Abstract
Abstract 1989
High-dose therapy (HDT) and ASCT is the standard of care in a variety of hematologic malignancies. Whereas for some indications a survival advantage for HDT and ASCT has been demonstrated, a benefit only in terms of better progression-free survival has been shown for CLL. Because of this the quality of life (QoL) deserves particular attention. QoL assessment was a major focus of a randomized controlled EBMT-Intergroup trial on the value of HDT compared to observation in first or second remission of CLL (Michallet, Blood, 2011).
222 patients were enrolled into the study and allocated to either ASCT or observation. In the transplant arm, 72% received HDT and ASCT (for those median time from randomization to transplant was 3.01 months); in the observation arm 9% received ASCT. QoL was assessed with the EORTC QLQ C30 version 3.0, a questionnaire that has to be filled in by the patients. The answers to the questions yielded 15 scores, each on a scale from 0 to 100. The scores represent 15 domains: global health status/QoL, 5 functional scales (100 representing perfect health) and 9 symptom scales (0 representing no complaints). QoL forms had to be completed at randomization and at months 4, 8, 12, and 24. Data on 56%, 53%, 54%, 61%, and 50% of the baseline patients are available for the respective periods. Missing forms were not systematically related to baseline variables or relapse. The numbers of drop out due to death at 2 years were 5 patients in the HDT arm and 4 patients in the control arm.
All QoL outcomes were analyzed with mixed models according to the intent to treat principle. Time (as factor), age, gender, treatment arm and the interaction of time and treatment arm were modelled as fixed effects, whereas individual random effects were added for the intercept.
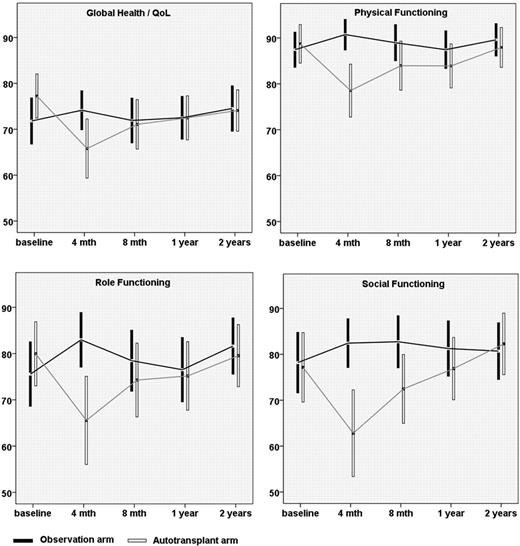
The mean values for global health status/QoL, physical functioning, role functioning and social functioning over time for the transplant and the observation group are shown in Figure 1. Global health status/QoL at 4 months (estimated effect from the multivariate model −7.15, p=0.034) was significantly inferior in the transplant cohort compared to the control group. At 8 months the estimated effect of HDT on global health status/QoL was −3.06 (p=0.36). This difference further diminished over the first year (estimate at 1 year −0.53, p=0.87). QoL did not decrease independently from the treatment during the first 2 years. The same global pattern of change over time was observed for physical functioning, role functioning and social functioning; however, the treatment impact was still significant at 8 months for physical functioning (-6.58; p=0.025) and social functioning (-11.18; p=0.014). No significant covariate effects could be delineated for either of these scales apart from age having a beneficial effect on social functioning.
Quality of life is affected multi-dimensionally in the first year after high-dose therapy and autologous stem cell support. The negative impact of HDT on QoL has disappeared after two years. Patients should be informed that HDT followed by ASCT impairs quality of life in the first year after transplantation.
Quality of life scores by treatment arm over time (means and 95%-confidence intervals).
Quality of life scores by treatment arm over time (means and 95%-confidence intervals).
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal