Abstract
Abstract 1172
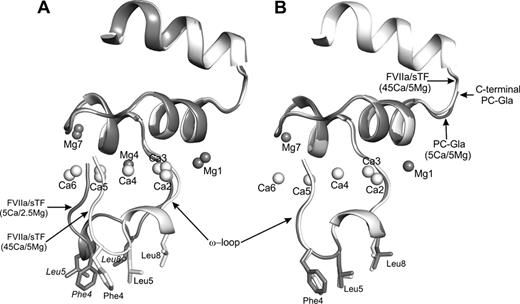
A) The superimposed structures of FVIIa Gla domains from 5Ca/2.5Mg (1.8 Å) and 45Ca/5Mg (1.7 Å) showing differences in the Ω-loops. B) The superimposed structures of Gla domain in FVIIa (45Ca/5Mg) and in 5Ca/5Mg PC-EPCR (1.6 Å) showing similar Ω-loop conformations.
A) The superimposed structures of FVIIa Gla domains from 5Ca/2.5Mg (1.8 Å) and 45Ca/5Mg (1.7 Å) showing differences in the Ω-loops. B) The superimposed structures of Gla domain in FVIIa (45Ca/5Mg) and in 5Ca/5Mg PC-EPCR (1.6 Å) showing similar Ω-loop conformations.
All seven metal sites in the structure of PC-Gla complexed to endothelial protein C receptor (EPCR) had been refined with Ca2+ despite the presence of 5 mM Ca2+ and 5 mM Mg2+ in the crystallization buffer (Oganesyan et al., J Biol Chem, 277, 24851–24854, 2002). Based on our findings with VIIa/sTF, we revisited the PC-Gla/EPCR structure. We determined that positions 1 and 7 are Mg2+ sites while 2, 3, 4, 5 and 6 are Ca2+ sites in the PC-Gla/EPCR structure (Fig. 1B). This contrasts to four Ca2+ and three Mg2+ bound to VIIa/TF at 5Ca/2.5Mg. Since five Ca2+ and two Mg2+ were seen in VIIa/sTF at 45Ca/5Mg, it would appear that position 4 can accommodate either Ca2+ or Mg2+ depending upon conditions. We propose that at near physiologic Ca2+ and Mg2+, ligation of the Gla domain with EPCR and possibly phospholipid (PL) membranes facilitates substitution of the Mg2+ bound at the position four with Ca2+. This metal switch may be essential to achieve a favorable conformation of the Gla domain Ω-loop for optimal ligand of PL binding.
Mg2+ also enhanced the Ca2+-dependent interaction of FVIIa or activated protein C (APC) to PL assessed by surface plasmon resonance. Binding of FVIIa and APC each was saturated by the physiological concentration of Ca2+ (1.1 mM) in the presence of physiological Mg2+ (0.6 mM). In contrast, only half-saturable binding was observed at the physiological concentration of Ca2+ in the absence of Mg2+. Further, 0.6 mM Mg2+ potentiated (∼2.5-fold) the PL-dependent activation of FX by FVIIa/TF at 1.1 mM Ca2+. Similarly, Mg2+ potentiated (∼3-fold) the activation of FX by FVIIa/TF assembled on the endotoxin-stimulated monocyte surface. PL-dependent inactivation of FVa by APC was also enhanced ∼3-fold by 0.6 mM Mg2+ at 1.1 mM Ca2+. At saturating Ca2+ (5 mM), the activation of FX by FVIIa/TF or the inactivation of FVa by APC was similar to that with 1.1 mM Ca2+/0.6 mM Mg2+. Thus Mg2+ at physiologic concentrations augments PL- or natural membrane-dependent coagulation and anticoagulation at the plasma concentration of Ca2+.
We propose that vitamin K-dependent clotting and anti-clotting proteins circulate in blood with four Ca2+ ions bound. The remaining three (or more in FIX and FX) divalent metal binding sites in each Gla domain are occupied by Mg2+. The conformation of the Ω-loop in circulating vitamin K- dependent proteins is not favorable for binding to PL but it is achieved by switching Mg2+ at position four to Ca2+. Thus, the metal ion at position four regulates PL-dependent coagulation and anticoagulation reactions.
No relevant conflicts of interest to declare.
KV and SA contributed equally to this work.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal