Abstract
Dendritic cells (DCs) and myeloid-derived suppressor cells (MDSCs) show opposing roles in the immune system. In the present study, we report that the establishment of a positive feedback loop between prostaglandin E2 (PGE2) and cyclooxygenase 2 (COX2), the key regulator of PGE2 synthesis, represents the determining factor in redirecting the development of CD1a+ DCs to CD14+CD33+CD34+ monocytic MDSCs. Exogenous PGE2 and such diverse COX2 activators as lipopolysaccharide, IL-1β, and IFNγ all induce monocyte expression of COX2, blocking their differentiation into CD1a+ DCs and inducing endogenous PGE2, IDO1, IL-4Rα, NOS2, and IL-10, typical MDSC-associated suppressive factors. The addition of PGE2 to GM-CSF/IL-4–supplemented monocyte cultures is sufficient to induce the MDSC phenotype and cytotoxic T lymphocyte (CTL)–suppressive function. In accordance with the key role of PGE2 in the physiologic induction of human MDSCs, the frequencies of CD11b+CD33+ MDSCs in ovarian cancer are closely correlated with local PGE2 production, whereas the cancer-promoted induction of MDSCs is strictly COX2 dependent. The disruption of COX2-PGE2 feedback using COX2 inhibitors or EP2 and EP4 antagonists suppresses the production of MDSC-associated suppressive factors and the CTL-inhibitory function of fully developed MDSCs from cancer patients. The central role of COX2-PGE2 feedback in the induction and persistence of MDSCs highlights the potential for its manipulation to enhance or suppress immune responses in cancer, autoimmunity, or transplantation.
Introduction
Dendritic cells (DCs) are key initiators and regulators of immune responses.1-3 Whereas the suppression of endogenous DC function has been shown to contribute to cancer progression, therapeutic targeting of DCs to suppress their function has been shown to be beneficial in mouse models of autoimmunity or transplantation.4
In contrast to DCs, myeloid-derived suppressor cells (MDSCs) suppress the ability of CD8+ T cells to mediate effective responses against cancer cells, but can be beneficial in controlling autoimmune phenomena or transplantation rejection.5-7 MDSCs express CD34, common myeloid marker CD33, macrophage/DC marker CD11b, and IL-4Rα (CD124), but lack expression of the lineage (Lin) markers of DCs and other mature myeloid cells.7,8 Human MDSCs are defined as CD33+Lin−HLA-DR−/low or CD33+CD14−HLA-DR−, with recent studies demonstrating a CD14+CD11b+HLA-DRlow phenotype of monocytic MDSCs in melanoma,9 prostate cancer,10 gastrointestinal malignancies,11 hepatocellular carcinoma,12,13 and glioblastoma,14 in addition to a CD15+ population of neutrophil-related immature MDSCs of similar biologic activity present in the peripheral blood.7 MDSCs express high levels of immunosuppressive factors such as indoleamine dioxygenase (IDO),15,16 IL-10,8 arginase,17,18 inducible nitric oxide synthase (NOS2),18 nitric oxide, and reactive oxygen species,19 and use these molecules to suppress T-cell responses,20,21 whereas their induction of natural killer cell anergy and reduced cytotoxicity is arginase independent12 but depends on TGFβ1.22 In addition, PD-L1/B7-H1, which is induced on MDSCs in the tumor microenvironment,23,24 suppresses antigen-specific immunity by activating regulatory T cells23 and reduces tumor clearance via enhanced T-cell IL-10 expression and reduced IFN-γ production.24
Molecular pathways involved in negative regulation of DC function remain largely unknown; however, they may involve the induction of the myeloid cell–expressed inhibitory immunoglobulin-like transcript receptors ILT-3 and ILT-4, which negatively regulate the activation of DCs, promoting T-cell tolerance.25,26
The development of functional MDSCs requires the inhibition of immunostimulatory APC development and the concomitant induction of suppressive functions.5 Such factors as GM-CSF, IL-6, or VEGF promote the expansion of immature myeloid cells (iMCs).20,27-29 An additional signal is required for the up-regulation of MDSC-associated immunosuppressive factors and for the establishment of their immunosuppressive function. Paradoxically, this signal can be delivered by the inflammatory molecules with nominally opposing functions within the immune system, such as IL-1β, IFNγ, prostaglandin E2 (PGE2), or TLR ligands.5
The absence of defined minimal requirements for MDSC development and the apparently multifactorial mode of induction of functional MDSCs raises obvious obstacles to the development of effective measures to suppress or promote MDSC development for therapeutic purposes.
PGE2 is a proinflammatory molecule produced by cancer cells, stroma, and infiltrating myeloid cells30 and by signaling via 4 G-protein–coupled receptors (EP1-EP4), of which EP2 and EP4 have also been shown to be involved in the elevation of cAMP.31 PGE2 can promote the final maturation of the already developed DCs, increasing their stimulatory function.32,33 However, the presence of PGE2 at early stages of DC development suppresses the differentiation of human monocytes into functional Th1-inducing CD1a+ DCs.34 PGE2 has also been shown to enhance the number of MDSCs in mouse models35-37 and the expression of arginase 1 in human MDSCs.17 Despite their diverse character and functions, numerous other factors implicated in MDSC development share the ability to induce cyclooxygenase 2 (COX2) expression and PGE2 production.38-40 Based on these considerations, we hypothesized that COX2/PGE2 expression represents the critical minimal requirement needed for the redirecting of DC development toward functionally stable MDSCs.
Methods
Media and reagents
Cells were cultured in IMDM (Invitrogen) supplemented with 10% FCS (Gemini). The PGE2 synthesis inhibitors celecoxib (BioVision) and NS398 (Sigma-Aldrich) were used at concentrations of 20μM unless stated otherwise, with similar results. Nor-NOHA and L-NMMA (Cayman Chemical) were used at 200μM; IDO inhibitor (Sigma-Aldrich) and neutralizing α-IL-10 mAb (R&D Systems) were used at 1.0 μg/mL; AH6809 (EP2/1 antagonist: EP2 antagonist known to be also a weak inhibitor of EP1), AH23848 (EP4 antagonist), and L798106 (EP3 antagonist) were all purchased from Sigma-Aldrich and used at 20μM. Sulprostone and butaprost, respective EP3/1 and EP2 agonists, were obtained from Sigma-Aldrich and used at 10μM concentrations. The EP4 agonist CAY10598 was obtained from Cayman Chemical and used at a 10nM concentration. The concentrations used did not have any significant impact on the viability of cultured cells, as determined by the live cell counts.
Generation of DCs, cancer-induced MDSCs, and PGE2-induced MDSCs
PBMCs were obtained from the blood of healthy donors using lymphocyte separation medium (Cellgro; Mediatech). Monocytes were isolated by positive magnetic selection using the EasySep CD14+ isolation kit (StemCell Technologies). Monocytes were cultured for 6 days in 24-well plates (BD Biosciences) at 5 × 105 cells per well in rhuGM-CSF and IL-4 (both 1000 U/mL; gifts from Schering Plough, Kenilworth, NJ) with or without PGE2 (PGE2d0; 10−6 M; Sigma-Aldrich), butaprost (10μM), sulprostone (10μM), CAY10598 (10nM), COX2-activating factors (ie, lipopolysaccharide [LPS] 20 ng/mL, IL-1β 25 ng/mL, or IFN-γ 1000 U/mL) or supplemented with cancer-infiltrating primary cell conditioned medium (CM; 1:1 ratio; respectively: PGE2-induced MDSCs and cancer-induced MDSCs; see experimental design in supplemental Figure 3, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
To demonstrate the time course of MDSC induction, PGE2 (10−6M) or cancer-infiltrating primary cell CM was added from day 0 (PGE2d0, OvCad0) or from day 6 (PGE2d6, OvCad6) and the cultures were extended until day 8.
Isolation of peripheral blood naive CD8+ T cells and CD3/CD28 in vitro effector generation
Naive CD8+CD45RA+CD45RO− T cells were isolated from PBMCs by negative selection using the naive CD8+ T-cell enrichment cocktail (StemCell Technologies), resulting in a uniform population of CD8+CD45RA+CD45RO− cells. CD8+ T cells were stimulated with CD3/CD28 Dynabeads (5 μL/mL; Invitrogen) in the presence or absence of the isolated MDSCs from cancer patients, total cancer ascites cells, cancer-induced MDSCs, PGE2d0/PGE2d6–induced MDSCs, or control DCs. CFSE staining of CD8+ T cells (Invitrogen) was performed according to the manufacturer's instructions. On days 4-5, expanded CD8+ T cells were analyzed for the expression of granzyme B expression and proliferation. Whereas in the preliminary experiments, we tested the impact of MDSCs on CTL development at MDSC:T-cell ratios of 1:1, 1:2, 1:4, and 1:8, in the subsequent standard experiments, we used a 1:4 ratio, which was determined to be optimal.
Isolation of cancer-infiltrating primary cells and MDSCs
After obtaining written informed consent in accordance with the Declaration of Helsinki, human ovarian cancer (OvCa) ascites were obtained intraoperatively from previously untreated patients with advanced epithelial OvCa in stage III or IV. The nature and possible consequences of the studies were explained. All specimens were provided under the protocols approved by the University of Pittsburgh Institutional Review Board (IRB0406147). The median age of patients was 56 (range 39-69). Twelve patients were white and 1 was African American. The majority of patients were International Federation of Gynecology and Obstetrics (FIGO) stage IIIC; one patient was stage IIA and one stage IIIA. Tumor histology was serous in 9 patients (69.2%), clear cell in 2 patients (15.4%), mixed histology in 1 patient (7.7%), and mucinous (7.7%) in 1 patient (see supplemental Table 1 for detailed information for each patient).
OvCa-infiltrating primary cells were harvested by centrifugation. CD11b+ (ie, MDSCs) were obtained after centrifugation of OvCa ascites, followed by RBC lysis and positive magnetic selection of CD11b+ cells (CD11b EasySep Isolation kit; StemCell Technologies). The isolated cells were > 95% CD11b+ pure. Control CD11b+ cells were isolated from buffy coats using the same method.
Flow cytometry
Two- and 3-color cell-surface and intracellular immunostaining analysis was performed using a Beckman Coulter Epics XL or an Accuri flow cytometer. DCs, cancer-induced cells, PGE2-induced cells, cancer-infiltrating primary cells, and cancer-infiltrating MDSCs were stained with the Abs DC-SIGN-FITC (Santa Cruz Biotechnology) and CD11b-FITC, CD14-PE, CD33-APC, CD34-Cy5, CD1a-PE, HLA-DR-PE, ILT2-PE, ILT3-FITC, ILT4-FITC, PDL1-PE/Cy7, and PDL2-APC (BD Biosciences and eBioscience). Rat IgG2α-PE, IgG1-FITC, IgG1-APC, IgG1-PE/Cy7 and IgG1-Cy5 isotype controls, and rat IgG2α-FITC isotype controls were from BD Pharmingen. IDO was labeled with α-IDO mAb (clone 10.1; Millipore) and anti-mouse Alexa Fluor 555–labeled secondary Ab. E prostanoid receptors EP1-4 were labeled with polyclonal rabbit α-EP1 and α-EP3 (Cayman Chemicals); anti–rabbit Alexa Fluor 488–labeled secondary Ab and α-EP2-PE (Cayman Chemicals); and α-EP4-PE (Abcam). To evaluate the specificity of COX2 induction, double staining for both COX1 and COX2 was performed using the α-COX-1–FITC/α-COX–2-PE staining kit (BD Biosciences) according to the manufacturer's protocol.
ELISA
ELISA analysis of IL-10 (R&D Systems) secretion by CD11b+ cells isolated from cancer ascites or cancer ascites CM–induced MDSCs was performed at 48 hours. The production of PGE2 was analyzed with a competitive parameter immunoassay according to the manufacturer's protocol (R&D Systems).
TaqMan analysis of mRNA expression
Four- to 6-hour mRNA expression for the cancer-, PGE2-, butaprost-, sulprostone-, CAY10598-, and COX2-activating factor–inducible phenotype was analyzed in CD11b+ cells (in the presence or absence of celecoxib) freshly isolated from cancer patients or after overnight incubation of the cancer ascites–isolated CD11b+ cells in the presence or absence of the COX2 inhibitor celecoxib or selective antagonists of PGE2 receptors. Persistence of the cancer- and PGE2-inducible phenotype was analyzed on day 6 of generation of cancer- and PGE2-induced MDSCs. TaqMan analysis was performed on the StepOnePlus system (Applied Biosystems). The expression of each gene was normalized to HPRT1 and is expressed as the fold increase (2−ΔCT), where ΔCT = CT(Target gene) − CT(HPRT1).
Statistical analysis
All data were evaluated using Prism Version 5 software (GraphPad) and analyzed using the Student t test (2 tailed), with P < .05 considered significant. A linear correlation between 2 continuous variables was tested with the R2 coefficient of determination. When indicated, the data from multiple different patients and control donors are expressed as means and SD from n donors (see the n values in the figure legends). The data identified as representative experiments were obtained from triplicate cultures with cells from individual donors. Each independent experiment was reproduced at least 3 times.
Results
Inhibition of DC differentiation by PGE2, proinflammatory cytokines, and TLR ligands is associated with the induction of endogenous COX2 and MDSC-associated suppressive factors
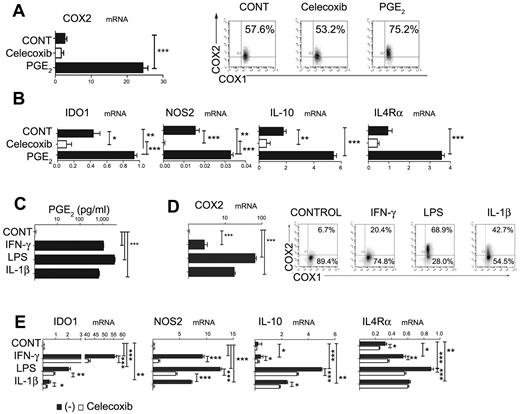
The differentiation of monocytes into functional CD1a+ DCs can be prevented by their exposure to PGE2 at early stages of DC development.34 Unexpectedly, we observed that, whereas monocytes constitutively expressed COX1, the PGE2-exposed monocytes also expressed endogenous COX2 (Figure 1A), indicating the ability of PGE2 to initiate COX2-mediated positive feedback in differentiating DC precursors. Furthermore, exposure to PGE2 also induced the expression of IDO1, NOS2, IL-10, or IL-4Rα, the factors typically associated with the differentiation of MDSCs (Figure 1B),
Inhibition of DC differentiation by PGE2, proinflammatory cytokines, and TLR ligands is associated with the induction of endogenous COX2- and MDSC-associated suppressive factors. (A) Expression of COX2 mRNA (panel A left) and protein (panel A right) levels is induced by synthetic PGE2, initiating a positive feedback loop in iMCs. Regulation of COX1 and COX2 expression by synthetic PGE2 was analyzed after 6-10 hours. (B) Induction of immunosuppressive factors IL-10, NOS2, IDO1, and IL-4Rα by synthetic PGE2. (C-D) Induction of PGE2 secretion (C) and COX2 mRNA (D left) and protein (D right) by the COX2 activators LPS (TLR4 ligand), IL-1β, and IFN-γ. (E) Celecoxib suppresses the induction of immunosuppressive factors (IDO1, IL-10, NOS2, and IL-4Rα) by COX2 activators. All data (panels A-E) were confirmed in at least 3 independent experiments. Histograms present data from a single representative experiment with different donors as mean ± SD. *P < .05; **P < .01; and ***P < .001.
Inhibition of DC differentiation by PGE2, proinflammatory cytokines, and TLR ligands is associated with the induction of endogenous COX2- and MDSC-associated suppressive factors. (A) Expression of COX2 mRNA (panel A left) and protein (panel A right) levels is induced by synthetic PGE2, initiating a positive feedback loop in iMCs. Regulation of COX1 and COX2 expression by synthetic PGE2 was analyzed after 6-10 hours. (B) Induction of immunosuppressive factors IL-10, NOS2, IDO1, and IL-4Rα by synthetic PGE2. (C-D) Induction of PGE2 secretion (C) and COX2 mRNA (D left) and protein (D right) by the COX2 activators LPS (TLR4 ligand), IL-1β, and IFN-γ. (E) Celecoxib suppresses the induction of immunosuppressive factors (IDO1, IL-10, NOS2, and IL-4Rα) by COX2 activators. All data (panels A-E) were confirmed in at least 3 independent experiments. Histograms present data from a single representative experiment with different donors as mean ± SD. *P < .05; **P < .01; and ***P < .001.
Interestingly, the inhibition of CD1a+CD14− DC development by early exposure of monocytes to such diverse factors as IL-1β, LPS, or IFN-γ was also partially reversible by COX2 inhibition (supplemental Figure 1), indicating the role of PGE2 in the ability of these factors to interfere with DC development. Indeed, the exposure of short-term cultured monocytes to IL-1β, LPS, or IFNγ in all cases induced their expression of COX2 and secretion of high levels of PGE2 (Figure 1C-D), accompanied by the induction of IDO1, NOS2, IL-10, and IL-4Rα. In accordance with the role of PGE2 as a mediator of the induction of such MDSC-related factors, these effects of LPS, IL-1β, and IFN-γ were in all cases at least partially inhibited by COX2 inhibition (Figure 1E).
PGE2 redirects DC differentiation and induces CD14+CD33+CD34+ cells with the phenotype and function of the patient-isolated monocytic MDSCs
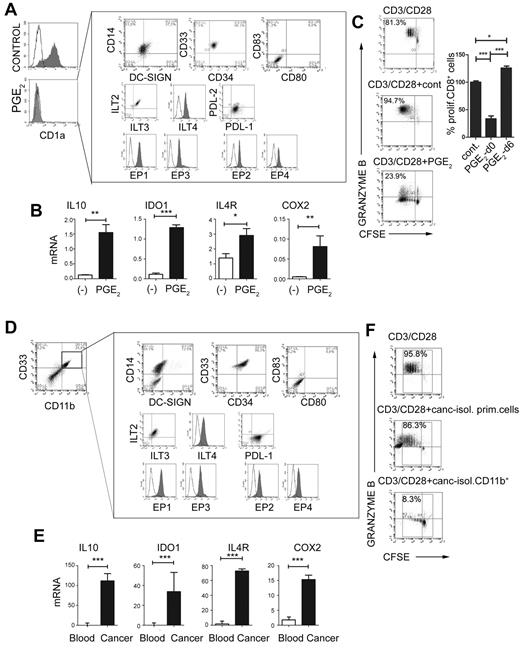
Prompted by the ability of PGE2 and other PGE2 inducers to initiate the PGE2-COX2–mediated positive feedback loop in monocytic precursors, and to induce the production of MDSC-associated suppressive factors, we investigated whether synthetic PGE2 is sufficient to redirect the differentiation of functional DCs toward monocytic MDSCs. As shown in Figure 2, the addition of PGE2 to the GM-CSF– and IL-4–supplemented cultures of differentiating monocytes, but not to the cultures of the already-developed immature CD1a+CD14− DCs (PGE2-conditioned DCs at day 6), abolished the induction of CD1a+CD14− DCs and promoted the development of CD1a−CD14+CD80−CD83− cells (Figure 2A-C). Both the naturally occurring monocytic MDSCs isolated from cancer patients and PGE2-induced cells expressed all EP1-EP4 receptors. Patient-isolated and PGE2-induced monocytic MDSCs displayed a suppressive phenotype marked by the expression of the inhibitory molecules ILT2, ILT3, ILT4, and PDL-1, which have been implicated previously in the suppressive functions of myeloid cells23,24 (Figure 2A vs D), and by the production of suppressive factors (Figure 2B and supplemental Figure 2A vs Figure 2E and supplemental Figure 2B) and suppressive functions (Figure 2C vs F).
PGE2 redirects DC differentiation and induces CD14+CD33+CD34+ cells with the phenotype and function of monocytic MDSCs isolated from cancer patients. (A) Phenotype of PGE2-induced CD1a−CD14+CD80−CD83− MDSCs expressing inhibitory molecules ILT2, ILT3, ILT4, PDL-1, but not PDL-2. PGE2-induced MDSCs express E-prostanoid receptors (labeled with α-EP1–, α-EP3–, sec.Alexa488, α-EP2–, and α-EP4–PE). (B) Expression of immunosuppressive factors IL-10, IDO1, IL-4Rα, and COX2 in PGE2-induced MDSCs (see supplemental Figure 1A for corresponding protein levels of IDO and IL-10). (C) Immunosuppressive effects of PGE2-induced MDSCs on allogeneic naive CFSE-labeled CD8+ T cells primed by CD3/CD28 and stained for granzyme B. Left panel: Percentages indicate the fraction of proliferating granzyme B+ (marker of CTL status) CD8+ cells. Right panel: Percentage of proliferating CD8+ T cells in the presence of PGE2-induced MDSCs (PGE2-d0) and PGE2-conditioned DCs (PGE2-d6). (D-F) MDSC phenotype and function of CD11b+ cells isolated from cancer ascites. (D) Characterization of cells from cancer ascites either before (left panel) or after (right panel box) isolation of CD11b+ cells. Note the high percentage of CD11b+ cells (8.9%-50.0%, mean 24.2%, n = 7) within the cancer-infiltrating primary cell population. (E) mRNA levels of IL-10, IDO1, IL-4Rα, and COX2 in CD11b+ cells isolated from cancer (see supplemental Figure 1B for corresponding protein levels of IDO and COX2) compared with CD11b+ cells isolated from blood (ascites-isolated, n = 7; control blood-isolated, n = 5). (F) Suppression of CFSE-labeled allogeneic naive CD8+ T-cell proliferation (CD3/CD28 stimulation) in the presence or absence of primary cells or CD11b+ cells isolated from cancer (ie, MDSCs isolated from cancer; n = 7). Percentages indicate the fraction of proliferating granzyme B+CD8+ cells. The gray squares represent the lymphocyte-specific gates used to exclude (CFSE-unlabeled) MDSCs. All data (panels A-F) were confirmed in at least 3 independent experiments. Histograms present data of a single representative experiment with different donors as means ± SD. *P < .05; **P < .01; and ***P < .001.
PGE2 redirects DC differentiation and induces CD14+CD33+CD34+ cells with the phenotype and function of monocytic MDSCs isolated from cancer patients. (A) Phenotype of PGE2-induced CD1a−CD14+CD80−CD83− MDSCs expressing inhibitory molecules ILT2, ILT3, ILT4, PDL-1, but not PDL-2. PGE2-induced MDSCs express E-prostanoid receptors (labeled with α-EP1–, α-EP3–, sec.Alexa488, α-EP2–, and α-EP4–PE). (B) Expression of immunosuppressive factors IL-10, IDO1, IL-4Rα, and COX2 in PGE2-induced MDSCs (see supplemental Figure 1A for corresponding protein levels of IDO and IL-10). (C) Immunosuppressive effects of PGE2-induced MDSCs on allogeneic naive CFSE-labeled CD8+ T cells primed by CD3/CD28 and stained for granzyme B. Left panel: Percentages indicate the fraction of proliferating granzyme B+ (marker of CTL status) CD8+ cells. Right panel: Percentage of proliferating CD8+ T cells in the presence of PGE2-induced MDSCs (PGE2-d0) and PGE2-conditioned DCs (PGE2-d6). (D-F) MDSC phenotype and function of CD11b+ cells isolated from cancer ascites. (D) Characterization of cells from cancer ascites either before (left panel) or after (right panel box) isolation of CD11b+ cells. Note the high percentage of CD11b+ cells (8.9%-50.0%, mean 24.2%, n = 7) within the cancer-infiltrating primary cell population. (E) mRNA levels of IL-10, IDO1, IL-4Rα, and COX2 in CD11b+ cells isolated from cancer (see supplemental Figure 1B for corresponding protein levels of IDO and COX2) compared with CD11b+ cells isolated from blood (ascites-isolated, n = 7; control blood-isolated, n = 5). (F) Suppression of CFSE-labeled allogeneic naive CD8+ T-cell proliferation (CD3/CD28 stimulation) in the presence or absence of primary cells or CD11b+ cells isolated from cancer (ie, MDSCs isolated from cancer; n = 7). Percentages indicate the fraction of proliferating granzyme B+CD8+ cells. The gray squares represent the lymphocyte-specific gates used to exclude (CFSE-unlabeled) MDSCs. All data (panels A-F) were confirmed in at least 3 independent experiments. Histograms present data of a single representative experiment with different donors as means ± SD. *P < .05; **P < .01; and ***P < .001.
Similar to the short-term–cultured monocytes, the monocytic MDSCs developing in 6-day-long, PGE2-supplemented cultures expressed high levels of COX2 (Figure 2B), demonstrating the establishment of long-term PGE2-COX2–mediated positive feedback loop in MDSCs.
PGE2 mediates the enhanced development of MDSCs in the human cancer environment
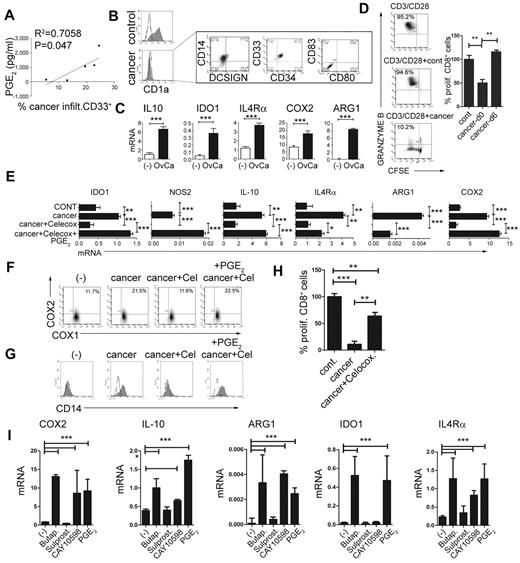
To evaluate the role of PGE2 in the physiologic and pathologic induction of MDSCs, we investigated the involvement of PGE2 in the development of MDSCs associated with the cancer microenvironment. As shown in Figure 3A, the frequencies of cancer-infiltrating CD33+ MDSCs (see Figure 2D for a complete phenotype) were closely correlated with the production of PGE2 in the ascites samples of the individual cancer patients. Similar to synthetic PGE2, cancer-infiltrating primary cells in the Transwell system or CM from cancer-ascites cells (see supplemental Figure 3 for the experimental design) redirected GM-CSF– and IL-4–driven monocyte differentiation, blocking their ability to develop into CD1a+CD14− DCs, and instead induced CD1a−CD14+ cells (Figure 3B left). Similar to the impact of synthetic PGE2 (Figure 1B and Figure 2B), the CM from cancer ascites cells induced high levels of the MDSC-associated immunosuppressive factors arginase 1, IDO1, NOS2, IL-4Rα, IL-10, and COX2 mRNA (Figure 3C) in differentiating monocytes. This effect was abrogated by the addition of COX2 inhibitor during the generation of CM from cancer ascites cells. Similar to PGE2-induced MDSCs, their cancer-induced counterparts, but not short-term cancer–conditioned DCs (OvCa-d6), potently suppressed CD8+ T-cell proliferation and their acquisition of granzyme B–containing cytolytic granules (Figure 3D).
PGE2 mediates the enhanced development of MDSCs in human cancer environment. (A) Correlation between the PGE2 production and the frequencies of cancer-infiltrating CD11b+CD33+ cells from different patients. The percentage of cancer-infiltrating CD11b+CD33+ cells was determined by flow cytometry (n = 5 patients). The regression line and corresponding R2 value are shown. (B) MDSC phenotype induced in GM-CSF+IL-4–cultured monocytes by membrane-permeable soluble factor(s) produced by cancer-infiltrating cells. Similar data were obtained using the CM from cancer-infiltrating cells and in a Transwell system (see supplemental Figure 3 for experimental design). Left panel: Suppression of DC differentiation by CM from cancer-infiltrating cells (manifested by loss of the DC marker CD1a). Right panel: Induction of the CD1a−CD14+DCSIGN−CD80−CD83− MDSC phenotype. (C) mRNA levels of IL-10, arginase 1 (ARG1), IDO1, IL-4Rα, and COX2 in cancer-induced MDSCs. (D) Suppressed CD3/CD28–induced proliferation of granzyme B+ CTL (percentages) in the presence of cancer-induced MDSCs. Left panel: Percentages indicate the fraction of proliferating granzyme B+CD8+ cells. Right panel: Percentage of proliferating CD8+ T cells in the presence of cancer-induced MDSCs (cancer d0) and cancer-conditioned DCs (cancer d6). (E) Induction of immunosuppressive factors by cancer-associated PGE2. (F) Induction of CD14+ MDSCs by CM from cancer-infiltrating cells is suppressed by COX2 inhibition and restored by synthetic PGE2. (G) Regulation of COX1 and COX2 expression by CM from cancer-infiltrating cells generated in the presence or absence of celecoxib and/or synthetic PGE2 and analyzed after 6-10 hours. (H) Immunosuppressive effects of cancer-induced MDSCs on naive CFSE-labeled CD8+ T cells primed by CD3/CD28 and stained for granzyme B. Cancer-infiltrating primary cell CM was generated in the presence or absence of the COX2 inhibitor celecoxib. (I) Induction of immunosuppressive factors by PGE2, the EP4 agonist CAY10598, the EP2 agonist butaprost, but not the EP3/1 agonist sulprostone. All data (panels A-I) were confirmed in 3-7 independent experiments. Histograms present data from a single representative experiment with different donors as mean ± SD.
PGE2 mediates the enhanced development of MDSCs in human cancer environment. (A) Correlation between the PGE2 production and the frequencies of cancer-infiltrating CD11b+CD33+ cells from different patients. The percentage of cancer-infiltrating CD11b+CD33+ cells was determined by flow cytometry (n = 5 patients). The regression line and corresponding R2 value are shown. (B) MDSC phenotype induced in GM-CSF+IL-4–cultured monocytes by membrane-permeable soluble factor(s) produced by cancer-infiltrating cells. Similar data were obtained using the CM from cancer-infiltrating cells and in a Transwell system (see supplemental Figure 3 for experimental design). Left panel: Suppression of DC differentiation by CM from cancer-infiltrating cells (manifested by loss of the DC marker CD1a). Right panel: Induction of the CD1a−CD14+DCSIGN−CD80−CD83− MDSC phenotype. (C) mRNA levels of IL-10, arginase 1 (ARG1), IDO1, IL-4Rα, and COX2 in cancer-induced MDSCs. (D) Suppressed CD3/CD28–induced proliferation of granzyme B+ CTL (percentages) in the presence of cancer-induced MDSCs. Left panel: Percentages indicate the fraction of proliferating granzyme B+CD8+ cells. Right panel: Percentage of proliferating CD8+ T cells in the presence of cancer-induced MDSCs (cancer d0) and cancer-conditioned DCs (cancer d6). (E) Induction of immunosuppressive factors by cancer-associated PGE2. (F) Induction of CD14+ MDSCs by CM from cancer-infiltrating cells is suppressed by COX2 inhibition and restored by synthetic PGE2. (G) Regulation of COX1 and COX2 expression by CM from cancer-infiltrating cells generated in the presence or absence of celecoxib and/or synthetic PGE2 and analyzed after 6-10 hours. (H) Immunosuppressive effects of cancer-induced MDSCs on naive CFSE-labeled CD8+ T cells primed by CD3/CD28 and stained for granzyme B. Cancer-infiltrating primary cell CM was generated in the presence or absence of the COX2 inhibitor celecoxib. (I) Induction of immunosuppressive factors by PGE2, the EP4 agonist CAY10598, the EP2 agonist butaprost, but not the EP3/1 agonist sulprostone. All data (panels A-I) were confirmed in 3-7 independent experiments. Histograms present data from a single representative experiment with different donors as mean ± SD.
In accordance with the critical requirement for PGE2 in the ability of the CM from cancer ascites cells to induce stable MDSCs and their function, the inhibition of COX2 completely abrogated the ability of CM from cancer ascites cells to induce COX2-PGE2 feedback (Figure 3E) and promote the development of the MDSC phenotype (Figure 3E-G) and function (Figure 3H).
Moreover, the EP2 agonist butaprost and the EP4 agonist CAY10598, but not the EP3/1 agonist sulprostone, induced high levels of the MDSC-associated immunosuppressive factors arginase 1, IDO1, NOS2, IL-4Rα, IL-10, and COX2 mRNA (Figure 3I), indicating that the induction of MDSCs by CM from cancer ascites cells involves both the EP2 and EP4 receptor signaling.
Intact PGE2-COX2 feedback loop and EP2 and EP4 signaling is critical to the stability of cancer ascites–isolated MDSCs
Prompted by the key role of a PGE2-COX2–dependent positive feedback loop in the de novo induction of functional MDSCs, we investigated whether such feedback is also needed for the stability of mature MDSCs isolated from cancer patients and their sustained immunosuppressive function.
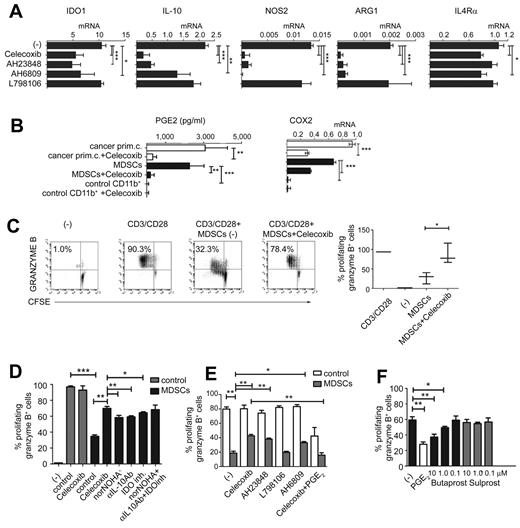
As shown in Figure 4, even a short-term exposure of the cancer ascites–isolated MDSCs (CD11b+CD14+DC-SIGN−CD33+CD34+ CD80−CD83−; see Figure 2D-E for the complete phenotype) to the COX2 inhibitor celecoxib profoundly reduced their production of IL-10, arginase 1, and NOS2, whereas the reduction of IDO1 (mRNA and protein levels) and IL-4Rα was less pronounced but also significant (Figure 4A and supplemental Figure 1B), indicating that the intact COX2-PGE2 axis is essential for the functional stability of even completely developed MDSCs. Similarly, the EP4 antagonist AH23848 and the EP2/1 antagonist AH6809, but not the EP3 receptor antagonist L798106, strongly suppressed the expression of immunosuppressive factors IL-10, arginase 1, and NOS2 (Figure 4A).
Functional stability of MDSCs isolated from cancer patients requires continuous PGE2-COX2 feedback involving EP2 and EP4 signaling. (A) Expression of immunosuppressive factors in cancer-isolated CD11b+ cells pretreated (24 hours) or not with celecoxib, the EP4 antagonist AH23848, the EP2/1 antagonist AH6809, and the EP3 antagonist L798106. (B) PGE2 production and COX2 expression in primary cells (white bars) and CD11b+ cells (black bars) isolated from cancer, compared with control CD11b+ cells isolated from blood treated or not with celecoxib. Measurements were performed in triplicates. (C) Celecoxib pretreatment of MDSCs isolated from cancer abolishes their suppressive impact on CD3/CD28–activated naive CD8+ T cells. (D) Inhibition of COX2, arginase-1, IL-10, or IDO1 counteracts the suppressive functions of MDSCs isolated from cancer (n = 3). (E) Celecoxib, the EP4 antagonist AH23848, and the EP2/1 antagonist AH6809, but not the EP3 antagonist L798106, similarly reverse the suppressive functions of MDSCs. The addition of synthetic PGE2 to celecoxib-pretreated MDSCs isolated from cancer restores immunosuppressive functions (n = 3). Neither celecoxib nor the EP antagonists showed any cytotoxic effects at the concentrations used. (F) CD3/CD28 activation of CD8+ T cells (absence of MDSCs) ± 1μM PGE2 or different concentrations of the commercially available agonists butaprost (EP2) or sulprostone (EP3/1 agonist, negative control). All data (panels A-F) were confirmed in at least 3 independent experiments. Histograms present data from a single representative experiment with different donors as means ± SD.
Functional stability of MDSCs isolated from cancer patients requires continuous PGE2-COX2 feedback involving EP2 and EP4 signaling. (A) Expression of immunosuppressive factors in cancer-isolated CD11b+ cells pretreated (24 hours) or not with celecoxib, the EP4 antagonist AH23848, the EP2/1 antagonist AH6809, and the EP3 antagonist L798106. (B) PGE2 production and COX2 expression in primary cells (white bars) and CD11b+ cells (black bars) isolated from cancer, compared with control CD11b+ cells isolated from blood treated or not with celecoxib. Measurements were performed in triplicates. (C) Celecoxib pretreatment of MDSCs isolated from cancer abolishes their suppressive impact on CD3/CD28–activated naive CD8+ T cells. (D) Inhibition of COX2, arginase-1, IL-10, or IDO1 counteracts the suppressive functions of MDSCs isolated from cancer (n = 3). (E) Celecoxib, the EP4 antagonist AH23848, and the EP2/1 antagonist AH6809, but not the EP3 antagonist L798106, similarly reverse the suppressive functions of MDSCs. The addition of synthetic PGE2 to celecoxib-pretreated MDSCs isolated from cancer restores immunosuppressive functions (n = 3). Neither celecoxib nor the EP antagonists showed any cytotoxic effects at the concentrations used. (F) CD3/CD28 activation of CD8+ T cells (absence of MDSCs) ± 1μM PGE2 or different concentrations of the commercially available agonists butaprost (EP2) or sulprostone (EP3/1 agonist, negative control). All data (panels A-F) were confirmed in at least 3 independent experiments. Histograms present data from a single representative experiment with different donors as means ± SD.
Whereas the MDSCs isolated from cancer patients expressed COX2 and secreted high levels of PGE2 (Figure 4B) compared with control CD11b+ cells from normal blood, their PGE2 secretion was blocked by celecoxib treatment (Figure 4B). Interestingly, although celecoxib interferes directly only with COX2 function rather than its expression, the elevated COX2 expression by total ascites cells and ascites-isolated MDSCs was significantly reduced by such short-term celecoxib treatment (Figure 4B and supplemental Figure 1B), confirming that endogenous PGE2 is important for the persistent ability of cancer-associated MDSCs to overexpress COX2 and maintain the COX2-PGE2 feedback that stabilizes MDSC function.
Celecoxib treatment also reversed the ability of ascites-isolated MDSCs to suppress the induction of CTLs (Figure 4C), being more potent than the inhibition of NOS2, arginase 1, IDO1 and IL-10 applied individually or combined (Figure 4D). The EP4 antagonist AH23848 and the EP2/1 antagonist AH6809, but not the EP3 antagonist L798106 generated results similar to celecoxib, indicating that binding of PGE2 to these 2 receptors is involved in its suppressive functions (Figure 4E). Interestingly, synthetic PGE2 and the EP2 agonist butaprost, but not the EP3/1 agonist sulprostone, directly suppressed CD8+ T-cell activation (Figure 4E-F), which is consistent with the possibility that MDSC-produced PGE2, in addition to its ability to induce other suppressive factors, can also directly suppress CTL responses; this also explains the potency of targeting this pathway in reversing MDSC function.
Our data indicate the involvement of EP2 and EP4 receptors, but not EP1 and EP3 receptors, in mediating the MDSC-promoting effects of PGE2.
Discussion
Our current data demonstrate that the establishment of a positive feedback loop between PGE2 and COX2 in differentiating monocytes is the necessary and sufficient factor in the redirecting of functional differentiation of DCs toward monocytic MDSCs. Our data also demonstrate that such a mechanism is used by the cancer microenvironment to promote local development of suppressive MDSCs, contributing to cancer-related immune dysfunction. Our observations showing that even short-term inhibition of COX2 profoundly affects the suppressive function of the established MDSCs isolated from OvCa patients further demonstrates the critical role of such feedback for the functional stability of the fully developed MDSCs, suggesting the potential for its therapeutic targeting in cancer immunotherapy.
The exposure of monocytes to PGE2 was sufficient to induce all of the other MDSC-associated immunosuppressive factors, IDO, arginase 1, IL-4Rα, NOS2, and IL-10. Moreover, PGE2 and other factors previously implicated in the induction of MDSCs all induce COX2, the key enzyme regulating PGE2 synthesis, leading to autocrine production of endogenous PGE2 in MDSC precursors and the establishment of a positive feedback loop inducing MDSCs and maintaining their functional stability. The ability of synthetic PGE2 alone to redirect the GM-CSF– and IL-4–driven differentiation of DCs toward MDSCs suggests the potential of stimulating the COX2-PGE2 pathway in the therapy of autoimmune diseases and transplantation rejection.
Local PGE2 production is correlated with frequencies of cancer-infiltrated CD11b+CD33+ myeloid cells, substantiating the physiologic (and pathologic) role of PGE2 during the development of human MDSCs, particularly in the cancer setting. Overexpression of the PGE2-producing enzyme COX2 has been detected previously in inflammatory tumor–infiltrated CD11b+ myeloid cells,41 and is associated with significant phenotypic and functional changes in myeloid cells37 ; however, the exact mechanism of the up-regulation of COX2 in tumor-infiltrated CD11b+ cells remains unclear. Recent studies suggest multiple mechanisms for COX2 induction, including hypoxia,42 TLR signaling,43 proinflammatory cytokines, or cell activation via adherence to the extracellular matrix. In the present study, we identified PGE2 as a primary tumor-associated inflammatory mediator responsible for the tumor-induced up-regulation of endogenous COX2 in tumor-infiltrated CD11b+ myeloid cells. Because overproduction of COX2 and PGE2 is a hallmark of many tumor types,44-46 the presently defined mechanism is likely applicable to the development of monocytic MDSCs in different inflammation-associated cancers,47 but the contribution of PGE2 to the development of different types of MDSC in other cancer settings needs to be determined individually. The possibility of the differential involvement of PGE2 to different aspects of MDSC function may be suggested by our observations that, whereas COX2 inhibition nearly completely suppressed MDSC expression of arginase 1, NOS, and IL-10, IDO, and IL-4Rα were suppressed to a lesser extent.
Based on the current observations, we propose that PGE2 produced by tumor cells (supplemental Figure 4) or induced by tumors instantaneously induces the additional MDSC-associated immunosuppressive factors in monocytes, as well as high expression of COX2 and de novo endogenous PGE2 production. PGE2, now produced at high levels by MDSCs, redirects DC differentiation toward MDSCs (supplemental Figure 5).
Whereas this PGE2-COX2 feedback mechanism can contribute to a vicious cycle amplifying the immune suppression within OvCa settings, the requirement for continued production of PGE2 for the functional stability of MDSCs allows for new modes of pharmacologic modulation. We observed that pharmacologic inhibition of PGE2 production not only prevents the induction of the MDSC phenotype in iMCs, but more importantly reverses the suppressive functions of the MDSCs previously formed in the cancer environment.
Our data show that the inhibition of endogenous PGE2 production using COX2-selective inhibitors reverses the suppressive effects of fully developed OvCa-isolated MDSCs. Similar results were also obtained using synthetic antagonists of the EP2/1 and EP4 receptors for PGE2.35 The ability of EP2 and EP4 agonists, but not EP3/1 agonists, to reproduce PGE2-induced effects demonstrates the key role of EP2 and EP4 in mediating the MDSC-promoting effects of PGE2 and suggests additional targets for pharmacologic targeting. Interestingly, COX2 inhibition gave superior results to IDO-1, arginase 1, IL-10, or NOS2 inhibition (Figure 4D). The reason for this particular potency of PGE2 targeting may be explained by the ability of PGE2 (and EP2 but not EP3/1 agonists) to exert a direct suppressive effect on CD8+ T cells (Figure 4F), apart from inducing the additional suppressive factors. These data highlight the mechanism of the previously reported involvement of PGE2 in MDSC differentiation17,35-37,48 and help to reconcile previous observations that the induction of MDSCs and MDSC-related factors in different models can be mediated by the COX2 pathway49 and EP436 or EP2 receptors,35 showing that these receptors have overlapping roles.
The current observations contribute to our understanding of the mechanism of myeloid cell alterations in the complex system of tumor-associated immune dysfunction and the role of PGE2 in this process. Because exposure to PGE2 proportionally affects all cell populations in a tumor-inflammatory setting, the current data suggest that even though a short-term exposure to PGE2 can induce the maturation of the already developed DCs, thus increasing their stimulatory function,32,33 the continued presence of PGE2 progressively affects the increasing proportion of differentiating DCs at early stages of their development, redirecting them toward MDSCs. In addition to the currently demonstrated central role of a PGE2-COX2 feedback mechanism in MDSC development, PGE2 is also known to suppress the production of IL-1233,34 and to prevent the development of type-1 immunity,33,34,50 all of which are essential for effective antitumor responses.
Our results explain the apparently multifactorial mechanism of MDSC induction and provide clinically feasible targets (COX2, EP2, and EP4) for counteracting MDSC-associated immune suppression in cancer. They also provide for a system to generate large numbers of MDSCs ex vivo, facilitating the development of additional MDSC-targeting strategies for the immunotherapies of cancer, autoimmunity, and other diseases.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs Michael Shurin, Shabaana Khader, Greg Lesinski, Anda Vlad, Julie Urban, and Jeff Wong for critical reading of the manuscript.
This study was supported by a grant from the National Institutes of Health (1PO1 CA132714 to P.K.) and by a Union for International Cancer Control Beginning Investigators Fellowship funded by the American Cancer Society (to N.O.).
National Institutes of Health
Authorship
Contribution: N.O. and P.K. designed the study and analyzed the data; N.O. and R.M. performed the experiments; R.P.E. and J.L. provided the human samples and participated in the manuscript preparation; and N.O. and P.K. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Pawel Kalinski, Department of Surgery, University of Pittsburgh, Hillman Cancer Center, UPCI Research Pavilion, Room 1.46, 5117 Center Ave, Pittsburgh, PA 15213-1863; e-mail: kalinskip@upmc.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal