Abstract
Inherited deficiency of major histocompatibility complex (MHC) class II molecules impairs antigen presentation to CD4+ T cells and results in combined immunodeficiency (CID). Autosomal-recessive mutations in the RFXANK gene account for two-thirds of all cases of MHC class II deficiency. We describe here the genetic, clinical, and immunologic features of 35 patients from 30 unrelated kindreds from North Africa sharing the same RFXANK founder mutation, a 26-bp deletion called I5E6-25_I5E6 + 1), and date the founder event responsible for this mutation in this population to approximately 2250 years ago (95% confidence interval [CI]: 1750-3025 years). Ten of the 23 patients who underwent hematopoietic stem cell transplantation (HSCT) were cured, with the recovery of almost normal immune functions. Five of the patients from this cohort who did not undergo HSCT had a poor prognosis and eventually died (at ages of 1-17 years). However, 7 patients who did not undergo HSCT (at ages of 6-32 years) are still alive on Ig treatment and antibiotic prophylaxis. RFXANK deficiency is a severe, often fatal CID for which HSCT is the only curative treatment. However, some patients may survive for relatively long periods if multiple prophylactic measures are implemented.
Introduction
Major histocompatibility complex (MHC) class II expression deficiency is a rare primary immunodeficiency (PID; MIM 209920) that is inherited as an autosomal-recessive trait. The lack of MHC class II expression leads to impaired antigen presentation by HLA-DR, HLA-DQ, and HLA-DP molecules on APCs such as dendritic cells and macrophages.1 This leads to defective CD4 T-cell development and function and a lack of Th cell–dependent Ab production by B cells. Hypogammaglobulinemia and low CD4 T-cell counts are found in most patients.2 Impaired T- and B-cell immunity results in susceptibility to a broad range of viral, bacterial, fungal, and protozoan infections.3-5 Infections generally occur in the first year of life and involve the respiratory and gastrointestinal tracts.2,4,5 Respiratory insufficiency, failure to thrive, and organ dysfunctions ensue, frequently causing death in childhood. Hematopoietic stem cell transplantation (HSCT) is the only available curative treatment, despite the high risk of toxicity in this particular setting.4,6-12
The MHC locus itself is intact in patients with MHC class II expression deficiency. Impaired transcription of the MHC class II genes has been shown to account for the lack of expression of DR, DP, and DQ MHC class II proteins on APCs.1 Somatic fusion experiments led to the description of 4 complementation groups (A, B, C, and D).1 Four disease-causing genes were subsequently identified and shown to encode regulatory factors controlling the transcription of MHC class II genes: CIITA for group A (MIM 600005),13 RFXANK for group B (MIM 603200),14 RFX5 for group C (MIM 601863),15 and RFXAP for group D (MIM 601861).16 The RFXANK, RFX5, and RFXAP proteins are 3 subunits of the ubiquitously expressed RFX complex, which binds directly to the promoters of all MHC class II genes and associates with other pleiotropic factors to form the MHC class II enhanceosome. CIITA is an inducible factor that controls MHC class II expression by binding the RFX complex and triggering transcription.17
More than 150 patients with MHC class II deficiency have been reported to date.2-12,14,15,18-39 Approximately two-thirds of these patients have complementation group B deficiencies, with most of the families being of North-African descent (Algeria, Morocco, and Tunisia).2,5,7,9,10,14,18,27-29,31,32,37 A recurrent mutation of the RFXANK gene, a 26-bp deletion at the boundary between intron 5 and exon 6 called I5E6-25_I5E6 + 1 (also known as 752delG-25), has been found in > 90% of North-African families and in more than one-half of all families with MHC II deficiency.28,37 Eight other mutations in RFXANK have been described in patients of different ethnic origins (The Netherlands, Italy, France, Spain, Tunisia, Turkey, and Saudi Arabia).14,27-29,31,32,40 Thirty families with group B MHC class II deficiency investigated at our center carry the I5E6-25_I5E6 + 1 mutation. We investigated whether this homozygous genotype confers a homogeneous disease phenotype by studying the clinical and immunologic features of a group of 35 North-African patients originating from 30 families and carrying a homozygous I5E6-25_I5E6 + 1 deletion.
Methods
Patients
Thirty-five patients with MHC class II expression deficiency born between 1970 and 2008 were referred to Necker Hospital in Paris between 1977 and 2010. Diagnosis was based on personal and family history and clinical and immunologic signs. Informed consent for participation in the study was obtained from adult patients or from the parents of children in accordance with the Declaration of Helsinki. All studies were approved by the Institutional Review Board of Necker Hospital. A homozygous I5E6-25_I5E6 + 1 deletion in the RFXANK gene was clearly demonstrated in all of the patients studied.28 This 26-bp deletion encompassing the intron 5/exon 6 boundary was detected by PCR amplification with the following primers: forward: TTg gCA gCA CTg ATA ggg g; reverse: CCA gCA gAC ACA gCC AAA AC. Genomic DNA RFXANK were amplified, sequenced, and analyzed on an ABI Prism 3700 apparatus (BigDye Terminator sequencing kit; Applied Biosystems).
Dating of the mutation
Founder-effect analyses were carried out on a subset of 24 available, apparently unrelated patients (19 from Algeria, 2 from Morocco, and 3 from Tunisia). Genotypes were obtained for > 250 000 single-nucleotide polymorphisms (SNPs) from the Affymetrix GeneChip Human Mapping 250K Nsp Array. SNPs with a 100% call rate were scanned for continuous stretches of homozygosity upstream and downstream from the RFXANK locus on chromosome 19p12. Pairwise comparisons within each mutation group revealed the limits of the longest shared haplotype and the positions of subsequent recombination break points. The likelihood-based ESTIAGE method was used to estimate the age of the most recent common ancestor (MRCA) for each mutation from the observed shared haplotypes; the recombination rates and haplotype frequencies were provided by the HapMap Project.41-43
Immunologic investigations
Lymphocyte subsets were evaluated by routine flow cytometry with the norms described by Shearer et al.44 Serum levels of IgM, IgA, and IgG and subclasses were assessed by standard nephelometry techniques. Total IgG Ab levels against tetanus toxoid and diphtheria, titers of antibodies against viruses and bacteria, including multiple pneumococcal serotypes (23 serotypes), and levels of IgG against Haemophilus influenzae PRP antigens were assessed by standard ELISA techniques. The expression of MHC class II and class I molecules by T and B cells was assessed and in vitro lymphocyte proliferation assays were carried out with cells from the patients, as described previously.2,7
Histology and immunohistochemistry
Rectal biopsies from patients and healthy controls were selected from the archives of the Department of Pathology at Necker-Enfants Malades Hospital (Paris, France). All cases were reviewed by 2 expert hematopathologists and the diagnoses were confirmed. Formalin-fixed paraffin-embedded tissue blocks were cut in 4-mm sections. Antigen retrieval was realized in citrate. The sections were then deparaffinized and rehydrated. Antibody used: HLA-DR-CMHII (Dako; Clone L243, dilution 1/200). Immunoperoxidase labeling was performed with the automatized technique by Menarini Diagnostics system. Pictures were realized with Leica Microscope at magnification ×200, Sony Camera DXC-650P and Tribun software.
Results
Epidemiologic features and founder-effect analysis
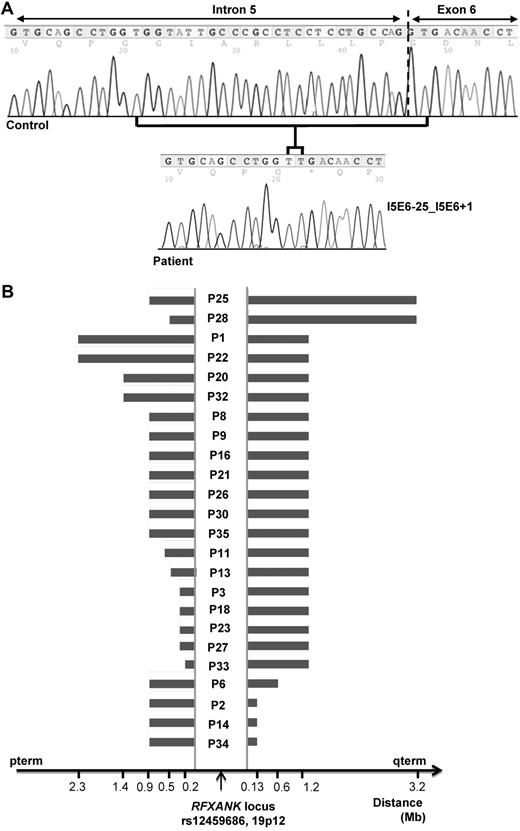
We studied 35 patients (16 male and 19 female patients) from 30 kindreds with RFX-ANK deficiency. This series includes 22 patients described in previous reports (belonging to 19 kindreds)2,7,10,28,31 and 13 newly diagnosed patients (belonging to 11 kindreds). All of the patients originated from North Africa (Algeria, 71%; Morocco, 14%; and Tunisia, 14%; Table 1). Most patients and their families were living in France, with the exception of 3 patients (P28, P29, and P32), who were living in their countries of origin. In all 30 probands, diagnosis was based on detection of the homozygous 26-bp deletion (I5E6-25_I5E6 + 1) in RFXANK accompanied by defective MHC class II antigen expression on monocytes and B cells (Figure 1A and supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Five relatives were also found to be homozygous for this mutation. The parents were consanguineous in 27 of the 30 kindreds. In the remaining 3 kindreds, the parents originated from the same country but were not known to be consanguineous. There were 23 sporadic cases (65%) and 12 (35%) familial cases (7 kindreds). An analysis of Affymetrix 250K Nsp Array data showed that patients carrying the I5E6-25_I5E6 + 1 deletion had a common homozygous haplotype around the RFXANK gene. The largest common haplotype identified was that between patients P25 and P28, extending > 3.7 megabases (corresponding to 160 SNPs). The smallest common haplotype was that between P33 and P34, encompassing 470 kb (corresponding to 11 SNPs; Figure 1B). The ESTIAGE method estimated the age of the MRCA to 90 generations (95% confidence interval [CI]: 68-121 generations). Assuming a generation time of 25 years, the MRCA of the patients therefore lived 2250 years ago (95% CI: 1700-3025 years).
Clinical characteristics of the 35 patients
| Kindred . | Patient . | Sex . | Origin . | Age at diagnosis . | Status . | Age at last follow-up, y . | Protracted diarrhea . | ENT infection . | Pneumonia . | Failure to thrive . | Severe viral Inf. . | P liver disease . | Cholangitis . | AI cytopenia . | Reference(s) . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | P1 | F | Algeria | 4 y | Dead | 10 | + | + | + | + | - | + | + | + | 2,31 |
| 2 | P2 | M | Algeria | 2 mo | HSCT/dead | 4 | + | + | + | + | + | + | + | - | 2,7,31 |
| 3 | P3 | F | Algeria | 3 mo | HSCT/dead | 6 | + | + | + | + | + | + | + | - | 2,7,31 |
| 3 | P4 | M | Algeria | 3 mo | Dead | 1 | - | + | + | + | + | - | - | - | 2,31 |
| 4 | P5 | M | Algeria | 3 y | HSCT/alive | 16 | + | + | + | + | - | - | - | - | 10,31 |
| 5 | P6 | M | Algeria | 5 y | Alive | 32 | + | + | + | + | + | + | - | + | 2,31 |
| 6 | P7 | F | Tunisia | 5 y | Dead | 5 | + | + | + | + | + | - | - | - | 2,31 |
| 7 | P8 | M | Algeria | 8 y | Dead | 17 | + | + | + | + | - | + | + | + | 2,31 |
| 8 | P9 | M | Algeria | 18 mo | HSCT/dead | 8 | + | + | + | + | - | - | - | - | 31 |
| 9 | P10 | M | Algeria | 2 y | HSCT/dead | 9 | + | + | + | + | + | - | - | + | 2,7,31 |
| 9 | P11 | M | Algeria | 1 mo | HSCT/dead | 1 | - | - | - | - | + | - | - | - | 2,7,31 |
| 10 | P12 | F | Morocco | 2 y | Dead | 2 | + | + | - | + | + | - | - | + | This report |
| 10 | P13 | F | Morocco | 1 y | HSCT/dead | 2 | + | + | + | + | - | - | - | - | 31 |
| 12 | P14 | M | Algeria | 2 y | Alive | 23 | + | + | + | + | - | + | - | - | 2,31 |
| 12 | P15 | F | Algeria | 1 mo | HSCT/dead | 1 | + | + | + | - | + | - | - | - | This report |
| 13 | P16 | F | Algeria | 1 y | HSCT/alive | 22 | + | + | + | + | - | + | - | - | 2,7,31 |
| 14 | P17 | F | Algeria | 5 mo | HSCT/alive | 18 | + | + | + | - | - | - | - | - | 7,31 |
| 15 | P18 | F | Algeria | 6 mo | HSCT/alive | 23 | + | + | + | + | - | + | - | - | 2,7,10,31 |
| 16 | P19 | F | Tunisia | 2 y | HSCT/alive | 26 | + | + | + | + | - | + | - | + | 2,7,31 |
| 17 | P20 | F | Tunisia | 1 mo | HSCT/dead | 5 | - | - | - | - | + | - | - | ND | 2,7,31 |
| 19 | P21 | M | Algeria | 2 y | HSCT/dead | 4 | + | + | + | + | + | + | - | - | 2,31 |
| 20 | P22 | F | Algeria | 1 y | HSCT/alive | 19 | + | + | + | + | - | + | - | - | 2,31 |
| 27 | P23 | M | Algeria | 4 y | HSCT/alive | 16 | + | + | + | + | - | - | - | - | 10,31 |
| 27 | P24 | M | Algeria | At birth | HSCT/alive | 4 | - | - | - | - | - | - | - | - | This report |
| 30 | P25 | F | Morocco | 6 mo | HSCT/dead | 4 | + | + | + | + | + | - | - | - | This report |
| 31 | P26 | M | Algeria | 9 mo | Alive | 6 | - | + | + | + | + | - | - | + | This report |
| 32 | P27 | F | Tunisia | 21 mo | HSCT/dead | 2.5 | + | - | + | + | + | - | - | + | 10 |
| 33 | P28 | F | Tunisia | 2.5 y | HSCT/dead | 4 | + | + | + | + | + | - | - | - | 10 |
| 34 | P29 | M | Morocco | 4.5 y | Alive | 9 | + | + | + | + | - | - | - | - | This report |
| 35 | P30 | F | Algeria | 7.5 y | HSCT/alive | 11 | + | + | - | + | + | + | - | - | This report |
| 36 | P31 | M | Algeria | 21 mo | HSCT/alive | 4 | + | + | - | + | + | + | - | - | This report |
| 37 | P32 | F | Algeria | 10 y | Alive | 12 | + | - | + | + | - | + | + | - | This report |
| 38 | P33 | F | Algeria | 7 mo | HSCT/alive | 2 | - | - | + | + | + | - | - | - | This report |
| 39 | P34 | F | Morocco | 5.5 y | Alive | 7 | + | + | + | - | + | + | - | - | This report |
| 40 | P35 | M | Algeria | 15 y | Alive | 16 | + | + | + | - | - | - | - | - | This report |
| Kindred . | Patient . | Sex . | Origin . | Age at diagnosis . | Status . | Age at last follow-up, y . | Protracted diarrhea . | ENT infection . | Pneumonia . | Failure to thrive . | Severe viral Inf. . | P liver disease . | Cholangitis . | AI cytopenia . | Reference(s) . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | P1 | F | Algeria | 4 y | Dead | 10 | + | + | + | + | - | + | + | + | 2,31 |
| 2 | P2 | M | Algeria | 2 mo | HSCT/dead | 4 | + | + | + | + | + | + | + | - | 2,7,31 |
| 3 | P3 | F | Algeria | 3 mo | HSCT/dead | 6 | + | + | + | + | + | + | + | - | 2,7,31 |
| 3 | P4 | M | Algeria | 3 mo | Dead | 1 | - | + | + | + | + | - | - | - | 2,31 |
| 4 | P5 | M | Algeria | 3 y | HSCT/alive | 16 | + | + | + | + | - | - | - | - | 10,31 |
| 5 | P6 | M | Algeria | 5 y | Alive | 32 | + | + | + | + | + | + | - | + | 2,31 |
| 6 | P7 | F | Tunisia | 5 y | Dead | 5 | + | + | + | + | + | - | - | - | 2,31 |
| 7 | P8 | M | Algeria | 8 y | Dead | 17 | + | + | + | + | - | + | + | + | 2,31 |
| 8 | P9 | M | Algeria | 18 mo | HSCT/dead | 8 | + | + | + | + | - | - | - | - | 31 |
| 9 | P10 | M | Algeria | 2 y | HSCT/dead | 9 | + | + | + | + | + | - | - | + | 2,7,31 |
| 9 | P11 | M | Algeria | 1 mo | HSCT/dead | 1 | - | - | - | - | + | - | - | - | 2,7,31 |
| 10 | P12 | F | Morocco | 2 y | Dead | 2 | + | + | - | + | + | - | - | + | This report |
| 10 | P13 | F | Morocco | 1 y | HSCT/dead | 2 | + | + | + | + | - | - | - | - | 31 |
| 12 | P14 | M | Algeria | 2 y | Alive | 23 | + | + | + | + | - | + | - | - | 2,31 |
| 12 | P15 | F | Algeria | 1 mo | HSCT/dead | 1 | + | + | + | - | + | - | - | - | This report |
| 13 | P16 | F | Algeria | 1 y | HSCT/alive | 22 | + | + | + | + | - | + | - | - | 2,7,31 |
| 14 | P17 | F | Algeria | 5 mo | HSCT/alive | 18 | + | + | + | - | - | - | - | - | 7,31 |
| 15 | P18 | F | Algeria | 6 mo | HSCT/alive | 23 | + | + | + | + | - | + | - | - | 2,7,10,31 |
| 16 | P19 | F | Tunisia | 2 y | HSCT/alive | 26 | + | + | + | + | - | + | - | + | 2,7,31 |
| 17 | P20 | F | Tunisia | 1 mo | HSCT/dead | 5 | - | - | - | - | + | - | - | ND | 2,7,31 |
| 19 | P21 | M | Algeria | 2 y | HSCT/dead | 4 | + | + | + | + | + | + | - | - | 2,31 |
| 20 | P22 | F | Algeria | 1 y | HSCT/alive | 19 | + | + | + | + | - | + | - | - | 2,31 |
| 27 | P23 | M | Algeria | 4 y | HSCT/alive | 16 | + | + | + | + | - | - | - | - | 10,31 |
| 27 | P24 | M | Algeria | At birth | HSCT/alive | 4 | - | - | - | - | - | - | - | - | This report |
| 30 | P25 | F | Morocco | 6 mo | HSCT/dead | 4 | + | + | + | + | + | - | - | - | This report |
| 31 | P26 | M | Algeria | 9 mo | Alive | 6 | - | + | + | + | + | - | - | + | This report |
| 32 | P27 | F | Tunisia | 21 mo | HSCT/dead | 2.5 | + | - | + | + | + | - | - | + | 10 |
| 33 | P28 | F | Tunisia | 2.5 y | HSCT/dead | 4 | + | + | + | + | + | - | - | - | 10 |
| 34 | P29 | M | Morocco | 4.5 y | Alive | 9 | + | + | + | + | - | - | - | - | This report |
| 35 | P30 | F | Algeria | 7.5 y | HSCT/alive | 11 | + | + | - | + | + | + | - | - | This report |
| 36 | P31 | M | Algeria | 21 mo | HSCT/alive | 4 | + | + | - | + | + | + | - | - | This report |
| 37 | P32 | F | Algeria | 10 y | Alive | 12 | + | - | + | + | - | + | + | - | This report |
| 38 | P33 | F | Algeria | 7 mo | HSCT/alive | 2 | - | - | + | + | + | - | - | - | This report |
| 39 | P34 | F | Morocco | 5.5 y | Alive | 7 | + | + | + | - | + | + | - | - | This report |
| 40 | P35 | M | Algeria | 15 y | Alive | 16 | + | + | + | - | - | - | - | - | This report |
ENT infection indicates ear nose and throat infections; P liver disease, progressive liver disease; AI cytopenia, autoimmune cytopenia; y, year(s); and mo, month(s).
Genetic analysis. (A) RFXANK mutation. Normal nucleotide sequence of the intron 5/exon 6 boundary of the RFXANK gene and the sequence from a patient with the homozygous 26-bp deletion I5E6-25_I5E6 + 1 (also known as 752delG-25). (B) Haplotype common to 24 unrelated patients with the homozygous 26-bp deletion in the RFXANK gene. An analysis of SNP Array 250 K data showed that patients carrying the I5E6-25_I5E6 + 1 deletion had a common homozygous haplotype around the RFXANK locus.
Genetic analysis. (A) RFXANK mutation. Normal nucleotide sequence of the intron 5/exon 6 boundary of the RFXANK gene and the sequence from a patient with the homozygous 26-bp deletion I5E6-25_I5E6 + 1 (also known as 752delG-25). (B) Haplotype common to 24 unrelated patients with the homozygous 26-bp deletion in the RFXANK gene. An analysis of SNP Array 250 K data showed that patients carrying the I5E6-25_I5E6 + 1 deletion had a common homozygous haplotype around the RFXANK locus.
Immunologic features
All 35 patients displayed a total absence of MHC class II (HLA-DR) molecule expression on monocytes (CD14+). In addition, in 25 of the 35 patients, no MHC class II molecules were detected on activated T (CD3+) and B (CD19+) cells. However, 10 of the 35 patients (29%) had residual levels of MHC class II molecules on B cells, generally after an infectious episode (n = 8). The percentage of positive cells and the density of MHC class II molecules was nonetheless lower on positive B-cell populations (< 6% of cells displaying MHC class II expression in 9 patients, with a mean fluorescence intensity 2 orders of magnitude lower than for the control) than on B cells from healthy controls. One patient (P32) with concomitant viral infection displayed MHC class II expression on 43% of B cells (CD19+), with a mean fluorescence intensity 2 orders of magnitude lower than for the control (supplemental Figure 1). The level of HLA class I molecule expression was lower than normal, to various extents, in 24 cases.
Low absolute CD4+ T-cell counts were found in 33 of the 35 patients (94%; Figure 2A). CD4+ T-cell counts were below 0.5 × 109 cells/L for 22 patients (63%) between the ages of 1 month and 15 years. The 5 patients studied showed variable decreases in the percentage of naive CD4+ T cells. CD8+ T-cell counts were low in 8 patients (23%) and high in another 9 patients (26%; Figure 2B). Natural killer cell (CD3−CD16+CD56+) counts were low in 3 of the 12 patients tested (25%). In vitro proliferative responses of T cells to mitogens were normal for all patients, but no proliferation in response to antigens was observed. B-cell immunity was also profoundly impaired. Nineteen of the 24 patients studied (79%) had normal numbers of circulating B lymphocytes, whereas the other 5 (21%) had fewer circulating B lymphocytes than normal. Before the initiation of IgG substitution therapy, 27 of 32 patients (84%) had low levels of IgG, 17 had low levels of IgA (53%), and 21 had low levels of IgM (65%). High IgM levels were found in 3 patients (9%). Three of the 5 patients with normal IgG levels were too young for the assessment of IgG production, and the other 2 were only 1 and 2 years of age. All 5 patients (P6, P8, P14, P32, and P35) who had reached puberty had hypogammaglobulinemia. No antibodies against viruses, bacteria, or protein vaccines could be detected in the 20 patients tested. However, 3 patients developed antibodies against Streptococcus pneumoniae, probably reflecting T cell–independent B-cell immunity: P1 after an acute episode of pneumonia episode and P34 and P35 4 years after immunization with a nonconjugated antipneumococcal vaccine. Immunologic data are summarized in Table 2.
T-cell immunophenotyping of the 35 patients. (A) CD4+ T-cell count in patients; the gray area indicates normal values as a function of age.44 (B) CD8+ T-cell count in patients; the gray area indicates normal values as a function of age.44
Immunologic characteristics of the 35 patients
| . | . | Patients (%) . |
|---|---|---|
| CD4+ T lymphocyte subset | Normal pts/tested pts (%) Pts with low level/tested pts (%) | 2/35 (6%) 33/35 (94%) |
| CD8+ T lymphocyte subset | Normal pts/tested pts (%) Pts with high level/tested pts (%) Pts with low level/tested pts (%) | 18/35 (51%) 9/35 (26%) 8/35 (23%) |
| B lymphocyte subset | Normal pts/tested pts (%) Pts with low level/tested pts (%) | 19/24 (79%) 5/24 (21%) |
| NK lymphocyte subset | Normal pts/tested pts (%) Pts with low level/tested pts (%) | 9/12 (75%) 3/12 (25%) |
| T cell proliferation in response to mitogens | Normal pts/tested pts (%) | 35/35 (100%) |
| T cell proliferation in response to antigens | Normal pts/tested pts (%) | 0/35 (0%) |
| IgG levels | Normal pts/tested pts (%) Pts with low level/tested pts (%) | 5/32 (16%) 27/32 (84%) |
| IgA levels | Normal pts/tested pts (%) Pts with low level/tested pts (%) | 15/32 (47%) 17/32 (53%) |
| IgM levels | Normal pts/tested pts (%) Pts with high level/tested pts (%) Pts with low level/tested pts (%) | 8/32 (25%) 3/32 (9%) 21/32 (66%) |
| IgE levels | Normal pts/tested pts (%) Pts with high level/tested pts (%) | 4/6 (67%) 2/6 (33%) |
| Specific antibodies against protein antigens (tetanus, diphtheria or polio) | Pts with low level/tested pts (%) | 20/20 (100%) |
| Antibody against S pneumonia | Normal pts/tested pts (%) Pts with abnormal response/tested pts (%) | 3/5(60%) 2/5(40%) |
| Allohemagglutinin | Normal pts/tested pts (%) Pts with low level/tested pts (%) | 3/5(60%) 2/5(40%) |
| . | . | Patients (%) . |
|---|---|---|
| CD4+ T lymphocyte subset | Normal pts/tested pts (%) Pts with low level/tested pts (%) | 2/35 (6%) 33/35 (94%) |
| CD8+ T lymphocyte subset | Normal pts/tested pts (%) Pts with high level/tested pts (%) Pts with low level/tested pts (%) | 18/35 (51%) 9/35 (26%) 8/35 (23%) |
| B lymphocyte subset | Normal pts/tested pts (%) Pts with low level/tested pts (%) | 19/24 (79%) 5/24 (21%) |
| NK lymphocyte subset | Normal pts/tested pts (%) Pts with low level/tested pts (%) | 9/12 (75%) 3/12 (25%) |
| T cell proliferation in response to mitogens | Normal pts/tested pts (%) | 35/35 (100%) |
| T cell proliferation in response to antigens | Normal pts/tested pts (%) | 0/35 (0%) |
| IgG levels | Normal pts/tested pts (%) Pts with low level/tested pts (%) | 5/32 (16%) 27/32 (84%) |
| IgA levels | Normal pts/tested pts (%) Pts with low level/tested pts (%) | 15/32 (47%) 17/32 (53%) |
| IgM levels | Normal pts/tested pts (%) Pts with high level/tested pts (%) Pts with low level/tested pts (%) | 8/32 (25%) 3/32 (9%) 21/32 (66%) |
| IgE levels | Normal pts/tested pts (%) Pts with high level/tested pts (%) | 4/6 (67%) 2/6 (33%) |
| Specific antibodies against protein antigens (tetanus, diphtheria or polio) | Pts with low level/tested pts (%) | 20/20 (100%) |
| Antibody against S pneumonia | Normal pts/tested pts (%) Pts with abnormal response/tested pts (%) | 3/5(60%) 2/5(40%) |
| Allohemagglutinin | Normal pts/tested pts (%) Pts with low level/tested pts (%) | 3/5(60%) 2/5(40%) |
Clinical features
Median follow-up was 5 years (range: birth-32 years) at the time of this report. Infections were the most prominent clinical feature of the disease, with all patients having had multiple infections. Mean age at first infection was 4.2 months (range: 1-14 months). The first clinical sign of the disease was gastrointestinal infection (15 cases, 43%) or pulmonary infection (12 cases, 34%) in most patients. The median age at diagnosis of MHC class II deficiency was 21 months (range: birth-15 years). Supportive treatment based on intravenous or subcutaneous IgG therapy and antibiotic prophylaxis (trimethoprim-sulfamethoxazole) was initiated after diagnosis in all patients. Regular administration of IgG markedly decreased the frequency of infectious episodes. The most common clinical signs in the patients are listed in Table 1.
Gastrointestinal and hepatic signs
Thirty-one patients (89%) had gastrointestinal infections. Diarrhea began during the first year of life and became protracted after 2 years in 22 of the patients (63%). All patients > 5 years of age who had not undergone HSCT suffered from protracted (or recurrent) diarrhea. Failure to thrive (SDs of −5 to −2 at the ages of 3 months-10 years) was observed in 28 patients (80%). Histologic study of intestinal mucosa was performed in 14 patients. In 10 patients, this study revealed various degrees of villous atrophy, frequently associated with intraepithelial infiltration by lymphocytes, and infiltration by eosinophils, macrophages, and plasma cells in the lamina propria, with no evidence of MHC class II expression in comparison with 1 healthy control (Figure 3A-F). The bacteria most frequently isolated from stools during episodes of diarrhea included Gram-positive (Staphylococcus aureus, n = 5; Enterococcus spp., n = 4) and Gram-negative (Escherichia coli, n = 5; Klebsiella pneumonia, n = 5; Campylobacter jejuni, n = 7; Proteus mirabilis, n = 4; Pseudomonas aeruginosa, n = 2; Salmonella enteritidis, n = 8; Shigella spp., n = 1; and Clostridium difficile, n = 1) bacteria. Viral infections were also a frequent cause of recurrent diarrhea: the viruses detected included adenovirus (n = 7), rotavirus (n = 1), CMV (n = 1), and enterovirus (n = 8). In inoculated patients with attenuated Poliovirus vaccine (n = 2), polio-virus was isolated in stools. Thirteen patients (37%) displayed chronic oral thrush, which was complicated by esophageal candidiasis in 1 patient (P14). Cryptosporidium parvum was recurrently found in the stools of 12 patients (34%) between the ages of 7 months and 10 years. Five patients displayed Giardia intestinalis colonization between the ages of 1 and 7 years. Hepatic abnormalities were also frequent, and 16 patients (46%) had hepatomegaly and/or high serum levels of liver enzymes. Beyond the age of 5 years, only 3 of the 12 patients who did not undergo HSCT (P6, P29, and P35) had no detectable liver disease. Patient P31 developed CMV hepatitis at the age of 18 months. In 5 patients (P1, P2, P3, P8, and P32), hepatic involvement resulted from cholangitis caused by C parvum, diagnosed on the basis of ultrasound and/or the results of biliary catheterization. Three of the 5 patients (P1, P2, and P8) died from liver failure at the ages of 4, 10, and 17 years, respectively.
Histologic features of the rectal mucosa. (A) Rectal biopsy of patient P26 showing very mild lamina propria infiltration by lymphocytes and macrophages. (B) No MHC class II expression on inflammatory and epithelial cells. (C) Rectal biopsy of patient P14 revealing moderate lamina propria infiltration by lymphocytes, eosinophils, macrophages, and plasma cells. (D) Absence of MHC class II expression on inflammatory and epithelial cells. Only eosinophils are stained because of endogenous peroxidase activity. (E) Normal histologic appearance of a control rectal biopsy. (F) Normal expression of MHC class II on lamina propria cells in a rectal biopsy.
Histologic features of the rectal mucosa. (A) Rectal biopsy of patient P26 showing very mild lamina propria infiltration by lymphocytes and macrophages. (B) No MHC class II expression on inflammatory and epithelial cells. (C) Rectal biopsy of patient P14 revealing moderate lamina propria infiltration by lymphocytes, eosinophils, macrophages, and plasma cells. (D) Absence of MHC class II expression on inflammatory and epithelial cells. Only eosinophils are stained because of endogenous peroxidase activity. (E) Normal histologic appearance of a control rectal biopsy. (F) Normal expression of MHC class II on lamina propria cells in a rectal biopsy.
Respiratory tract infections
Recurrent pneumonia was reported in 29 patients (83%), and occurred before the age of 1 year in 22 of these patients (76%). Eight patients (23%) developed acute hypoxic pneumonia requiring mechanical ventilation between the ages of 7 months and 5 years (before the initiation of IgG substitution in 6 of these cases). The pneumonia was caused by Pneumocystis jiroveci (n = 4), CMV (n = 2), or adenovirus (n = 2) infection and was fatal in 1 case (P7). P jiroveci was detected in 3 other patients with mild symptoms. Respiratory syncytial virus was identified in 2 patients (P33 and P22). Ten patients (28%) developed documented bacterial pneumonia between the ages of 7 months and 29 years caused by S pneumoniae (n = 2), H influenza (n = 4, associated with M catarrhalis in 1 case), S aureus (n = 3, associated with Proteus mirabilis in 1 case), K pneumoniae (n = 1), and Mycoplasma pneumonia (n = 1). In these 10 patients, the incidence of bacterial pneumonia was 3/100 patients/year in the absence of IgG treatment and 0.6/100 patients/year with IgG treatment. Ten patients (29%) developed chronic bronchopneumopathy with bronchiectasis between the ages of 1 and 12 years (mean: 4.5 years). Sputum cultures were positive for M catarrhalis (n = 7), S pneumoniae (n = 8), S aureus (n = 3), H influenzae (n = 8), P mirabilis (n = 1), Klebsiella oxytoca (n = 1), K pneumoniae (n = 1), P aeruginosa (n = 3), and M pneumonia (n = 1). Chronic sinusitis was documented in 3 patients > 3 years of age (P1, P8, and P30). Sixteen patients (46%) had recurrent acute otitis media beginning before the age of 2 years and requiring a trans-tympanic drain in 3 patients (P23, P30, and P35). H influenza was responsible for these infections in 3 patients, but the causal pathogens were not documented in the other cases.
Other infections
Seven patients were vaccinated with Bacillus Calmette-Guérin (BCG) and none developed BCGitis. Five patients (14%) had acute pyelonephritis caused by E coli (n = 2), K pneumoniae (n = 2), or P aeruginosa (n = 1). Eleven patients (31%) had sepsis because of S aureus (n = 2), S epidermidis (n = 3), S pneumonia (n = 3), and P aeruginosa (n = 3), occurring after central venous line colonization in 5 cases. One case of arthritis was observed. Five patients had CNS infections. P6 presented with meningitis caused by Listeria monocytogenes at the age of 26 years and CMV retinitis at the age of 29 years. Two siblings (P3 and P4) developed neurologic manifestations ∼ 6 months after oral immunization with an attenuated live poliovirus strain. These patients developed slowly progressive muscle weakness without recovery until their deaths, and had recurrent poliovirus in their stools (Tables 3 and 4). Patient P12 suffered from chronic lymphocytic meningitis caused by HSV type 2 at the age of 14 months and eventually died from this meningoencephalitis at the age of 2 years. P20 had chronic lymphocytic meningitis caused by adenovirus at the age of 4 years, which led to death at the age of 5 years. Cutaneous chickenpox was observed in 8 patients, with a favorable outcome in the absence of treatment in all cases. HSV caused recurrent gingivostomatitis in 5 patients beginning in the first 2 years of life in all cases.
Outcome of the patients undergoing transplantation
| Kindred . | Patient . | Sex . | Year of HSCT . | Age at last follow-up, y . | HLA identity of stem cell donor . | Engraftment . | Period post-HSCT . | Outcome . | Current status or cause of death . |
|---|---|---|---|---|---|---|---|---|---|
| 2 | P2 | M | 1985 | 4 | Haploidentical | - | 3 y | Dead | Liver failure and sepsis |
| 3 | P3 | F | 1985 | 6 | Haplo identical | + | 1 mo | Dead | Viral infection |
| 4 | P5 | M | 1997 | 16 | Geno identical | + | 13 y | Alive | Well without treatment |
| 8 | P9 | M | 2000 | 8 | Haplo identical | - | 6 y | Dead | Medullary and liver failure |
| 9 | P10 | M | 1991 | 9 | Haplo identical | + | 2 mo | Dead | CMV infection |
| 9 | P11 | M | 1985 | 1 | Haplo identical | + | 2 mo | Dead | Enterovirus infection |
| 10 | P13 | F | 1997 | 2 | Haplo identical | + | 3 mo | Dead | Viral meningitis |
| 12 | P15 | F | 2000 | 1 | Haplo identical | + | 3 mo | Dead | CMV infection |
| 13 | P16 | F | 1991 | 22 | Haplo identical | + | 19 y | Alive | Well without treatment |
| 14 | P17 | F | 1993 | 18 | Haplo identical | + | 17 y | Alive | Well without treatment |
| 15 | P18 | F | 1988 | 23 | HLA identity (5/6) | + | 22 y | Alive | Well without treatment |
| 16 | P19 | F | 1986 | 26 | Haplo identical | + | 24 y | Alive | Well without treatment |
| 17 | P20 | F | 1987 | 5 | Haplo identical | - | 4.5 y | Dead | Viral meningoencephalitis |
| 19 | P21 | M | 1994 | 4 | Haplo identical | + | 4 mo | Dead | GVHD |
| 20 | P22 | F | 1993 | 19 | HLA identity (5/6) | + | 17.5 y | Alive | Well without treatment |
| 27 | P23 | M | 1999 | 16 | Geno identical | + | 11 y | Alive | Well without treatment |
| 27 | P24 | M | 2007 | 4 | Haplo identical | Partial 5% | 3 y | Alive | Well without treatment |
| 30 | P25 | F | 2001 | 4 | Haplo identical | + | 4 mo | Dead | CMV and C albicans infection |
| 32 | P27 | F | 2004 | 2.5 | Geno identical | + | 5 mo | Dead | GVHD - adenovirus infection |
| 33 | P28 | F | 2006 | 4 | Geno identical | + | 3 mo | Dead d | GVHD - adenovirus infection |
| 35 | P30 | F | 2008 | 11 | Geno identical | + | 2 y | Alive | Well without treatment |
| 36 | P31 | M | 2009 | 4 | Cord blood (9/10) | Partial 1% | 15 mo | Alive | AHAI, poor condition |
| 38 | P33 | F | 2009 | 2 | HLA identity (9/10) | + | 12 mo | Alive | Well without treatment |
| Kindred . | Patient . | Sex . | Year of HSCT . | Age at last follow-up, y . | HLA identity of stem cell donor . | Engraftment . | Period post-HSCT . | Outcome . | Current status or cause of death . |
|---|---|---|---|---|---|---|---|---|---|
| 2 | P2 | M | 1985 | 4 | Haploidentical | - | 3 y | Dead | Liver failure and sepsis |
| 3 | P3 | F | 1985 | 6 | Haplo identical | + | 1 mo | Dead | Viral infection |
| 4 | P5 | M | 1997 | 16 | Geno identical | + | 13 y | Alive | Well without treatment |
| 8 | P9 | M | 2000 | 8 | Haplo identical | - | 6 y | Dead | Medullary and liver failure |
| 9 | P10 | M | 1991 | 9 | Haplo identical | + | 2 mo | Dead | CMV infection |
| 9 | P11 | M | 1985 | 1 | Haplo identical | + | 2 mo | Dead | Enterovirus infection |
| 10 | P13 | F | 1997 | 2 | Haplo identical | + | 3 mo | Dead | Viral meningitis |
| 12 | P15 | F | 2000 | 1 | Haplo identical | + | 3 mo | Dead | CMV infection |
| 13 | P16 | F | 1991 | 22 | Haplo identical | + | 19 y | Alive | Well without treatment |
| 14 | P17 | F | 1993 | 18 | Haplo identical | + | 17 y | Alive | Well without treatment |
| 15 | P18 | F | 1988 | 23 | HLA identity (5/6) | + | 22 y | Alive | Well without treatment |
| 16 | P19 | F | 1986 | 26 | Haplo identical | + | 24 y | Alive | Well without treatment |
| 17 | P20 | F | 1987 | 5 | Haplo identical | - | 4.5 y | Dead | Viral meningoencephalitis |
| 19 | P21 | M | 1994 | 4 | Haplo identical | + | 4 mo | Dead | GVHD |
| 20 | P22 | F | 1993 | 19 | HLA identity (5/6) | + | 17.5 y | Alive | Well without treatment |
| 27 | P23 | M | 1999 | 16 | Geno identical | + | 11 y | Alive | Well without treatment |
| 27 | P24 | M | 2007 | 4 | Haplo identical | Partial 5% | 3 y | Alive | Well without treatment |
| 30 | P25 | F | 2001 | 4 | Haplo identical | + | 4 mo | Dead | CMV and C albicans infection |
| 32 | P27 | F | 2004 | 2.5 | Geno identical | + | 5 mo | Dead | GVHD - adenovirus infection |
| 33 | P28 | F | 2006 | 4 | Geno identical | + | 3 mo | Dead d | GVHD - adenovirus infection |
| 35 | P30 | F | 2008 | 11 | Geno identical | + | 2 y | Alive | Well without treatment |
| 36 | P31 | M | 2009 | 4 | Cord blood (9/10) | Partial 1% | 15 mo | Alive | AHAI, poor condition |
| 38 | P33 | F | 2009 | 2 | HLA identity (9/10) | + | 12 mo | Alive | Well without treatment |
Age indicates age at death or last follow-up at the time of writing; and GVHD, graft-versus-host disease.
Outcome of patients who did not undergo transplantation
| Kindred . | Patient . | Sex . | Age at last follow-up, y . | Status . | Cause of death . | Complications . |
|---|---|---|---|---|---|---|
| 1 | P1 | F | 10 | Dead | Liver failure | ENT infections, pneumonia, bacterial sepsis severe viral infection, chronic diarrhea, liver disease, AIHA |
| 3 | P4 | M | 1 | Dead | Encephalomyelitis | ENT infections, severe viral infection |
| 5 | P6 | M | 32 | Alive | ENT infections, pneumonia, severe viral infection, bacterial meningitis liver disease, AIHA | |
| 6 | P7 | F | 5 | Dead | Severe pneumonia | ENT infections, pneumonia, chronic diarrhea |
| 7 | P8 | M | 17 | Dead | Liver failure | ENT infections, pneumonia, bacterial sepsis, chronic diarrhea, chronic intestinal cryptosporidiosis, liver disease, AIHA |
| 10 | P12 | F | 2 | Dead | Meningoencephalitis | ENT infections, bacterial sepsis, severe viral infection |
| 12 | P14 | M | 23 | Alive | ENT infections, pneumonia, recurrent gastrointestinal infections, liver disease | |
| 31 | P26 | M | 6 | Alive | ENT infections, severe viral infection | |
| 34 | P29 | M | 9 | Alive | ENT infections, pneumonia, chronic diarrhea | |
| 37 | P32 | F | 12 | Alive | Pneumonia, chronic diarrhea, liver disease | |
| 39 | P34 | F | 7 | Alive | Pneumonia, bronchiectasis, chronic intestinal cryptosporidiosis, liver disease | |
| 40 | P35 | M | 16 | Alive | Pneumonia, bronchiectasis |
| Kindred . | Patient . | Sex . | Age at last follow-up, y . | Status . | Cause of death . | Complications . |
|---|---|---|---|---|---|---|
| 1 | P1 | F | 10 | Dead | Liver failure | ENT infections, pneumonia, bacterial sepsis severe viral infection, chronic diarrhea, liver disease, AIHA |
| 3 | P4 | M | 1 | Dead | Encephalomyelitis | ENT infections, severe viral infection |
| 5 | P6 | M | 32 | Alive | ENT infections, pneumonia, severe viral infection, bacterial meningitis liver disease, AIHA | |
| 6 | P7 | F | 5 | Dead | Severe pneumonia | ENT infections, pneumonia, chronic diarrhea |
| 7 | P8 | M | 17 | Dead | Liver failure | ENT infections, pneumonia, bacterial sepsis, chronic diarrhea, chronic intestinal cryptosporidiosis, liver disease, AIHA |
| 10 | P12 | F | 2 | Dead | Meningoencephalitis | ENT infections, bacterial sepsis, severe viral infection |
| 12 | P14 | M | 23 | Alive | ENT infections, pneumonia, recurrent gastrointestinal infections, liver disease | |
| 31 | P26 | M | 6 | Alive | ENT infections, severe viral infection | |
| 34 | P29 | M | 9 | Alive | ENT infections, pneumonia, chronic diarrhea | |
| 37 | P32 | F | 12 | Alive | Pneumonia, chronic diarrhea, liver disease | |
| 39 | P34 | F | 7 | Alive | Pneumonia, bronchiectasis, chronic intestinal cryptosporidiosis, liver disease | |
| 40 | P35 | M | 16 | Alive | Pneumonia, bronchiectasis |
Other manifestations
Autoimmune hemolytic anemia (with positive Coombs-type IgG and complement) occurred in 3 patients at the ages of 5, 10, and 31 years (P1, P8, and P6, respectively), requiring treatment with corticosteroids and splenectomy at the age of 9 years in 1 patient (P1) and treatment with corticosteroids in the other 2 cases. Four other patients (P10, P16, P26, and P27) had peripheral neutropenia with no detectable autoantibodies. Four children (P2, P8, P15, and P18) had transient hypereosinophilia (range: 3-10 × 109 cells/L) associated with eczema or cryptosporidiosis. Eczema was observed in a total of 4 patients (P9, P15, P18, and P31). Finally, an allergic cutaneous reaction was observed in 4 patients (P6 and P23 after amoxicillin treatment and P26 and P35 after trimethoprim-sulfamethoxazole treatment).
Outcome and treatment
HSCT.
HSCT is the only known curative treatment for MHC class II expression deficiency. In our series, 23 (66%) of the 35 patients underwent HSCT (Figure 4A and Table 3). The indications for HSCT mostly related to clinical status (age and infection state). Myeloablative conditioning regimens combining busulfan with cyclophosphamide were used in all patients undergoing HSCT. Most patients receiving grafts from an unrelated or HLA-mismatched donor were given in vivo immunosuppressive treatment (antithymocyte globulin, Campath IG, or anti-LFA1 with or without CD2, depending on the conditioning protocol in routine use at the time). Ten of the 23 patients who underwent HSCT (43%) are alive and have been cured, with the recovery of almost normal immune functions (Table 3). These patients received HSCT between the ages of 1 and 9 years (mean: 2.8 years). Follow-up is currently 1-24 years after HSCT (mean: 11.8 years). Complete engraftment was observed in 9 patients and partial engraftment was observed in another. One patient (P33) who underwent HSCT with cord blood with a single mismatch currently displays poor engraftment and little immunologic recovery 1 year after HSCT. Twelve of 23 patients who underwent HSCT (52%) died after transplantation (1 month-6 years after HSCT; mean: 1.3 years; Table 3). These patients underwent transplantation between the ages of 6 months and 9 years (mean: 2.9 years). Nine patients died in the period immediately after HSCT (1-5 months after HSCT) from severe viral and/or fungal infections (n = 6), severe GVHD (n = 1), or both severe viral infections and severe GVHD (n = 2). An absence of engraftment was noted in 3 patients receiving haploidentical grafts. These patients died between 4.5 and 6 years after HSCT from liver failure and sepsis, viral meningoencephalitis, and liver and medullary failure.
Survival curves (Kaplan-Meier). (A) Survival curve for patients who underwent HSCT. (B) Survival curve for patients who did not undergo HSCT.
Survival curves (Kaplan-Meier). (A) Survival curve for patients who underwent HSCT. (B) Survival curve for patients who did not undergo HSCT.
Outcome of patients who did not undergo HSCT.
Twelve patients did not undergo HSCT (Table 4). Clinical course was unfavorable in these patients, with the progression of infectious complications to death in 5 patients (42%; range: 1-17 years; mean age at death: 7 years; Figure 4B). The causes of death were severe viral infections before the age of 5 years, with 2 cases of viral meningoencephalitis. P7 died from severe undocumented interstitial pneumonia. Liver failure was responsible for the death of 2 patients (P1 and P8) > 5 years of age (Table 4). P8 had severe progressive respiratory, gut, and hepatic dysfunctions and failure to thrive, resulting in death at the age of 17 years. Seven patients who did not undergo HSCT (58%) are still alive at the time of this report at a median age of 12 years (range: 6-32 years; mean age: 15 years). All of these patients except one are living in France and have respiratory and/or gastrointestinal and/or hepatic symptoms (Table 4). Four patients (P6, P14, P32, and P35) have reached puberty and are still alive on supportive care (IgG treatment and antibiotic prophylaxis with trimethoprim-sulfamethoxazole; Table 4).
The oldest of these patients is 32 years of age (P6) and has had symptoms since the age of 7 months. He was diagnosed with MHC class II deficiency at the age of 5 years. His symptoms improved between the ages of 10 and 20 years, with a decrease in the frequency and severity of infections. After the age of 20 years, P6 presented symptomatic respiratory syncytial virus infection and recurrent HSV1 infections, cholestasis, and moderate cytolysis. A liver biopsy showed granulomatous lesions associated with moderate portal fibrosis, but no pathogens were detected. P6 developed meningitis caused by L monocytogenes at the age of 26 years, CMV colitis at the age of 27 years, and CMV retinitis at of the age 29 years. He had autoimmune hemolytic anemia requiring steroid treatment at the age of 31 years. He also has recurrent gastroenteritis (C jejuni, C difficile, and Salmonella spp.), recurrent gastritis caused by Helicobacter pylori, ear-nose-throat (ENT) infections, and bacterial pneumonia (H influenzae), with chronic bronchopneumonia. He has a Karnofsky Performance Scale Index score of 90%.
The second patient (P14) has had symptoms since the first year of life and was diagnosed with MHC class II deficiency at the age of 2 years. He has recurrent ENT infections (H influenzae) and bacterial pneumonia (S pneumoniae), with chronic bronchopneumonia since the age of 1 year. He also had CMV, varicella zoster virus, and zoster infections with a favorable outcome during childhood. He has recurrent bacterial diarrhea caused by Salmonella spp. and C jejuni. Since the age of 9 years, this patient has suffered from liver dysfunction related to C parvum infection. He is now 23 years old and has a Karnofsky Performance Scale Index score of 90%.
The third patient (P32) was diagnosed at the age of 10 years. She is now 12 years old and has a Karnofsky Performance Scale Index score of 60%. She has had recurrent diarrhea and chronic bronchopneumonia requiring oxygen treatment since the age of 1 year. Hepatic involvement due to cholangitis caused by C parvum was diagnosed at the age of 10 years. This patient is one of those who displayed residual MHC class II expression on B cells, with no expression on monocytes. Despite this residual expression, she had severe CD4+ T-cell lymphopenia, defective T-cell proliferation in response to antigens, and profound hypogammaglobulinemia.
The fourth patient (P35) was diagnosed at the age of 15 years and is now 16 years old, with a Karnofsky Performance Scale Index score of 90%. He has no severe viral infections and no liver involvement. He has had symptoms since the age of 3 months, with recurrent ENT infections, undocumented pneumonia (3 episodes per year), and intermittent moderate diarrhea with normal growth since the first year of life. At the age of 15 years, he presented with chronic bronchopneumonia complicated by localized bronchiectasis.
Discussion
We describe here the clinical and immunologic signs of 35 patients with MHC class II deficiency assigned to complementation group B and sharing the same genotype. The presence of the same mutation in another 15 families from North Africa is consistent with the observation that ∼ 70% of all cases of MHC class II deficiency belong to complementation group B.2-12,14,15,18-39 All of the families in this cohort were of North-African origin and had the same homozygous 26-bp deletion in the RFXANK gene called I5E6-25_I5E6 + 1. A founder effect was demonstrated for this mutation and was dated to ∼ 2250 years ago (95% CI: 1700-3025 years). Simulation studies have shown that the ESTIAGE method provides excellent age estimates for the MRCA from small numbers of individuals, and the precision of this method has been greatly increased by the advent of high-resolution genotyping.41,43 This ancestor therefore lived before the Arabic invasion of North Africa, which occurred in the 7th century, and probably belonged to the Berber civilization. Indeed, the estimated age of the MRCA, the claims to pure Berber ancestry of approximately one-half of the patients, and the observation that no patient from Saudi Arabia has ever been found to carry the I5E6-25_I5E6 + 1 mutation are all consistent with this hypothesis.
MHC class II deficiency is associated with a poor prognosis, with most patients having a life expectancy of only a few years. In most of these patients, clinical signs and outcome were similar to those reported in other groups of patients with MHC class II deficiency.1,2,4,5,37 The clinical signs included pulmonary, ENT, gastrointestinal and urinary tract infections, septicemia and severe viral infections, generally with a fatal outcome in the absence of curative HSCT. Infections with viruses such as CMV, adenovirus, HSV, and enterovirus were associated with a poor prognosis, with the patient generally dying during childhood.2,7 Symptomatic and prophylactic supportive care of these patients decreased the frequency of clinical infectious complications. However, such care did not prevent progressive organ dysfunction, particularly of the liver. Indeed, hepatic tract involvement appears to be a factor associated with poor prognosis. Most of the patients with Cryptosporidium infection suffered progressive liver failure. Viral hepatitis, cholangitis, and the use of hepatotoxic drugs may have contributed to this problem. Severe liver disease had a marked influence on mortality after the age of 5 years. None of the patients developed Toxoplasma infection or BCGitis. The absence of BCGitis may be accounted for by the presence of residual immunity in the form of CD8+ T and natural killer cells. The presence of signs of autoimmunity in 20% (7 of 35) of the patients highlights the role of MCH class II proteins in the autoimmunity and tolerance check point.45
Most of patients studied were diagnosed in the first year of life, but some of the patients were diagnosed later, at ages of up to 15 years. This may be because of a milder onset in some patients with few infections and/or delays in the referral of patients to specialized centers. Moreover, 5 of the patients in this cohort lived beyond the age of 12 years despite recurrent infections and 4 are still alive. In particular, P6 and P14 had a clinical course similar to that of other patients in early childhood, but displayed fewer symptoms during their teens. The frequency of infection in these patients increased again when they reached adulthood. Immunologic investigations revealed no significant differences from other patients. Unknown genetic factors may influence innate or CD8+ T cell–mediated immunity, potentially accounting for this more favorable outcome. Genetic predisposition factors and environmental factors such as medical care and family hygiene may be responsible for differences in the clinical expression of the disease state.
In addition, some variability in immunologic phenotype was observed in 10 patients in our cohort who displayed residual MHC class II molecule expression on B cells and/or activated T cells, generally at the time of or immediately after an infectious episode. However, none of these patients displayed any detectable HLA-DR expression on monocytes. This observation suggests that the cellular expression of HLA-DR is regulated differently in monocytes and lymphocytes. However, none of these patients was infection-free; like the other patients, all had CD4+ T-cell lymphopenia and no specific response to antigens. A less severe clinical course has been reported in a 32-year-old patient with a homozygous splicing mutation of RFXANK leading to an absence of HLA-DR cellular expression.32 Three adult sisters with CIITA deficiency and homozygous missense mutations have been reported to have a leaky clinical phenotype, with residual MHC class II molecule expression on monocytes and B cells.30 None of the patients in our cohort displayed such a leaky immunologic phenotype on monocytes. The reasons for this variability in immunologic phenotype in this setting are unknown. A reversion of the mutation, as reported in X-linked gammaC SCID,46 ADA deficiency,47 and Wiskott-Aldrich syndrome48 with substitution or insertion mutations, is unlikely given the size of the deletion (26 bp) and the absence of HLA class II expression.
HSCT is the only curative treatment for this PID, but residual adaptive immunity, the high rate of GVHD, and severe viral infections render HSCT very difficult in these patients. In this cohort, 12 of the 23 patients died after HSCT. The incidence and severity of GVHD appears to be associated with the presence of ongoing viral infection before HSCT.10 Aggressive anti-infectious treatment before HSCT, such as preemptive treatments against viral infections caused by adenovirus, CMV, or enterovirus, may therefore be beneficial in such patients. HSCT has a limited success rate in these patients, not exceeding 50%, in contrast to what has been observed for other T-cell PIDs.4,7,10,11,49,50 Better results have recently been obtained for HSCT with grafts from unrelated donors and a lower-intensity conditioning regimen.12,50 However, unrelated compatible donors for these patients are rare because of the genetic origin of the disease. MHC class II–deficient patients undergoing HSCT seem to have persistently small numbers of CD4+ T cells with a moderate decrease in naive CD4+ T cells. This finding is consistent with impaired thymic maturation due to defective MHC class II expression on thymic epithelia. Despite their CD4+ T-cell lymphopenia, patients with complete or partial engraftment (> 5%) display a normalization of antigen-specific T-cell stimulation and Ab production in response to immunization antigens.
In conclusion, we have identified and dated a founder event responsible for the RFXANK deletion I5E6-25_I5E6 + 1 in the North-African population. This deletion is the most frequent mutation responsible for MHC class II deficiency. We show that patients with this homozygous mutation have a poor prognosis, with a life expectancy of a few years in most cases, although some patients may survive much longer, into their teens. Supportive care and environmental factors may influence this particular clinical outcome.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank members of Inserm U768 and the Immuno-Hematology Unit of Necker Hospital for helpful discussions; Frédéric Rieux-Laucat, Dr Anne-Sophie Lascaux, Dr Françoise Bernaudin, Pr Olivier Lortholary, and Pr Nicole Brousse for their collaboration; Marie-Claude Fondaneche, Nathalie Lambert, Virginie Grandin, Corinne Jacques, Chantal Harre, Stéphanie N′daga, and Aminata Diabate for excellent technical assistance; and Christine Bole-Feysot from the genomic platform of the IMAGINE Foundation at Necker Hospital.
The Inserm U768 laboratory is supported by Inserm funding. The Laboratory of Human Genetics of Infectious Diseases is supported by the March of Dimes, the Dana Foundation, and Inserm.
Authorship
Contribution: M.O. collected, analyzed, and interpreted the data and wrote the manuscript; Q.B.V. and L.A. performed the genetic analysis, did the research, and wrote the manuscript; P.F., F.T., S.S., M.B., A.B., Y.L., M.D., and S.B. provided clinical information and patient samples; D.C. and J.B. performed and interpreted the histologic data; B.L.-G., J.-L.C., and A.F. provided essential clinical information from patients, interpreted the data, and wrote the manuscript; and C.P. designed the research, interpreted the genetic and immunologic data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Capucine Picard, Study Center for Primary Immunodeficiencies, AP-HP, Necker Hospital, 149 rue de Sèvres, 75015 Paris, France; e-mail: capucine.picard@inserm.fr.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal