Abstract
Abstract 2034
Despite the survival improvements with the advent of hypomethylating treatment (HMT), stem cell transplantation (SCT) remains the only curative treatment strategy in patients with myelodysplastic syndrome (MDS). Recent reports suggest that HMT could be a feasible bridge to SCT, thereby stabilizing the natural course of the disease. In the current study, we analyzed the influences of treatment response of HMT on the transplant outcome to clarify the optimal time point to proceed to SCT during HMT.
We retrospectively analyzed 56 consecutive patients at a median 49 (18–70) years who received grafts from 23 sibling, 24 unrelated, or 9 alternative donors following a median 4 (1–13) cycles of HMT for MDS. Response assessment followed the standard criteria of IWG 2006 but two criteria were applied for disease progression (DP), for which blast counts at the start or of HMT (BL-start) or maximal response (BL-max) were used as reference blast level. Nineteen patients received SCT in continuous response (COR), which included 2 CR, 15 mCR with or without HI, and 2 HI. For remaining 37 patients, 21 were in stable disease (SD-start) and 16 in DP (DP-start) when BL-start was used whereas SD-start was reclassified to DP in 6 patients when BL-max was applied, who were designated as loss of response (LOR). DP included 12 AML, of whom 5 were in mCR at SCT.
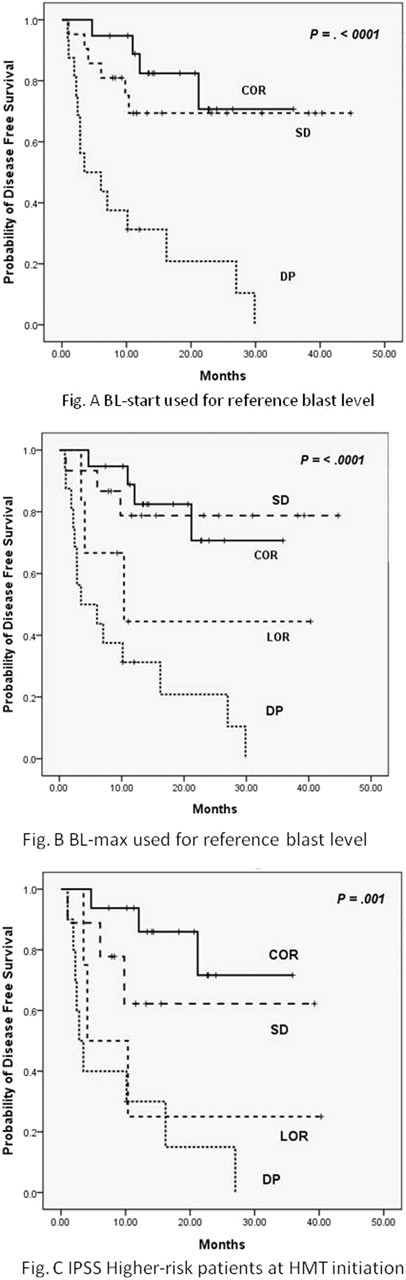
Successful engraftment was achieved in all cases, and the cumulative incidence of acute GVHD (°ÃII) at day 100 and chronic GHVD at 2 years among evaluable patients were 32.1% and 66.0%, respectively. After a median follow-up of 18.3 months for survivors, overall survival (OS), disease free survival (DFS), cumulative incidence of relapse (CIR) and treatment related mortality (CITRM) at 2 years were 60.8%, 55.6%, 32.2, and 22.2%, respectively. Although statistically significant differences in survival were demonstrated whether BL-start or BL-max was used (Fig. A & B), the latter response model was better predictive and used for following analysis. When patients were dichotomized into COR/SD-max versus LOR/DP-start, the latter group was a significantly poor predictor for 2-year OS (77.1% versus 34.8%, p=<.0001), DFS (74.5% versus 26.0%, p=<.0001) and CIR (9.1% vs 64.5%, p=<.0001) while CITRM was non-significant (16.3% versus 26.8%, p=.270). Multivariate analysis by adjusting for BM blast counts, cytogenetic risks at SCT, CD 34+ cell dose infused, hematopoietic cell transplantation specific comorbidity index and history of AML, DPmax and poor cytogenetic risk were powerful factors predicting worse DFS: DPmax, HR=4.09, p=.009,; HR=3.65, p=.004). This effect of response remained significant when analysis was confined in cohort excluding the patients with history of AML. Unexpected predictive power of response in the comparison of blast count at SCT was strengthened by the significant differences in DFS between COR/SD-max and LOR/DP-start in patients having the same blast level (5–19%). When influences of response were analyzed according to the IPSS risk group at HMT, COR showed better DFS compared to SD-max, LOR, or PD-start in INT-2/high group (Fig.C).
This analysis demonstrates response to HMT and karyotype are the significant predictors in allogeneic SCT with pre-transplant HMT for MDS rather than the disease status before HMT or BM blast count at the time of SCT. Our data also suggests that carrying out SCT in MDS should be performed before LOR as well as PD and that DP is not just a progressed disease phase exerted by increased BM blast count but a condition led by evolved MDS clones with alternated characteristic features affected by HMT. This current study demonstrates the significance of response to HMT on transplant outcomes and therefore necessitates continued studies to further illustrate the significance.
Kim:Janssen: Family, Honoraria, Research Funding; Celgene: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal