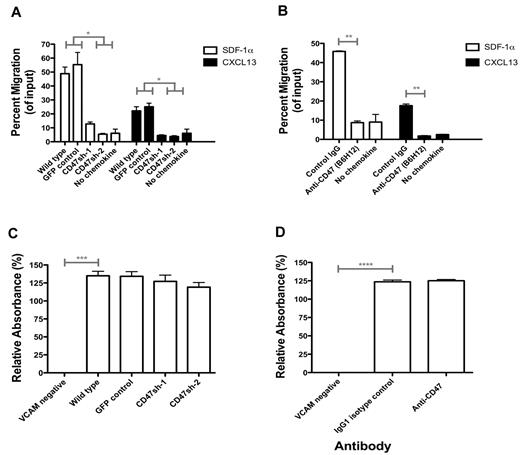
In addition to phagocytosis, CD47 has been implicated in integrin-mediated adhesion and cell migration,14 which we investigated as potential mechanisms for regulating lymphoma dissemination. First, control and CD47 knockdown Raji cells were assessed for their ability to migrate toward known lymphoma chemoattractants (SDF-1α17,18 and CXCL1317,18 ) in a transwell migration assay in vitro. Knockdown Raji cells exhibited decreased migration in response to both SDF-1α and CXCL13 compared with control cells (Figure 6A). Migration differences between Raji cell lines were not the result of differential expression of SDF-1α and CXCL13 receptors (CXCR4 and CXCR5, respectively) as all cell lines expressed similar levels of both (supplemental Figure 2). We next investigated the effect of anti-CD47 antibody blockade on Raji cell migration. Compared with isotype control, blocking anti-CD47 antibody reduced the migration of Raji cells to both SDF-1α and CXCL13 (Figure 6B) in a dose-dependent manner (supplemental Figure 3).
Anti-CD47 antibody inhibits chemokine-mediated migration of lymphoma but does not affect integrin-mediated adhesion. (A) Raji cells engineered with reduced CD47 expression levels (as in Figure 1) were assessed in a transwell migration assay with chemokines, either human SDF-1α (10nM) or CXCL13 (1 μg/mL) for 3 hours at 37°C. (B) Wild-type Raji cells were preincubated with the indicated antibodies at 10 μg/mL for 20 minutes and then assessed in a transwell migration assay for 3.5 hours at 37°C with the indicated chemokine. (A-B) The percentage of migrating cells relative to input was determined. Experiments were performed in duplicate and repeated. (C) Raji cell subclones were incubated on plates coated with human VCAM at 1 μg/mL unless indicated (VCAM negative = BSA-coated plates). (D) Raji cells were incubated in similar conditions to panel C with the indicated antibodies at 10 μg/mL. Adhesion of cells was determined by measuring absorbance at 490 nm, and relative absorbance to BSA-coated plates is shown ± SEM. No differences in VCAM-coated conditions were observed in either panel C or D. *P < .05 (t test). **P < .01 (t test). ***P < .005 (t test). ****P < .0001 (t test).
Anti-CD47 antibody inhibits chemokine-mediated migration of lymphoma but does not affect integrin-mediated adhesion. (A) Raji cells engineered with reduced CD47 expression levels (as in Figure 1) were assessed in a transwell migration assay with chemokines, either human SDF-1α (10nM) or CXCL13 (1 μg/mL) for 3 hours at 37°C. (B) Wild-type Raji cells were preincubated with the indicated antibodies at 10 μg/mL for 20 minutes and then assessed in a transwell migration assay for 3.5 hours at 37°C with the indicated chemokine. (A-B) The percentage of migrating cells relative to input was determined. Experiments were performed in duplicate and repeated. (C) Raji cell subclones were incubated on plates coated with human VCAM at 1 μg/mL unless indicated (VCAM negative = BSA-coated plates). (D) Raji cells were incubated in similar conditions to panel C with the indicated antibodies at 10 μg/mL. Adhesion of cells was determined by measuring absorbance at 490 nm, and relative absorbance to BSA-coated plates is shown ± SEM. No differences in VCAM-coated conditions were observed in either panel C or D. *P < .05 (t test). **P < .01 (t test). ***P < .005 (t test). ****P < .0001 (t test).
Regulation of lymphoma adhesion by CD47 was investigated using an in vitro static adhesion assay of Raji cells on VCAM, which plays an essential role in integrin-mediated adhesion, mobilization of hematopoietic cells, and tumor metastasis.23,24 Indeed, Raji cells express the VCAM receptor, VLA-4 (also known as α4β1 integrin; supplemental Figure 2), suggesting that the VCAM/VLA-4 pathway could play a role in mediating Raji cell adhesion. Whereas Raji cells adhered to human VCAM-coated plates in vitro, no differences were observed in adhesion of CD74 knockdown Raji cells compared with controls (Figure 6C). In addition, wild-type Raji cells incubated with anti-CD47 antibody did not modulate VCAM-mediated adhesion compared with isotype control (Figure 6D).
Macrophages are necessary for anti-CD47 antibody-mediated inhibition of lymphoma dissemination
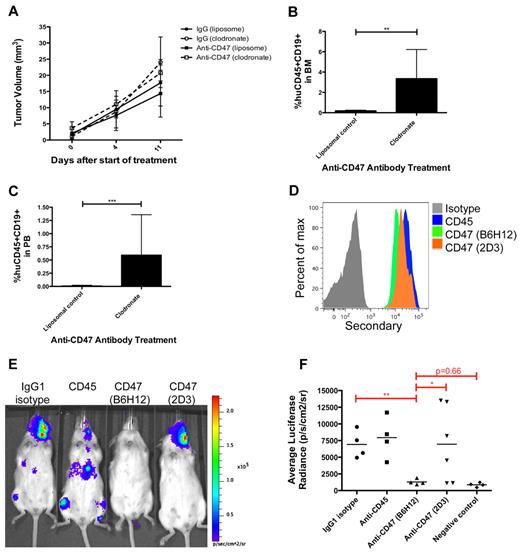
We previously demonstrated that macrophages are the principal immune effector cell type mediating anti-CD47 antibody-induced phagocytosis of human NHL cells in vitro and in vivo.15 Therefore, we investigated whether macrophages also mediated anti-CD47 antibody inhibition of lymphoma dissemination. Primary DLBCL cells were transplanted subcutaneously into NSG mice and divided into control IgG and anti-CD47 antibody treatment groups that were further separated into cohorts receiving either liposomal clodronate, a bisphosphonate that depletes phagocytes, or a liposomal control. Clodronate was used as previously described,15 resulting in an average reduction of F4/80+ macrophages to 11% of liposomal control-treated mice (data not shown). Mice were followed serially for tumor volume and the presence of lymphoma in the bone marrow and peripheral blood. Macrophage depletion partially abrogated the minimal effect of anti-CD47 antibody in reducing primary tumor volume; however, this effect was not statistically significant (Figure 7A). No difference in tumor volume was observed with or without macrophage depletion in control IgG-treated mice (Figure 7A). In contrast, macrophage depletion completely abrogated anti-CD47 antibody-mediated inhibition of hematogenous lymphoma dissemination (Figure 7B-C), as evidenced by lymphoma cells in the bone marrow and peripheral blood, but had no effect on dissemination in mice treated with control IgG (supplemental Figure 4).
Inhibition of lymphoma dissemination by anti-CD47 antibody requires macrophages and blockade of SIRPα. (A) Subcutaneously engrafted DLBCL mice with palpable flank tumors (3 weeks after transplantation) were treated with control IgG or anti-CD47 antibody for 11 days in the presence of liposomal clodronate or liposomal control. Tumor volume was assessed and indicated that clodronate administration partially abrogated anti-CD47 antibody-mediated reduction in tumor volume compared with control (P = .25). No difference was observed between clodronate and liposomal control administration in control IgG-treated mice (P = .62). In mice treated with liposomal control, anti-CD47 antibody partially reduced tumor volume compared with control IgG treatment, but this difference was not statistically significant (P = .49). Statistical analysis was conducted with a 2-way ANOVA test. (B-C) The presence of lymphoma in the bone marrow (B) and peripheral blood (C) of anti-CD47 antibody-treated mice was assessed in respective treatment cohorts. Lymphoma dissemination to the bone marrow and peripheral blood was observed in anti-CD47 antibody-treated mice receiving clodronate macrophage depletion, but not in controls. Statistical analysis was conducted with Fisher exact test. Each treatment group consisted of 3 or 4 mice. (D) Luciferase-labeled Raji cells were coated ex vivo with the indicated primary antibodies, labeled with a fluorescently conjugated secondary antibody, and analyzed for binding by flow cytometry. (E) Raji cells incubated ex vivo with the indicated antibodies were transplanted intravenously into adult NSG mice. One week later, mice were analyzed by bioluminescent imaging for engraftment, and representative mice are shown. (F) The luciferase signal was determined for a cohort of mice transplanted with ex vivo antibody-coated cells where each point represents a single mouse. Blocking anti-CD47 (B6H12.2) antibody inhibited tumor engraftment compared with the nonblocking anti-CD47 (2D3) antibody and antibody controls. Statistical analysis was conducted with Fisher exact test. Data are mean ± SD. *P < .05. **P < .005. ***P < .0005.
Inhibition of lymphoma dissemination by anti-CD47 antibody requires macrophages and blockade of SIRPα. (A) Subcutaneously engrafted DLBCL mice with palpable flank tumors (3 weeks after transplantation) were treated with control IgG or anti-CD47 antibody for 11 days in the presence of liposomal clodronate or liposomal control. Tumor volume was assessed and indicated that clodronate administration partially abrogated anti-CD47 antibody-mediated reduction in tumor volume compared with control (P = .25). No difference was observed between clodronate and liposomal control administration in control IgG-treated mice (P = .62). In mice treated with liposomal control, anti-CD47 antibody partially reduced tumor volume compared with control IgG treatment, but this difference was not statistically significant (P = .49). Statistical analysis was conducted with a 2-way ANOVA test. (B-C) The presence of lymphoma in the bone marrow (B) and peripheral blood (C) of anti-CD47 antibody-treated mice was assessed in respective treatment cohorts. Lymphoma dissemination to the bone marrow and peripheral blood was observed in anti-CD47 antibody-treated mice receiving clodronate macrophage depletion, but not in controls. Statistical analysis was conducted with Fisher exact test. Each treatment group consisted of 3 or 4 mice. (D) Luciferase-labeled Raji cells were coated ex vivo with the indicated primary antibodies, labeled with a fluorescently conjugated secondary antibody, and analyzed for binding by flow cytometry. (E) Raji cells incubated ex vivo with the indicated antibodies were transplanted intravenously into adult NSG mice. One week later, mice were analyzed by bioluminescent imaging for engraftment, and representative mice are shown. (F) The luciferase signal was determined for a cohort of mice transplanted with ex vivo antibody-coated cells where each point represents a single mouse. Blocking anti-CD47 (B6H12.2) antibody inhibited tumor engraftment compared with the nonblocking anti-CD47 (2D3) antibody and antibody controls. Statistical analysis was conducted with Fisher exact test. Data are mean ± SD. *P < .05. **P < .005. ***P < .0005.
Inhibition of lymphoma dissemination is dependent on blockade of the CD47-SIRPα interaction
Lastly, we determined whether anti-CD47 antibody-mediated inhibition of NHL dissemination was specifically dependent on blockade of the CD47-SIRPα interaction. Luciferase-labeled Raji cells were incubated ex vivo with antibody controls or an anti-CD47 antibody capable or not capable of blocking the CD47-SIRPα interaction (clones B6H12.2 and 2D3, respectively). Both anti-CD47 antibodies bound Raji cells similarly (Figure 7D), consistent with prior results.15 Antibody-coated Raji cells were then transplanted intravenously into adult NSG mice and analyzed for lymphoma engraftment by bioluminescent imaging to determine the effect of antibody coating on disease dissemination. Consistent with our prior report,15 the blocking anti-CD47 antibody (B6H12.2) inhibited tumor dissemination in 100% of mice compared with IgG1 isotype and anti-CD45 antibody controls (Figure 7E-F). In contrast, the nonblocking anti-CD47 antibody (2D3) had a minimal effect on lymphoma dissemination compared with antibody controls, as 66% (4 of 6) mice developed detectable engraftment (Figure 7E-F).
Discussion
We report here that extranodal dissemination of lymphoma requires CD47 and is inhibited by blockade of the CD47-SIRPα. We developed 2 in vivo models of human NHL (Burkitt lymphoma and DLBCL), whereby disease from primary subcutaneous sites disseminated to multiple secondary sites. Although treatment of these mice with a blocking anti-CD47 antibody had a minimal effect on reducing disease at the primary site, anti-CD47 antibody robustly inhibited dissemination of disease to secondary sites in both models. CD47 expression was required for NHL cells to spread in vivo as knockdown of CD47 decreased the invasive potential of NHL cells; furthermore, increased CD47 expression was observed on disseminated NHL cells compared with cells at the primary site. These results demonstrate that CD47 is required for NHL dissemination and that this secondary spread can be therapeutically targeted with a blocking anti-CD47 antibody.
The migratory role of CD47 is conserved in normal physiology and tumor metastasis
We hypothesize that dissemination of lymphoma cells regulated by CD47 resembles the physiologic role of CD47 in the normal migration of hematopoietic cells. Indeed, CD47 plays a migratory role in multiple hematopoietic cell types. For example, CD47 is required for granulocyte and T-cell recruitment to sites of infection25,26 and for dendritic cell trafficking to secondary lymphoid organs.27 In mouse melanoma, CD47 has been implicated in tumor metastasis to bone.28 The physiologic and tumorigenic role of CD47 in migration may be the result of several distinct mechanisms given that CD47 interacts with several proteins, including SIRPα, integrins, and thrombospondin-1.14,29 First, CD47-mediated regulation of cell migration may be integrin- or chemokine-dependent as CD47 has been shown to bind several integrins, including αvβ3, αiibβ3, and α2β1.14,30 In this way, CD47 may mediate normal and tumor cell migration through integrin activation and cell attachment. We show here (Figure 6A-B) that down-regulation of CD47 expression as well as administration of a blocking anti-CD47 antibody inhibited chemotaxis of lymphoma cells to 2 chemokines (SDF-1α and CXCL13) that have been shown to trigger integrin activation of LFA-1, α4β1, and αvβ3.31-33 In another study, antibodies to CD47 and αvβ3 inhibited in vitro chemotaxis of melanoma and prostate cancer cell lines.34 Although CD47 plays a role in chemokine-mediated migration of lymphoma cells, we did not observe a role for CD47 in integrin-mediated adhesion. Modulation of CD47 did not impact VCAM/VLA-4 adhesion (Figure 6B-C); however, CD47 may play a role in other integrin-mediated adhesion pathways.
Apart from integrins, the function of CD47 in regulating cell migration may be mediated by inhibition of phagocytosis through ligation of SIRPα on phagocytes. We have previously shown that mobilization of mouse HSCs from the bone marrow to the periphery requires transient up-regulation of CD47.35 During mobilization, HSCs must navigate through and past perviascular and sinusoidal macrophages in blood vessels, lymph nodes, and other tissues, raising the possibility that increased CD47 expression is necessary to evade phagocytosis during the migratory process. We show that this potential mechanism is probably conserved in tumor cell migration based on 5 lines of evidence. First, lymphoma cells engineered with reduced CD47 expression exhibited decreased dissemination compared with wild-type cells (Figure 1). Second, antibody blockade of CD47 inhibited lymphoma dissemination to secondary sites in 2 different models (Figures 2,Figure 3–4). Third, lymphoma cells that disseminated to secondary sites expressed higher levels of CD47 compared with cells at the primary site (Figure 5), and this increased expression was required for dissemination (Figure 1). Fourth, macrophages played an essential role in lymphoma dissemination in a CD47-dependent manner. When macrophages were depleted, the anti-CD47 antibody-mediated inhibition of lymphoma dissemination was completely abrogated (Figure 7A-C). Fifth, inhibition of dissemination was observed only with an anti-CD47 antibody capable of blocking the CD47-SIRPα interaction (clone B6H12.2), whereas a nonblocking anti-CD47 antibody (clone 2D3) had no effect (Figure 7D-F).
Anti-CD47 antibody therapy is effective in preventing NHL dissemination
Human lymphomas display a wide spectrum of growth patterns, from limited to widespread invasion. In NHL, subtypes are partly classified in terms of anatomic distribution: nodal versus extranodal lymphomas. These differences often correlate with clinical prognosis as extranodal lymphomas resident in the brain, testicles, and eye generally confer a worse prognosis and are less responsive to standard treatment.36 In addition, advanced stage, which factors in degree of both anatomic involvement and extranodal involvement, confers a worse clinical prognosis across multiple NHL subtypes.37 Many of the aggressive NHL subtypes (DLBCL, Burkitt lymphoma, and peripheral T-cell lymphoma) that confer a poor prognosis also have a high tendency to disseminate.
Of specific clinical concern with dissemination is CNS involvement, which in NHL can occur in up to 30% of some lymphoma subtypes38 and carries an extremely poor prognosis despite the administration of aggressive therapies. Although combination chemotherapy with the anti-CD20 antibody rituximab has significantly improved outcomes in NHL,39 it is well known that rituximab and many standard chemotherapeutic agents (cyclophosphamide, doxorubicin, vincristine, and prednisone) do not effectively penetrate the blood-brain barrier and reach the CNS.40 Moreover, the addition of rituximab to standard therapy has not shown a definitive reduction in CNS involvement.41-44 Thus, intravenous and/or intrathecal administration of cytotoxic agents is routinely used for prophylaxis against secondary CNS disease and is often associated with significant systemic toxicity.
In the present study, we demonstrate that anti-CD47 antibody is able to prevent the dissemination of NHL from its primary site in 2 xenograft models. Although anti-CD47 antibody treatment had only a partial effect on debulking the primary solid tumor site (Figures 2A and 4A), antibody therapy was completely effective at inhibiting or eliminating invasion of lymphoma cells to major organs, including the CNS (Figures 2 and 3). These preclinical results suggest that anti-CD47 antibody may be an effective therapy in the prophylaxis of CNS lymphoma in contrast to standard antibody therapy with rituximab. In addition, we have previously shown that anti-CD47 antibody has a minimal preclinical toxicity profile15,19,22 and thus could also be an attractive alternative to CNS prophylaxis with toxic chemotherapies. Lastly, anti-CD47 antibody could be used as an adjunct in combination with standard therapy. For example, cytoreduction of bulky lymph node disease could be achieved with standard chemotherapy or radiation, whereas administration of anti-CD47 antibody could be used to either prevent disease spread in early-stage NHL or for the treatment of late-stage NHL where lymphoma has already disseminated to other organs.
In conclusion, we find that CD47 plays an essential role in the dissemination of human lymphoma and that a blocking anti-CD47 antibody may have clinical utility in preventing or eliminating disease spread. Given the shared role of CD47 in the biology and pathogenesis of many cancers, including solid tumors, targeting of CD47 may be a potential therapeutic strategy to prevent dissemination and metastasis of multiple tumor types.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Jonathan Irish and June Helen Myklebust for providing NHL samples and corresponding clinical information, Adriane Mosley for animal handling, and Theresa Storm and Feifei Zhao for excellent laboratory management.
This work was supported by the National Institutes of Health (grant P01CA139490, I.L.W.) and the Ludwig Foundation. M.P.C. and C.T. are supported by a medical fellowship from the Howard Hughes Medical Institute. M.P.C. is supported by the National Cancer Institute, DHHS (PHS grant CA09302). R.K.P. is supported by an ASCO Young Investigator Award and California Breast Cancer Research Program Fellowship. R.M. holds a Career Award for Medical Scientists from the Burroughs Wellcome Fund.
National Institutes of Health
Authorship
Contribution: M.P.C., C.T., R.K.P., and R.C. performed the experiments and analyzed results; and M.P.C., R.M., and I.L.W. designed experiments and wrote the manuscript.
Conflict-of-interest disclosure: I.L.W., M.P.C., and R.M. have filed US Patent Application Serial No. 12/321,215 entitled “Methods For Manipulating Phagocytosis Mediated by CD47.” The remaining authors declare no competing financial interests.
Correspondence: Mark P. Chao, Stanford Institute for Stem Cell Biology and Regenerative Medicine, Lorry Lokey Research Bldg, 256 Campus Dr, Rm G3005, Stanford, CA 94305; e-mail: mpchao@stanford.edu.
References
Author notes
R.M. and I.L.W. contributed equally to this study.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal