Abstract
Patients with core binding factor acute myeloid leukemia (CBF-AML) benefit from more intensive chemotherapy, but whether both the t(8;21) and inv(16)/t (16;16) subtypes requires intensification remained to be determined. In the 2 successive studies (AML-BFM-1998 and AML-BFM-2004), 220 CBF-AML patients were treated using the same chemotherapy backbone, whereby reinduction with high-dose cytarabine and mitoxantrone (HAM) was scheduled for these cohorts only in study AML-BFM-1998 but not in AML-BFM-2004 against the background to minimize overtreatment. Five-year overall survival (OS) and event-free survival (EFS) were significantly higher and the cumulative incidence of relapse (CIR) lower in t(8;21) patients treated with HAM (n = 78) compared with without HAM (n = 53): OS 92% ± 3% versus 80% ± 6%, plogrank0.047, EFS 84% ± 4% versus 59% ± 7%, plogrank0.001, and CIR 14% ± 4% versus 34% ± 7%, p(gray)0.006. These differences were not seen for inv(16) (n = 43 and 46, respectively): OS 93% ± 4% versus 94% ± 4%, EFS 75% ± 7% versus 71% ± 9% and CIR 15% ± 6% versus 23% ± 8% (not significant). The subtype t(8;21), but not inv(16), was an independent predictor of worse outcome without HAM reinduction. Based on our data, a 5-year OS of > 90% can be expected for CBF-AML, when stratifying t(8;21), but not inv(16), patients to high-risk chemotherapy, including HAM reinduction.
Introduction
Core binding factor (CBF) acute myeloid leukemia (AML) include 2 major subtypes with the translocation (8;21)(q22;q22) [= t(8;21)] and inversion of chromosome 16 (p13q22) and its variant t(16;16)(p13;q22) [= inv(16)] that occur predominantly in younger patients and are associated with a relative favorable prognosis. Overall survival (OS) rates have improved after intensification of the treatment by different approaches, to reach between 60% and 70% in adults and ∼ 80% in children.1-5 The incidence of t(8;21) is 12% to 14% in children and adolescents3,4,6 and ∼ 7% in adults with AML younger than 60 years.2,7 Inversion 16 is less frequent: children 7% to 9%3,4 and adults 4%.2,7
In particular, intensive postremission chemotherapy with high-dose cytarabine (HDAC) improved prognosis in patients with CBF leukemia.8,9 More recent studies indicate that CBF-AML with t(8;21) may be associated with less favorable prognosis than with inv(16).9,10 The question whether patients with both CBF-AML subtypes benefit from treatment intensification and which treatment strategy is optimal is still inconclusively addressed.5
The evolution of the AML treatment strategy in 2 subsequent pediatric trials from the AML-BFM study group now provides the opportunity to address the importance of a second induction with HDAC and mitoxantrone (HAM) for these CBF-AML subtypes.
Based on the superior outcome with an intensified second induction with HAM in the AML-BFM 93 study, which was introduced for high-risk patients only, excluding all patients with t(8;21) and inv(16),11 HAM treatment was also introduced for patients with CBF-AML in the subsequent AML-BFM-1998 study, given its tolerable acute toxicity profile. However, based on a preliminary analysis that failed to show a benefit of treatment intensification for this AML subtype and with a concern to avoid toxicity, second induction with HAM was omitted in the current AML-BFM-2004 trial for CBF-AML patients (Figure 1). Here we report that reduction of treatment intensity by omission of HAM resulted in an unexpected and marked decrease in event-free survival (EFS) and OS in patients with t(8;21), but not in patients with inv(16), providing compelling evidence to suggest that the 2 subtypes of CBF-AML should be considered separately and that the addition of HAM is beneficial for patients with t(8,21) in the context of the AML-BFM chemotherapy backbone.
Treatment schedules of studies AML-BFM-1998 and AML-BFM-2004 in standard-risk patients. Induction: AIE indicates cytarabine/idarubicin/etoposide or randomized with ADxE; and ADxE, cytarabine/liposomal daunorubicin/etoposide. Second induction: HAM indicates high-dose cytarabine [3 g/m2]/mitoxantrone. Consolidation: AI indicates cytarabine [0.5 g/m2]/idarubicin; and hAM, high-dose cytarabine [1 g/m2]/mitoxantrone. Intensification: HAE indicates high-dose cytarabine [3 g/m2]/etoposide; and CNS-RT, cranial irradiation.
Treatment schedules of studies AML-BFM-1998 and AML-BFM-2004 in standard-risk patients. Induction: AIE indicates cytarabine/idarubicin/etoposide or randomized with ADxE; and ADxE, cytarabine/liposomal daunorubicin/etoposide. Second induction: HAM indicates high-dose cytarabine [3 g/m2]/mitoxantrone. Consolidation: AI indicates cytarabine [0.5 g/m2]/idarubicin; and hAM, high-dose cytarabine [1 g/m2]/mitoxantrone. Intensification: HAE indicates high-dose cytarabine [3 g/m2]/etoposide; and CNS-RT, cranial irradiation.
Methods
Patients
Between July 1998 and December 2009, 1280 AML patients younger than 18 years were enrolled in studies AML-BFM-1998 and AML-BFM-2004. Of these, 131 (10%) presented with t(8;21) or the molecular genetic equivalent AML1/ETO alone, and 91 (7%) with inv(16) or the molecular genetic equivalent CBFB-MYH11 alone. Two patients were excluded from the analysis because they died before the second therapy course. Patients treated in study AML-BFM-1998 and in the AML-BFM-1998 interim phase (amendment to study AML-BFM-1998 implemented after closure of study AML-BFM-1998 (July 2003 until March 2004) are summarized as AML-BFM-1998 (n = 110) in the following analysis. Their data were compared with those of study AML-BFM-2004 (n = 110). In study AML-BFM-1998, a second induction with HAM was scheduled, but neither in the interim phase nor in study AML-BFM-2004. For the main analysis, results were compared according to treatment (HAM vs no HAM) because, in both studies, treatment differed in some patients: study AML-BFM-1998, 15 patients without HAM from the AML-BFM-1998 interim phase; study AML-BFM-2004, 9 patients with > 5% BM blasts on day 15, 2 patients with FLT-ITD, and 15 other patients (9 with > 5% BM blasts on day 28) with HAM. The study was approved by the ethical committee of the University of Muenster.
The studies were performed in Germany, Austria, Switzerland, and the Czech Republic. Written informed consent from patients, parents, or guardians was obtained at study entry in accordance with the Declaration of Helsinki.
Central review of BM morphology and cytogenetics was performed. Comprehensive cytogenetic data from 119 of 131 (91%) of the patients with t(8;21) and 79 of 89 (89%) with inv(16) were available. Cytogenetic analysis was performed as previously described.4 Routine testing for FLT3-ITD at initial diagnosis has been performed since study AML-BFM-2004, when stratification to the standard risk group was, among others, based on negativity for FLT3-ITD.
Treatment plan
All patients were treated according to regimens AML-BFM-1998, AML-BFM-1998-Interim, or AML-BFM-2004,12,13 which were similar for most parts (Figure 1).12 The AML-BFM-1998 protocol, with the exception of the AML-BFM-1998-Interim phase, included HAM after induction with cytarabine, idarubicin, and etoposide (AIE) for all patients. In AML-BFM-2004, HAM was exclusively given to high-risk patients after induction with AIE or with cytarabine, liposomal daunorubicin, and etoposide. Standard risk, including t(8;21) and inv(16), patients with BM blasts > 5% on day 15, were restratified to the high-risk group and treated with HAM. After first induction (study AML-BFM-2004) or after HAM (study AML-BFM-1998), 2 additional short cycles with medium (0.5 g/m2) and HDAC (1-3 g/m2) and anthracyclines were administered (Figure 1). Intensification and maintenance regimens were similar during both study periods, with no indication for allogeneic hematopoietic stem cell transplantation in CR1 in standard-risk patients.13 Intrathecal cytarabine was given 11 times following age-dependent dosage regimens.
Definition and statistics
Risk classification was based on morphology, cytogenetics, molecular genetics, and response to treatment: Standard risk indicates French-American-British (FAB) M1/2 with Auer rods, FAB M4 with atypical eosinophils (M4Eo), FAB M3 and/or favorable cytogenetics, such as t(8;21) and/or AML1-ETO, t(15;17), and/or PML-RARA and inv(16) or t(16;16) and/or CBFB/MYH1, if there was no persistence of BM blasts (> 5%) on day 15 or FLT3-ITD positivity (FAB M3 excluded). All others were classified as high-risk patients. Since study AML-BFM-2004, FLT3-ITD mutation analysis was part of the risk group stratification because the negative impact of this mutation was found to be in general a strong and independent adverse prognostic factor in pediatric AML.14
CR was defined according to the CALGB criteria15 and needed to be achieved at the end of intensification treatment. EFS was calculated from the date of diagnosis to last follow-up or first event (failure to achieve remission, relapse, secondary malignancy, or death of any cause). Survival was calculated from the date of diagnosis to death of any cause or last follow-up. Probabilities of survival were estimated using the Kaplan-Meier method with SEs according to Greenwood16 and compared with the log-rank test. Cumulative incidence functions of relapse and secondary malignancy were constructed by the method of Kalbfleisch and Prentice.16 Functions were compared with Gray's test. Toxicity was assessed according to the NCI Common Toxicity Criteria Version 2.0 (www.ctep.cancer.gov/reporting).
Univariate analysis was conducted by the Wilcoxon test for quantitative variables and Fisher exact test for qualitative variables. When frequencies were sufficiently large, χ2 statistics were used. Computations were performed using SAS (Statistical Analysis System Version 9.1; SAS Institute).
Results
Patient characteristics
Table 1 shows patient characteristics corresponding to the cytogenetic subgroups and to the treatment with or without HAM. Translocation (8;21) patients were older by trend (see interquartile range) than inv(16) patients (P = .06, Table 1). Median WBC count at diagnosis was significantly higher in inv(16) patients (P < .001). Translocation (8;21)/AML1/ETO was associated with the FAB subtype M2 with Auer rods (101 of 131, 77%) and inv(16)/CBFB-MYH11 with FAB subtype M4Eo (85 of 89, 96%). Eighteen of 127 t(8;21) patients (14%), but only 2 of 83 (2%) of those with inv(16) presented with more than 5% BM blasts on day 15 (P = .001). Initial CNS involvement occurred more frequently among inv(16) patients (20 of 86, 23%) than among t(8;21) patients (9 of 131, 7%, P = .0005). Seventeen of the t(8;21) patients presented with orbital involvement, 3 of them combined with a CNS manifestation, in contrast to inv(16) patients (only 2 with orbital involvement, one combined in the CNS), whereas other extramedullary leukemia was found equally often in both groups. For 37 of 119 patients (31%), t(8;21) was the sole aberration, whereas 82 of 119 patients (69%) also harbored secondary cytogenetic abnormalities, such as loss of sex chromosomes (LOS; n = 57 of 119, 48%, Table 2). In 12 patients, only the AML1/ETO rearrangement was examined. In 26 of 79 (33%) patients with inv(16), additional aberrations occurred; the most common was trisomy 22 (11 of 79, 14%).
Initial basic data of patients with t(8;21) and inv(16) treated with or without HAM
| . | t(8;21) . | inv(16) . | Total t(8;21) . | Total inv(16) . | P (χ2) . | ||||
|---|---|---|---|---|---|---|---|---|---|
| HAM . | No HAM . | P (χ2) . | HAM . | No HAM . | P (χ2) . | ||||
| No. of patients | 78 | 53 | 43 | 46 | 131 | 89 | |||
| Median age, y | 9.7 | 11.6 | .22 | 11.6 | 9.8 | .65 | 10.5 | 10.1 | .06 |
| Q1-Q3 | 6.5-14.0 | 8.8-13.9 | 4.9-13.0 | 2.8-13.8 | 7.6-14.0 | 3.3-13.6 | |||
| Leukocytes, × 109μL, median | 9100 | 11 020 | .36 | 34 900 | 50 300 | .15 | 10 400 | 41 900 | < .001 |
| Q1-Q3 | 5600-17 050 | 5700-22 500 | 21 400-108,000 | 15 000-18 200 | 5600-21 000 | 19 735-93 950 | |||
| Sex, no. (%) | |||||||||
| Male | 44 (56) | 27 (52) | .54 | 20 (47) | 25 (54) | .46 | 71 (54) | 45 (51) | .60 |
| Female | 34 (44) | 26 (48) | 23 (53) | 21 (46) | 60 (46) | 44 (49) | |||
| CNS involvement, no. (%) | 5 (6) | 4 (8) | .80 | 14 (35) | 6 (13) | .016 | 9 (7) | 20 (23) | .001 |
| Extramedullary organ involvement, no. (%) | 19 (24) | 12 (23) | .90 | 11 (26) | 11 (24) | .81 | 31 (23) | 22 (25) | .82 |
| Blasts day 15 > 5%,* no. (%) | 18 (23) | 0 (0) | < .001 | 2 (5) | 0 (0) | .14 | 18 (14) | 2 (2) | .005 |
| Cytogenetic data, no. (%) | 71 (91) | 48 (91) | 1.00 | 43 (100) | 36 (78) | 119 (91) | 79 (89) | ||
| Molecular genetic only, no. (%) | 7 (9) | 5 (9) | 1.00 | 0 (0) | 10 (22) | 12 (9) | 10 (11) | ||
| Risk groups, no. (%) | |||||||||
| SR | 54 (69) | 52 (98) | < .001 | 37 (86) | 44 (96) | .11 | 106 (81) | 81 (91) | .04 |
| HR† | 24 (31) | 1 (2) | 6 (14) | 2 (4) | 25 (19) | 8 (9) | |||
| . | t(8;21) . | inv(16) . | Total t(8;21) . | Total inv(16) . | P (χ2) . | ||||
|---|---|---|---|---|---|---|---|---|---|
| HAM . | No HAM . | P (χ2) . | HAM . | No HAM . | P (χ2) . | ||||
| No. of patients | 78 | 53 | 43 | 46 | 131 | 89 | |||
| Median age, y | 9.7 | 11.6 | .22 | 11.6 | 9.8 | .65 | 10.5 | 10.1 | .06 |
| Q1-Q3 | 6.5-14.0 | 8.8-13.9 | 4.9-13.0 | 2.8-13.8 | 7.6-14.0 | 3.3-13.6 | |||
| Leukocytes, × 109μL, median | 9100 | 11 020 | .36 | 34 900 | 50 300 | .15 | 10 400 | 41 900 | < .001 |
| Q1-Q3 | 5600-17 050 | 5700-22 500 | 21 400-108,000 | 15 000-18 200 | 5600-21 000 | 19 735-93 950 | |||
| Sex, no. (%) | |||||||||
| Male | 44 (56) | 27 (52) | .54 | 20 (47) | 25 (54) | .46 | 71 (54) | 45 (51) | .60 |
| Female | 34 (44) | 26 (48) | 23 (53) | 21 (46) | 60 (46) | 44 (49) | |||
| CNS involvement, no. (%) | 5 (6) | 4 (8) | .80 | 14 (35) | 6 (13) | .016 | 9 (7) | 20 (23) | .001 |
| Extramedullary organ involvement, no. (%) | 19 (24) | 12 (23) | .90 | 11 (26) | 11 (24) | .81 | 31 (23) | 22 (25) | .82 |
| Blasts day 15 > 5%,* no. (%) | 18 (23) | 0 (0) | < .001 | 2 (5) | 0 (0) | .14 | 18 (14) | 2 (2) | .005 |
| Cytogenetic data, no. (%) | 71 (91) | 48 (91) | 1.00 | 43 (100) | 36 (78) | 119 (91) | 79 (89) | ||
| Molecular genetic only, no. (%) | 7 (9) | 5 (9) | 1.00 | 0 (0) | 10 (22) | 12 (9) | 10 (11) | ||
| Risk groups, no. (%) | |||||||||
| SR | 54 (69) | 52 (98) | < .001 | 37 (86) | 44 (96) | .11 | 106 (81) | 81 (91) | .04 |
| HR† | 24 (31) | 1 (2) | 6 (14) | 2 (4) | 25 (19) | 8 (9) | |||
Patients with data.
Restratification to high-risk (HR), because of blasts > 5% on day 15 or FLT3-ITD positivity.
Most common cytogenetic data of 119 patients with t(8;21)* and 79 patients with inv(16) treated with or without HAM
| . | With HAM . | No HAM . | P . | Total . | |||
|---|---|---|---|---|---|---|---|
| n . | % . | N . | % . | n . | % . | ||
| t(8;21) [solely] | 19 | 27 | 18 | 4 | .31 | 37 | 31 |
| t(8;21) −Y† | 22 | 31 | 11 | 23 | .37 | 33 | 28 |
| t(8;21) −X† | 16 | 22 | 8 | 17 | .47 | 24 | 20 |
| t(8;21) del(9q) † | 5 | 7 | 8 | 17 | .10 | 13 | 11 |
| t(8;21) +8† | 6 | 9 | 3 | 6 | .66 | 9 | 8 |
| inv(16) [solely] | 28 | 65 | 25 | 69 | .68 | 53 | 65 |
| inv(16) +22† | 5 | 12 | 6 | 17 | .52 | 11 | 14 |
| inv(16) + other‡ | 10 | 23 | 5 | 14 | .29 | 15 | 19 |
| . | With HAM . | No HAM . | P . | Total . | |||
|---|---|---|---|---|---|---|---|
| n . | % . | N . | % . | n . | % . | ||
| t(8;21) [solely] | 19 | 27 | 18 | 4 | .31 | 37 | 31 |
| t(8;21) −Y† | 22 | 31 | 11 | 23 | .37 | 33 | 28 |
| t(8;21) −X† | 16 | 22 | 8 | 17 | .47 | 24 | 20 |
| t(8;21) del(9q) † | 5 | 7 | 8 | 17 | .10 | 13 | 11 |
| t(8;21) +8† | 6 | 9 | 3 | 6 | .66 | 9 | 8 |
| inv(16) [solely] | 28 | 65 | 25 | 69 | .68 | 53 | 65 |
| inv(16) +22† | 5 | 12 | 6 | 17 | .52 | 11 | 14 |
| inv(16) + other‡ | 10 | 23 | 5 | 14 | .29 | 15 | 19 |
Of 119 patients with data on cytogenetics, 17 patients had other secondary aberrations, including multiple nomination.
As a sole aberration or with additional aberrations.
Further aberrations.
In both studies, patient characteristics, including cytogenetic findings, were comparable in the respective groups with t(8;21) and inv(16) (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) and in the corresponding treatment groups with or without HAM (Tables 1 and 2).
Study periods
For t(8;21) patients treated in study AML-BFM-1998, EFS was significantly higher compared with study 2004 (81% ± 5% vs 66% ± 6%, plogrank = 0.046), whereas the cumulative incidence of relapse (CIR) was lower (16% ± 5% vs 28% ± 6%, p(gray) = 0.009; supplemental Figure 1A-C). This was different for patients with inv(16) (EFS 74% ± 7% vs 74 ± 9%, plogrank = 0.98, CIR 14% ± 5% vs 26% ± 9%, p(Gray) = 0.26; supplemental Table 2).
Treatment with or without HAM
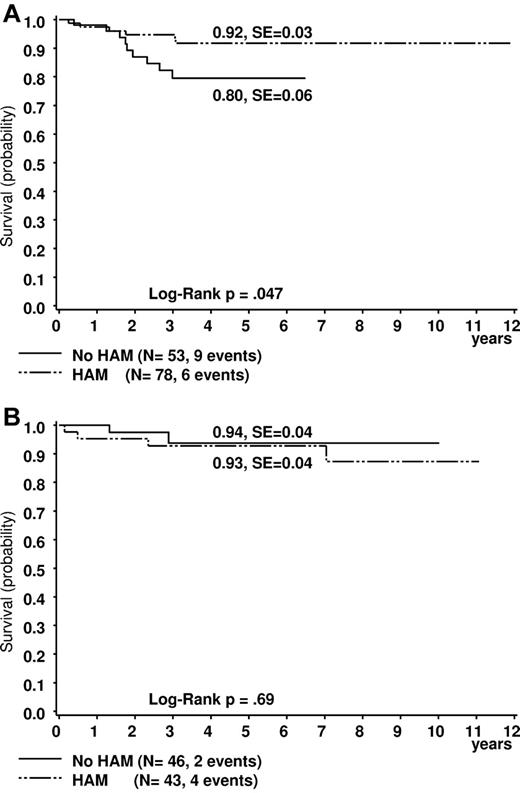
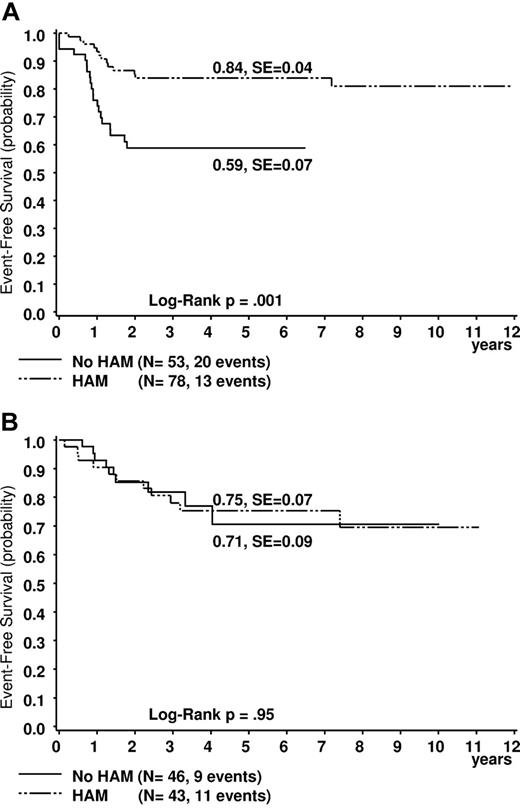
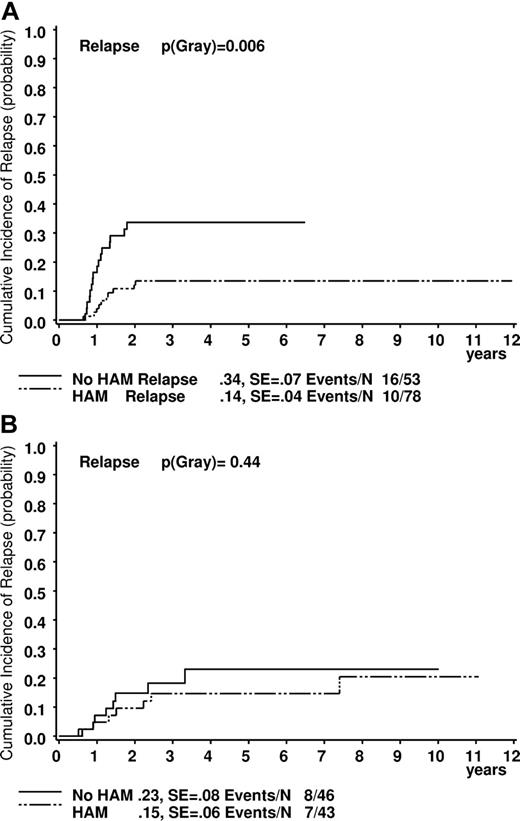
Comparing results after treatment with or without HAM (see “Patients”), the differences in outcome of t(8;21) patients were even more obvious. Five-year survival was significantly higher in the cohort who received HAM (n = 78) than in the one without HAM (n = 53; 92% ± 3% vs 80% ± 6%, plogrank = 0.047; Figure 2A). EFS data strongly confirmed these observations (84% ± 4% vs 59% ± 7%, plogrank = 0.001; Figure 3A). The CIR was also lower for patients who received HAM (14% ± 4% vs 34% ± 7%, p(gray) = 0.006; Figure 4A). This was different for patients with inv(16) (survival, 93% ± 4% vs 94 ± 4; EFS: 75% ± 7% vs 71% ± 9%; and CIR 15% ± 6% vs 23% ± 8%; all P values not significant, Figures 2B, 3B, and 4B).
Estimated probability of 5-year survival in patients. (A) Patients with t(8;21). (B) Patients with inv(16). Treated with or without HAM.
Estimated probability of 5-year survival in patients. (A) Patients with t(8;21). (B) Patients with inv(16). Treated with or without HAM.
Estimated probability of 5-year EFS in patients. (A) Patients with t(8;21). (B) Patients with inv(16). Treated with or without HAM.
Estimated probability of 5-year EFS in patients. (A) Patients with t(8;21). (B) Patients with inv(16). Treated with or without HAM.
Estimated probability of the cumulative incidence of relapse at 5 years in patients. (A) Patients with t(8;21). (B) Patients with inv(16). Treated with or without HAM.
Estimated probability of the cumulative incidence of relapse at 5 years in patients. (A) Patients with t(8;21). (B) Patients with inv(16). Treated with or without HAM.
In study AML-BFM-2004, there were 3 nonresponders among the t(8;21) patients (in the “no-HAM” group). Three patients died in CR, one in the no-HAM and 2 in the HAM group. All inv(16) patients reached a CR. Two patients died in remission after HAM because of severe infections (Table 3).
Results of patients with t(8;21) or inv(16) treated with or without HAM
| . | t(8;21) . | inv(16) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HAM . | No HAM . | P . | HAM . | No HAM . | P . | |||||
| n . | % . | n . | % . | n . | % . | n . | % . | |||
| No. of patients | 78 | 53 | 43 | 46 | ||||||
| Nonresponders | 0 | 0 | 3 | 6 | .08 | 0 | 0 | 0 | 0 | |
| CR achieved | 78 | 100 | 50 | 94 | .24 | 43 | 98 | 46 | 100 | .30 |
| Death in CCR | 2 | 3 | 1 | 2 | .55 | 2 | 5 | 0 | 0 | .14 |
| Relapse (cumulative incidence) | 14 ± 4 | 34 ± 7 | .006 | 15 ± 6 | 23 ± 8 | .44 | ||||
| Secondary malignancies | 1 | 1 | 0 | 0 | 2 | 5 | 1 | 2 | ||
| P (survival 5 y) | 92 ± 3 | 80 ± 6 | .047 | 93 ± 4 | 94 ± 4 | .69 | ||||
| P (EFS 5 y) | 84 ± 4 | 59 ± 7 | .001 | 75 ± 7 | 71 ± 9 | .95 | ||||
| . | t(8;21) . | inv(16) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HAM . | No HAM . | P . | HAM . | No HAM . | P . | |||||
| n . | % . | n . | % . | n . | % . | n . | % . | |||
| No. of patients | 78 | 53 | 43 | 46 | ||||||
| Nonresponders | 0 | 0 | 3 | 6 | .08 | 0 | 0 | 0 | 0 | |
| CR achieved | 78 | 100 | 50 | 94 | .24 | 43 | 98 | 46 | 100 | .30 |
| Death in CCR | 2 | 3 | 1 | 2 | .55 | 2 | 5 | 0 | 0 | .14 |
| Relapse (cumulative incidence) | 14 ± 4 | 34 ± 7 | .006 | 15 ± 6 | 23 ± 8 | .44 | ||||
| Secondary malignancies | 1 | 1 | 0 | 0 | 2 | 5 | 1 | 2 | ||
| P (survival 5 y) | 92 ± 3 | 80 ± 6 | .047 | 93 ± 4 | 94 ± 4 | .69 | ||||
| P (EFS 5 y) | 84 ± 4 | 59 ± 7 | .001 | 75 ± 7 | 71 ± 9 | .95 | ||||
After relapse
Survival rates of patients who had a relapse are generally better in CBF-AML patients than in other AML cohorts.17 The former were comparable in studies AML-BFM-1998 and AML-BFM-2004 (50% ± 16% vs 64% ± 13%, plogrank = 0.57) and also for patients who did or did not receive HAM (60% ± 16% vs 57% ± 13%, plogrank = 0.85). This was also seen in inv(16) patients: studies AML-BFM-1998 and AML-BFM-2004 (66% ± 19% vs 85% ± 13%, plogrank = 0.35), with or without HAM (83% ± 15% vs 73% ± 17%, plogrank = 0.66). These results also imply that pediatric CBF-AML patients are salvageable after first relapse.
BM blast count on day 15
Early response to induction treatment evaluated by analysis of day 15 BM was one of the most important prognostic factors for AML.18,19 In study AML-BFM-1998, EFS results were similar for t(8;21) patients with BM blasts ≤ 5% or > 5% on day 15 (all of them treated with HAM; 81% ± 5% vs 78% ± 14%, plogrank = 0.89). However, in study AML-BFM-2004, there was a marked difference in EFS in favor of patients with > 5% BM blasts on day 15 (ie, in favor of exclusively those who were restratified to the HR group and thus received HAM, n = 9: 100% vs 61% ± 7%, plogrank = 0.05). There were only 2 inv(16) patients in study 98 with > 5% BM blasts on day 15 (both in remission, treated with HAM).
WBC
High WBC count is of negative impact on outcome in adults with t(8;21).10,20 However, this was not seen in our pediatric t(8;21) patient cohort (EFS: WBC < 20 000/μL vs ≥ 20 000/μL = 74% ± 5% vs 76% ± 8%, plogrank = 0.82). There was also no difference for patients with inv(16), EFS: WBC < 20 000/μL vs ≥ 20 000/μL = 73% ± 10% vs 76% ± 6%, plogrank = 0.83).
Additional cytogenetic aberrations and cooperating mutations
There is a remarkable association of t(8;21) and LOS, and conflicting results have been reported in adults with LOS: a somewhat better CR and better results in male patients with −Y,2 but also an inferior outcome was reported.10 In our t(8;21) patients, secondary cytogenetic abnormalities had a favorable effect compared with patients with t(8;21) as sole aberration (EFS 78% ± 5% vs 58% ± 8%, plogrank = 0.06; supplemental Figure 2). This effect was especially apparent in patients with LOS (EFS 83% ± 5% vs 64% ± 6%, plogrank = 0.047). Patients with t(8;21) with additional cytogenetic abnormalities showed a trend to benefit from reinduction with HAM (EFS 90% ± 4% vs 68% ± 11% in patients with a sole t(8;21) abnormality, plogrank = 0.07). In contrast, no differences between these cytogenetic subgroups could be detected when HAM was omitted (EFS 59% ± 10% vs 46% ± 12%, plogrank = 0.54). Patients with LOS showed a lower CIR (11% ± 4% vs 34% ± 6%, p(Gray) = 0.005) and higher EFS rates (83% ± 5% vs 64% ± 6%, plogrank = 0.047) compared with others. The same trend of improved outcome for patients with LOS was also seen in the subgroups with or without HAM.
Trisomy 22 was the most common additional recurrent abnormality in inv(16) and associated with better survival rates.21 However, in our series, EFS rates were similar for trisomy 22 patients compared with others (76% ± 16% vs 74% ± 6%, plogrank = 0.78).
One of 73 patients with (1) t(8;21) and 2 of 51 patients with (2) inv(16) were FLT3-ITD–positive: (1) no event, and (2) 1 of 2 events. Nine of 36 (25%) patients with t(8;21) analyzed for c-KIT mutations were c-KIT–positive (EFS 67% ± 9% vs 75% ± 15%, plogrank = 0.69). Only one of 24 patients with inv(16) proved to be c-KIT–positive (no event).
Multivariate analysis
To confirm the effect of HAM in patients with t(8;21), we performed a multivariate analysis using a Cox regression analysis of EFS and relapse-free survival, including the following risk factors: inv(16), t(8;21) without HAM (interaction term), age more than or equal to 10 years, and BM blast count after induction (> 5%). Only t(8;21) without HAM treatment (EFS: hazard risk ratio [RR] = 2.83, 95% confidence interval, 1.33-6.02, p(chi) = 0.007; relapse-free survival: RR = 3.82 [1.66-8.80], p(chi) = 0.002) was of prognostic significance.
Taken together, our results strongly support the notion that CBF-AML patients with t(8;21), but not with inv(16), benefit from reinduction with high-dose cytarabine and mitoxantrone on the AML-BFM treatment protocol.
Discussion
Although it is clear that patients with CBF-AML have benefited from intensification of AML treatment, it remains unclear which components of the therapy have the most impact on this subtype and whether patients with t(8,21) and inv(16) should be considered as separated entities for treatment. Here we report, based on the analysis of 2 large cohorts of CBF-AML patients, that omission of reinduction with HAM resulted in a marked decrease in EFS for patients with t(8;21). This provides conclusive evidence that t(8;21) and inv(16) constitute distinct subtypes in the context of a chemotherapy strategy that relies on HDAC. This observation is consistent with previous reports from adult studies, suggesting that t(8;21) patients had a less favorable outcome and a lower response to salvage therapy than inv(16) patients.9,10,21
In the AML-BFM-1983 study, we identified 2 subtypes by morphology that benefited most from intensified induction chemotherapy: patients with FAB M1/M2 with Auer rods and patients with FAB M4Eo, which were shown retrospectively to include in ∼ 80% of the patients with t(8;21) and inv(16).18 These observations indicate that the intensity of chemotherapy has a major impact on the favorable outcome of CBF-AML. During the 1990s, studies of the Cancer and Leukemia Group B (CALGB) in adults younger than 60 years established HDAC (defined as ≥ 1 g/m2 per single cytarabine dose) as an effective part of AML treatment.22 These studies also suggested that patients with CBF leukemia benefit most from an intensive post remission chemotherapy with 3 or 4 HDAC courses or hematopoietic stem cell transplantation.8,9 Analogical data for pediatric AML are scarce. A retrospective analysis of patients with t(8;21) treated in the St Jude Children's Research Hospital between 1980 and 1996 revealed a relatively poor outcome for this group of patients (6-year survival of 55% ± 9% and EFS of 33% ± 7%) on a treatment protocol in which cytarabine was only used at a lower dose, never exceeding 500 mg/m2. The major difference in comparable studies with more favorable outcome for this subgroup is the use of cytarabine at 1 to 3 g/m2.23 For example, the Japanese Childhood AML Cooperative Study Group reported recently a 5-year survival of 86% ± 6% and EFS of 71% ± 8% for low-risk patients, which mainly included patients with t(8;21) on the AML99 trial.24 These patients were treated with 5 consolidation courses, including cytarabine with a dosage up to 3 g/m2. Similarly, the 7-year survival for CBF-AML patients [18 patients with t(8;21) and 10 patients with inv(16)] on the AML-93 trial of the Nordic Society for Paediatric Hematology and Oncology was 77% ± 10%, using a regimen including 4 courses of HDAC-based therapy.25 However, the dose-response relationship between 1 and 3 g/m2 has not been well defined. A very recent study of 860 adult patients younger than 60 years showed comparable outcome when using cytarabine 1 g/m2 or up to 3 g/m2, suggesting a plateau in the dose-response effect > 1 g/m2.26 In this study, there was no selective advantage for CBF-AML with 3 g/m2, although the numbers were small. Taken together, our data and the data reported in the literature indicate that higher doses of cytarabine are a major component in the treatment regimen for patients with CBF-AML.
Importantly, marked improvement of outcome for CBF-AML has also been achieved by other treatment intensification approaches. In particular, treatment on the Medical Research Council AML10 and AML12 trials, which have been based on high cumulative doses of anthracyclines, but only one course with HDAC, resulted in comparable outcome for CBF-AML, with a 10-year survival of 80% in 86 children with t(8;21) and 43 with inv(16).3 In addition, the addition of gemtuzumab ozogamicin to standard anthracycline-based chemotherapy was associated with marked benefit for this group of patients in the adult Medical Research Council AML15 trial.27 As in many other instances, such questions have to be evaluated in the context of each particular chemotherapy backbone, but these results indicate that overall treatment intensity, rather than a specific effect of cytarabine, is critical for patients with t(8;21), a finding that is consistent with findings in adults.9 On the other hand, specifically reinduction at an early phase of treatment in combination with mitoxantrone was required in our study to improve outcome. This observation and the fact that patients with inv(16) have a comparably good outcome without reinduction are new findings, which will be considered in our future treatment regimen.
During the last years, it appeared that the day 15 blasts had no impact on the outcome of patients with favorable karyotypes.28,29 Assessment of in vivo response to treatment at day 15 was not sufficient to capture a group of patients with t(8;21) who would benefit from more intensive treatment. Our results show that the few patients with inferior response fared even better than those with < 5% blasts in study AML-BFM-2004, apparently because they were treated with HAM, which is an additional finding supporting the benefit of reinduction for this subtype.
Similarly, there is evidence that treatment intensity has to be high also in subgroups of t(8;21) with additional cytogenetic abnormalities who seem to have a favorable outcome, such as LOS. Patients with t(8;21) and additional cytogenetic abnormalities did show a trend for better outcome, which is consistent with similar observations in adults,2,21 and they did appear to benefit as well from reinduction therapy. Our data are consistent with previous reports in adult cohorts, in which the incidence of secondary cytogenetic aberrations was significantly higher in t(8;21) than in inv(16) cases.9,21 Recurrent secondary aberrations appear to be different in the 2 CBF-AML subgroups, including LOS and del(9q) in t(8;21) cases and +22 in inv(16) cases. It remains to be explored to which extent distinct cooperative events may contribute to the different phenotypes of t(8;21) and inv(16) cases in the context of this treatment regimen. According to the data of our and other study groups, the FLT3-ITD mutation, which was shown to be an independent prognostic factor in the total group of AML patients, does not appear to be associated more frequently with t(8;21).14,30 According to different authors, approximately one-third of adult and pediatric CBF-AML patients show c-KIT mutations.30-32 This is of potential therapeutic value as c-KIT mutations can be treated with novel tyrosine kinase inhibitors. Recently, the CALGB group reported an increased risk of relapse in adults presenting with c-KIT mutations.32 However, this was not seen in another study with a heterogeneous group of adult and pediatric patients where only a trend was seen toward an increased risk of relapse for patients with t(8;21) and c-KIT mutations.31 There was also no prognostic significance of c-KIT in children with CBF leukemia,30 which was confirmed in our study.
In conclusion, our results identified a marked biologic difference between the 2 subtypes of CBF-AML. The present analysis has shown that HAM, an intensive combination of high-dose cytarabine with mitoxantrone, given for second induction promotes superior outcome for children with t(8;21) AML, but not for those with inv(16). Using this strategy, a 5-year survival rate of > 90% can be achieved for these pediatric patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the colleagues, data managers, and technicians of the participating hospitals for their valuable cooperation, Jans-Enno Müller for competent data management, and Gesche Tallen and Ursula Bernsmann for their help in preparing the manuscript.
This work was supported by the Deutsche Krebshilfe e.V. and partly by the Czech Ministry of Education (grant MSM0021620813).
Authorship
Contribution: U.C., M.Z., J.-P.B., M.N.D., and D.R. designed and performed research and wrote and edited the manuscript; M.Z., U.C., and D.R. analyzed the data; C.v.N., A. Sander, A.T.-S., and S.C. performed diagnostic studies and edited the manuscript; and A. Schrauder and J.S. provided study materials or patients and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ursula Creutzig, Klinik und Poliklinik für Kinderheilkunde, Pädiatrische Hämatologie/Onkologie, Albert-Schweitzer-Campus 1, D-48149 Münster, Germany; e-mail: ursula@creutzig.de.

![Figure 1. Treatment schedules of studies AML-BFM-1998 and AML-BFM-2004 in standard-risk patients. Induction: AIE indicates cytarabine/idarubicin/etoposide or randomized with ADxE; and ADxE, cytarabine/liposomal daunorubicin/etoposide. Second induction: HAM indicates high-dose cytarabine [3 g/m2]/mitoxantrone. Consolidation: AI indicates cytarabine [0.5 g/m2]/idarubicin; and hAM, high-dose cytarabine [1 g/m2]/mitoxantrone. Intensification: HAE indicates high-dose cytarabine [3 g/m2]/etoposide; and CNS-RT, cranial irradiation.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/118/20/10.1182_blood-2011-07-364661/4/m_zh89991181870001.jpeg?Expires=1767747992&Signature=GTlMuuzBV8Ld2BFpdiitFxLT~HxM2P-gEJ4G0O8NbzLCuebI9XfPQ6OQd~hIl04DkZONW5uZe-9ZYeJ2fEIvH-S2bEzbFixFxrTe88Vt5J-5ykMI05TRQ0UEDqGsCYQb1dBc9tKrt8E~JyEl38oFc3GS~BqRokbrxhWz7fP2p2WAeeBJN9ZGREXMDvNNgRluLggjr1KHpQjIXtX6C-zXJ05dBi3W-vOp6055FjrQPvsz4M3t30Pnaj1I6eGpjLzSDZ01Rj~T6pMyalhiYvBFrG7ceHcX3TLno2uFG3llP-0R6gL4tgnN-6tXKpJFunUK5~wC50S9lMYVv1DiEImqcw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal