Abstract
HSCs are defined by their ability to self-renew and maintain hematopoiesis throughout the lifespan of an organism. The optical clarity of their embryos and the ease of genetic manipulation make the zebrafish (Danio rerio) an excellent model for studying hematopoiesis. Using flow cytometry, we identified 2 populations of CD41-GFP+ cells (GFPhi and GFPlo) in the whole kidney marrow of Tg(CD41:GFP) zebrafish. Past studies in humans and mice have shown that CD41 is transiently expressed in the earliest hematopoietic progenitors and is then silenced, reappearing in the platelet/thrombocyte lineage. We have transplanted flow-sorted GFPhi and GFPlo cells into irradiated adult zebrafish and assessed long-term hematopoietic engraftment. Transplantation of GFPhi cells did not reconstitute hematopoiesis. In contrast, we observed multilineage hematopoiesis up to 68 weeks after primary and secondary transplantation of GFPlo cells. We detected the CD41-GFP transgene in all major hematopoietic lineages and CD41-GFP+ cells in histologic sections of kidneys from transplant recipients. These studies show that CD41-GFPlo cells fulfill generally accepted criteria for HSCs. The identification of fluorescent zebrafish HSCs, coupled with our ability to transplant them into irradiated adult recipients, provide a valuable new tool to track HSC homing, proliferation, and differentiation into hematopoietic cells.
Introduction
Since the classic experiments of McCulloch and Till, HSCs have been defined by their ability to self-renew and maintain hematopoiesis after transplantation into irradiated recipients.1-3 HSCs have been identified in murine and human BM by flow cytometry using combinations of cell-surface markers or the efflux of the fluorescent vital dye Hoechst 33342.4-9 Although there are techniques for the short-term and long-term culture of human and murine hematopoietic progenitors and HSCs, the reconstitution of multilineage hematopoiesis after transplantation of a putative stem cell into an irradiated recipient remains the generally accepted best definition of a HSC.
The zebrafish, Danio rerio, is becoming an increasingly popular model for the study of hematopoietic development, although to date, there have been no definitive studies characterizing zebrafish HSCs. Murayama and colleagues have tracked the movement of zebrafish CD41-GFP+ cells from the ventral wall of the dorsal aorta (aortic gonadal mesonephric region [AGM]) to a newly discovered posterior blood island thought to be the site of definitive hematopoiesis—analogous to mammalian fetal liver, and then to the developing mesonephros.10 The same group traced the migration of zebrafish CD41-GFP+ cells from the AGM, via the axial vein, to the caudal hematopoietic organ and then, to the fetal thymus.11 Bertrand and colleagues demonstrated the migration of CD41+c-myb+ cells to the zebrafish pronephros and tracked their migratory route along the pronephric tubules to the interstitial space which serves as the marrow niche in fish.12 Two recent studies have used zebrafish to visualize the movement of putative HSCs from the aortic endothelium into the circulation.13,14 These studies all suggest, but do not prove, that a subset of zebrafish CD41-GFP+ cells is likely to be either a HSC or an early hematopoietic progenitor.
The studies by Murayama et al10 and Kissa et al11 and the recent study by Bertrand at al13 were carried out with a transgenic zebrafish strain developed in our laboratory. The strain was engineered by driving expression of the GFP reporter with the platelet/thrombocyte-specific CD41 promoter. In our original publication, we noted 2 populations of CD41-GFP+ cells in suspensions of whole kidney marrow and reported that they could be separated by flow cytometry based on their different intensity of fluorescence.15 We also reported that GFPhi cells looked like mature thrombocytes by morphology, while the GFPlo cells looked like immature cells of uncertain lineage.
Since previous studies in humans and mice have shown that CD41 is transiently expressed in the earliest hematopoietic progenitors and is then silenced16,17 ; Robin et al showed that CD41 is developmentally regulated and differentially expressed on mouse HSCs.18 We reasoned that the GFPlo cells we had previously identified might either be hematopoietic progenitors or HSCs. To test this hypothesis, we have transplanted flow-sorted GFPhi and GFPlo cells into γ-irradiated adult AB strain zebrafish. The GFPhi cells were no longer detected 1 month after transplantation. In contrast, we have observed long-term engraftment and multilineage reconstitution for over a year after transplantation of GFPlo cells into zebrafish, conditioned with 25 Gy γ-irradiation from a 137Cesium source. In this study, we will present evidence that CD41-GFPlo cells are bona fide HSCs capable of sustaining long-term hematopoiesis in both primary and secondary zebrafish recipients.
Methods
Zebrafish strains and maintenance
Zebrafish, Danio rerio, were laboratory-bred and reared at 28.5°C under a photoperiod of 14 hours of light/10 hours of darkness and fed commercial feed. Four- to 10-month-old wild-type AB fish were used as transplant recipients. The Tg(CD41:GFP) line, which was engineered onto an AB background, were donors. After irradiation and/or transplantation, fish were isolated for 24-48 hours without food in static aquarium water containing 250 μL/L “Stress Coat” (Aquarium Pharmaceuticals Inc) and were then transferred into isolation cages. All studies using the zebrafish model were approved by the Brigham and Women's Hospital Animal Care and Use Committee.
Gamma irradiation
Recipient zebrafish were anesthetized with 0.01% tricaine, then irradiated using a 137Cesium source (Gammacell 1000; Best Theratronics). Irradiation was conducted 24-72 hours before transplantation.19
Cell collection
Donor kidneys were dissected from CD41-GFP zebrafish as previously described.19 Wild-type AB zebrafish whole kidney marrow (WKM) was harvested and admixed with flow-sorted CD41-GFPlo cells in some of the transplant experiments. Citrate phosphate dextrose solution (CPD) was used as anticoagulant. WKM cells were processed by successive filtration, centrifugation and washing in 0.9× PBS supplemented with 5% FCS and CPD.
Isolation of CD41-GFP+ cells by flow cytometry
Propidium iodide (PI; Sigma-Aldrich) was added to single-cell suspensions of WKM at a final concentration of 1 μg/mL. FACS analysis and sorting were performed based on PI exclusion, and forward and side light scattering using a FACSVantage flow cytometer (BD Biosciences) at the FACS Core Facility, Children's Hospital Boston. Cells were kept on ice after sorting and before transplantation. Peripheral blood and kidney samples from transplanted fish were isolated, washed, and resuspended, as described in “Cell collection,” and analyzed by flow cytometry. Controls included cells collected from wild-type nontransplanted and wild-type fish that had been transplanted but had not engrafted.
Analysis of Hoechst staining by flow cytometry
WKM cells were obtained from 15-40 Tg(CD41:GFP) zebrafish, as previously described.20 Erythrocytes were depleted by briefly pipetting the WKM cells in 1-5 mL of sterile water, followed by immediate addition of 2 volumes of 2× cold 0.9× PBS/2% FBS. Before staining, cells were resuspended at a concentration of 106/mL. Hoechst 33342 dye (Sigma-Aldrich) was added at a final concentration of 1, 3, 5, 7.5, and 10 μg/mL.21,22 All samples were incubated for 60, 90, and 120 minutes at 28°C or 37°C in a waterbath, protected from light. In parallel, 1 × 106 cells were stained with Hoechst 33342 along with 50μM verapamil (Sigma-Aldrich). PI was added to all samples at 2 μg/mL final concentration to exclude nonviable cells. SP cells were detected in a FACSVantage flow cytometer (BD Biosciences). Flow cytometry data were analyzed using FlowJo software Version 7.6 (TreeStar Inc).
Adult zebrafish transplantation
Anesthetized fish were placed on a sponge, with the heart exposed by lightly squeezing the animal near the pectoral fins. Donor cells were transplanted by intracardiac injection with a 10-μL syringe (Hamilton Company) equipped with a 31-gauge needle. Initially, 16 000 flow-purified GFPlo or GFPhi cells were transplanted along with 0.8 × 106 unsorted wild-type WKM “carrier” cells. Subsequent transplantation of flow-sorted GFPlo cells was carried out after 25 or 35 Gy irradiation with from 10 to 9000 CD41-GFPlo cells per fish. Each of the surviving fish was anesthetized with 0.01% tricaine 30 days after the transplantation and examined under an inverted fluorescent microscope (Leica DM-IRE2) for the presence of circulating GFP+ cells. The presence of GFP+ cells in transplant recipients was taken as evidence of engraftment.
Genomic DNA extraction and PCR analysis
Genomic DNA was extracted from peripheral blood, spleen, and whole kidney marrow as well as from flow-sorted cell populations using the REDExtract-N-Amp Tissue and Blood PCR Kit (Sigma-Aldrich). The primers used were specific for the CD41-GFP transgene as previously reported.15 Amplification conditions were 94°C for 3 minutes followed by 32 cycles at 94°C for 30 seconds, 55°C for 1 minute, 72°C for 1 minute, and a final extension at 72°C for 10 minutes. The amplification products were examined by electrophoresis on 0.8% agarose gel and stained with ethidium bromide. All samples were analyzed with a negative control (wild-type AB fish), a positive control (CD41-GFP fish), and samples from irradiated transplanted fish that had not engrafted. We also tested all samples with primers for the zebrafish elongation factor 1 α (EF1α) gene. The EF1α primers were: (1) forward primer: cggtgacaacatgctggagg; (2) reverse primer: accagtctccacacgaccca.
The percentage of chimerism was determined by quantitative real-time PCR using an ABI PRISM 7700 Real Time PCR System (Applied Biosystems). The SYBR Green ER Supermix reaction mixtures contained diluted genomic DNA, 2× SYBR Green ER Supermix, 7.5 mmol/liter of each gene-specific primer and nuclease-free water to a final volume of 20 μL. PCR thermal-cycle conditions were as follows: an initial step at an initial step at 95°C for 10 minutes, followed by 40 cycles at 95°C for 15 seconds, 60°C for 60 seconds, and 72°C for 30 seconds. The specificity of the PCR products was controlled by melting curve analysis. The comparative threshold cycle (CT) method and an internal control (EF1α) were used to normalize donor-specific gene expression.
Cytology
For cytologic studies, 0.5∼1 × 105 cells were centrifuged onto glass slides using the Shandon Cytospin 3 centrifuge (GMI Inc). Sorted GFP+ cells were kept in 0.9× PBS with 5% FCS, centrifuged at 200g onto poly-L-lysine–coated cover slips placed at the bottom of the wells of a 48-well microtiter plate. The coverslips were immediately transferred onto a microscope slide that allowed the cells to be kept in media while being observed under an inverted fluorescent microscope (Leica DM-IRE2).
Thin-section electron microscopy
Cells were fixed with 1.5% glutaraldehyde in 0.1M cacodylate buffer, pH 7.4, for 8 hours. Cells were dehydrated through a series of alcohols, infiltrated with propylene oxide, and embedded in epoxy resin in an inverted beam capsule. Ultrathin sections were stained with uranyl acetate and lead citrate, and examined with a Tecnai G2 Spirit BioTWIN transmission electron microscope (Dymek Company) at an accelerating voltage of 80 kV. Images were recorded with an AMT 2k CCD camera (Advanced Microscopy Techniques).
Histology, in situ hybridization, and immunohistochemistry
Tissues were processed and embedded and paraffin sections (4-μm thick) were prepared by the Specialized Histopathology Core Facility at Dana-Farber/Harvard Cancer Center. Riboprobes were prepared by in vitro transcription of the linearized plasmid containing the CD41 promoter linked to the EGFP cDNA. The final concentration of the cDNA templates was 1 mg/ml. Riboprobes were synthesized and labeled using a DIG RNA labeling kit (Roche Molecular Biochemicals). The efficiency of transcription and the incorporation of digoxigenin-UTP into the riboprobes were evaluated by 5% acrylamide-urea gel electrophoresis and dot blotting on nylon membranes, respectively (DIG Nucleic Acid Detection Kit-NBT/BCIP; Roche Molecular Biochemicals).
Automated in situ hybridization (ISH) was performed on the Discovery System (Ventana Medical Systems). After paraffin removal, slides were fixed in 4% paraformaldehyde for 10 minutes and digested in a 10 μg/mL solution of proteinase K for 10 minutes at 37°C. Slides were hybridized at 65°C for 6 hours, washed twice in 0.1× SSC buffer at 75°C for 6 minutes. Transcripts were detected with biotinylated antidigoxigenin Ab (Biogenex) followed by streptavidin-alkaline-phosphatase conjugate and visualized by the NBT/BCIP substrate reaction (Ventana BlueMap Detection Kit; Ventana Medical Systems). For immunohistochemistry, slides were incubated with 1:1500 anti-GFP Ab (monoclonal mouse raised against full-length GFP Living Colors A.v. Antibody; Clontech) for 1 hour and then developed with the DAKO Envision Detection Kit.
Imaging
Flow-sorted GFP+ cells were observed at room temperature on a Nikon TE 2000 Eclipse microscope equipped with either a 10× (NA 0.3) or 60× (NA 1.4) Nikon objective and a 100-W mercury lamp. Images were acquired with a Hamamatsu Orca IIER CCD camera. Electronic shutters and image acquisition were under the control of Molecular Devices Metamorph software (MDS Analytical Technologies). Images were acquired by fluorescence microscopy with an image capture time of 200-500 ms.
Freshly dissected kidneys of Tg(CD41:GFP) fish were analyzed at room temperature using a PerkinElmer UltraView Vox spinning disk confocal microscope at Children's Hospital Boston IDDRC Imaging Core Facility. Images were acquired on a Hamamatsu EMCCD C9100-50 camera equipped with either 10× Nikon CFI Plan Fluor dry (NA 0.3) or 60× Nikon CFI Plan Apo oil (NA 1.4). Data were processed using Volocity 5.2 (PerkinElmer).
Statistics
The Kaplan-Meier survival analysis with the log-rank test, along with all statistic analysis was carried out in GraphPad Prism 5 (GraphPad Software).
Results
Flow-sorting of CD41-GFP+ thrombocytes and progenitors
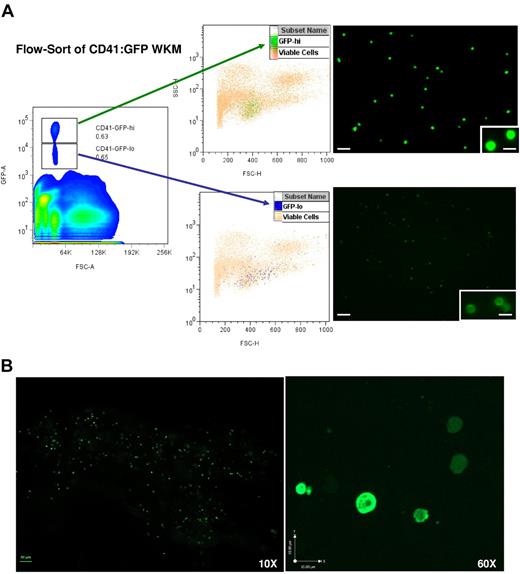
Figure 1 shows the results of flow-sorting single-cell suspensions of whole kidney marrow (WKM) obtained from Tg(CD41:GFP) donor fish. As shown in the left panel, there were 2 major populations of GFP+ cells. We have previously reported that the more intensely fluorescent (GFPhi) cells were thrombocytes, the zebrafish equivalent of mammalian platelets. For the transplantation studies outlined here, we collected the GFPhi and GFPlo cell populations separately for subsequent injection. Each of the flow-sorted samples was routinely reanalyzed to ensure that it contained 85%-90% GFP+ cells. Some WKM preparations required 2 or 3 rounds of sorting to reach this degree of purity.
Isolation of GFPhi and GFPlo cells from CD41-GFP transgenic zebrafish by flow cytometry. (A) The left panel shows the distribution of CD41-GFP+ cells in single-cell suspension of WKM derived from Tg(CD41:GFP) zebrafish. The frames outline the gatings used to define the CD41-GFPlo and CD41-GFPhi subsets. Viable cells were selected based on propidium iodide exclusion. FSC-A indicates forward scatter; and SSC-H, side scatter. The middle panels locate the CD41-GFPlo and CD41-GFPhi cells on scatter plots of viable cells derived from WKM. FSC-H indicates forward scatter; and GFP-A, GFP-positive. The right panels are fluorescent micrographs at 10× and 60× (inset) of flow-sorted CD41-GFPlo and CD41-GFPhi cells. (B) The direct visualization of CD41-GFPlo and CD41-GFPhi cells in the kidney of Tg(CD41-GFP) fish at 10× (scale bar = 50 μm) and 60× magnification (scale bar = 10 μm).
Isolation of GFPhi and GFPlo cells from CD41-GFP transgenic zebrafish by flow cytometry. (A) The left panel shows the distribution of CD41-GFP+ cells in single-cell suspension of WKM derived from Tg(CD41:GFP) zebrafish. The frames outline the gatings used to define the CD41-GFPlo and CD41-GFPhi subsets. Viable cells were selected based on propidium iodide exclusion. FSC-A indicates forward scatter; and SSC-H, side scatter. The middle panels locate the CD41-GFPlo and CD41-GFPhi cells on scatter plots of viable cells derived from WKM. FSC-H indicates forward scatter; and GFP-A, GFP-positive. The right panels are fluorescent micrographs at 10× and 60× (inset) of flow-sorted CD41-GFPlo and CD41-GFPhi cells. (B) The direct visualization of CD41-GFPlo and CD41-GFPhi cells in the kidney of Tg(CD41-GFP) fish at 10× (scale bar = 50 μm) and 60× magnification (scale bar = 10 μm).
The middle panel of Figure 1 superimposes the sorted GFPhi and GFPlo subpopulations on the complete spectrum of viable WKM cells. Consistent with previously published data, 0.81 ± 0.41% of the WKM cells were GFPhi cells, while 0.79 ± 0.30% of the cells were the more dimly fluorescent GFPlo cells.15 The GFPhi population is fairly homogeneous, while the GFPlo subpopulation is somewhat more heterogeneous, with wider variation in cell size as measured by forward light scatter. The right panel contains fluorescent photomicrographs of the 2 subpopulations, showing the difference in fluorescence intensity at low (10×) and high (60×) magnification. The observation was reproduced when the kidney of a Tg(CD41:GFP) fish was visualized by confocal imaging (Figure 1B).
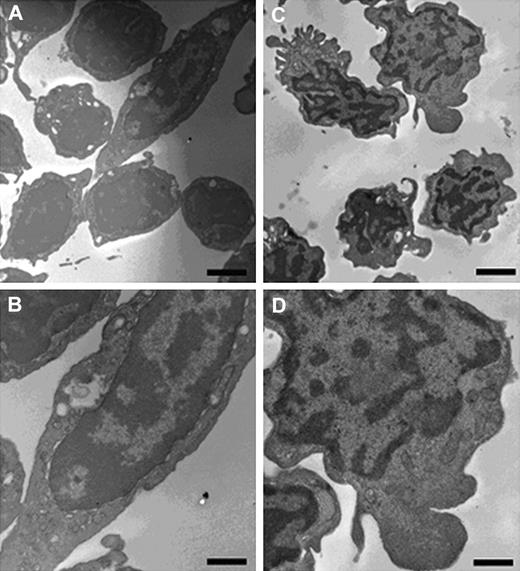
When viewed under the electron microscope (Figure 2), CD41hi cells showed the key features expected of a mature thrombocyte, including the characteristic surface-connected canalicular system and a cytoplasm packed with numerous small granules (Figure 2A-B). The CD41lo cells, in contrast, had an irregular shape with small protrusions (Figure 2C-D). Ribosomes and mitochondria were observed at the rim of cytoplasm surrounding the nucleus. These cells lacked the granules observed in the CD41hi population. The nuclei were more irregular in shape and there were prominent bands of heterochromatin at the margin of the nucleus seen in noncycling cells.23 The heterochromatin is more fragmented throughout the nucleus and less marked at the margin. In short, CD41lo cells exhibited more undifferentiated ultrastructural morphology.
Ultrastructural morphology of CD41hi and CD41lo cells. Transmission electron microscopy of CD41hi cells (A, low magnification; B, high magnification) shows the key features of thrombocytes, including the characteristic surface-connected canalicular system and a cytoplasm packed with numerous small granules. Ultrastructural examination of CD41lo cells (C, low magnification; D, high magnification) shows an immature phenotype. Cells were generally homogeneous in appearance showing an irregular shape with small protrusions. Ribosomes and mitochondria were observed in the rim of cytoplasm surrounding the nucleus. However, these cells lacked the numerous granules observed in the more mature CD41hi thrombocytes. The nuclei were more irregular in shape and a prominent band of heterochromatin at the margin of the nucleus was observed in the majority of cells. The heterochromatin appears more fragmented through the nucleus and less marked at the margin. Scale: A,C: bar = 2 μm; B,D: bar = 1 μm.
Ultrastructural morphology of CD41hi and CD41lo cells. Transmission electron microscopy of CD41hi cells (A, low magnification; B, high magnification) shows the key features of thrombocytes, including the characteristic surface-connected canalicular system and a cytoplasm packed with numerous small granules. Ultrastructural examination of CD41lo cells (C, low magnification; D, high magnification) shows an immature phenotype. Cells were generally homogeneous in appearance showing an irregular shape with small protrusions. Ribosomes and mitochondria were observed in the rim of cytoplasm surrounding the nucleus. However, these cells lacked the numerous granules observed in the more mature CD41hi thrombocytes. The nuclei were more irregular in shape and a prominent band of heterochromatin at the margin of the nucleus was observed in the majority of cells. The heterochromatin appears more fragmented through the nucleus and less marked at the margin. Scale: A,C: bar = 2 μm; B,D: bar = 1 μm.
Transplantation of GFPlo cells provides radioprotection and improves the survival of lethally irradiated fish
In our initial studies, we conditioned recipient fish with either 25 or 35 Gy total body irradiation (TBI) and then transplanted 105 flow-sorted GFPlo cells along with 5-8 × 105 wild-type WKM “carrier” cells to provide short-term radioprotection. However, despite hematopoietic engraftment, the cohort of fish that had received 35 Gy TBI had very high mortality and only 5% of these fish survived 30 days after transplantation (data not shown). In contrast, 60% of the fish that were conditioned with 25 Gy survived 30 days after transplantation.
In all of the subsequent studies, we used the 25Gy “sublethal” conditioning dose of radiation and transplanted of flow-sorted GFPlo cells without the addition of any WKM carrier cells. We assumed that the surviving engrafted fish would be hematopoietic chimeras with varying mixtures of donor (GFP+) and recipient (GFP−) hematopoietic cells. We also assumed that any cells derived from transplanted GFPlo cells would carry the CD41-GFP transgene, but that only HSCs and thrombocytes or, perhaps, their precursors (prothrombocytes) would express the GFP protein and, therefore, fluoresce. Transplant recipients were scored as having engrafted, if there were circulating green fluorescent cells (thrombocytes) 1 month after transplantation. We recognize that this may underestimate the number of engrafted fish as we might have missed fish with low numbers of circulating thrombocytes.
As shown in Table 1, when 16 000 GFPlo cells were transplanted along with 8 × 105 WKM “carrier” cells, 15 of 18 fish (83%) were alive 3 months after transplantation and 10 of the 15 survivors (67%) had engrafted. As progressively fewer GFPlo cells were transplanted, without any “carrier” marrow cells, mortality increased. For example, 3 months after the infusion of 100 GFPlo cells, only 19% of the transplanted fish were alive. However, 64% of the surviving fish had engrafted. In subsequent studies, we found that 44% of the surviving fish receiving 50 GFPlo cells engrafted, and 15% of surviving fish that had received only 10 GFPlo cells engrafted.
Transplantation with flow-sorted CD41-GFPlo cells
| GFPlo cells/recipient . | Survival (%) . | Engrafted (%) . |
|---|---|---|
| 16 000* | 15/18 (83) | 10/15 (67) |
| 4000* | 19/30 (40) | 12/19 (63) |
| 2700 | 39/64 (61) | 16/39 (41) |
| 2000* | 61/137 (45) | 50/61 (82) |
| 1000 | 58/119 (49) | 23/58 (48) |
| 200 | 23/84 (27) | 11/23 (48) |
| 100 | 14/72 (19) | 9/14 (64) |
| 50 | 23/109 (21) | 10/23 (44) |
| 10 | 20/95 (21) | 3/20 (15) |
| GFPlo cells/recipient . | Survival (%) . | Engrafted (%) . |
|---|---|---|
| 16 000* | 15/18 (83) | 10/15 (67) |
| 4000* | 19/30 (40) | 12/19 (63) |
| 2700 | 39/64 (61) | 16/39 (41) |
| 2000* | 61/137 (45) | 50/61 (82) |
| 1000 | 58/119 (49) | 23/58 (48) |
| 200 | 23/84 (27) | 11/23 (48) |
| 100 | 14/72 (19) | 9/14 (64) |
| 50 | 23/109 (21) | 10/23 (44) |
| 10 | 20/95 (21) | 3/20 (15) |
Transplantation with addition of 5-8 × 105 wild-type WKM cells.
In contrast, when 10 000 unsorted WKM cells (Table 2) from Tg(CD41:GFP) donor fish were transplanted, 18 of 51 fish (35%) survived 3 months after transplantation, half the number surviving after the infusion of a similar number of flow-sorted GFPlo cells. After the infusion of 1000 unsorted WKM cells, survival fell to 17% and only a single fish engrafted (Table 2). In contrast, after the infusion of 4000 GFPlo cells (Table 1), 40% of the fish were alive at 3 months with 63% of the survivors engrafted; and after infusion of 1000 GFPlo cells, 49% of the transplant recipients were alive at 3 months with 48% of the survivors engrafted. Although the majority of fish were killed 3 months after transplantation, we have followed some fish for as long as 68 weeks after transplantation, which is over half the lifespan of a zebrafish, and they have all retained their grafts.
Transplantation with unfractionated CD41-GFP WKM cells
| Cell dose/recipient . | Survival (%) . | Engrafted (%) . |
|---|---|---|
| 10 000 | 18/51 (35) | 7/18 (39) |
| 1000 | 10/59 (17) | 1/10 (10) |
| Cell dose/recipient . | Survival (%) . | Engrafted (%) . |
|---|---|---|
| 10 000 | 18/51 (35) | 7/18 (39) |
| 1000 | 10/59 (17) | 1/10 (10) |
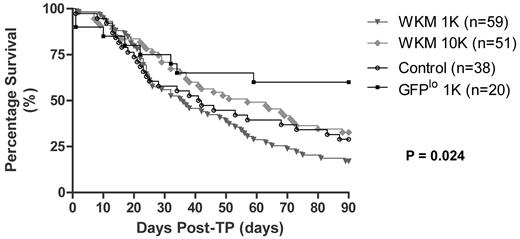
Figure 3 shows Kaplan-Meier plots of the survival curves for irradiated fish receiving either 1000 or 10 000 unfractionated WKM cells compared with survival after transplantation with 1000 flow-sorted GFPlo cells. Fish that had received low doses of WKM continued to die up to 90 days after TBI and received little or no radioprotection from the WKM infusion. In contrast, there was a significant survival advantage to receiving 1000 GFPlo cells, with few deaths occurring 30 days after transplantation. The enhanced survival was paralleled by the high percentage of engrafted fish after the infusion of GFPlo cells. The log-rank P value of .024 indicated that the survival curves were significantly different.
Survival of irradiated zebrafish transplanted with unfractionated WKM or flow-sorted CD41-GFPlo cells calculated by the method of Kaplan and Meier. This figure depicts survival at 90 days of fish transplanted with either varying numbers of CD41-GFP WKM cells or flow-sorted CD41-GFPlo cells. Fish that were transplanted with 1000 CD41-GFPlo cells showed much higher survival rate compared with control and the cohorts of fish received varying numbers of unfractionated CD41-GFP WKM cells. The P value .024 is for the log-rank test that indicates the survival distributions are significantly different.
Survival of irradiated zebrafish transplanted with unfractionated WKM or flow-sorted CD41-GFPlo cells calculated by the method of Kaplan and Meier. This figure depicts survival at 90 days of fish transplanted with either varying numbers of CD41-GFP WKM cells or flow-sorted CD41-GFPlo cells. Fish that were transplanted with 1000 CD41-GFPlo cells showed much higher survival rate compared with control and the cohorts of fish received varying numbers of unfractionated CD41-GFP WKM cells. The P value .024 is for the log-rank test that indicates the survival distributions are significantly different.
Engrafted CD41-GFPlo cells can repopulate secondary hosts
The primary allografts continued to function over 6 months after transplantation, suggesting that the infused GFPlo cells were likely to be HSCs. Because one additional property of a HSC is its ability to repopulate the BM of secondary recipients, we carried out secondary transplants by flow-sorting CD41-GFPlo cells from the kidneys of engrafted fish, 3 months after primary transplantation. Table 3 shows the result of injecting small numbers of flow-sorted cells into secondary hosts. In these experiments, 100% of the recipients transplanted with 1000 flow-sorted GFPlo cells from the WKM of primary hosts survived and 17% had engrafted. Eighty-three percent of the fish receiving 500 GFPlo cells survived and 20% engrafted. These secondary grafts persisted for at least an additional 6 months.
Transplanted CD41-GFPlo cells reconstitute the major zebrafish hematopoietic lineages
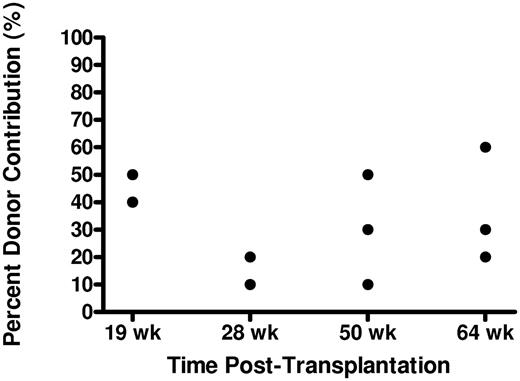
Our next question was: do CD41-GFPlo cells reconstitute major hematopoietic lineages? We first examined the percentage donor contribution of cells in the peripheral blood of individual transplanted fish 19, 28, 50, and 64 weeks after transplantation. As shown in Figure 4, the percentage could be as low as 10%, regardless of time after transplantation, either at 28 weeks or at 50 weeks; or as high as 50% at 19 weeks, 60% at 64 weeks, with considerable individual variation.
Donor chimerism in peripheral blood of transplanted recipients over time. The graph depicts the contribution of donor DNA to total genomic DNA in unfractionated peripheral blood cells sampled from zebrafish 19, 28, 50, and 64 weeks after transplantation with 103 CD41-GFPlo cells. Each round dot represents data from an individual recipient fish.
Donor chimerism in peripheral blood of transplanted recipients over time. The graph depicts the contribution of donor DNA to total genomic DNA in unfractionated peripheral blood cells sampled from zebrafish 19, 28, 50, and 64 weeks after transplantation with 103 CD41-GFPlo cells. Each round dot represents data from an individual recipient fish.
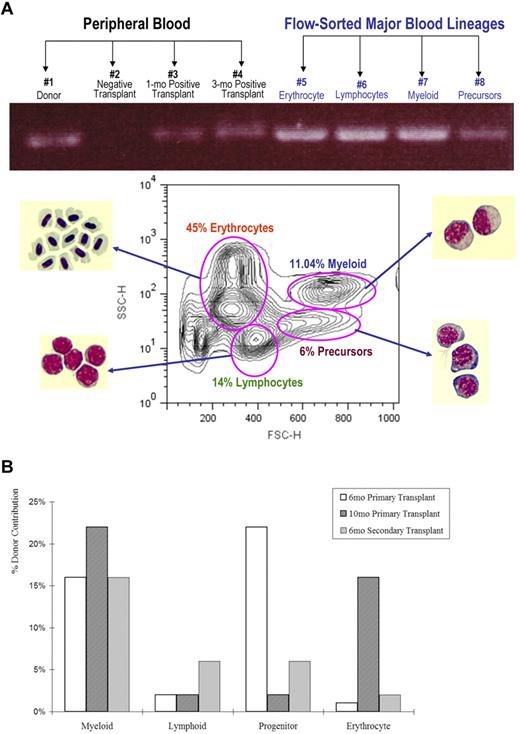
Figure 5A documents that CD41-GFPlo cells give rise to the major zebrafish hematopoietic lineages. The CD41-GFP transgene was detected in genomic DNA extracted from un-fractionated peripheral blood samples 1 month and 3 months after transplantation, as well as from flow-sorted myeloid, erythroid, lymphoid, and progenitor cell pools. As expected, the CD41-GFP transgene was present in peripheral blood and WKM of donor fish, but not in nonengrafted wild-type recipients.
Multilineage reconstitution after transplantation of CD41-GFPlo cells into irradiated primary and secondary recipients. (A) The top panel analyzes donor-specific signals by PCR in the genomic DNA of unfractionated peripheral blood cells 1-3 months after transplantation and flow-sorted WKM cells 3 months after transplantation. The bottom panel depicts a plot of the forward and side scatter of WKM cells and the location, as determined by microscopic examination, of the major blood cell lineages. The pink ovals mark the windows for erythroid, myeloid, lymphoid, and various precursor populations in zebrafish WKM determined by examining Wright-Giemsa stains of cytospins derived from the flow-sorted cells. The percentages vary slightly with each WKM preparation but the ones shown here are representative. FSC-H indicates forward scatter; and SSC-H, side scatter. The bar chart in panel B shows the percentage of total genomic DNA extracted from flow-sorted cells that contains the donor-specific signal (GFP) gated according to the windows depicted in panel A. Two fish per group were examined 6 and 10 months after primary transplantation and 6 months after secondary transplantation. As shown, the donor contribution percentage varied among lineages, with time after transplantation and among individual fish.
Multilineage reconstitution after transplantation of CD41-GFPlo cells into irradiated primary and secondary recipients. (A) The top panel analyzes donor-specific signals by PCR in the genomic DNA of unfractionated peripheral blood cells 1-3 months after transplantation and flow-sorted WKM cells 3 months after transplantation. The bottom panel depicts a plot of the forward and side scatter of WKM cells and the location, as determined by microscopic examination, of the major blood cell lineages. The pink ovals mark the windows for erythroid, myeloid, lymphoid, and various precursor populations in zebrafish WKM determined by examining Wright-Giemsa stains of cytospins derived from the flow-sorted cells. The percentages vary slightly with each WKM preparation but the ones shown here are representative. FSC-H indicates forward scatter; and SSC-H, side scatter. The bar chart in panel B shows the percentage of total genomic DNA extracted from flow-sorted cells that contains the donor-specific signal (GFP) gated according to the windows depicted in panel A. Two fish per group were examined 6 and 10 months after primary transplantation and 6 months after secondary transplantation. As shown, the donor contribution percentage varied among lineages, with time after transplantation and among individual fish.
We next evaluated the percentage donor chimerism in transplant recipients using quantitative PCR. As shown in Figure 5B, there was considerable variability in the degree of chimerism in different hematopietic lineages. The extent of donor chimerism varied over time, within each lineage and among various donors. For example, 16% of myeloid cells contained the CD41-GFP transgene 6 months after primary transplantation, 22% of myeloid cells had the transgene 10 months after a primary transplantation and 16% of myeloid cells 6 months after a secondary transplantation. In contrast, the degree of red cell chimerism was 1%, 16%, and 2% in the same animals and marrow precursor chimerism was 22%, 3%, and 6%.
Localization of CD41-GFP+ cells in zebrafish kidney
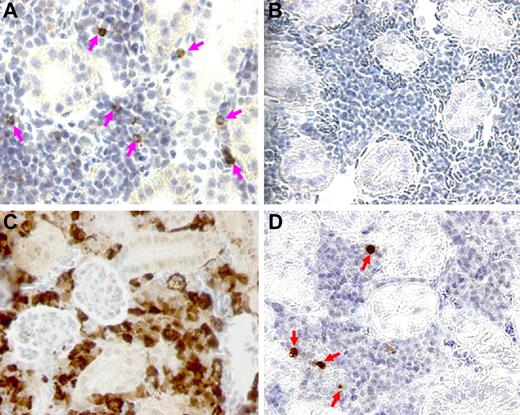
We prepared paraffin sections of kidney from transplant donors and recipients and examined them for the presence of cells that reacted with anti-GFP Ab, as indicated by brown-staining cells observed in panels A, C, and D in Figure 6. The zebrafish kidney section is populated with epithelial lined tubules and the renal interstitium where hematopoietic elements are located. Panel A shows the staining pattern of Tg(CD41:GFP) donor fish with peroxidase-positive cells scattered among the densely packed renal interstitial cells. In contrast, panel B shows a wild-type nontransplanted kidney section, where no staining was detected. In panel C, we observed numerous brown staining cells in a fish that was transplanted with 0.5 × 106 unfractionated WKM cells from a Tg(β-actin:GFP) donor. Virtually all of the interstitial cells plus some renal tubules were peroxidase positive, indicating a significantly higher amount of engrafted cells. This is not surprising as the Tg(β-actin:GFP) donor is known for its ubiquitously expressed promoter. Panel D shows the staining pattern of a fish that had received a transplant of 1000 CD41-GFPlo cells. There are a small number of peroxidase positive cells, many fewer than in the Tg(CD41:GFP) donor kidney, in keeping with the chimeric state of the recipient.
Immunohistochemical staining of sections of zebrafish kidney with anti-GFP Ab. Staining was performed on paraffin-embedded kidney sections using a 1:1500 dilution of anti-GFP Ab and peroxidase-conjugated secondary Ab. Brown-staining cells were observed in panels A, C, and D. (A) In donor Tg(CD41:GFP) kidney, representative CD41-GFP+ cells are indicated by pink arrows. (B) In wild-type untransplanted fish, there was no staining detected. (C) Irradiated fish that were transplanted with 0.5 × 106 β-actin:GFP+ cells from Tg(β-actin:GFP) WKM showed engrafted cells of multiple lineages throughout the renal tubules and interstitium, as exhibited by brown signals galore in the kidney section. (D) In transplanted fish that received 103 flow-sorted CD41-GFPlo cells, engrafted cells were marked by red arrows in the hematopoietic cell portion of the recipient kidney.
Immunohistochemical staining of sections of zebrafish kidney with anti-GFP Ab. Staining was performed on paraffin-embedded kidney sections using a 1:1500 dilution of anti-GFP Ab and peroxidase-conjugated secondary Ab. Brown-staining cells were observed in panels A, C, and D. (A) In donor Tg(CD41:GFP) kidney, representative CD41-GFP+ cells are indicated by pink arrows. (B) In wild-type untransplanted fish, there was no staining detected. (C) Irradiated fish that were transplanted with 0.5 × 106 β-actin:GFP+ cells from Tg(β-actin:GFP) WKM showed engrafted cells of multiple lineages throughout the renal tubules and interstitium, as exhibited by brown signals galore in the kidney section. (D) In transplanted fish that received 103 flow-sorted CD41-GFPlo cells, engrafted cells were marked by red arrows in the hematopoietic cell portion of the recipient kidney.
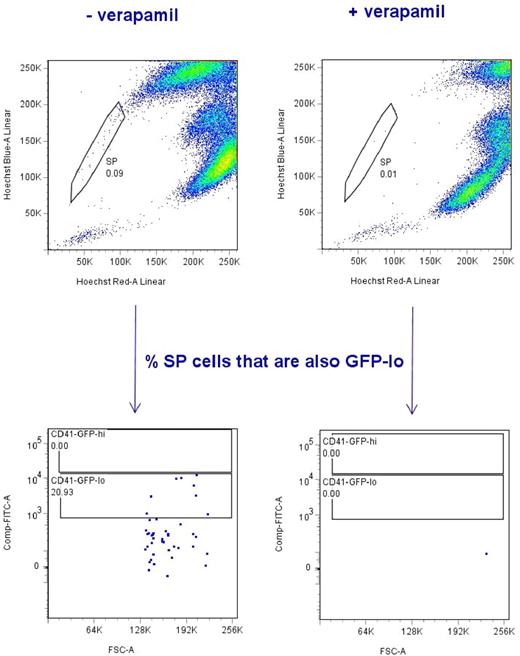
CD41-GFPlo cells overlap with side population cells in zebrafish WKM
Two groups have reported that zebrafish kidney marrow contains a “side population” of cells that efflux Hoechst 33342 dye.22,24 This is of interest, as the efflux of Hoechst 33342 is thought to be a property of stem cells derived from many tissues. Although this property is highly sensitive to dye concentration, incubation time, cell concentration and temperature present during staining.25 We wanted to determine whether CD41-GFPlo cells overlapped with the kidney marrow side population (SP) cells. As shown in Figure 7, a small fraction of CD41-GFP WKM cells effluxed Hoechst 33342 dye. Efflux was inhibited by 50μM verapamil. In 3 separate experiments, 22.48% + 7.87% (mean + SD, n = 6) of the SP population overlapped with the CD41-GFPlo cells. This finding is in agreement with mouse studies showing the coexistence of SP and non-SP HSCs in the mouse BM.26
Side population (SP) cells from Tg(CD41:GFP) zebrafish overlaps with CD41-GFPlo subpopulation. The top panel flow cytometric diagrams show typical SP profile derived from lymphocyte and progenitor subsets of CD41-GFP WKM cells after incubation with the Hoechst 33342 dye in presence (on the right) or absence of verapamil (on the left). Vertical axis shows blue Hoechst fluorescence; horizontal axis shows red Hoechst fluorescence. The SP cells are indicated within the gate. The lower 2 panels indicate the percentage of SP cells that are also CD41-GFPlo. Here the vertical axis shows FITC fluorescence; horizontal axis shows forward scatter. Two gates mark CD41-GFPlo and CD41-GFPhi subsets, respectively. The SP cells overlap with CD41-GFPlo by > 20%.
Side population (SP) cells from Tg(CD41:GFP) zebrafish overlaps with CD41-GFPlo subpopulation. The top panel flow cytometric diagrams show typical SP profile derived from lymphocyte and progenitor subsets of CD41-GFP WKM cells after incubation with the Hoechst 33342 dye in presence (on the right) or absence of verapamil (on the left). Vertical axis shows blue Hoechst fluorescence; horizontal axis shows red Hoechst fluorescence. The SP cells are indicated within the gate. The lower 2 panels indicate the percentage of SP cells that are also CD41-GFPlo. Here the vertical axis shows FITC fluorescence; horizontal axis shows forward scatter. Two gates mark CD41-GFPlo and CD41-GFPhi subsets, respectively. The SP cells overlap with CD41-GFPlo by > 20%.
Discussion
A HSC is generally defined as a cell that can: (1) affect the long-term reconstitution of all hematopoietic lineages; (2) be serially transplanted into naïve recipients and recapitulate hematopoiesis; (3) reconstitute hematopoiesis after infusion of as little as a single cell into an irradiated host. The CD41-GFPlo cells described here fulfill all of these criteria. CD41-GFPlo cells efficiently home to a niche in the kidney of irradiated zebrafish, proliferate, differentiate, and contribute to all the major lineages present in zebrafish peripheral blood. The stem cell grafts have persisted for > 68 weeks and contribute to as much as 45% of the peripheral blood cells in some of the transplant chimeras.
In these studies, candidate HSCs have been defined solely by the expression of CD41, as there are no other zebrafish stem cell markers like lin, sca, kit or CD34, that are known to identify HSCs. We tested the hypothesis that weak expression of CD41, which we had noted in previous studies of zebrafish thrombopoiesis, might mark HSCs, by transplanting CD41-GFPlo cells into irradiated recipients.
Transplantation remains the universally accepted way to characterize HSCs. Although we believe that the transplant data presented here supports our hypothesis that CD41-GFPlo cells are true HSCs, there are a few potential problems with the data. The CD41-GFPlo cell population we used for transplantation is heterogeneous with respect to its light scattering properties—a surrogate for cell size. It is possible that cells of different size might have different biologic properties. However, reconstitution of hematopoiesis in our system requires the transplantation of only a small number (10-100) CD41-GFPlo cells, suggesting that the majority, if not all of the cells in the CD41-GFPlo fraction, irrespective of size, are HSCs. Second, small numbers of CD41-GFPlo cells reduce the mortality of irradiated recipient fish compared with the infusion of a much larger number of unfractionated WKM cells, which also supports the conclusion that the CD41-GFPlo cell population is enriched in HSCs.
Finally, it is reassuring that we can differentiate CD41lo and CD41hi cells within the WKM of Tg (CD41-GFP) fish by confocal fluorescence microscopy. This suggests that weak fluorescence is not an artifact of cell isolation or flow cytometry. Previous studies used CD41-GFP zebrafish embryos and conventional fluorescence microscopy to trace the migration of CD41+ cells but could not distinguish between the CD41-GFPhi and CD41-GFPlo subpopulations in the developing kidney. Although not the major focus of the current report, confocal imaging of Tg (CD41:GFP) kidney may allow the direct visualization of HSCs in a vertebrate hematopoietic niche.
Because the degree of GFP fluorescence was the only criteria used to delineate putative HSCs for transplantation, we sought an additional independent method to validate that the cells we selected for transplantation were HSCs. We labeled cells with Hoechst 33342 dye and colocalized GFPlo cells with the verapamil-sensitive Hoechst-effluxing cells—the so-called SP cells.
The SP phenotype was originally described in murine BM preparations and is highly enriched in long-term repopulating HSCs.21 The fidelity of the Hoechst dye as a stem cell probe resides in the ability of the dye to be effluxed by membrane pumps of the ATP-binding cassette (ABC) transporter superfamily.27 SP cells typically represent 0.05% to 0.10% of viable cells from murine BM, we found 0.09% of the cells in zebrafish WKM had the SP phenotype. More than 20% of the CD41-GFPlo cells share the SP phenotype. The overlap between the SP and the CD41:GFPlo-expressing cells provides additional evidence that the cells used for transplantation were HSCs.
Although many studies have linked the SP phenotype to HSCs, a recent report has indicated that BM HSCs are equally divided between the SP and the non-SP fractions with no significant difference in long-term repopulating activity.26 Thus, the SP phenotype does not mark all HSCs at any one time and may explain why only a fraction of CD41-GFPlo cells have the SP phenotype. In addition, we have no way to be certain that SP cells that do not also weakly express GFP might be stem cells for nonhematopoietic tissues, such as the renal nephron. We have recently isolated and characterized an lhx1+ renal progenitor that forms fully functional rephrons in the adult zebrafish kidney.28
Because AB strain zebrafish are not isogenic, the CD41-GFPlo cells were likely transplanted across a histocompatibility barrier. This could explain the low percentage of engraftment in the studies using small numbers of CD41-GFPlo cells, as well as some of the deaths that occurred during the first 30 days after stem cell infusion. However, we have not observed any late graft loss or any evidence of graft versus host disease in the long-term graft recipients. As previously mentioned the transplanted fish remained healthy and retained their grafts for well over a year after HSC transplantation. We assume that despite the MHC mismatch, there is no detectable GVHD because we are transplanting highly purified HSCs without any contaminating donor T lymphocytes. There is ample evidence that T-cell depletion reduces the incidence and severity of GVHD.29-31
The dose of TBI needed for conditioning zebrafish is higher than the dose used to condition mammals for peripheral blood stem cell or marrow transplantation. Prior studies have shown that between 20 and 35 Gy of irradiation is needed for hematopoietic engraftment in zebrafish.19,20 Initially, we used 35 Gy, which is a lethal conditioning dose without hematopoietic rescue. However, we noted high mortality in engrafted fish, suggesting damage to other organs from the radiation. Seeking a balance between sufficient irradiation to provide immunosuppression and the lowest possible irradiation dose we settled on 25 Gy. This permits both efficient engraftment as well as survival of the fish for at least a year after transplantation. Although the engrafted fish are chimeras, the mortality after 25 Gy irradiation stem cell infusion is twice that of 25 Gy followed by GFPlo cell infusion. The data shown in Figure 3 suggest that the GFPlo cells provide some degree of radioprotection.
Most studies of zebrafish hematopoiesis have been carried out on embryos, using whole mount in situ hybridization (WISH) with hematopoietic probes or transgenic fish with fluorescent cellular markers in hematopoietic cells. These approaches have been quite informative and have helped to define both the timing and the anatomic location of embryonic and definitive hematopoiesis. Although inferences are possible about putative stem cells by marker expression, the techniques do not permit assessment of a cell's biologic properties. In addition, it is difficult to study definitive hematopoiesis with these techniques, as the onset of definitive hematopoiesis in WKM does not occur until 5 to 7 days after fertilization, when WISH or the detection of cell fluorescence in an internal organ, like the kidney, is constrained by the size of the fish, the loss of optical clarity and the development of skin and organ pigmentation.
There may be ways to circumvent some of these limitations by the use of confocal microscopy combined with pigment mutant strains that remain transparent into adult life.32-35 However, the definitive functional assay, used extensively in the mouse and applied as a therapeutic tool in humans, is stem cell transplantation. Our ability to carry out stem cell transplants with fluorescently tagged HSCs opens up several possible experiments. By combining transplantation with fluorescence microscopy and possibly carrying out transplants with pigment mutant fish, it should be possible to analyze stem cell homing to the mesonephros or perhaps to other tissue like the spleen, niche selection and differentiation. It may also be possible to follow the fate of GFP tagged tumor initiating or cancer “stem cells” derived from hematopoietic or other neoplasms after intracardiac injection into irradiated fish.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Len Zon and Barry Paw for their helpful discussions, Donna S. Neuberg and Sandra L. Dabora for assistance on statistical analysis, Elizabeth A Mayhall for demonstration of adult zebrafish transplantation, and Christian Lawrence for his superb management of the aquatics facility.
This work was supported by a grant from the Department of Medicine at the Brigham and Women's Hospital, the Jock Adams Fund for Discovery, and National Institutes of Health (NIH) grant HL-092231. The Children's Hospital Boston Intellectual and Developmental Disabilities Research Center (IDDRC) Imaging Core Facility was supported by NIH-P30-HD-18 655.
National Institutes of Health
Authorship
Contribution: D.M. designed and performed experiments, analyzed the data, and wrote the manuscript; J.Z. performed the MO knockdown experiments; H.-f.L. provided critical constructs and primers; J.I. performed the EM experiments; and R.I.H. designed the research, and wrote and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Robert I. Handin, MD, Hematology Division, Brigham and Women's Hospital, Karp Family Research Building, Rm 6210, One Blackfan Circle, Boston, MA 02115; e-mail: rhandin@partners.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal