Abstract
Absent in peripheral tissues during homeostasis, human plasmacytoid dendritic cells (pDCs) are described in inflamed skin or mucosa. Here, we report that, unlike blood pDCs, a subset of tonsil pDCs express functional CCR6 and CCR10, and their respective ligands CCL20 and CCL27are detected in inflamed epithelia contacting blood dendritic cell antigen 2+ pDCs. Moreover, pDCs are recruited to imiquimod-treated skin tumors in WT but not CCR6-deficient mice, and competitive adoptive transfers reveal that CCR6-deficient pDCs are impaired in homing to inflamed skin tumors after intravenous transfer. On IL-3 culture, CCR6 and CCR10 expression is induced on human blood pDCs that become responsive to CCL20 and CCL27/CCL28, respectively. Interestingly, unlike myeloid DC, blood pDCs initially up-regulate CCR7 expression and CCL19 responsiveness on IL-3 ± CpG-B and then acquire functional CCR6 and CCR10. Finally, IL-3–differentiated CCR6+ CCR10+ pDCs secrete high levels of IFN-α in response to virus. Overall, we propose an unexpected pDCs migratory model that may best apply for mucosal-associated lymphoid tissues. After CCR7-mediated extravasation into lymphoid tissues draining inflamed epithelia, blood pDCs may be instructed to up-regulate CCR6 and/or CCR10 allowing their homing into inflamed epithelia (in mucosae or skin). At this site, pDCs can then produce IFN-α contributing to pathogen clearance and/or local inflammation.
Introduction
Plasmacytoid dendritic cells (pDCs) play an important role in innate antiviral immunity by rapidly secreting abundant type I IFNs after exposure to various RNA or DNA viruses.1 This unique ability is mediated through their selective expression of TLR7 and TLR9,2 involved in pathogen sensing. After activation, pDCs differentiate into a distinct type of mature DCs directing T-cell responses with high flexibility.1 Thus, pDCs play a critical role at the interface between innate and adaptive immunity.
pDCs are commonly detected in peripheral blood and lymphoid organs.1 Unlike myeloid DC (mDCs), they are absent from peripheral epithelial tissues under steady-state conditions, fail to migrate in response to inflammatory chemokines in vitro, and are constitutively recruited from the blood to the lymph nodes through high endothelial venule, a process involving CD62L, CCR7, ChemR23, and CXCR3/4.3-10 On maturation, both DC subsets up-regulate CCR7 expression and respond to the lymph node-homing chemokines CCL19 and CCL21,5,7,8 allowing their recruitment in T-cell areas where they initiate adaptive immune responses. It was recently shown in mice that CCR7 plays an essential role for the homing of pDCs, regardless of their activation status, to lymph node under both steady-state and inflammatory conditions.10
However, pDCs also accumulate in inflamed epithelial tissues during noninfectious and infectious disorders9,11-18 and participate in inflammatory chronic diseases, such as psoriasis and systemic lupus erythematosus.14,19,20 Moreover, pDC leukemia/lymphoma is often associated with isolated cutaneous lesions because of skin accumulation of leukemic pDCs.21 Inducible CXCR3 ligands (CXCL9/10/11) and chemerin, expressed on inflamed endothelium, have been reported to direct pDC extravasation to peripheral inflamed tissues.3,5,8,9,12,22 However, the whole sequence of migratory events governing pDC recruitment to inflamed tissues remains still unknown.
CCL20 is the main chemokine expressed by inflamed skin, mucosal epithelium, and mucosal-associated lymphoid tissue epithelium participating in the recruitment of CCR6-expressing Langerhans cell precursors.23-26 Among other chemokine receptors involved in immune cell trafficking to epithelial sites, CCR10 is selectively expressed on a subset of memory T cells and IgA-secreting B cells, respectively, homing to the skin and the gut.27-29 Both CCR10 ligands are expressed in peripheral epithelial sites. CCL27 is up-regulated in inflamed skin,27 whereas CCL28 is selectively expressed in intestinal epithelium.30 In addition, a population of T cells specifically secreting high levels of IL-22, termed Th22 cells, was recently reported to express both CCR6 and CCR10 allowing their skin homing.31,32
We report here, for the first time, a role for CCR6 and CCR10 ligands in pDC recruitment to inflamed epithelia. Furthermore, an unexpected sequence of chemokine receptor expression was observed, suggesting that consecutive to an initial CCR7-mediated recruitment from blood into lymphoid tissues draining inflamed epithelia, pDCs might be conditioned to acquire CCR6 and CCR10 expression, endowing them with the capacity to migrate into inflamed epithelia of mucosae or skin. Such a scenario enables pDCs to play an effector role through IFN-α production at inflamed epithelial sites during viral/bacterial entry or inflammatory/autoimmune disorders.
Methods
Patients
Human blood and tonsil specimens were respectively provided anonymously from the Etablissement Français du Sang and hospitals after obtaining informed consent, according to law. Skin biopsies were obtained from either healthy persons undergoing plastic surgery (n = 3) or patients with psoriasis (n = 5) or verrucae vulgaris (n = 3). This study was approved by the Comité d'Evaluation Commun au Centre Léon Bérard, á l'Animatérie de transit de l'ENS, au PBES et Laboratoire P4 ethics committee.
Mice
Female C57Bl/6 mice (Charles River Laboratories) and CCR6-deficient mice33 were kept under specific pathogen-free conditions and provided with food and water ad libithum. All animal experiments were approved by the local animal ethics committee according to French legislation on animal experiments.
In vivo mobilization of pDCs
Wild-type (WT) and CCR6-deficient mice at 8 to 12 weeks of age were subcutaneously injected with 1 × 106 B16 melanoma cells. At day 7, B16 tumors were topically treated on the skin covering the transplanted tumors with 5% imiquimod cream (Aldara; 3M Pharmaceuticals) for 6 consecutive days. Control mice were similarly treated with a vehicle cream. After treatments, mice were killed, and tumors were harvested and processed.
Adoptive transfer of labeled cells
B6 and CCR6-deficient mice received subcutaneously 5 × 106 B16-FL cells, a murine melanoma tumor cell line engineered to stably produce murine Flt-3L.34 After 12 days, animals were killed. pDCs were enriched from splenocytes from B16-FL tumor-carrying B6 or CCR6-deficient mice by negative selection using an adapted protocol of the mouse pDCs isolation kit II (Miltenyi Biotec) to obtain approximately 30% of pDCs among purified cells. pDC-enriched cells were labeled with CFSE (green fluorescent) and CellTrace violet (violet fluorescent). Cell populations were adjusted to contain equal numbers of pDCs. For adoptive transfers, 4 × 106 pDCs for both colors were intravenously injected retro-orbitally in B16-carrying B6 recipients as a wt/wt or ko/wt pool. After 18 hours, recipients were killed and cells were isolated from the tumors and spleens.
Preparation of blood, tonsil, and B16 tumor cell suspensions
Human tonsils and mice B16 harvested melanoma were minced into small pieces and digested with an enzymatic cocktail containing collagenase Ia (1 μg/mL) and DNase I (0.1 mg/mL; Sigma-Aldrich) for 1 hour at 37°C with gentle agitation. The resulting cell suspensions were filtered, washed, and resuspended in RPMI 1640 medium (Invitrogen) supplemented with 10% FCS (Lonza) and antibiotics (Complete RPMI) for further analyses. Mouse tumor mononuclear cells were as PBMCs obtained by Pancoll density gradient centrifugation (Dominique Dutscher) for 20 minutes at 620g. Finally, immune cells contained in B16 tumors were selectively purified using the CD45 isolation kit provided by Miltenyi Biotec and then further analyzed by flow cytometry.
Flow cytometric analysis
Three- and 4-color staining was performed on human PBMCs, tonsil mononuclear cells, and mouse B16 tumor cells by flow cytometry. Human pDCs identified with anti–blood dendritic cell antigen (BDCA)-2 FITC (Miltenyi Biotec) and anti–CD123 PE-Cy5 (BD Biosciences) or purified pDCs were stained with PE-coupled or biotinylated anti-CCR6 (BD Biosciences), -CCR7 and -CCR10 antibodies (R&D Systems).27 Mouse tumor pDCs were identified among viable DAPI− or 7-AAD− cells, as CD11b−, CD11c+, B220/CD45RB+, and BST2+/mPDCA1+ using anti–CD11b FITC or PE, -CD11c APC or PE-Cy7, -B220/CD45RB PerCP-Cy5.5 or APC-Cy7 (BD Bioscience), and -BST2/mPDCA1 PE or APC (Miltenyi Biotec) antibodies. At least 1 × 105 total events were acquired either on FACScan (BD Biosciences), Cyan (Dako North America), or Fortessa (BD Biosciences) cytometers and analyzed using BD Cell Quest Pro, TreeStar FlowJo, and BD FACS Diva software.
Immunohistochemistry
Frozen 6-μm tonsil or skin sections from healthy donors or patients with psoriasis or verrucae vulgaris were (1) fixed with acetone, (2) preprocessed with 0.3% H2O2 (Sigma-Aldrich) followed by an avidin and biotin blocking step (Blocking kit, Vector Laboratories), (3) incubated with blocking serum for 30 minutes, (4) stained with antibodies against hCCL20 (goat IgG, 10 μg/mL), hCCL27 (124302, mIgG2a, 10 μg/mL; R&D Systems), and hCD123/IL-3Rα (9F5, mIgG1, 10 μg/mL; BD Biosciences) for 1 hour, and (5) revealed by biotinylated rabbit anti–goat IgG (Vector Laboratories) plus extravidin-peroxidase (Sigma-Aldrich) or sheep anti-mIgG1 (Binding Site) plus mouse alkaline phosphatase-anti–alkaline phosphatase complexes (Dako North America) and/or sheep anti-mIgG2a coupled to peroxidase (Binding Site) for 30 minutes. The peroxidase and alkaline phosphatase activities were revealed using 3-amino 9-ethyl carbazole substrate and alkaline phosphatase substrate III (Vector Laboratories), respectively. Some tissue sections were also incubated with anti–BDCA2 antibody (104C12, mIgG1, Dendritics, 10 μg/mL), revealed with anti–mouse Ig peroxidase kit using ImmPRESS system (Vector Laboratories) according to the supplier's instructions and as previously reported35 and DAB as substrate (Dako North America). The latter sections were then stained with either the anti-hCCL20 (goat IgG, 10μg/mL) or the anti-hCCL27 (124302, mIgG2a, 10μg/mL; R&D Systems) antibodies that were revealed with ImmPRESS anti–goat and anti–mouse Ig peroxidase kit and Histogreen as substrate (Abcys). In addition, negative controls using isotype control primary antibodies were also performed.
pDC purification, culture, and IFN-α production
Purified human blood pDCs (90%-98%) using either BDCA-4 cell isolation kit (Miltenyi Biotec) or FACS sorting (lineage− CD4+ CD11c−) were cultured with IFN-α (Schering-Plough, 1000 U/mL) or IL-3 (R&D Systems, 20 ng/mL) ± CpG-B oligodeoxynucleotide (ODN) 2006 (InvivoGen; 5 μg/mL) for different time periods, and their expression of CCR6, CCR7, and CCR10 was analyzed by flow cytometry. IL-3–treated pDCs were also activated with formaldehyde-inactivated influenza virus (A/Wisconsin/67/05, 100 HAU/mL, Sanofi Pasteur) and CpG-A ODN2336 (Coley 5 μg/mL) for an additional 24 hours. IFN-α was measured in supernatants using human IFN-α2 ELISA kit (Bender MedSystem).
Chemotaxis assay in transwells
Non-T cells isolated from digested tonsils by eliminating CD2+ cells by Ficoll-Rosetting method as well as fresh or IL-3–cultured purifed pDCs were first preincubated 1 hour at 37°C and then resuspended at 2 to 5 × 105 cells/well in 100 μL of RPMI medium 1640 containing 2% FCS and 20 ng/mL IL-3 and loaded into 5-μm pore size inserts (6.5-mm diameter, Costar) that were placed in 24-well plates containing 600 μL control medium or medium supplemented with various concentrations of human CCL19, CCL20, CCL27, and CCL28 (R&D Systems). For migration assays using non-T cells enriched from tonsils, at least 3 wells containing 5 × 105 cells per condition were pooled in triplicate to obtain enough migrated pDCs. After 2 hours of incubation at 37°C, the migrated cells were collected and pDC migration was quantified by double staining gated on BDCA-4+/HLA-DR+ for purified pDCs or triple staining gated on CD19− CD123+ BDCA2+ cells for non-T cells and counting using flow cytometry. Each experimental point was determined in triplicates, and < 10% variation was observed in these experiments. The ratio of the number of pDCs that migrated in the presence of chemokine to the number of cells that migrated to control medium was calculated and is given as the migration index or is presented as the percentage of migrated cells relative to the input.
Intracellular cytokine staining
Purified blood pDCs primed 96 hours in IL-3 were activated by inactivated influenza virus during 3 hours, followed by Golgi Plug (BD Biosciences) addition for 3 hours. After cell surface labeling with anti-CCR6 or anti-CCR10 antibodies, cells were fixed in paraformaldehyde 2% and permeabilized in saponin 0.5% (Sigma-Aldrich). Finally, cells were stained with a combination of mAbs to IFN-α FITC (Miltenyi Biotec), TNF-α PE-Cy7, and CXCL10 PE (BD Biosciences) and analyzed by flow cytometry.
Statistics
Statistical significance was determined using the nonparametric Wilcoxon test and was retained for P values < .05.
Results
A subset of tonsil pDCs express functional CCR6 and CCR10
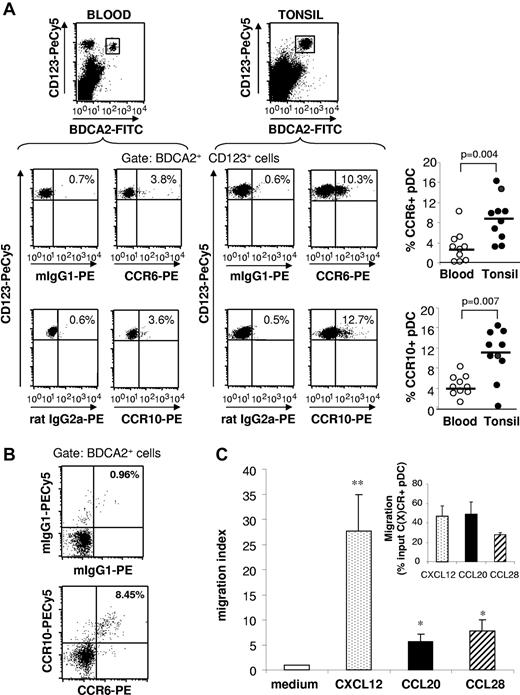
We observed that, in human tonsils, CCR6 and CCR10 are present on lymphocytes and a small cell population lacking lineage markers (not shown). The latter cell subset expressed pDC specific markers CD123 and BDCA-2 (Figure 1A). Nevertheless, tonsil pDCs expressed a lower level of CCR6 and CCR10 than B or T lymphocytes (not shown). CCR6 was expressed on 3% to 16% (8.6% ± 4.4%, n = 10) and CCR10 on 0% to 16% (10.3% ± 5%, n = 10) of tonsil pDCs (Figure 1A). In contrast, a significantly lower percentage of peripheral blood pDCs expressed CCR6 and CCR10 (Figure 1A). CCR6 and CCR10 were mostly coexpressed on BDCA-2+ tonsil pDCs, suggesting that the cells expressing both receptors represent a separate subset of pDCs (Figure 1B). In vitro transwell migration assays using cells enriched in non-T cells by eliminating CD2+ cells from tonsil mononuclear cells (resulting in 2%-3% pDCs) revealed that, in respect to the percentage of pDCs expressing the respective chemokine receptors, pDCs were induced to migrate toward CCL20 and CCL28 but with a lower potency than toward CXCL12 (the ligand for CXCR4), identified before as a potent chemoattractant for pDCs.8 Nevertheless, we observed a similar efficacy of CCL20 and CCL28 compared with CXCL12 in specifically attracting pDCs expressing their respective receptors (Figure 1C inset).
A subset of tonsil pDCs express functional CCR6 and CCR10. (A) CCR6 and CCR10 expression was analyzed by flow cytometry on pDCs from healthy blood and tonsil mononuclear cells. Representative staining (left) and the percentages of CCR6+ and CCR10+ cells among CD123+ BDCA2+ pDCs in blood and tonsil are shown (right). Each point represents an independent donor (n = 10); and horizontal bars, group medians. The P value was obtained by a nonparametric Wilcoxon test. (B) Percentages of CCR6+ CCR10+ cells among BDCA2+ pDCs from tonsil mononuclear cells are indicated, and data are representative of 3 independent experiments. (C) pDCs were enriched from tonsils by eliminating CD2+ cells. Chemotactic activity of tonsil pDCs among enriched cells toward CXCL12, CCL20, and CCL28 was analyzed. Results are expressed as migration index or percentage input according to the number of C(X)CR-expressing pDCs of the input population (inset). **P < .01 (Wilcoxon test). *P < .05 (Wilcoxon test).
A subset of tonsil pDCs express functional CCR6 and CCR10. (A) CCR6 and CCR10 expression was analyzed by flow cytometry on pDCs from healthy blood and tonsil mononuclear cells. Representative staining (left) and the percentages of CCR6+ and CCR10+ cells among CD123+ BDCA2+ pDCs in blood and tonsil are shown (right). Each point represents an independent donor (n = 10); and horizontal bars, group medians. The P value was obtained by a nonparametric Wilcoxon test. (B) Percentages of CCR6+ CCR10+ cells among BDCA2+ pDCs from tonsil mononuclear cells are indicated, and data are representative of 3 independent experiments. (C) pDCs were enriched from tonsils by eliminating CD2+ cells. Chemotactic activity of tonsil pDCs among enriched cells toward CXCL12, CCL20, and CCL28 was analyzed. Results are expressed as migration index or percentage input according to the number of C(X)CR-expressing pDCs of the input population (inset). **P < .01 (Wilcoxon test). *P < .05 (Wilcoxon test).
In situ, pDCs colocalize with CCR6 and CCR10 ligand expression in inflamed epithelia
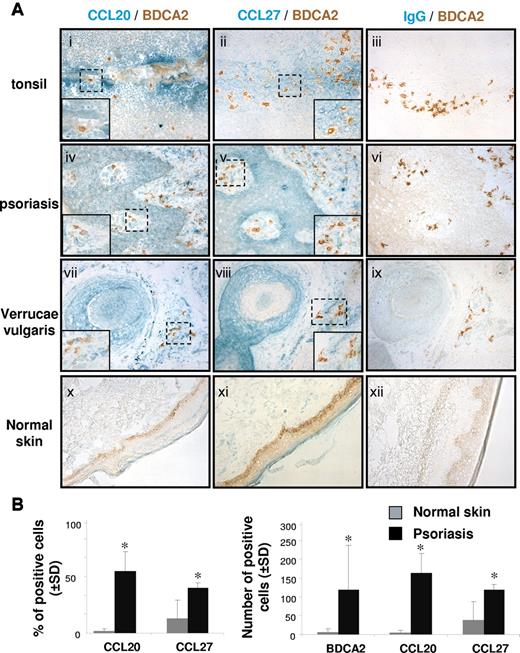
On tonsil serial sections, we observed isolated BDCA2+ and CD123+ pDCs in epithelial areas adjacent to CCL20- and CCL27-producing sites (Figure 2Ai-ii; supplemental Figure 1A-B, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). CCL20 was strongly expressed in differentiated epithelial cells lining the lumen of the crypt, whereas CCL27 was intensely expressed in basal epithelial cells. In psoriatic and virally induced verrucae vulgaris skin lesions, pDCs accumulated in perivascular pockets and were evenly distributed in other compartments of the dermis, such as dermal papilla and dermis area contacting sweat gland epithelium (Figure 2Aiv-v,vii-viii; supplemental Figure 1C-F), whereas they were absent from the epidermis. As we reported previously,23,24,26 CCL20 expression was up-regulated in these areas (Figure 2Aiv,vii; supplemental Figure 1C,E) compared with normal skin (Figure 2Ax). In line with our previous observation,27 CCL27-expressing cells are found in the epithelial area but also in the dermis and in dermal papilla in psoriatic (Figure 2Av; supplemental Figure 1D) and verrucae vulgaris skin lesions (Figure 2Aviii; supplemental Figure 1F), where pDCs are present. In contrast, only a few CCL27-immunoreactive cells were detected in the dermis of healthy skin (Figures 2Axi). The quantification of CCL20+, CCL27+, and BDCA-2+ cells in the different experimental conditions is reported in Figure 2B. These results suggested that CCL20 and CCL27, which are induced or increased in epithelia exposed to the external milieu during infectious or sterile inflammation,13,24,27,36-38 contribute to the recruitment of CCR6+/CCR10+ pDCs in epithelia.
pDCs are in close contact with CCR6 and CCR10 ligands in inflamed epithelia of tonsil, and psoriatic and viral skin lesions. (A) Immunohistochemical stainings on 6-μm acetone fixed sections of tonsils, psoriatic skin, normal skin, and biopsies of verrucae vulgaris showing CCL20 (i,iv,vii,x), CCL27 (ii,v,viii,xi), and control IgG (iii,vi,ix,xii) (green) in epithelial sites together with BDCA-2+ pDCs (brown; original magnification ×20). (Inset) Original magnification ×40;these stainings are representative of 4 independent patients. (B) Positive cells were enumerated in the dermis of psoriatic and normal skin sections (n = 3). Slides were analyzed blind by 2 observers, and positive cells were counted in 5 adjacent fields (original magnification ×40). Results are expressed as the percentage or mean number of positive cells ± SD. *P < .05.
pDCs are in close contact with CCR6 and CCR10 ligands in inflamed epithelia of tonsil, and psoriatic and viral skin lesions. (A) Immunohistochemical stainings on 6-μm acetone fixed sections of tonsils, psoriatic skin, normal skin, and biopsies of verrucae vulgaris showing CCL20 (i,iv,vii,x), CCL27 (ii,v,viii,xi), and control IgG (iii,vi,ix,xii) (green) in epithelial sites together with BDCA-2+ pDCs (brown; original magnification ×20). (Inset) Original magnification ×40;these stainings are representative of 4 independent patients. (B) Positive cells were enumerated in the dermis of psoriatic and normal skin sections (n = 3). Slides were analyzed blind by 2 observers, and positive cells were counted in 5 adjacent fields (original magnification ×40). Results are expressed as the percentage or mean number of positive cells ± SD. *P < .05.
CCR6 deficiency in mice impairs pDC recruitment into epithelial sites after TLR7-ligand-induced inflammation
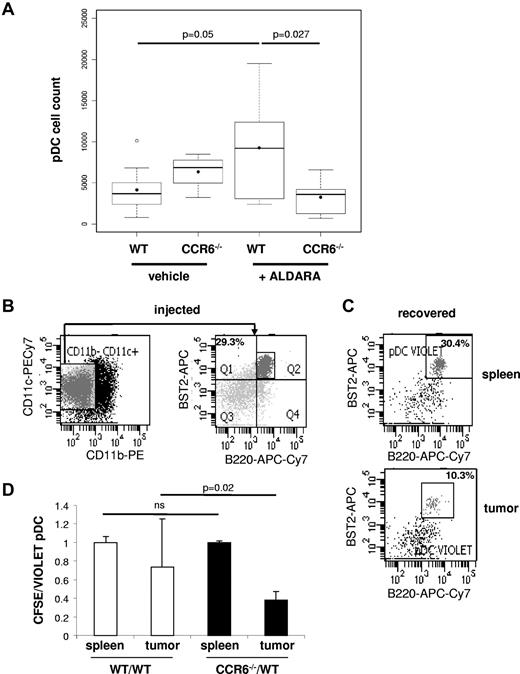
We next addressed the role of CCR6 in pDC recruitment into inflamed epithelial tissues in vivo. In agreement with previous reports,39,40 we observed that a 6-day topical application of imiquimod (Aldara) on the skin covering subcutaneous transplanted B16 tumors enhances the global immune infiltrate characterized by higher percentages of CD45+ cells in B6 mice (supplemental Figure 2A). Among CD45+ cells, pDCs were identified as CD11c+ CD11b− BST2+ B220+ cells (supplemental Figure 2B). We observed that pDC numbers increased in B16 tumors treated with Aldara in B6 mice (Figure 3A). Interestingly, this imiquimod-induced active pDC recruitment was strongly reduced in CCR6−/− mice (Figure 3A). Indeed, the numbers of pDCs among total cells in imiquimod-treated B16 tumors from CCR6−/− mice (3234 ± 2098, n = 9) was significantly lower (P < .05) than the frequency of pDCs infiltrating imiquimod-treated B16 tumors in WT mice (9256 ± 5933, n = 9) and equivalent to the basal level of pDCs infiltrating nontreated B16 tumors in B6 animals (4157 ± 2877, n = 9), whereas there were no such differences in liver, spleen, lymph nodes, and bone marrow in WT and CCR6-deficient mice (supplemental Figure 3). Of note, the numbers of CD45+ and CD11c+ cells were also reduced in imiquimod-treated B16 tumors from CCR6−/− mice compared with B6 mice (supplemental Figure 2C-D). The specific requirement for CCR6 on pDCs for their recruitment to skin tumors during inflammatory processes was confirmed by adoptive transfer experiments. pDCs enriched up to 30% from splenocytes of CCR6−/− and WT mice that carried a Flt3-L-secreting B16 tumor for 12 days were labeled differentially with either CFSE or CellTrace Violet. Equal numbers of CCR6−/− (CFSE) and WT (violet) pDCs were mixed at a ratio 1:1 and then adoptively transferred intravenously into B16 tumor-carrying WT recipients that were previously treated with Aldara for 6 days as described in Figure 3B. After 18 hours of transfer, 30% and 10% of adoptively transferred cells recovered from the spleen and B16 tumor fractions, respectively, were pDCs (Figure 3C). These findings demonstrated that transferred pDCs easily access the spleen, whereas only one-third of them are able to reach the tumor. Of most importance, CCR6-deficient pDCs were impaired in their capacity to migrate to the tumor as reflected by the low migration ratio of CCR6-deficient versus WT pDCs found in the tumor preparation, whereas both pDC populations migrated with similar efficiency to the spleen (Figure 3D). There was no impact of cell labeling on these experiments because transferred pDCs in the mixture of WT (CFSE) and WT (violet) cells migrate with the same efficiency to the tumors (Figure 3D). Together, these results indicate that imiquimod-induced inflammation promotes local accumulation of pDCs in and around skin tumors via a CCR6-dependent mechanism.
pDC recruitment to B16 melanoma after Aldara treatment is CCR6-dependent. (A) B16 melanoma tumors of WT and CCR6-deficient mice were treated daily with Aldara or vehicle cream for 6 consecutive days. At day 7, harvested tumors after purification of CD45+ immune cells by MACS sorting were analyzed for the frequency of B220+ BST2+ pDC infiltration after gating on viable (DAPI−) and CD11c+/CD11b− DCs (see supplemental Figure 2B). The numbers of CD11c+ CD11b− BST2+ B220+ pDCs among total viable tumor cells were compared using R2.13.2 software (R foundation for Statistical Computing, Vienna, Austria, url:http://www.R-project.org) between WT animals and CCR6-deficient animals treated with Aldara or vehicle cream (n = 9 animals). (B) pDC-enriched cells from in vivo Flt3-L-expanded splenocytes of B6 donors were analyzed for the presence of pDCs (CD11c+ CD11b− BST2+ B220+). (C) pDC-enriched cells from WT Flt3-L-expanded splenocytes were labeled with CellTrace Violet and intravenously injected into recipients. The occurrence of donor pDCs in the recipient's spleen (top) and B16 tumor (bottom) preparation was analyzed 18 hours later (gate on CellTrace violet+ cells). (B-C) Data shown are representative for 5 animals of 2 independent experiments. (D) pDC-enriched cells from splenocytes of Flt3-L–treated WT or CCR6-deficient mice were labeled with CellTrace Violet and CFSE, respectively, for the pool CCR6−/−/WT (black bars). As a control, pDC-enriched cells from splenocytes of Flt3-L–treated B6 were labeled either with CellTrace Violet or CFSE, respectively, for the pool WT/WT (white bars). Cells were adjusted to equal number of pDCs and injected at a ratio 1:1 in B16-carrying B6 recipients that were previously treated with Aldara as described in panel A. After 18 hours, recipients were killed, tumors were processed as described in panel A, and the ratio of donor B6 (violet) and CCR6-deficient (CFSE) pDCs or donor B6 (violet) and donor B6 (CFSE) pDCs was analyzed in the recipient's spleen and tumors (mean ± SD; n = 5 recipients). Statistical significance was obtained with a nonparametric Wilcoxon test.
pDC recruitment to B16 melanoma after Aldara treatment is CCR6-dependent. (A) B16 melanoma tumors of WT and CCR6-deficient mice were treated daily with Aldara or vehicle cream for 6 consecutive days. At day 7, harvested tumors after purification of CD45+ immune cells by MACS sorting were analyzed for the frequency of B220+ BST2+ pDC infiltration after gating on viable (DAPI−) and CD11c+/CD11b− DCs (see supplemental Figure 2B). The numbers of CD11c+ CD11b− BST2+ B220+ pDCs among total viable tumor cells were compared using R2.13.2 software (R foundation for Statistical Computing, Vienna, Austria, url:http://www.R-project.org) between WT animals and CCR6-deficient animals treated with Aldara or vehicle cream (n = 9 animals). (B) pDC-enriched cells from in vivo Flt3-L-expanded splenocytes of B6 donors were analyzed for the presence of pDCs (CD11c+ CD11b− BST2+ B220+). (C) pDC-enriched cells from WT Flt3-L-expanded splenocytes were labeled with CellTrace Violet and intravenously injected into recipients. The occurrence of donor pDCs in the recipient's spleen (top) and B16 tumor (bottom) preparation was analyzed 18 hours later (gate on CellTrace violet+ cells). (B-C) Data shown are representative for 5 animals of 2 independent experiments. (D) pDC-enriched cells from splenocytes of Flt3-L–treated WT or CCR6-deficient mice were labeled with CellTrace Violet and CFSE, respectively, for the pool CCR6−/−/WT (black bars). As a control, pDC-enriched cells from splenocytes of Flt3-L–treated B6 were labeled either with CellTrace Violet or CFSE, respectively, for the pool WT/WT (white bars). Cells were adjusted to equal number of pDCs and injected at a ratio 1:1 in B16-carrying B6 recipients that were previously treated with Aldara as described in panel A. After 18 hours, recipients were killed, tumors were processed as described in panel A, and the ratio of donor B6 (violet) and CCR6-deficient (CFSE) pDCs or donor B6 (violet) and donor B6 (CFSE) pDCs was analyzed in the recipient's spleen and tumors (mean ± SD; n = 5 recipients). Statistical significance was obtained with a nonparametric Wilcoxon test.
CCR6 and CCR10 are induced on blood pDCs exposed to IL-3 in vitro
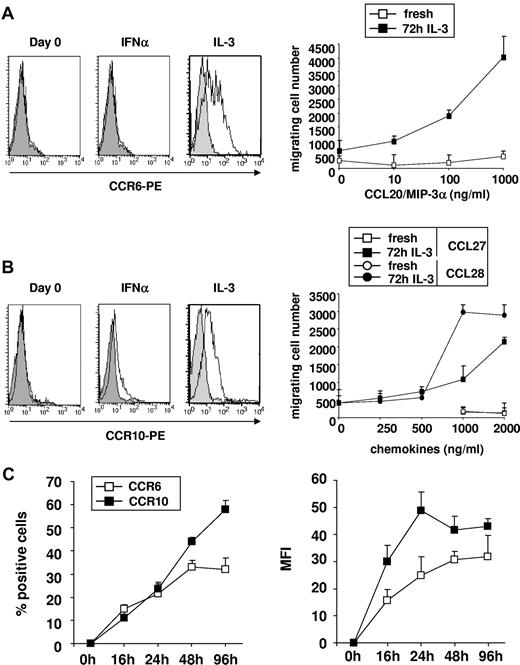
In agreement with their low CCR6 and CCR10 expression, freshly isolated blood pDCs did not migrate in response to CCL20 (Figure 4A) and CCL27/CCL28 (Figure 4B), respectively. However, we observed that, in response to IL-3 but not to IFN-α, both CCR6 and CCR10 were strongly induced on blood pDCs at 72 hours (Figure 4A-B). IL-3-induced CCR6 and CCR10 expression on pDCs was variable, ranging from 9.6% to 76.9% (41.5% ± 15.9%, n = 21) and from 9% to 78.4% (35.7% ± 17%, n = 17), respectively. Furthermore, CCL20, CCL27, and CCL28 induced potent migration of IL-3–cultured blood pDCs in a dose-dependent manner (Figure 4A-B). Optimal blood pDC expression of CCR6 and CCR10 was reached after 48- to 96-hour culture in presence of IL-3 (Figure 4C; supplemental Figure 4). Of note, CCR9 that was shown to mediate pDC recruitment into intestinal mucosa under steady-state and inflammatory conditions41 was not induced on human pDCs under these experimental conditions (not shown).
IL-3 induces CCR6 and CCR10 expression on purified blood pDCs as well as their migration in response to CCL20, CCL27, and CCL28 in vitro. (A) CCR6 and (B) CCR10 were measured on freshly isolated blood pDCs and cultured in the presence of IFN-α or IL-3 during 72 hours. Shading represents isotype control. Dose-response to (A) CCL20 and (B) CCL27 or CCL28 of freshly isolated and 72-hour IL-3–cultured blood pDCs in transwell assay. Results are expressed as number of migrating cells (mean of duplicate experimental points). (C) Percentages (left panel) and mean fluorescence intensity (right panel) of freshly isolated and IL-3–treated blood pDCs expressing CCR6 and CCR10 at various time points after culture. Data are representative of 3 independent experiments. In the experiment shown, pDC viability in IL-3 was 60%, 60%, 68%, and 57% at 16, 24, 48, and 96 hours, respectively.
IL-3 induces CCR6 and CCR10 expression on purified blood pDCs as well as their migration in response to CCL20, CCL27, and CCL28 in vitro. (A) CCR6 and (B) CCR10 were measured on freshly isolated blood pDCs and cultured in the presence of IFN-α or IL-3 during 72 hours. Shading represents isotype control. Dose-response to (A) CCL20 and (B) CCL27 or CCL28 of freshly isolated and 72-hour IL-3–cultured blood pDCs in transwell assay. Results are expressed as number of migrating cells (mean of duplicate experimental points). (C) Percentages (left panel) and mean fluorescence intensity (right panel) of freshly isolated and IL-3–treated blood pDCs expressing CCR6 and CCR10 at various time points after culture. Data are representative of 3 independent experiments. In the experiment shown, pDC viability in IL-3 was 60%, 60%, 68%, and 57% at 16, 24, 48, and 96 hours, respectively.
The response of cultured blood pDCs to CCL19 precedes that to CCL20
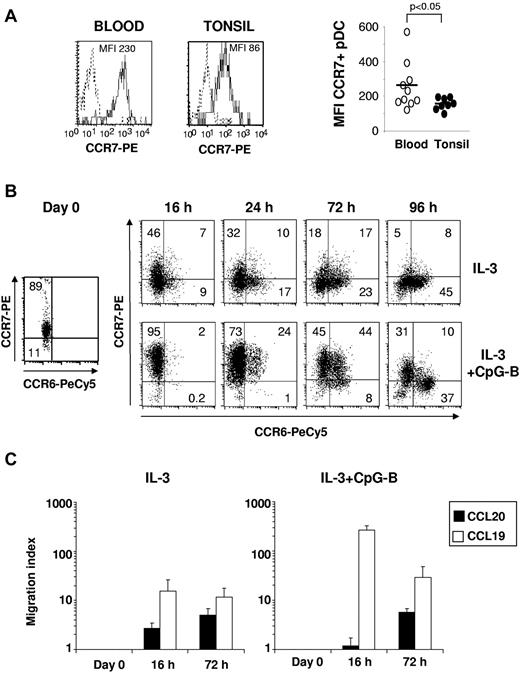
Blood pDCs expressed higher levels of CCR7 and CD62L (not shown) than tonsil pDCs (blood CCR7 mean fluorescence intensity = 263 ± 138 vs tonsil CCR7 mean fluorescence intensity = 153 ± 34, n = 8-10, P < .05; Figure 5A), suggesting the involvement of those receptors in pDC recruitment from blood into tonsils, as proposed for lymph nodes.4,6 Despite their constitutive CCR7 expression, freshly isolated blood pDCs did not migrate in response to CCL197 (Figure 5C). CCR7 was progressively down-regulated on blood pDCs after 24 hours in culture with IL-3– whereas CCR6 (Figure 5B) and CCR10 (supplemental Figure 5A) expression was detected after 16 hours and reached its maximun at 96 hours. After activation by IL-3 together with CpG-B, CCR7 expression by pDCs was highly increased and maximal already at 16 hours (Figure 5B-C). This rapid up-regulation of CCR7 preceded the acquisition of CCR6 and CCR10 to a lesser extent, which were optimally observed after 3 days of culture (Figures 4C and 5B; supplemental Figure 5A). CCR6 (Figure 5B) and CCR10 (supplemental Figure 5A) expression at these late time points was preferentially observed on pDCs that have lost their CCR7 expression. Consistent with CCR7 up-regulation, a strong response to CCL19 after 16 hours in IL-3 plus CpG-B ODN was observed (Figure 5B-C). Although IL-3 alone has no major effect on CCR7 expression, it also confers responsiveness to CCL19, but at lesser extent than IL-3 plus CpG-B (Figure 5C). Conversely, in correlation with CCR6 and CCR10 expression, a significant migration toward CCL20 and CCL28 was observed in both culture conditions only at later time points (72 hours), when CCR7 expression starts to decrease (Figure 5B-C; supplemental Figure 5B). Thus, unlike circulating mDC, blood pDCs seem to initially migrate in response to CCR7 ligands and then acquire the ability to respond to CCR6 and CCR10 ligands on exposure to IL-3 in tissues.
CCR7 expression and responsiveness to CCL19 are acquired before CCR6 expression and responsiveness to CCL20 on cultured blood pDCs. (A) Representative staining (left) and the percentages of CCR7+ cells (right) among CD123+ BDCA2+ pDCs in blood and tonsil are shown. Each point represents an independent donor (n = 8-10); and horizontal bars, means. The P value was obtained by a nonparametric Wilcoxon test. (B) CCR7 and CCR6 expression and (C) migratory capacities to CCL19 and CCL20 (1 μg/mL) were measured on freshly isolated, IL-3, and IL-3 + CpG-B-treated blood pDCs at various times after culture by flow cytometry and transwell assay, respectively. Percentages of positive cells are indicated in each dot plot (data are representative of 3 independent experiments), and pDC migration is shown as migration index (mean ± SD for 6 independent experiments).
CCR7 expression and responsiveness to CCL19 are acquired before CCR6 expression and responsiveness to CCL20 on cultured blood pDCs. (A) Representative staining (left) and the percentages of CCR7+ cells (right) among CD123+ BDCA2+ pDCs in blood and tonsil are shown. Each point represents an independent donor (n = 8-10); and horizontal bars, means. The P value was obtained by a nonparametric Wilcoxon test. (B) CCR7 and CCR6 expression and (C) migratory capacities to CCL19 and CCL20 (1 μg/mL) were measured on freshly isolated, IL-3, and IL-3 + CpG-B-treated blood pDCs at various times after culture by flow cytometry and transwell assay, respectively. Percentages of positive cells are indicated in each dot plot (data are representative of 3 independent experiments), and pDC migration is shown as migration index (mean ± SD for 6 independent experiments).
pDCs expressing CCR6 and CCR10 produce IFN-α in response to virus
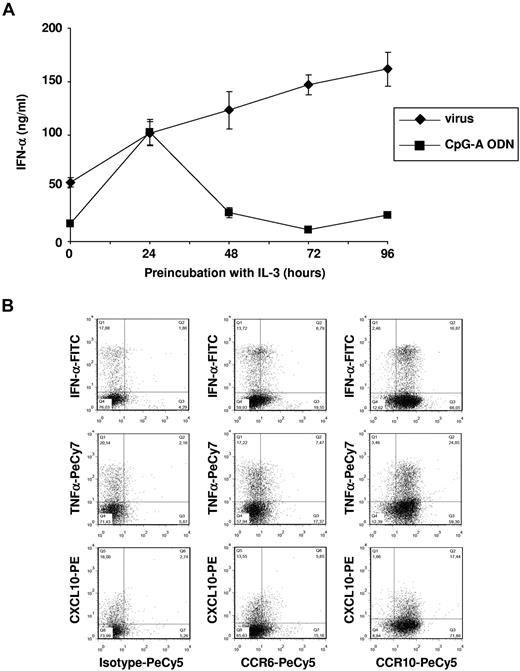
We next investigated the ability of pDCs to produce IFN-α in response to TLR ligands after culture in the presence of IL-3 inducing CCR6 and CCR10 expression. IFN-α production triggered by inactivated flu virus was already high at day 0 and persisted until day 4 (Figure 6A) when CCR6 and CCR10 expression was optimal (Figure 4A). In contrast, CpG-A–induced IFN-α production by freshly isolated pDCs was almost completely abolished 48 hours after culture with IL-3. This might be because of a differential effect of IL-3 up-regulating TLR7 and down-regulating TLR9 expression in cultured pDCs (not shown). In addition, we observed by intracellular staining that, besides IFN-α, IL-3–differentiated CCR6+ and CCR10+ pDCs produced TNF-α and CXCL10 in response to flu virus stimulation after 96 hours (Figure 6B). Altogether, these observations indicate that IL-3–differentiated pDCs, which acquire the ability to migrate in response to both CCR6 and CCR10 ligands, preserve their capacity to produce IFN-α, inflammatory cytokines, and chemokines on viral activation.
IL-3–differentiated CCR6+ CCR10+ blood pDCs preserve their ability to produce high levels of IFN-α, TNF-α, and CXCL10 in response to flu virus. (A) Freshly purified blood pDCs were cultured in IL-3 for different time periods before flu virus and CpG-A ODN2336 were added for another 24 hours. IFN-α was measured in the supernatants by ELISA. Data are mean ± SD of duplicate wells of culture and are representative of 3 independent experiments. (B) Intracellular production of IFN-α, TNF-α, and CXCL10 assessed by flow cytometry. pDCs were cultured in IL-3 for 96 hours and then activated with flu virus for an additional 6 hours. Percentages of each population are indicated in dot plots, and data are representative of 3 independent experiments.
IL-3–differentiated CCR6+ CCR10+ blood pDCs preserve their ability to produce high levels of IFN-α, TNF-α, and CXCL10 in response to flu virus. (A) Freshly purified blood pDCs were cultured in IL-3 for different time periods before flu virus and CpG-A ODN2336 were added for another 24 hours. IFN-α was measured in the supernatants by ELISA. Data are mean ± SD of duplicate wells of culture and are representative of 3 independent experiments. (B) Intracellular production of IFN-α, TNF-α, and CXCL10 assessed by flow cytometry. pDCs were cultured in IL-3 for 96 hours and then activated with flu virus for an additional 6 hours. Percentages of each population are indicated in dot plots, and data are representative of 3 independent experiments.
Discussion
pDC biology is central to the regulation of a wide range of inflammatory immune responses, yet relatively little is known about the dynamics of their accumulation in inflamed peripheral tissues, where they may participate in the maintenance of chronic inflammation or to the clearance of viral infections.42
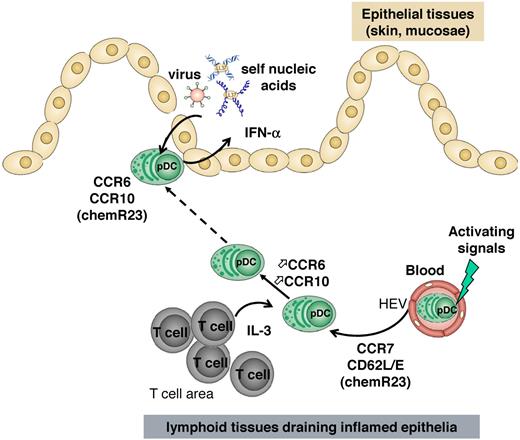
In the present study, we demonstrated for the first time that a subset of tonsil pDCs and IL-3–cultured blood pDCs express functional CCR6 and CCR10 chemokine receptors that may participate in their recruitment into inflamed epithelial tissues expressing their respective ligands CCL20 and CCL27/CCL28. Why a minute percentage of tonsil pDCs express CCR6/CCR10, whereas the great majority of pDCs exposed in vitro to IL-3 ± CpG become CCR6/CCR10-positive is still unknown. This could be explained by (1) the absence of inflammation when tonsils are usually resected, (2) less accessibility to IL-3 in vivo than in vitro, and (3) integration of different signals from the environment by pDCs in vivo that are more complex than on in vitro culture. Indeed, the microenvironment could deliver negative signals counteracting the induction of CCR6 and CCR10 by IL-3, as already reported for NKp44 induction on pDCs.43 We also provide the primary evidence that, unlike mDCs, the response of pDCs to CCR7 ligand (CCL19) precedes that to CCR6/10 ligands (CCL20 and CCL27). Such findings lead us to suggest the following model (Figure 7): consecutive to an initial CCR7-mediated recruitment from blood into lymphoid tissues draining inflamed epithelia, pDCs might be conditioned to acquire CCR6 and CCR10 expression, which in turn orchestrate their migration to mucosal or skin inflamed epithelia. In such tissues, pDCs could play an effector function through their persistent IFN-α production during viral/bacterial entry or inflammatory reactions. This mechanistic understanding is particularly relevant because of the pivotal role of pDCs in the triggering and maintenance of inflammatory reactions associated with autoimmune diseases, such as psoriasis and systemic lupus erythematosus.14,19,20 Furthermore, considering the expression of CCL21 on activated dermal vessels in some T-cell infiltrative autoimmune diseases of the skin, such as atopic dermatitis and lichen planus, CCR7 could also support blood pDC recruitment to the inflamed skin directly.44 However, CCL21 expression was not detected in dermal vessels of psoriatic skin.45 Thus, according to the disease, CCR7 may be involved or not in pDC recruitment to inflamed skin.
Model for pDC recruitment into inflamed epithelial sites. On their recruitment into lymphoid organs in a manner that involves CCR7 and CD62L, pDCs might be instructed through IL-3 released by local T cells to express CCR6 and CCR10. Such instructed pDCs thus respond to CCL20 and CCL27/CCL28 mediating their migration into epithelial areas of mucosa or skin where they can sense viruses or self nucleic acids and produce IFN-α participating in viral clearance or inflammatory reactions.
Model for pDC recruitment into inflamed epithelial sites. On their recruitment into lymphoid organs in a manner that involves CCR7 and CD62L, pDCs might be instructed through IL-3 released by local T cells to express CCR6 and CCR10. Such instructed pDCs thus respond to CCL20 and CCL27/CCL28 mediating their migration into epithelial areas of mucosa or skin where they can sense viruses or self nucleic acids and produce IFN-α participating in viral clearance or inflammatory reactions.
Among DC subsets, CCR6 expression is known to be restricted to immature mDCs characterized by their skin or mucosal homing capacity.23,26,33,46 In addition, both CCR6 and CCR10 are expressed on a subset of memory T cells exhibiting skin homing properties24,27,28,47 as well as on mucosal epithelial tissue IgA-secreting B cells.29 The present study supports the idea that such chemokine receptors also regulate pDC recruitment into inflamed epithelial tissues. In this line, results provided here demonstrate that: (1) ex vivo a subset of human pDCs coexpress CCR6 and CCR10 in inflamed tonsils, (2) in situ human pDCs are in close vicinity to CCL20- and CCL27-expressing epithelial cells in inflamed tissues (tonsils and psoriatic and virally induced skin lesions), (3) the recruitment of CCR6-deficient pDCs to skin tumors is impaired under inflammatory conditions, and (4) pDCs derived from CCR6-deficient donors are impaired in homing to skin tumors once adoptively transferred to WT recipients, in accordance with the hypothesis that CCR6 is required for pDC skin homing. Our observations are in agreement with the recent description of CCR6 up-regulation on (1) leukemic pDCs shown to home to cutaneous lesions21 and (2) blood pDCs from melanoma patients48 whose tumors expressing high levels of CCL20 are known to be infiltrated by pDCs.49 Furthermore, as we show that CCL20 and CCL27 gradients are distinct in epithelial tissues (basal vs differentiated epithelial cells), CCR6 and CCR10 may not have completely redundant roles. Therefore, it seems possible that CCR10 ligands drive pDC recruitment at dermal/epidermal junction, whereas CCL20 gradient allows skin epithelium infiltration. Indeed, immobilization of CCL27 on dermal extracellular matrix and fibroblasts may sustain a chemokine gradient directing tissue-infiltrating pDCs from perivascular pockets to subepidermal locations, as for skin-infiltrating T cells.27 This nonredundancy could also be relevant in mucosa, as a recent study has shown a marginal role, if any, for CCR6 in mediating pDC homing into inflamed lungs on RSV infection.50 In this model, CCL28 might overcome the need for CCR6 for an efficient pDC recruitment as its expression was reported to be increased in both human and mouse lung inflammation during asthma51 and viral bronchiolitis.52 Furthermore, given (1) the role of CCR9 in controlling pDC migration to the intestinal mucosa under both steady-state and inflammatory conditions41 and (2) the identification of a CCR9-expressing pDC subset that resides in peripheral lymphoid tissues,41,53 we control the expression of CCR9 on human pDCs. We were not able to detect any CCR9 expression on human pDCs isolated from blood or tonsil and whatever their activation status, excluding a role for this receptor in our system. Thus, together with their expression of skin and mucosal addressin CLA and α4β7 (data not shown),8,12 pDCs are well equipped to reach inflamed epithelial tissues.
Our study also reveals an unexpected sequence of CCR7 versus CCR6/CCR10 up-regulation on pDCs that contrasts with that observed on mDCs. Indeed, during their differentiation process, mDCs express CCR6 involved in their recruitment into inflamed peripheral tissues.26 There, on activation they down-regulate CCR6 and acquire CCR7, allowing their entry into the lymph stream and their migration to the draining lymph node through gradient of CCR7 ligands.23 Our observations that (1) blood pDCs express constitutively high levels of CCR7, in agreement with recent observations in mice showing that already nonstimulated naive pDCs express CCR710 and (2) pDCs rapidly (< 6 hours) up-regulate CCR7 expression and responsiveness to its ligands after activation while their optimal expression of CCR6 and CCR10 required > 2 days suggest an opposite scenario. Indeed, during viral infection in peripheral epithelial tissues, inflammatory mediators, cellular or viral genetic material (CpG-containing DNA, RNA or DNA virus) may reach blood CCR7+ CD62L+ pDCs, inducing rapid responsiveness to CCR7 ligands. After rolling and attachment to high endothelial venule through E and L selectins (CD62E and CD62L),54 sensitized pDCs may transmigrate into lymphoid tissues in response to CCL21 exposed in the blood lumen of high endothelial venule, as demonstrated in lymph nodes6,9 and home to T-cell areas.4,6 Within T-cell areas, recruited pDCs can be instructed through IL-3 released by local memory CD8 T cells43 or pDCs-primed T cells through ICOS/ICOS-L interaction55 to express CCR6 or CCR10 enabeling their trafficking to the mucosal-associated lymphoid tissue epithelium, such as tonsillar epithelial crypts. This is consistent with (1) the low frequency of CCR6+ and CCR10+ pDCs in the blood and (2) the expression of both CCR6 and CCR10 on a subset of tonsillar pDCs. Whether pDCs migrating into lymph nodes may, like naive T cells, have the capacity to egress through the efferent lymph stream and the thoracic duct into the blood circulation is an important issue that remains to be investigated. On infection or inflammation, a scenario comparable with primed memory T cells acquiring CCR6 and CCR1056 may apply to pDCs. In lymph nodes, instructed pDCs acquiring CCR6 and CCR10 would afterward reach the bloodstream where they may represent the small subset of blood pDCs expressing both CCR6 and CCR10, and then access the inflamed epithelia and enter the site of viral dissemination or inflammation. Indeed, it was recently described in melanoma patients that blood pDCs exhibit a high CCR6 expression, which was proposed to mediate their homing to the tumor site.48 Because CCR6- and CCR10-expressing pDCs keep their ability to produce IFN-α, after their recruitment in peripheral epithelia, they might play their effector function limiting viral spreading and increasing local inflammation. pDCs, through IFN-α production and Ag presentation, are thought to be important in bridging innate and adaptive immune responses; thus, rapid recruitment to sites of inflammation might be critical for this role. In this context, it is interesting to note that pDCs have been recently shown to induce the differentiation of naive CD4+ T cells into IL-22–producing T cells that are skin-homing memory CD4+ T cells expressing CCR6 and CCR10.31,32 The present scenario of pDC migration challenges the potential capacity of pDCs to present antigens from inflamed peripheral sites to T cells in the draining lymphoid organs.
In conclusion, we identified CCR6 and CCR10 as new chemokines receptors governing pDC trafficking into inflamed epithelia and also an intriguing migratory sequence for pDCs requiring their recruitment into lymphoid tissues before their redistribution into epithelial sites. The functional role of pDCs in several physiopathologic situations related to tolerance induction or chronic inflammation depending on the context is well established.19,42 Therefore, understanding how pDCs are recruited from the blood to tissues opens new opportunities for the development of therapeutic strategies mobilizing pDCs to modulate immunity.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank doctors and colleagues from clinics and hospitals who provide us with blood and tissues samples, Dr Jenny Valladeau-Guilemond and Michael Gobert for their critical reading of the manuscript, and Anthony Besse for help with statistical analyses.
This work was supported by Association pour la Recherche contre le Cancer, Comités départementaux de Saône-et-Loire et du Rhône de la Ligue nationale contre le cancer, Institut National du Cancer (grants INCA ACI-63-04, ACI 2007-2009, PL116, and PL-969-017), Breast Cancer Research Foundation, and BioPole program DEMINAP. V.S. was supported by the Région Rhône-Alpes and Association pour la Recherche sur le Cancer.
Authorship
Contribution: V.S., N.V., B.V., T.D., I.P., G.T., A.P., J.-Y.B., C.C., and N.B.-V. conceived and designed the experiments; V.S., N.V., B.V., T.D., I.P., and N.B.-V. performed the experiments; V.S., N.V., B.V., T.D., G.T., B.D., C.C., and N.B.-V. analyzed the data; B.H., E.P.B., B.D., D.K., and S.A.L. contributed reagents/materials/analysis tools; and V.S., C.C., and N.B.-V. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Nathalie Bendriss-Vermare, Centre de Recherche en Cancérologie de Lyon, Unité Mixte de Recherche Inserm 1052–Centre National de la Recherche Scientifique 5286, Centre Léon Bérard, 28 rue Laennec, 69373 Lyon cedex 08, France; e-mail: nathalie.bendriss-vermare@lyon.unicancer.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal